Abstract
Attenuated activity of the central nervous melanocortin system causes obesity and insulin resistance. Obese rodents treated with melanocortins exhibit improvements in obesity and metabolic homeostasis that are not mutually dependent, suggesting metabolic actions that are independent of weight changes. These responses are generally thought to involve G-protein-coupled receptors expressed in the brain. Melanocortin-4 receptors (MC4Rs) regulate satiety and autonomic nervous system and thyroid function. MC3Rs are expressed in hypothalamic and limbic regions involved in controlling ingestive behaviors and autonomic function. Mc3r−/− mice exhibit increased adiposity and an accelerated diet-induced obesity. While this phenotype is not dependent on hyperphagia, data on the regulation of food intake by MC3Rs are inconsistent. Recent investigations by our laboratory suggest a unique combination of behavioral and metabolic disorders in Mc3r−/− mice. MC3Rs are critical for the expression of the anticipatory response and metabolic homeostasis when food intake occurs outside the normal voluntary rhythms driven by photoperiod. Using a Cre-Lox strategy, we can now investigate MC3Rs expressed in different brain regions and organ systems in the periphery. While focusing on the functions of neural MC3Rs, early results suggest an additional layer of complexity with central and peripheral MC3Rs involved in the defense of body weight.
1. INTRODUCTION
The rise in the prevalence of obesity is a significant public health issue as it is associated with insulin resistance and increased risk for type 2 diabetes and cardiovascular disease. Indeed, less than half of the population in the United States has a healthy body weight (a body mass index between 18.5 and 24.9 kg/m2).1 Lifestyle changes causing weight loss and to prevent development of obesity are thus increasingly considered as imperatives. Unfortunately, strong behavioral responses to caloric restriction make adhering to lifestyle changes extremely difficult in obese individuals. Clinical studies also indicate other counter responses to limit weight loss, with reduced resting metabolic rate and physical activity.2-4 In individuals where the metabolic stress associated with obesity is severe, surgical and/or pharmacological interventions are therefore often required to obtain weight loss. Gastric bypass procedures are highly effective and also rapidly improve metabolic homeostasis.5 However, the options for pharmacological intervention are limited.
Body weight exhibits remarkable stability over time, even in the severely obese. This suggests that mechanisms exist to sense energy balance and prevent excess weight gain through the control of appetite and energy expenditure and/or nutrient partitioning. A popular concept for “energy homeostasis” has evolved whereby body weight is maintained by neuronal circuits that emanate from the hypothalamus that connect appetite and the autonomic and neuroendocrine control of metabolism with signals of metabolic status.6-9 As mentioned previously, caloric restriction produces powerful behavioral and metabolic counter responses to limit weight loss. However, the nature of the “anti-obesity” mechanisms beyond the control of appetite in situations of nutrient abundance is still being debated.10-16 In situations where calorie-dense diets are freely available, the mechanisms for the defense of body weight are less robust when compared to the powerful counter-regulatory responses to calorie restriction.17
It has been known for nearly a century that damage to the hypothalamus or mutations in certain genes can result in obesity.18-20 Much of the research into the mechanisms that control the balance of energy intake and expenditure over the last two decades has also focused on the hypothalamus. Strong evidence links altered activity of the central nervous melanocortin system that is centered within the hypothalamus with human obesity, and a model for how the system functions to maintain body weight has been proposed.6,21 Melanocortins regulate both sides of the energy balance equation, regulating satiety and systems that govern energy expenditure. Much has been learnt about the “first order” melanocortin neurons releasing the endogenous melanocortin ligands that are regulated by signals of metabolic state. Considerable attention has also been given to one of melanocortin receptors that are widely expressed in the brain (melanocortin-4 receptor, MC4R). The functions of the other melanocortin receptors expressed in the brain (melanocortin-3 receptor, MC3R) that is also known to affect body weight and composition have received far less attention.
This review begins by providing an overview of our understanding of the neural systems and the involvement of melanocortins in metabolic homeostasis and prevention of obesity. It will then focus on the most poorly understood component of the melanocortin system, the MC3R, in regulating body weight and metabolic homeostasis.
