Abstract
Antibody-mediated blockade of the adhesion molecule very late antigen-4 (VLA-4) has been shown to ameliorate disease in human multiple sclerosis (MS) patients and experimental autoimmune encephalomyelitis (EAE) animal models. We wanted to determine whether anti-VLA-4 antibody treatment affected the function and persistence of autoreactive T cells in mice with EAE. Unexpectedly, we observed a high level of mortality in anti-VLA-4 mAb (PS/2) treated mice with actively induced EAE despite decreased disease severity. Investigation of the underlying mechanism showed that injection of PS/2 mAb in combination with pertussis toxin (PTX) resulted in anaphylaxis and mortality. Furthermore, the data showed that CD4+ T cells were required for this effect and suggested a role for IL-1β and TNF-α in the underlying pathology. The results reveal a previously not appreciated deleterious effect of anti-VLA-4 antibody treatment in combination with exposure to PTX.
Keywords: Pertussis toxin, anti-VLA-4, mice, EAE
Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune disease which predominantly affects young adult females (1,2). It is commonly believed that myelin-reactive CD4+ T cells play an important role in the disease process by infiltrating the central nervous system (CNS) and triggering inflammation and demyelination via the release of proinflammatory cytokines (3,4). EAE is a widely accepted animal disease model of MS (5). EAE can be induced by active immunization with myelin antigens or by adoptive transfer of myelin-specific T cells (3,6,7). Active induction of EAE usually requires co-injection of Pertussis toxin (PTX) to facilitate and enhance the disease.
Blockade of leukocyte trafficking into the CNS by targeting of specific adhesion molecules has been viewed as a viable strategy to prevent disease relapses and slow the progression of MS (8,9). In particularly, VLA-4, an integrin heterodimer composed of an α4 (CD49d) subunit paired with a β1 (CD29) chain has been shown to be critical for leukocyte migration into the CNS (10,11). VLA-4 expression increases after T-cell activation and it interacts with vascular cell adhesion molecule 1 (VCAM-1) on activated endothelium. VLA-4 is important for recruiting activated effector T cells into target sites, especially across the blood brain barrier (BBB) (12,13). Blockade of VLA-4 by monoclonal antibodies has been shown to ameliorate clinical disease in MS patients and in EAE models (14–17). It is known that autoreactive T cells still persist in the periphery of anti-VLA-4 mAb treated individuals, but it has remained unresolved for how long and whether their function is altered (18,19). To begin to address these issues we used the EAE model in C57BL/6 and SJL mice and treated the animals with anti-VLA-4 mAb.
Unexpectedly, we observed that anti-VLA-4 mAb treatment resulted in high mortality, as compared with control animals, despite overall decreased EAE severity. The results showed that injection of PTX in combination with the PS/2 mAb was required to induce anaphylaxis and mortality. Additionally, CD4+ T cells were required for PS/2 plus PTX induced morbidity and mortality, as both SCID and CD4+ T cell-deficient MHC class II knockout mice were protected.
Materials and Methods
Mice
Female C57BL/6 and SJL/J mice (6 – 8 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions and all animal procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at San Antonio.
EAE induction
Active EAE was induced in female C57BL/6 and SJL/J mice by subcutaneous (s.c.) injection of 200 µg MOG35–55 peptide (United Biochemical Research) or 100 µg PLP139–151 peptide (Princeton BioMolecules Corporation), respectively, in 50 µl of CFA. Mice also received intraperitoneal (i.p.) injections of 200 ng PTX on day 0 and day 1. For induction of EAE by adoptive transfer, female SJL/J mice were immunized s.c. with 100 µg of PLP139–151 in CFA. Splenocytes and draining lymph nodes (DLN) were collected from donor mice 9 days later and restimulated with 30 µg/ml of PLP139–151 peptide in complete DMEM containing 20 ng/ml of mouse recombinant IL-23 (eBioscience) for 4 days at 37°C. Recipient mice received 1.2 × 107 restimulated donor cells by i.p. injection.
EAE evaluation: Mice were monitored and graded daily for clinical signs of EAE using the following scoring system (20): 0, no abnormality; 1, limp tail; 2, moderate and hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia or premoribund state; 5, death.
Generation of monoclonal antibodies
PS/2 mAb was generated as previously described (21). In brief, hybridoma cell lines (anti-VLA-/4 integrin α4 antibody, clone PS/2; rat IgG2b isotype control antibody, clone SFR3-DR5; both from ATCC®) were cultured in serum-free medium (Ultraculture, Hyclone, Fisher Scientific) and the supernatant was filtered through a 0.22 µm filter and adjusted to pH 7.5 before passing through a protein G column (Upstate Fastflow, Millipore). Concentrated mAb was eluted at pH 2.5, and dialyzed in PBS to remove NaN3 and excessive ions. Purified mAbs were aliquoted and stored at −80°C. Endotoxin content of the mAb was determined by using a Limulus Amebocyte Lysate (LAL) assay kit (QCL-100, Cambrex) and found to be generally less than 0.0025 ng endotoxin per µg of protein.
Pertussis toxin and antibody treatment
Groups of immunized or naive mice were injected i.p. with 200 ng of PTX or PTX B-oligomer on days 0 and 1 as indicated. PS/2 (anti-VLA-4) mAb, or isotype control antibody, was injected i.p. one to three times per week as indicated, starting on day 4 or day 7 after the first PTX injection.
Serum cytokine ELISA
Blood was collected from the animals and centrifuged at 1,500 rpm for 20 min. Serum or tissue culture supernatants were stored at −80°C and analyzed as indicated. Mouse ELISA Ready-Set-Go kits (eBioscience) for TNF-α, IL-1 β and IL-6 were used to detect cytokine levels in serum or supernatants following the manufacturer's instructions. After development, plates were read at OD 450 nm to acquire data (Multi-Detection Microplate Reader, Synery HT, Bio-TEK).
