Abstract
Highly regenerative adult tissues are supported by rare populations of stem cells that continuously divide to self-renew and generate differentiated progeny. This process is tightly regulated by signals emanating from surrounding cells to fulfill the dynamic demands of the tissue. One of the hallmarks of aging is slow and aberrant tissue regeneration due to deteriorated function of stem and supporting cells. Several Drosophila regenerative tissues are unique in that they provide exact identification of stem and neighboring cells in whole-tissue anatomy. This allows for precise tracking of age-related changes as well as their targeted manipulation within the tissue. In this review we present the stem cell niche of Drosophila testis, ovary and intestine and describe the major changes and phenotypes that occur in the course of aging. Specifically we discuss changes in both intrinsic properties of stem cells and their microenvironment that contribute to the decline in tissue functionality. Understanding these mechanisms in adult Drosophila tissues will likely provide new paradigms in the field of aging.
Keywords: Aging, Adult stem cells, Niche, Drosophila.
INTRODUCTION
It is evident that a scratch along the skin heals faster in young people and that the healing capacity and the normal cell turnover gradually decline with age. The skin is one example of several regenerative tissues, which include blood, gut, mammary epithelium and testis that undergo routine cell turnover. The maintenance and repair of these adult tissues relies upon rare populations of tissue specific stem cells that replenish cells in normal homeostasis or damage [1].
While tissue stem cells share more similarities with their lineage cells than with stem cells among other tissues, all stem cells share the common ability to generate self-renewed stem cell and differentiated cells. This property is common to embryonic stem cells. However, in contrast to embryonic stem cells that completely differentiate throughout development, tissue stem cells persist in adults and maintain a balance between self-renewal and differentiation processes. An age-related decline in tissue regeneration is determined partly by changes in these mitotic stem cells and their lineage-restricted differentiated cells but also by non-dividing microenvironment niche supporting cells [2-4].
THE STEM CELL NICHE
In many tissues, the niche is an anatomical compartment, composed of unique cell populations that provide the source for growth factors and adhesion molecules that determine whether the cell will continue to self-renew, or whether itwill differentiate into a committed cell and lose its stem cell properties [1, 5, 6]. Importantly, several niches such as the neurogenic niche of neural stem cells, are found in close proximity to blood vessels where they enable circulating systemic factors to signal to stem cells in order to meet the dynamic tissue demands [7]. Therefore, the niche acts as a "control-unit" that determines stem cell proliferation rate, the fate of its daughter cells and protects the overall stem cell pool from depletion. In a given tissue, stem cells change their properties to respond to stress conditions and to match age-related changes in tissue growth. Throughout development, stem cells divide frequently to support the rapid growth. However during adulthood, growth is arrested and most stem cells divide occasionally to support tissue homeostasis or to replace damaged cells. Since the in vivo context of tissue stem cells determines their properties, the age-related decline in stem cell functionality is the sum of the overall cellular aging mechanisms of the niche, the systemic milieu and the stem cells themselves [3].
The small percentage of stem cells and the complexity of mammalian tissues present a big obstacle in the unequivocal identification of the rare stem cells and their niche. In recent years Drosophila melanogaster has been established as an excellent model to study stem cells in six distinct tissues: neuroblasts [8], hematopoietic [9], intestinal [10], malpighian tubules [11] and male and female germlines comprised of both somatic and germline stem cells [12]. In all of these tissues, the niche, stem cells and their immediate daughter cells are identified at a single cell resolution in the context of whole tissue anatomy. While neuroblasts and hematopoietic stem cells lose their stemness properties in adult flies, intestinal, male and feamale germline stem cells support gut, testis and ovaries homeostasis respectively, throughout adulthood and aging. As such, these three tissues provide attractive models to study stem cell function throughout normal aging (Fig. 1). The genetic ammenability and the abundance of available tools to specifically mark cell populations, allowing for visualization of phenotypes in the context of the endogenous stem cell niche have significantly enhanced our understanding of stem cells mechanisms that are modulated during aging. In this review we discuss cellular aging mechanisms of tissue stem cells, niche and systemic signals that were studied in Drosophila intestine, male and female germlines.