2. THE “LEPTIN-MELANOCORTIN” PATHWAY: A CANONICAL PATHWAY LINKING ENERGY STORES WITH NEURAL OUTPUTS REGULATING ADAPTATION TO ENERGY REQUIREMENTS
The “modern era” of obesity research that began to elucidate the mechanisms orchestrating metabolic homeostasis and weight control originated with mouse genetics that was used to identify and characterize loci associated with hyperphagia and increased adiposity. The characterization of the obese (ob/ob) and diabetic (db/db) mice, which are carriers of recessive mutations causing severe obesity, was a landmark in the development of models explaining how body weight might be controlled. This research also provided a fundamental insight into how organisms adapt behavior and metabolism to fasting. Parabiosis experiments performed by Douglas Coleman and colleagues over 40 years ago suggested that ob/ob mutant mice lacked a secreted factor produced by a gene on chromosome 6, while db/db mice lacked a receptor for a satiety factor likely expressed in the hypothalamus that is produced by a gene on chromosome 4.22 Positional cloning of the mouse obese gene identified leptin as a 16 kDa peptide hormone belonging to the cytokine superfamily secreted from adipocytes.23 Shortly thereafter an expression cloning strategy identified the leptin receptor as a member of the single transmembrane-spanning cytokine receptor family.24 Genetic screens of morbidly obese humans subsequently identified homozygous carriers of “loss of function” mutations in genes encoding leptin (LEP) or the leptin receptor (LEPR).25 These individuals exhibited a ravenous appetite and severe obesity from early childhood.
Leptin levels in the circulation are proportional to fat mass, while the actions of leptin inhibit food intake and reduce fat.26 Circulating leptin may therefore function as an input into a negative feedback loop that maintains energy reserves in adipose tissue at stable levels. Heterozygous carriers of null LEP alleles exhibit increased adiposity, consistent with a compensatory increase in fat mass to increase leptin levels and thereby raising the “settling point” of adiposity.27 The discovery of LEP and LEPR genes has, however, not led to effective obesity therapies owing to leptin resistance in obesity.28
Leptin regulates behavioral and metabolic programs that are important for survival during times of caloric insufficiency (Fig. 4.1).36 The effect of leptin on energy balance is generally “inhibitory” (i.e., suppression of appetite, stimulation of energy expenditure), loss or attenuation of action therefore has the potential to cause weight gain and obesity. Conditional targeting of the Lepr gene in mice indicates that the actions of leptin in the central nervous system are sufficient and obligatory to maintain energy balance.37-42 Leptin secretion in well-fed conditions provides the brain with a signal of caloric sufficiency, attenuating appetite while maintaining energy expenditure. A decline in leptin secretion from adipocytes with fasting signals caloric deprivation,43 and instigates behavioral programs including increased food seeking and an attenuated response to satiety signals. Leptin-deficiency also promotes neuroendocrine and autonomic responses to instigate metabolic adaptation to caloric restriction (e.g., reducing energy expenditure).2,44-46 The decline in leptin with fasting and the triggering of a counter response to a negative energy balance are likely the raison d’etre for this hormone.43
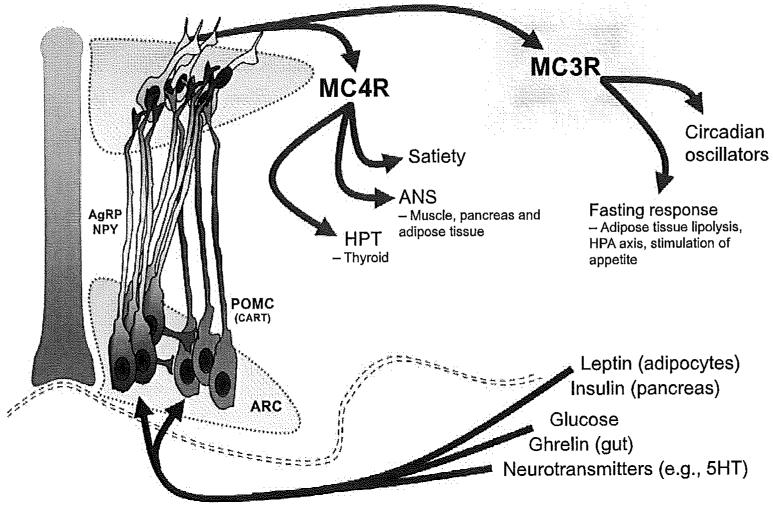
Figure 4.1.