Adoptive transfer of splenic CD4+ T cells
Spleens from Wt C57BL/6 mice were harvested and single-cell suspensions generated. Splenic CD4+ T cells were negatively selected using CD4+ T cell isolation kits and an AutoMacs separator system (Miltenyi Biotec) following the manufacturer’s instructions. Purified CD4+ T cells were injected i.p. into TNFα−/− recipient mice at 3–5 × 106 cells per mouse. After 48 h, recipient mice were treated with PTX followed by PS/2 mAb injections as indicated.
Results
Anti-VLA-4 mAb treatment results in anaphylaxis and high mortality in mice with actively induced EAE
Conceivably, pathogenic T lymphocytes in anti-VLA-4 mAb treated individuals could persist for extended periods of time in the immune periphery and mediate relapses upon reactivation. To begin to investigate this possibility, we induced active or passive EAE in C57BL/6 or SJL/J mice and treated the animals with anti-VLA-4 antibody (PS/2).
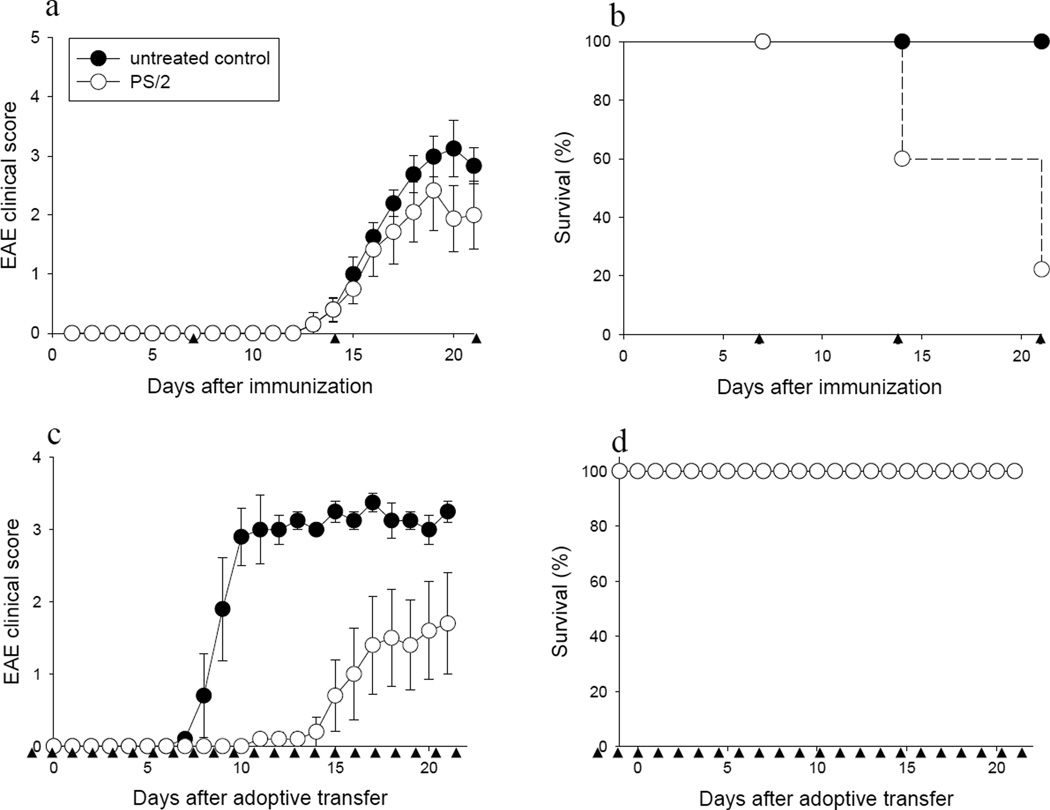
As shown in Fig. 1a, treatment of C57BL/6 mice with MOG35–55-induced EAE with PS/2 mAb ameliorated EAE symptoms, as compared with the untreated control group, consistent with previous reports (17). Similarly, EAE was ameliorated in SJL mice with PLP139-15-induced EAE following treatment with PS/2 mAb (data not shown). Unexpectedly, a high rate of mortality was consistently observed in PS/2 mAb-injected mice, as compared with control mice, despite exhibiting overall lower EAE scores (Fig. 1b). Death of the animals started to occur after the second injection of PS/2 mAb and reached 80% by the third injection. Importantly, death of the animals usually occurred within one to two hours after the last PS/2 injection, and the mice exhibited an anaphylactic-type picture characterized by a significant drop in body temperature (>5°C) and respiratory distress. Histopathological evaluation did not show significant changes, which may have been due to the short interval between the last injection and the death of the animals (not shown).
Figure 1. High mortality of mice with actively induced EAE treated with anti-VLA-4 mAb.
Active or passive EAE was induced in groups of female C57BL/6 or SJL/J mice with MOG35–55 or PLP139–151 peptide, respectively, as described in Materials and Methods. Mice were monitored and scored daily for clinical disease and survival was recorded in (a & b) C57BL/6 mice with actively induced EAE and (c & d) SJL/J mice with passive EAE induced by adoptive transfer. a & b, Mice were injected i.p. with PTX on day 0 and 1 with 200 ng PTX, and with 200 µg of PS/2 mAb starting on day 7 after immunization, once a week (●, untreated control; ○, PS/2; n = 10 each group). c & d, Adoptive transfer recipients were injected i.p. with 200 µg PS/2 mAb on day −1 before transfer, and with 100 µg of PS/2 daily thereafter (●, untreated control; ○, PS/2; n = 5 each group). ▲ indicates PS/2 mAb injection. a & c, shown are daily EAE scores (mean ± SEM). Data are representative of 4 – 6 independent experiments.