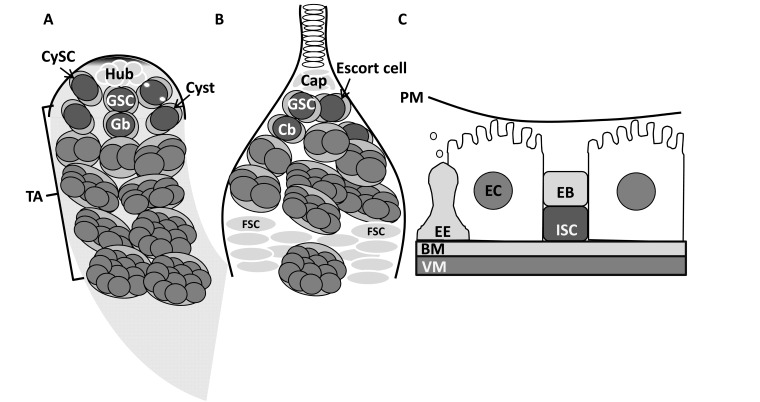
Fig. (1).
Adult regeneration models in Drosophila. A) The male germline stem cell (GSC) niche is located at the apical tip of the testis. Three representative GSCs (of ~eight) intermingle with cyst stem cells (CySCs) to surround and contact the hub. The centrosomes (depicted by white dots) of the right GSC are aligned perpendicularly to the hub. The progenitor gonialblast (Gb) cell, which is encapsulated by two cyst cells, transiently amplifies (TA) four times prior to terminal differentiation. B) The female GSC niche is located at the tip of the germarium. Anteriorly, terminal filament cells (white) and cap cells form the somatic niche. Two GSCs are bound to the cap cells. The progenitor cystoblast (Cb) undergoes four mitotic divisions. Two follicle stem cells (FSCs) are located in the middle of the germarium. C) The intestinal stem cell (ISC) of the posterior midgut differentiates into a progenitor enteroblast (EB) that further differentiates either into a big enterocyte (EC) absorptive cell or to enteroendocrine (EE) secretory cell. Visceral muscle (VM); basal lamina (BL); peritrophic membrane (PM).
THE MALE GERMLINE STEM CELL NICHE
The Drosophila testis is a transparent coiled tube in which the germ cells are organized in a clear gradient of differentiation from the apical to the basal edge where short-lived mature sperm are formed and are constantly released from the testis [13]. The male germline stem cell (GSC) niche is located at the apical tip of the testis and is comprised of three cell types: GSCs, cyst stem cells (CySCs) and hub cells (Fig. 1). GSCs coordinate signals with the niche to self-renew and to continually supply differentiated germ cells. GSCs and CySCs form a rosette-like structure around the hub, a cluster of 8-16 post-mitotic somatic cells. The hub secrets the Unpaired (Upd) ligand that locally activates the JAK-STAT pathway to promote stem cell self-renewal and niche-maintenance in the closely neighboring CySCs and GSCs [14-16]. CySCs act as a niche for GSCs and produce Hedgehog to self-renew and bone morphogenetic proteins (BMPs) to regulate GSCs [17]. As a result of GSC asymmetric division, one daughter cell remains within the niche for self-renewal, while the other, a displaced progenitor cell (gonialblast), begins the transit amplification (TA) program. In many stem cell lineages, daughter cells destined for differentiation undergo TA divisions prior to initiating terminal differentiation, thus enabling amplification of the rare and infrequently dividing stem cells. In the testis, four synchronized cycles of TA divisions with incomplete cytokinesis, generate progenitor spermatogonia [18]. The resulting sixteen germ cells spermatogonia undergo a terminal differentiation program and meiosis. Each GSC has a limited number of asymmetric divisions and a half-life-time of fourteen days [19], after which the GSC disconnects from the niche and differentiates. Spermatogonia cells also dedifferentiate to replace GSC loss [20-22]. Thus, germline maintenance relies on an intricate balance of renewal/differentiation decisions in multiple co-developing and spatially interlacing cell populations, including the hub, GSCs, CySCs and progenitor cells.