Schematic showing the central role played by arcuate (ARC) melanocortin neurons to integrate signals of metabolic status with outputs involved in metabolic homeostasis. The figure shows the two groups of “primary” melanocortin neurons that reside in the ARC and send projections throughout the central nervous system. POMC neurons (red) express the preprohormone processed to produce α- and γ-MSH (not shown). Activation of POMC neurons has been suggested to have “catabolic” actions that limit weight gain by reducing food intake and promoting energy expenditure. AgRP neurons (green) also express neuropeptide Y (NPY); both neuropeptides are orexigenic and have inhibitory effects on outputs that maintain energy expenditure (i.e., ANS and HPT). AgRP and POMC neurons integrate several signals of metabolic state and vigilance. Note, however, that the populations of POMC and AgRP neurons are probably heterogeneous, exhibiting differential responses to some or all of the inputs shown here.29 Central administration of α-MSH inhibits food intake, a response requiring functional MC4Rs.30 MC4Rs also regulate the autonomic nervous system (ANS) and the hypothalamo-pituitary-thyroid axis (HPT). Activation of MC4Rs stimulates insulin action to affect glucose disposal and lipid metabolism through effects on the ANS. Activation of MC4Rs also contributes to maintaining normal levels of thyroid activity in fed conditions.31 The role of MC3Rs in the central nervous system is less clear. However, Mc3r−/− mice display reduced activity of circadian oscillators suggesting a role in maintaining rhythms in the central nervous system.32-34 It was recently reported that Mc3r−/− mice also exhibit reduced behavioral and metabolic adaptation to fasting.35 Stimulation of AgRP expression and appetite is Mc3r dependent, as is the stimulation of adipose tissue lipolysis.
While leptin levels do increase with weight gain, this is considered an indicator of leptin resistance as opposed to an effective counter response for preventing further weight gain. Indeed, desensitization of hypothalamic neurons to leptin, including the primary melanocortin neurons releasing the endogenous melanocortin agonists,47 likely contributes to the deterioration of metabolic homeostasis that is frequently observed in obesity. Several mechanisms have been suggested to explain leptin resistance with weight gain, including attenuation of a saturable transporter system across the blood–brain barrier, increased activity of inhibitory signaling proteins (e.g., protein tyrosine phosphatase IB, suppressor of cytokine signaling 3), inflammation, and other cellular stress responses linked to insulin resistance.28 However, results from a recent study suggest that receptor desensitization, which describes the phenomenon whereby the response of the receptor declines over time despite the presence of stimulus, may be critical for the development of leptin resistance. When leptin levels are “clamped” in C57BL/6J (B6) mice subjected to diet-induced obesity, weight gain was normal.48 Hyperleptinemia therefore does not prevent further weight gain. These mice also did not develop leptin resistance with obesity. Receptor desensitization through upregulation of inhibitory signaling proteins (and perhaps saturation of leptin transport across the blood brain barrier) may therefore be obligatory for the development of leptin resistance. On the other hand, we have also observed that in conditions of extreme obesity, hyperleptinemia may actually exacerbate the obese phenotype. Alleviation of severe hyperleptinemia in B6 mice with obesity due to a combination of reduced MC4R signaling and high fat diet was not associated with further gain, but actually attenuated the obese phenotype.49
Mouse genetics also facilitated the neurochemical identification of the first group of hypothalamic neurons involved in mediating the actions of leptin. Mice carrying the dominant agouti alleles lethal yellow (AY) or viable yellow (AVY) had been known for nearly a century to develop an “adult onset” obesity.50 Positional cloning of the agouti locus identified the agouti-signaling protein (ASIP)51,52 subsequently shown to function as a competitive antagonist for two melanocortin receptors (MC1R, MC4R).53 The yellow color observed in AY and AVY mice results from antagonism of MC1Rs; obesity was hypothesized to result from antagonism of MC4Rs expressed in the central nervous system.54-56 The similarities in the phenotype of Mc4r− /− and AY mice (increased longitudinal growth, obesity, insulin resistance, and hy-perphagia) supported this hypothesis.56 Genetic screens using morbidly obese individuals identified homo- and heterozygous carriers of MC4R mutations. These individuals have a severe hypherphagic obesity syndrome from a very early age.57,58 Unlike the case with leptin and its receptor, however, mutations in the MC4R gene turned out to be the most common form of monogenic obesity, occurring in 1–6% of obese individuals.