To investigate further the mechanism underlying the deaths in PS/2 mAb injected mice, we tested the antibody preparation for endotoxin by LAL assay. The results showed that the PS/2 antibody generally contained less than 0.003 ng of endotoxin per µg of antibody (approximately 0.005 EU endotoxin; data not shown). This concentration of endotoxin is approximately 20,000-fold lower than the typical LD50 of endotoxin for mice. Thus, the results strongly argued against endotoxin-mediated adverse effects produced by the PS/2 antibody preparation.
To further test the PS/2 antibody for toxicity we injected naïve C57BL/6 mice with varying doses of the PS/2 mAb as high as 1 mg per mouse once, or 200 µg daily for 10 days (data not shown). However, animals injected in such a way did not show any signs of morbidity or mortality, further arguing against toxicity of the antibody preparation.
Importantly, mortality observed upon PS/2 mAb injection was only observed in mice with actively induced EAE (immunized with neuroantigen in CFA and injected with PTX), but not in mice with passively induced disease produced via adoptive transfer of myelin-reactive T cells (Fig. 1, c & d, open circles).
Taken together, our results showed an unexpected high rate of anaphylactic-type mortality in mice with actively induced EAE upon treatment with PS/2 mAb. Furthermore, our data showed that the observed mortality was not due to endotoxin contamination or direct toxicity of the PS/2 antibody preparation.
PTX precipitates mortality in PS/2 mAb treated mice
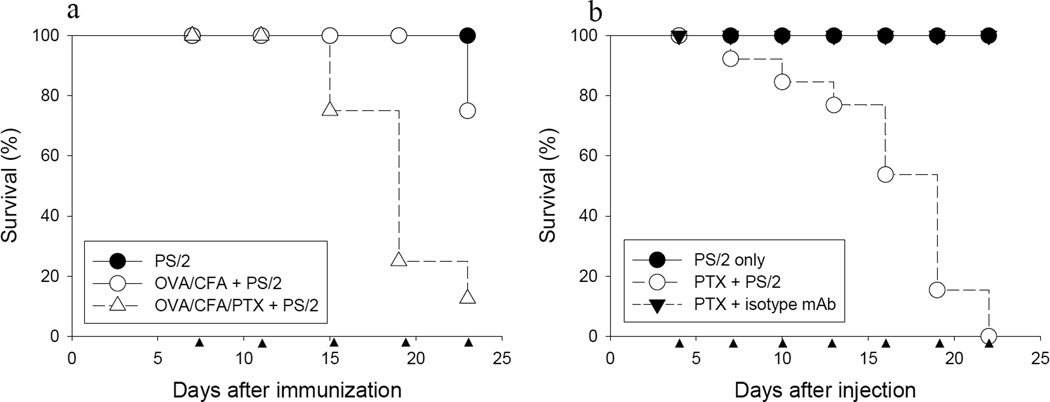
Conceivably, mortality observed in PS/2 mAb treated mice with actively induced EAE could be due to pathology induced by encephalitogenic T cells. To address this issue, C57BL/6 mice were immunized with the foreign antigen ovalbumin (OVA) with or without co-injection of PTX, and then treated with PS/2 mAb starting on day 7 after immunization.
The results show that mice injected with PS/2 mAb alone did not show anaphylactic symptoms or mortality (Fig. 2a, filled circles). In strong contrast, mice immunized with OVA/CFA and PTX followed by PS/2 mAb treatment exhibited anaphylaxis and mortality comparable to mice injected with myelin antigens (Fig. 2a, open triangles versus Fig. 1b, open circles). Importantly, mortality of the animals was dramatically reduced in the absence of PTX co-injection (Fig. 2a, open circles).
Figure 2. PTX but not autoreactive T cells are critically required for mortality in PS/2-injected mice.
a, Groups of female C57BL/6 mice were immunized with OVA/CFA and injected i.p. with (△) or without (○) PTX on days 0 and 1 as described in Materials and Methods. Mice in each group were injected i.p. with 200 µg PS/2 mAb twice per week, starting on day 7 after immunization (●, PS/2; n = 8 each group). Shown are pooled data from 2 independent experiments. b, Groups of naïve female C57BL/6 mice were injected i.p. with PTX on day 0 and 1 as described in Materials and Methods. Mice were injected i.p. with 200 µg PS/2 mAb or isotype control rat IgG2b mAb twice per week, starting on day 4 after PTX injection (●, PS/2, n = 10; ○, PTX + PS/2, n = 13; ▼, PTX + isotype mAb, n = 14). Shown are pooled data from 3 independent experiments. ▲ indicates PS/2 mAb injection.
To directly test the requirement of PTX for mortality in this model, we injected naive C57BL/6 mice with PTX followed by injection with PS/2 or isotype matched control mAb. Importantly, the results show that injection of PS/2 mAb in combination with PTX induced a high rate of anaphylaxis and mortality (Fig. 2b, open circles), similar to that observed in mice with EAE treated with the mAb. In strong contrast, mice injected with isotype control antibody and PTX, or mice injected with PS/2 mAb alone, did not show morbidity or mortality (Fig. 2b, filled symbols). Taken together, the results show that injection of PS/2 mAb in combination with PTX precipitated anaphylactic-type reactions and mortality.