AGE-RELATED CHANGES OF THE MALE GERMLINE STEM CELL NICHE
Drosophila aging compromises testis function resulting in morphological changes of significantly smaller testis with reduced stem cells and differentiated progeny, and in physiological changes that eventually arrest sperm production [23]. One of the striking phenotypes of aging is a gradual reduction in GSC mitotic division frequency. The plane of the mitotic division of a given GSC is perpendicular to the hub, allowing gonialblast progenitor germ cell to break away from the niche and to initiate differentiation [24]. To restrict the division to this stereotypical orientation, GSCs obtained a unique centrosome checkpoint. The GSC mother centrosome is attached to the hub boundary, whereas the daughter centrosome migrates to the opposite side ([25], Fig. 1). In young adults, the GSC's centrosomes remain oriented towards the hub throughout the cell cycle. However, the number of GSCs with misoriented centrosomes, where neither centrosome is located in the GSC-hub interface, increases gradually throughout aging, reaching about 40% of total GSCs in 30 day old flies [21]. The age-related accumulation in GSCs bearing misoriented centrosomes is attributed mainly to the dedifferentiation process whereby the spermatogonia dissociate and migrate to the niche to replenish the stem cell pool [20, 26]. These GSCs with misoriented centrosomes do not enter mitosis but they can restart cell division once the centrosomes restore their perpendicular positioning. However, reorientation result in slower GSC regeneration function and is a main cause for the decline in stem cell activity and spermatogenesis during aging. It is still unknown whether a similar checkpoint regulates asymmetric divisions of stem cells in other tissues and in other organisms.
An additional cell cycle mechanism that is modulated during aging involves changes in String (Stg), the Drosophila homolog of the dual-specificity phosphatatse Cdc25 that promotes entry and progression of the cell cycle. In young adults, Stg is highly expressed in both GSCs and CySCs and rapidly decreases in differentiating progeny. Loss and gain of function analyses showed that Stg is required for stem cells maintenance and division. Throughout aging, Stg expression specifically declines in GSCs, but not in CySCs. Although constitutive expression of Stg in the germline rescues the age-related decrease in GSC division and CySC number, it promotes tumor development in testes of aged males, suggesting that reduced Stg expression contributes to the decline in spermatogonia during aging, but may have an important role in tumor-suppression [27].
Remarkably, aging compromises not only the dividing stem and progenitor cells but also the hub cells that represent a major component of the stem cell niche. Age-related changes in RNA and protein composition of the fully-committed post-mitotic hub cells significantly contribute to the decline in the number of GSCs of aged males. These changes are controlled by a post-transcriptional network that regulates maintenance and aging of the hub. Elevated levels of the heterochronic microRNA let-7 [28, 29] in the hub of aged males initiate a cascade of post-transcriptional events in the stem cell niche that result in reduced levels of the self-renewal factor Upd leading to an overall decrease in stem cells that reside within the niche [23, 30]. let-7 negatively regulates the expression of Imp, an evolutionarily conserved RNA binding protein that acts as a self-renewal factor of the hub. Elevated levels of let-7 in the hub of aged males target Imp 3’UTR and inhibit Imp protein translation. In young males, Imp binds the 3'UTR of upd and stabilizes its mRNA levels. This interaction protects upd from degradation mediated by endo-siRNAs and thus contributes to the maintenance of the niche in young adults. In older males, upd mRNA loses its Imp protection, an event which renders it susceptible to degradation that leads to reduced niche function and GSCs loss. Interestingly, ectopic expression of either Upd or Imp that cannot be targeted by let-7 rescue the age-dependent decline in the number of GSCs that reside within the niche [23, 30]. Therefore, changes in the RNA levels of post-mitotic niche cells generate an active stem cell microenvironment that supports distinct self-renewal programs at different ages.