Mortality in PS/2 treated mice requires the enzymatically active PTX
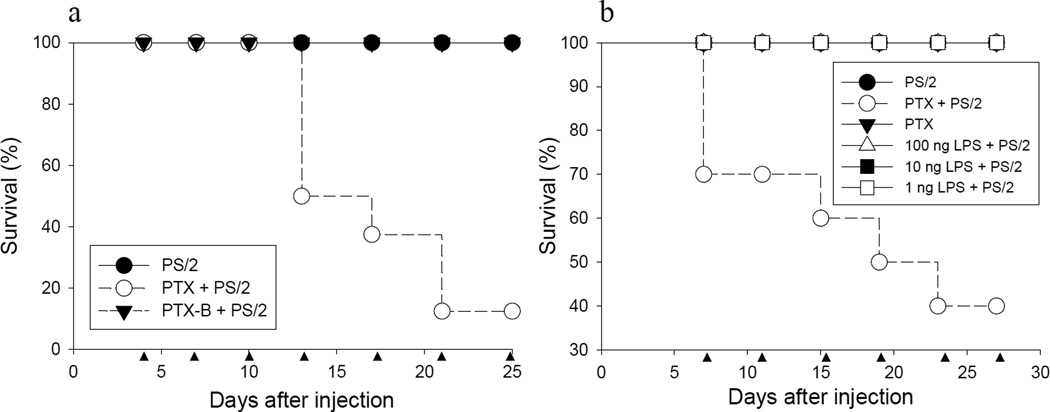
PTX is composed of a non-covalently linked enzymatically active PTX-A monomer and a PTX-B oligomer (22). The PTX-A subunit catalyzes ADP-ribosylation of G-proteins and inhibits G protein-mediated signaling in mammalian cells. The PTX-B oligomer binds to cell surface receptors and delivers the PTX-A monomer into cells (23,24). To investigate whether the G protein inhibitory activity of the PTX-A subunit played a role in the mortality observed in this model, we injected naïve C57BL/6 mice with purified PTX-B oligomer, followed 4 days later by injection with the PS/2 mAb. As controls, C57BL/6 mice were injected with PTX and PS/2 mAb, or with PS/2 mAb alone. As shown earlier, mice injected with PTX and PS/2 mAb began to succumb after several injections (Fig. 3a, open circles). In contrast, no animals died in the PS/2 mAb injected control group (Fig. 3a, filled circles). Of note, mice injected with the PTX-B oligomer and PS/2 mAb showed complete survival without any clinical symptoms (Fig. 3a, filled triangles). Furthermore, animals injected with PS/2 mAb plus heat-inactivated PTX holotoxin also did not show morbidity or mortality (data now shown).
Figure 3. Enzymatically active PTX holotoxin is required for induction of mortality in PS/2 mAb injected mice.
a, Groups of female C57BL/6 mice were injected i.p. with PTX or PTX-B oligomer (PTX-B) and 4 days later with PS/2 mAb as indicated (●, PS/2, n = 8; ○, PTX + PS/2, n = 8; ▼, PTX-B + PS/2 group, n = 9). Shown are pooled data from 2 independent experiments. b, Groups of female C57BL/6 mice were injected with PTX (○ and ▼) or LPS as indicated (△ 100 ng LPS, ■ 10 ng LPS and □ 1 ng LPS). Mice received i.p. injections twice weekly with 200 µg of PS/2 mAb (except ▼, PTX only group), beginning on day 7 after PTX or LPS injection (●, PS/2; n = 10 each group). Shown are pooled data from 2 independent experiments. ▲ indicates PS/2 mAb injection.
Together, the data suggested that the G protein inhibitory activity of the PTX-A protomer was required for morbidity and mortality in PS/2 mAb injected animals.
Since PTX has been reported to mediate its proinflammatory effects via TLR4 (25,25), we tested whether mortality mediated by the toxin in combination with PS/2 mAb could be mimicked by endotoxin, which signals via TLR4. Naive C57BL/6 mice were injected with PTX or different doses of lipopolysaccharide (LPS) (1 ng to 100 ng), followed by bi-weekly injections of PS/2 mAb.
The results showed that injection of PS/2 mAb in combination with PTX resulted in high mortality of the animals, confirming our earlier data (Fig. 3b, open circles). In strong contrast, injection of mice with PS/2 mAb in combination with LPS failed to cause morbidity or mortality (Fig. 3b, open triangles, open and filled squares). Similarly, injection of PS/2 mAb, PTX, or LPS alone did not cause mortality (data not shown).
Therefore, the data argue against a role of TLR4 in the PTX-mediated mortality in PS/2 injected mice.
Mortality in PS/2 mAb plus PTX treated mice requires CD4+ T cells
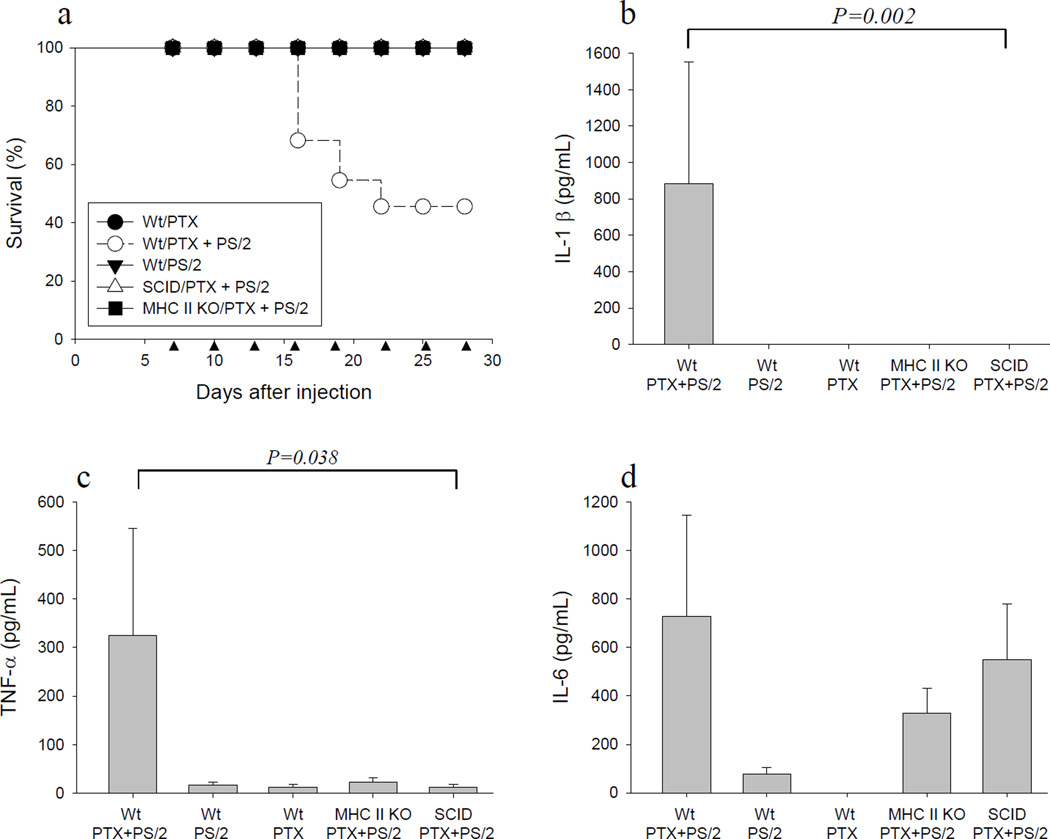
PS/2 mAb binds VLA-4 on lymphocytes, monocytes and neural crest-derived cells (11,26). Therefore, we asked which cell type was required for the mortality observed in mice injected with PTX plus PS/2 mAb. To address this question, PS/2 mAb and PTX were injected into C57BL/6 Wt, C57BL/6 SCID (lacking T and B cells), and C57BL/6 MHC class II knockout (KO; lacking CD4+ T cells) mice as described earlier. The mice were observed for up to 30 days for morbidity and mortality. As control, each strain of mice was injected with PS/2 mAb or PTX alone, respectively.
As expected, C57BL/6 Wt mice injected with both PTX and PS/2 mAb showed a high rate of mortality (Fig. 4a, open circles). In contrast, no animals died when injected with PS/2 mAb or PTX alone (Fig. 4a, filled circles and triangles). Importantly, no deaths were observed in SCID mice or MHC class II KO mice injected with both PTX and PS/2 mAb (Fig. 4a, open triangles and filled squares). The absence of mortality induced by PS/2 in combination with PTX in SCID and MHC class II KO mice showed that CD4+ T cells, but not CD8+ T cells or innate immune cells were required for pathology in this model.
Figure 4. CD4+ T cells are required for induction of mortality in mice injected with PS/2 in combination with PTX.
a, Groups of female C57BL/6 Wt, SCID and MHC II KO mice were injected i.p. with PTX and 200 µg PS/2 mAb as indicated (●, Wt/PTX, n= 8; ○, Wt/PTX + PS/2, n = 22; ▼, Wt/PS/2, n = 10; △, SCID/PTX + PS/2, n = 13; ■, MHC II KO/PTX + PS/2, n = 21). Shown are pooled data from 3 independent experiments. ▲ indicates PS/2 mAb injection. Cytokine ELISAs were performed to determine the concentration of IL-1β (b), TNF-α (c), or IL-6 (d) in serum from the different groups. (Mean ± SEM, ANOVA. Wt/PTX, n = 6; Wt/PTX + PS/2, n = 14; Wt/PS/2, n = 9; SCID/PTX + PS/2, n = 8; MHC II KO/PTX + PS/2, n = 7). Shown are pooled data from 2 independent experiments.
IL-1β and TNF-α have been shown to contribute to anaphylactic reactions, for example in LPS-mediated toxic shock (27–29). Similarly, IL-6 can be induced by LPS and modulate acute phase responses (30). Therefore, we determined serum levels of IL-1β, TNF-α and IL-6 in this model. C57BL/6 Wt, SCID, and MHC class II KO mice were injected with both PTX and PS2 mAb, PS/2 mAb alone, or PTX alone as outlined earlier. Serum was obtained from mice that exhibited clinical signs of anaphylaxis, or from representative control animals, and analyzed by cytokine ELISA assays as outlined in Materials and Methods.
As shown in Fig. 4, b & c, serum levels of IL-1β and TNF-α were significantly increased in Wt mice injected with both PS/2 mAb and PTX, as compared to Wt control animals. In contrast, serum levels of IL-1β and TNF-α were significantly decreased in MHC class II KO and SCID mice treated with PTX and PS/2 mAb as compared to treated Wt mice (Fig. 4, b & c). Finally, IL-6 serum levels were significantly increased in Wt mice treated with both PTX and PS/2 mAb, compared with control animals injected with PS/2 mAb or PTX alone (Fig. 4d). However, no significant differences in the levels of IL-6 were noted between Wt and SCID or MHC II KO mice treated with both PTX and PS/2 mAb (Fig. 4d). Thus, the results suggested that IL-1β, TNF-α, and IL-6 may have contributed to morbidity and mortality in this model.
To further investigate the role of these cytokines we tested whether combined PTX and PS/2 mAb treatment induced mortality in TNF-α knockout (TNFα−/−) mice.