In addition to reduction in the self-renewal program dictated by the hub, there is also a decline in the adhesion signals that emanate from the hub to retain the stem cells within the niche [23]. High levels of cell adhesion molecules are found in the junctions among the hub cells and in the interface between hub cells and the two stem cell populations that surround it, namely CySCs and GSCs. They include Fasciclin 3 (Fas3, [31]) and the Drosophila homologs of E-cadherin, (DE-cad) and neural cadherin (DN-cad, [32]). In aged males there is a slight decrease in the number of hub cells, changes in the distribution of Fas3 and reduction in the DE-Cad levels that contribute to the reduced number of stem cells resident within the niche [19, 23].
THE FEMALE GERMLINE STEM CELL NICHE
The female Drosophila has two ovaries, each composed of sixteen ovariole units that all are terminated by a germarium unit. At the tip of each germarium there is a female GSCs niche that is comprised of supporting niche somatic cap cells that emanate short-range signals to regulate GSCs self-renewal and maintenance in the niche (Fig. 1). As in males, the differentiation program of the cystoblast involves four cycles of mitotic divisions that form of a 16-cell cyst [33]. However, these are not TA divisions since only one cell eventually becomes an egg and the rest of the cells function as supporting nurse cells. The growing cytoblasts move down to the posterior edge of the germarium and either interchange connections with somatic escort cells [34] or dedifferentiate to replace GSCs [35]. The GSCs in the ovary also use DE-cad-rich adherens junctions to connect to the niche cap cells. DE-cad is expressed by both GSCs and the cap cells to enable anchorage and GSCs self-renewal [36, 37]. However, each female niche is much smaller and occupies two-three GSCs and the main self-renewal ligands dictated by the niche are the decapentaplegic (Dpp) and glass-bottomed boat (Gbb). These secreted factors activate bone morphogenetic protein (BMP) signaling in the neighboring GSCs to repress transcription of the key differentiation factor, bag-of-marbles (bam) [38]. Therefore, the stem cell identity acquires an active program to repress differentiation that is the default of the cells, unless they are located within the niche. An additional stem cell population, the follicle stem cells (FSCs, Fig. 1) is located farther away from the GSCs niche in the mid-germarium and gives rise to polar and stalk cells to form the follicle [34, 39].
AGE-RELATED CHANGES OF THE FEMALE GERMLINE STEM CELL NICHE
In aged females, BMP signaling undergoes an age-dependent decline, resulting in a small but significant reduction in the number of both GSCs and cap cells [40, 41]. In addition to the reduced number of GSCs in the niche, their proliferation capacity significantly declines during aging. This reduction together with a significant increase in the incidents of apoptotic cell death in the ovary of aged females, culminates in a progressive decline in egg production [41]. Gbb ectopic expression in the niche or ectopic expression of BMP receptor intrinsically in GSCs rescues the age-related GSCs loss and proliferation rate. Similarly to males, aging appears to reduce the levels of DE-Cad in the anchorage junctions between GSCs and cap cells [40]. Remarkably, studies of the female niche clearly show that reducing reactive oxygen species (ROS) delays the stem cell aging phenotypes. ROS, the byproducts of oxidative energy metabolism, have long been suggested to be a main damaging substance that causes cellular aging in different organisms [4]. Overexpression of an antioxidant enzyme, superoxide dismutase (Sod) that catalyzes ROS into less reactive species, either in GSCs or in niche cap cells, prolongs GSCs and cap cells maintenance in the niche and extends their proliferation rate [40].