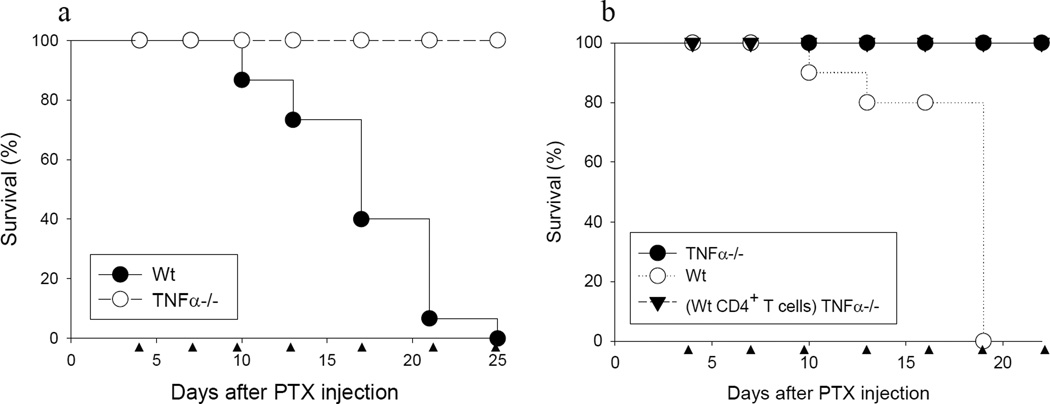
As expected, Wt mice injected with PTX and PS/2 mAb exhibited a high rate of mortality starting with the third injection of PS/2 mAb (Fig. 5a, closed symbols). In strong contrast, all TNFα−/− mice survived over the full observation period (Fig. 5a, open symbols). Reconstitution of TNFα−/− mice with Wt CD4+ T cells did not restore mortality observed in Wt mice (Fig. 5b). Thus, the results showed that TNF-α was important for mortality in this model. Furthermore, the results suggested that in addition to CD4+ T cell-derived TNF-α, production of this cytokine by other cells, for example innate immune cells, contributed to mortality.
Figure 5. TNF-α promotes induction of mortality in PTX and PS/2 mAb injected mice.
a, Groups of Wt C57BL/6 or TNFα−/− mice were injected i.p. with PTX and 4 days later with continued PS/2 mAb injections as indicated (●, Wt, n = 15; ○, TNFα−/−, n = 17). Shown are pooled data from 3 independent experiments. b, Wt C57BL/6, TNFα−/− or TNFα−/− recipient mice which received Wt CD4+ T cells were injected i.p. with PTX and 4 days later with PS/2 mAb injections as indicated (●, TNFα−/−, n = 12; ○, Wt, n = 10; (Wt CD4+ T cells) TNFα−/−, n = 10). Shown are pooled data from 2 independent experiments. ▲ indicates PS/2 mAb injection.
Discussion
The results of this study show that co-injection of anti-VLA-4 mAb PS/2 with PTX resulted in anaphylactic-type mortality in mice, and that CD4+ T cells were a critical component for pathology. The results suggest that anti-VLA-4 blockade may amplify the immune activating properties of PTX, and that this may result in detrimental side effects of this treatment.
PTX typically is used in models of actively induced EAE in rodents to facilitate the induction of disease. The underlying mechanism was originally thought to be due to the toxin opening up the blood-brain-barrier to inflammatory leukocytes (31,32). In addition, recently it was shown that the toxin has a remarkable propensity to activate APCs and promote the induction of Th1 and Th2 immune responses (20,33–36).
Our observation of high mortality in Wt mice treated with PS/2 mAb in combination with PTX suggests a CD4+ T cell-mediated mechanism by which anti-VLA-4 blockade in combination with a microbial toxin-induced proinflammatory stimulus can lead to pathology.
The critical role for CD4+ T cells in this model raises the question as to whether PS/2 and PTX combine to directly affect T cells, or whether this effect is mediated indirectly, for example via APCs. The literature supporting direct effects of PTX on T cells is inconclusive (37–39). Alternatively, PTX may mediate its effects in PS/2 mAb injected mice by acting on innate immune cells or non-immune cells (e.g. vascular endothelial cells) resulting in the subsequent activation of CD4+ T cells and death of the animals. This view is consistent with studies showing a direct effect of PTX on APCs leading to the upregulation of costimulatory molecules and enhanced cytokine production (35,36,40).
Irrespective, our data show that the mechanism by which PTX exerts its effects in this model is dependent on the presence of the enzymatically active PTX-A subunit. Injection of mice with both PS/2 mAb and PTX-B or with heat-inactivated PTX did not cause mortality in mice. In turn, this suggested that the G-protein inhibitory activity of the PTX-A subunit was required. Along these lines, PTX-mediated G-protein inhibition is well known to affect intracellular signaling, for example via modulation of protein kinase C and phospholipase C activity (41,42).
Of note, injection of PTX alone did not induce morbidity or mortality in the absence of PS/2 mAb treatment, suggesting that the two molecules must function in concert to induce pathology. VLA-4 has been reported to signal in T cells, and signaling through this molecule on T cells has been shown to antagonize apoptosis (43). Enhanced activation status of peripheral blood T cells has been reported in MS patients treated with natalizumab (44,45). Thus, it is conceivable that the combination of PS/2 mAb with PTX further enhanced T cell activation and production of proinflammatory cytokines, ultimately leading to pathology.
In support of this view, we observed that serum levels of IL-1β and TNF-α were significantly enhanced in PS/2 mAb and PTX injected Wt mice. Both of these cytokines have been shown to play a critical role in endotoxin-mediated lethal shock, and the clinical picture observed in our studies is consistent with this interpretation. The lack of mortality in TNF-α knockout mice upon treatment with PS/2 mAb and PTX further supported a role for this cytokine. However, since reconstitution of Wt CD4+ T cells into TNF-α knockout mice was not sufficient to induce mortality, it is likely that TNF-α produced by other cells, for example innate immune cells, played a role.
Along these lines, IL-6 was enhanced in Wt mice injected with PTX and PS/2 mAb, but also in SCID and MHC Class II KO mice, which are both deficient in CD4+ T cells. IL-6 is a proinflammatory cytokine with diverse biological activities which can be induced in many cell types, including peripheral blood monocytes, by stimuli such as endotoxin and IL-1β (30). Conceivably, IL-6 could have synergized with TNF-α and IL-1β to induce mortality (46). The lack of mortality in TNF-α knockout mice is in agreement with this view. However, the role of IL-6 in toxic shock is still not fully resolved, and a protective role for this cytokine has been reported (47).
Of note, pathology in this model was not dependent on the activation of autoreactive T cells, since mortality was similarly observed after co-injection of PS/2 mAb and PTX in naive mice, or in OVA immunized animals. Anti-VLA-4 mAb blockade is used for the treatment of MS, and it is therefore justified to ask whether our results may have ramifications for the treatment of MS patients. Studies in animal models have limitations as to their extrapolation to human disease conditions. Thus, it must be cautioned that we have not tested whether human T cells are similarly activated by anti-VLA-4 mAb and PTX. Furthermore, the PS/2 mAb used in our studies is not identical to the antibody in human patients used to block anti-VLA-4 (natalizumab). Finally, the function of VLA-4 in mouse and human T cells, as well as the effect of PTX in humans might be different. To our knowledge, anaphylactic reactions after natalizumab treatment in humans have not been reported. Concerns upon natalizumab treatment have so far centered on progressive multifocal leukoencephalopathy (PML) and the reactivation of JC virus, conceivably due to lack of immunosurveillance in the CNS (19,48). Nevertheless, our results point out that certain microbial protein in combination with anti-VLA-4 mAb treatment could potentially result in previously unrecognized detrimental side effects. As a consequence, it may not advisable to treat patients undergoing secondary infections with natalizumab.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- VLA-4
very late antigen-4
- PTX
Pertussis toxin
- BBB
blood-brain-barrier
Footnotes
This work was supported by grant NS-52177 from the National Institute of Health, and grants RG3499 and RG3701 from the National Multiple Sclerosis Society (T.G.F.).
Reference List
- 1.Compston A, Coles A. Multiple sclerosis (vol 359, pg 1221, 2002) Lancet. 2002;360:648. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Medical progress: Multiple sclerosis. New England Journal of Medicine. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Mokhtarian F, Mcfarlin DE, Raine CS. Adoptive Transfer of Myelin Basic Protein-Sensitized T-Cells Produces Chronic Relapsing Demyelinating Disease in Mice. Nature. 1984;309:356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 5.Gijbels K, Engelborghs S, De Deyn PP. Experimental autoimmune encephalomyelitis: An animal model for multiple sclerosis. Neuroscience Research Communications. 2000;26:193–206. [Google Scholar]
- 6.Wisniewski HM, Keith AB. Chronic Relapsing Experimental Allergic Encephalomyelitis - Experimental-Model of Multiple-Sclerosis. Annals of Neurology. 1977;1:144–148. doi: 10.1002/ana.410010207. [DOI] [PubMed] [Google Scholar]
- 7.Stone SH, Lerner EM. Chronic Disseminated Allergic Encephalomyelitis in Guinea Pigs. Annals of the New York Academy of Sciences. 1965;122:227-&. doi: 10.1111/j.1749-6632.1965.tb20206.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: The use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review) - Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773. doi: 10.1212/01.wnl.0000320512.21919.d2. [DOI] [PubMed] [Google Scholar]
- 9.Schreiner B, Kieseier BC, Hartung HR, Hohlfeld R, Wiendl H. Blocking adhesion molecules with natalizumab in multiple sclerosis. Nervenarzt. 2005;76:999–1005. doi: 10.1007/s00115-005-1900-2. [DOI] [PubMed] [Google Scholar]
- 10.Springer TA. Adhesion Receptors of the Immune-System. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 11.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. Vcam-1 on Activated Endothelium Interacts with the Leukocyte Integrin Vla-4 at A Site Distinct from the Vla-4 Fibronectin Binding-Site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte Interactions with Endothelial-Cells. Immunology Today. 1992;13:106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 13.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface Expression of Alpha-4 Integrin by Cd4 T-Cells Is Required for Their Entry Into Brain Parenchyma. Journal of Experimental Medicine. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: Natalizumab. Nature Reviews Drug Discovery. 2005;4 doi: 10.1038/nrd1752. 510-5U3. [DOI] [PubMed] [Google Scholar]
- 15.Yednock TA, Cannon C, Fritz LC, Sanchezmadrid F, Steinman L, Karin N. Prevention of Experimental Autoimmune Encephalomyelitis by Antibodies Against Alpha-4-Beta-1 Integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 16.Kent SJ, Karlik SJ, Cannon C, Hines DK, Yednock TA, Fritz LC, Horner HC. A Monoclonal-Antibody to Alpha-4 Integrin Suppresses and Reverses Active Experimental Allergic Encephalomyelitis. Journal of Neuroimmunology. 1995;58:1–10. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- 17.Theien BE, Vanderlugt CL, Eagar TN, Nickerson-Nutter C, Nazareno R, Kuchroo VK, Miller SD. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. Journal of Clinical Investigation. 2001;107:995–1006. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niino M, Bodner C, Simard ML, Alatab S, Gano D, Kim HJ, Trigueiro M, Racicot D, Guerette C, Antel JP, Fournier A, Grand'Maison F, Bar-Or A. Natalizumab effects on immune cell responses in multiple sclerosis. Annals of Neurology. 2006;59:748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 19.Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Annals of Neurology. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund's adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. Journal of Immunology. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 21.Schneider YJ. Optimization of Hybridoma Cell-Growth and Monoclonal-Antibody Secretion in A Chemically Defined, Serum-Free and Protein-Free Culture-Medium. Journal of Immunological Methods. 1989;116:65–77. doi: 10.1016/0022-1759(89)90314-1. [DOI] [PubMed] [Google Scholar]
- 22.Merritt EA, Hol WGJ. Ab(5) Toxins. Current Opinion in Structural Biology. 1995;5:165–171. doi: 10.1016/0959-440x(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 23.Kaslow HR, Burns DL. Pertussis Toxin and Target Eukaryotic Cells - Binding, Entry, and Activation. Faseb Journal. 1992;6:2684–2690. doi: 10.1096/fasebj.6.9.1612292. [DOI] [PubMed] [Google Scholar]