Lastly, in Drosophila it is not completely clear whether signals from the gonad regulate whole-organism aging. In C. elegans there is a balance between longevity promoting signals from the somatic gonad and aging signals from the germline. Germline ablation extends lifespan by reducing insulin signaling and activating DAF-16, a forkhead transcription factor (Foxo) [42, 43]. In Drosophila, ectopic expression of Bam in male and female GSCs, leads to a one-way GSCs differentiation and results in Foxo activation and lifespan extension [44]. On the contrary, a failure to form primordial germ cells in grandchildless-like mutants (oskar, tudor, germ cell-less) that preserve the soma does not extant lifespan [45]. Thus, unlike the clear tradeoff between reproduction and longevity of the worm, how reproductive systems modulate aging in Drosophila remains poorly understood.
THE INTESTINAL STEM CELLS
The Drosophila gut is parallel to the human gastrointestinal system in both its structural and functional properties. It is divided into three distinct compartments: foregut, midgut and hindgut. The posterior midgut bears similarities to the small intestine and serves as the main site for food digestion and nutrient absorption [46, 47]. Intestinal stem cells (ISCs) are located at the base of the intestinal epithelium and support epithelium renewal and weekly replacement of the intestinal spent cells (Fig. 1, [48]). In addition, stressors that damage the gut are associated with an increase in stem cell division to compensate for the loss of spent cells.
Unlike GSCs that are grouped in designated locations within a stromal niche, ISCs are scattered along the basement membrane [47]. Like GSCs, ISCs respond to a variety of systemic signals and cues emanating from neighboring cells [10]. Each ISC divides asymmetrically to give rise to one self-renewed ISC and one committed progenitor Enteroblast (EB) cell that further differentiates into one of two cell types: enterocyte (EC) absorptive cell or enteroendocrine (EE) secretory cell (Fig. 1). ECs endoreplicate their genome and grow into large cells that comprise the bulk of the midgut epithelium [10]. The main signaling pathway that regulates this asymmetric division is the canonical Delta-Notch pathway. The ligand Delta determines the alternative differentiation outcome of an EB cell in a dose-dependent manner. ISCs that express high levels of the ligand Delta and presumably lead to high activation of Notch in EBs determine the fate of EC and inhibit ISCs proliferation [47, 49], whereas low Delta levels in ISCs specify EE cell fate [48].
AGE-RELATED CHANGES OF THE INTESTINAL EPITHELIUM
Aging alters adult midgut properties in several major ways including: ISC hyperproliferation, misregulated differentiation and compartmentalization defects.
In aging flies, ISCs hyperproliferate and cause aberrant lineage differentiation and accumulation of mis-differentiated daughter cells [50-53]. Experimentally, excessive ISC proliferation can be detected by BrdU incorporation that reveal DNA synthesis or by excessive staining with the cell cycle marker phospho-histone H3 since ISCs are the only mitotic cells in the midgut [50, 52]. These changes are largely caused by accumulation of oxidative stress and attributed to age-related changes in two signaling pathways: vascular endothelial growth factor (VEGF) and Jun N-terminal kinase (JNK) [50, 52, 54, 55]. PVF2, the Drosophila homologue of VEGF is expressed in ISCs and their daughter EBs and its expression increases during normal aging and upon induction of oxidative stress. Similarly, a phenotype of excess ISCs was observed upon ROS induction [52]. These results further support the connection between VEGF and ROS levels and the role of ROS as a cause of cellular aging [52]. In addition to the increased number of ISCs, aging is also characterized by accumulation of mis-differentiated cells that express ISC markers and present EC morphology [50, 51]. Similarly to PVF2, oxidative stress also increases signaling in the JNK pathway of ISCs and EBs of aged flies. Manipulation of the JNK pathway specifically within these cells was found to postpone the aging phenotypes [50, 51]. The Delta-Notch pathway is also misregulated in aged flies. In young midguts, Delta expression in ISCs rapidly decays in daughter EBs. However, high levels of both Delta and active-Notch are observed at the same cells of aged midguts. Interestingly, Notch activation in ISCs and progenitor EBs restricts stress or JNK-induced ISCs proliferation, suggesting that age-related increase in Notch signaling may compensate ISCs hyperproliferation [50]. Since ROS levels highly correlate with mitochondrial function, improving mitochondrial function by overexpressing the transcriptional co-activator PGC1 or by ectopically expressing the single-subunit yeast alternative NADH dehydrogenase, ndi1, delays age-related changes in the intestine and improves homeostasis function [54, 55].