- 24.Su SB, Silver PB, Zhang MF, Chan CC, Caspi RR. Pertussis toxin inhibits induction of tissue-specific autoimmune disease by disrupting G protein-coupled signals. Journal of Immunology. 2001;167:250–256. doi: 10.4049/jimmunol.167.1.250. [DOI] [PubMed] [Google Scholar]
- 25.Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends in Immunology. 2005;26:289–291. doi: 10.1016/j.it.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Vonderheide RH, Springer TA. Lymphocyte Adhesion Through Very Late Antigen-4 - Evidence for A Novel Binding-Site in the Alternatively Spliced Domain of Vascular Cell-Adhesion Molecule-1 and An Additional Alpha-4 Integrin Counter-Receptor on Stimulated Endothelium. Journal of Experimental Medicine. 1992;175:1433–1442. doi: 10.1084/jem.175.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann T, Buscher D, Hipskind RA, Krautwald S, Lohmannmatthes ML, Baccarini M. Lipopolysaccharide Induces Activation of the Raf-1/Map Kinase Pathway - A Putative Role for Raf-1 in the Induction of the Il-1-Beta and the Tnf-Alpha Genes. Journal of Immunology. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 28.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. Role of Tumor Necrosis Factor-Alpha in Lipopolysaccharide-Induced Pathological Alterations. American Journal of Pathology. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 29.Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: Correlation with inhibition of cytokine secretion. Infection and Immunity. 1996;64:825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosato G, Jones KD. Interleukin-1 Induces Interleukin-6 Production in Peripheral-Blood Monocytes. Blood. 1990;75:1305–1310. [PubMed] [Google Scholar]
- 31.Linthicum DS, Munoz JJ, Blaskett A. Acute Experimental Autoimmune Encephalomyelitis in Mice .1. Adjuvant Action of Bordetella-Pertussis Is Due to Vasoactive Amine Sensitization and Increased Vascular-Permeability of the Central Nervous-System. Cellular Immunology. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 32.Yong T, Meininger GA, Linthicum DS. Enhancement of Histamine-Induced Vascular Leakage by Pertussis Toxin in Sjl/J Mice But Not Balb/C Mice. Journal of Neuroimmunology. 1993;45:47–52. doi: 10.1016/0165-5728(93)90162-r. [DOI] [PubMed] [Google Scholar]
- 33.de Jong EC, Vieira PL, Kalinski P, N. Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. Journal of Immunology. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 34.Hou WQ, Wu YD, Sun SH, Shi M, Sun Y, Yang CH, Pei G, Gu YD, Zhong CP, Sun B. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. Journal of Immunology. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 35.Ryan M, McCarthy L, Rappuoli R, Mahon BP, G. Mills KH. Pertussis toxin potentiates T(h)1 and T(h)2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. International Immunology. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 36.Shive CL, Hofstetter H, Arredondo L, Shaw C, Forsthuber TG. The enhanced antigen-specific production of cytokines induced by pertussis toxin is due to clonal expansion of T cells and not to altered effector functions of long-term memory cells. European Journal of Immunology. 2000;30:2422–2431. doi: 10.1002/1521-4141(2000)30:8<2422::AID-IMMU2422>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 37.Wakatsuki A, Borrow P, Rigley K, Beverley PCL. Cell-surface bound pertussis toxin induces polyclonal T cell responses with high levels of interferon-gamma in the absence of interleukin-12. European Journal of Immunology. 2003;33:1859–1868. doi: 10.1002/eji.200323675. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Howard OMZ, Oppenheim JJ. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. Journal of Immunology. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 39.Schneider OD, Weiss AA, Miller WE. Pertussis toxin utilizes proximal components of the T-cell receptor complex to initiate signal transduction events in T cells. Infect. Immun. 2007;75:4040–4049. doi: 10.1128/IAI.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denkinger CM, Denkinger MD, Forsthuber TG. Pertussis toxin-induced cytokine differentiation and clonal expansion of T cells is mediated predominantly via costimulation. Cellular Immunology. 2007;246:46–54. doi: 10.1016/j.cellimm.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luttrell LM, Hawes BE, vanBiesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and G beta gamma subunit-mediated activation of mitogen-activated protein kinases. Journal of Biological Chemistry. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 42.Piiper A, Gebhardt R, Kronenberger B, Giannini CD, Elez R, Zeuzem S. Pertussis toxin inhibits cholecystokinin- and epidermal growth factor-induced mitogen-activated protein kinase activation by disinhibition of the cAMP signaling pathway and inhibition of c-Raf-1. Molecular Pharmacology. 2000;58:608–613. doi: 10.1124/mol.58.3.608. [DOI] [PubMed] [Google Scholar]
- 43.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nature Reviews Immunology. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- 44.Kivisakk P, Healy B, Quintana F, Weiner H, Khoury S. Increased expression of proinflammatory cytokines by peripheral blood leukocytes during natalizumab treatment in patients with multiple sclerosis. Journal of Neuroimmunology. 2008;203:268. [Google Scholar]
- 45.Kivisakk P, Healy BC, Viglietta V, Quintana FJ, Hootstein MA, Weiner HL, Khoury SJ. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 2009;72:1922–1930. doi: 10.1212/WNL.0b013e3181a8266f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krakauer T, Buckley MJ, Fisher D. Proinflammatory Mediators of Toxic Shock and Their Correlation to Lethality. Mediators of Inflammation. 2010 doi: 10.1155/2010/517594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao H, Kohanawa M. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infection and Immunity. 2005;73:3745–3748. doi: 10.1128/IAI.73.6.3745-3748.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuve O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. Journal of the Neurological Sciences. 2008;274:39–41. doi: 10.1016/j.jns.2008.03.022. [DOI] [PubMed] [Google Scholar]