The increased proliferation of ISCs during aging is largely due to an alteration of the local environment caused by bacterial infections and changes in commensal (bacteria that benefits the gut without affecting it) microflora populations. The significant increase in the bacterial load found in the lumen of the gut [56], suggests an age-related decline in the ability of ECs to manage the microflora. ECs use two major pathways to manage pathogens and commensal flora: ROS generation by dual oxidase (Duox), which initiates an oxidative burst response [57] and expression of antimicrobial peptides (AMPs) by the immune deficiency (IMD/Relish) pathway [58]. The Relish pathway is compromised during aging due to an increase in an upstream regulator Foxo in ECs, resulting in imbalanced levels of commensal bacteria and dysplasia [59]. This microenvironment triggers an inflammatory response by activating Duox and ROS production, thus leading to ISC hyperproliferation.
ISC differentiation is characterized by a progressive relocation of the daughter EB cell from the basal membrane to the lumen of the midgut [51]. This respective apical-basal location of ISCs and EBs that is clearly observed in young midguts is disrupted in aged midguts and the abnormal cells that express ISCs markers and EC morphology accumulate at the basal membrane. These clusters of abnormal cells also appear upon activation of the JNK signaling [52].
Lastly, the three major compartments of the gut can be further subdivide into regional compartmentalization according to distinct morphogical, histological, and genetic properties [46, 60]. These subdivisions are maintained in young adults and remain stable in the course of different diets. However, in aged organisms 90% of intestinal gene expression patterns are altered, indicating that the regionalization of the intestine declines with age. Similarly to the aging phenotype, knocking-down transcription factors that regulate gut compartmentalization reduces homeostasis and increases ISCs proliferation [46].
Studies of ISC regeneration of the midgut during aging also provided the interesting observation that genetic manipulations that improve midgut homeostasis, such as reduced JNK signaling or improved mitochondrial function or improved host-commensal interactions all extend the organism lifespan [51, 54, 55, 59]. These findings indicate that midgut homeostasis and chronological age are mutually affecting each other.
In summary, Drosophila regenerative tissues enable thorough examination of mechanisms that regulate stem cell aging. The genetic amenability and the adequate characterization of stem and neighboring cells present tools to examine and manipulate age-related changes in subsets of cells to determine their contribution to tissue homeostasis. These studies provide detailed verifications of hypothesis-driven mechanisms that regulate aging such as accumulation of oxidative stress and stem cell hyperproliferation during aging [50, 52]. Furthermore these studies also demonstrate the contribution of previously unsuspected mechanisms that regulate declined regeneration function of stem cells in aged tissues. These include changes in the checkpoints of GSCs centrosome orientation and changes in non-coding RNAs of post-mitotic niche cells [21, 30]. Although these mechanisms partly explain the declined regenerative capacity of stem cell during aging, considerable research remains to establish the comprehensive network that regulates stem cell aging.
ACKNOWLEDGEMENTS
We thank Dr. L. Barki-Harrington and the anonymous reviewers for careful examination of the review manuscript. H. Toledano is supported by FP7-PEOPLE-2011-IIF: Marie Curie Action: “International Incoming Fellowship”.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat. Cell. Biol. 2011;13(5):506–12. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441(7097):1080–6. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 4.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12 (2):152–65. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008;9(1):11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 6.Shim J, Gururaja-Rao S, Banerjee U. Nutritional regulation of stem and progenitor cells in Drosophila. Development. 2013;140 (23):4647–56. doi: 10.1242/dev.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139(23):4297–310. doi: 10.1242/dev.080515. [DOI] [PubMed] [Google Scholar]
- 9.Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14(4):394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Edgar BA. Intestinal stem cells in the adult Drosophila midgut. Exp. Cell Res. 2011;317(19):2780–8. doi: 10.1016/j.yexcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1(2):191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316(5823):402–4. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 13.Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3(11):a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 15.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 2010;12(8):806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294(5551):2546–9. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 17.Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140(1):56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc. Natl. Acad. Sci. U S A. 2009;106(52):22311–6. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5(4):297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 20.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304(5675):1331–4. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456(7222):599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138(16):3367–76. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1(4):470–8. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301(5639):1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315(5811):518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5(2):191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inaba M, Yuan H, Yamashita YM. String (Cdc25) regulates stem cell maintenance, proliferation and aging in Drosophila testis. Development. 2011;138(23):5079–86. doi: 10.1242/dev.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambros V. MicroRNAs and developmental timing. Curr. Opin. Genet. Dev. 2011;21(4):511–7. doi: 10.1016/j.gde.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sempere LF, Dubrovsky EB, Dubrovskaya VA, Berger E M, Ambros V. The expression of the let-7 small regulatory RNA is controlled by ecdysone during metamorphosis in Drosophila melanogaster. Dev. Biol. 2002;244(1):170–9. doi: 10.1006/dbio.2002.0594. [DOI] [PubMed] [Google Scholar]
- 30.Toledano H, D'Alterio C, Czech B, Levine E, Jones DL. The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature. 2012;485(7400):605–10. doi: 10.1038/nature11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124(21):4361–71. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- 32.Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev. Biol. 2006;294(1):92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Gilboa L, Lehmann R. How different is Venus from Mars?.The genetics of germ-line stem cells in Drosophila females and males. Development. 2004;131(20):4895–905. doi: 10.1242/dev.01373. [DOI] [PubMed] [Google Scholar]
- 34.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138(11):2207–15. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428(6982):564–9. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 36.Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. U S A. 2002;99(23):14813–8. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296(5574):1855–7. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 38.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 2003;13(20):1786–91. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184(2):503–15. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1(4):458–69. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–54. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 42.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C.elegans. Nature. 1999;399(6734):362–6. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 43.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–5. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 44.Flatt T, Min KJ, D'Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U S A. 2008;105(17):6368–73. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes AI, Boone JM, Jacobson J, Partridge L, Chapman T. No extension of lifespan by ablation of germ line in Drosophila. Proc. Biol. Sci. 2006;273(1589):939–47. doi: 10.1098/rspb.2005.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, Lemaitre B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3(5):1725–38. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 48.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315(5814):988–92. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 49.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 50.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6(10):e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7(3):318–34. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YJ, Hwang MS, Park JS, Bae SK, Kim YS, Yoo M A. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochim. Biophys. Acta. 2008;1780(10):1093–100. doi: 10.1016/j.bbagen.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Hur JH, Bahadorani S, Graniel J, Koehler CL, Ulgherait M, Rera M, Jones DL, Walker DW. Increased longevity mediated by yeast NDI1 expression in Drosophila intestinal stem and progenitor cells. Aging (Albany NY) 2013;5(9):662–81. doi: 10.18632/aging.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr, Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14(5):623–34. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6(2):144–52. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat. Immunol. 2009;10(9):949–57. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 58.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23(19):2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Karpac J, Tran SL, Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156(1-2):109–22. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marianes A, Spradling AC. Physiological and stem cell compartmentalization within the Drosophila midgut. Elife. 2013;2:e00886. doi: 10.7554/eLife.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]