Regulation of satiety is a major factor in the defense of body weight by MC4Rs.56,59,60 However, as is case the for leptin, studies using rodent models indicate a metabolic component. Prevention of hyperphagia in Mc4r− /− mice only attenuates obesity,61 while analysis of the response ofMc4r− /− mice during the transition to diets with increased fat content suggests deficits in energy expenditure and nutrient partitioning.60,62-64 MC4Rs in the brain regulate glucose and lipid metabolism via neuroendocrine and autonomic outputs, and their activation promotes energy expenditure during periods of caloric sufficiency.60,61,64-69 Studies using a Cre-Lox strategy to selectively target Mc4r expression in mice suggest that the expression of this receptor in the nervous system is sufficient to restore body weight.70 These studies also suggest a functional divergence. Expression of Mc4rs in the paraventricular nucleus (PVN) of the hypothalamus is sufficient to restore normal regulation of satiety. However, it does not restore the regulation of energy expenditure.70 On the other hand, deficits in energy expenditure and insulin sensitivity (but not food intake) are rescued by expression of Mc4r in preganglionic sympathetic neurons in the hindbrain.65 These results need to be interpreted with caution, however. For example, several laboratories have reported a role for Mc4rs in the brainstem in the regulation of satiety.71-76 The actions of these receptors, while important, may thus not be sufficient to overcome loss of Mc4r activity in PVN neurons. Conversely, intracerebroventricular (icv) injections of melanocortin agonists into the hypothalamus can stimulate thermogene-sis.77 One interpretation of these results is a distributed network involving hypothalamic and brainstem sites governing appetite and metabolism. Within this network are critical “nodes” where MC4R activity is sufficient and necessary to regulate one or the other function, but not both. The results from studies using the same genetic strategy to explore leptin signaling in the central nervous system have shown similar outcomes.78
Strong evidence from basic research and clinical genetics supporting a role for MC4Rs in preventing obesity in humans has led to several campaigns by pharmaceutical companies and academia to identify small molecule and peptide analogs with activity at this receptor.79-84 However, the response of obese volunteers to compounds targeting MC4Rs in the clinic has thus far been discouraging due to limited effectiveness and side effects on cardiovascular function.85 MC4Rs are not, however, the only member of the melanocortin receptor family involved in metabolic homeostasis. Mc3r− /− mice display an obese phenotype,32,62,63,86-90 attenuated behavioral, and metabolic adaptations when food intake is limited both calorically and temporally (to the mid-light cycle),33,34,91,92 and to fasting.35 And, although obese Mc4r−/− mice treated with melanocortin analogs do not exhibit reduced food intake and weight loss, reductions in fasting insulin and glucose, suggesting improvements in glucose homeostasis, were still observed (Fig. 4.2, Table 4.1).93 This experiment did not determine the subtype of melanocortin receptor that is involved. There is evidence suggesting that other melanocortin receptors play a role in metabolic homeostasis. MC5Rs expressed in skeletal muscle regulate activity of AMPK, suggesting a role in regulating glucose and fatty acid oxidation.94 However, with a caveat that human receptors were used to characterize the pharmacology, the melanocortin analog administered to Mc4r−/− mice had low affinity for MC5Rs (Ki 7 μM, EC50=4 μM). This suggests that actions involving stimulation of MC3Rs and/or MC1Rs result in improvements in glucose homeostasis in obese mice. As shall be discussed later in the review, data from our laboratory and others suggest that the functions of MC3Rs can impact on glucose homeostasis.92,95
Table 4.1.
Blood chemistries of WT, Mc3rKO, and Mc4rKO treated with BIM-22511 compared to controls
| Genotype: | WT | Mc3rKO | Mc4rKO | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Treatment: | Saline | BIM-22511 | Saline | BIM-22511 | Saline | BIM-22511 |
| Glucose (mg/dL) |
100±7 | 129±20 | 164±22 | 193±28 | 153±10 | 119±15 |
|
| ||||||
| Insulin (pg/ml) |
138±30 | 145±40 | 124±21 | 310±55 | 3588±812 | 1899±160* |
|
| ||||||
| HOMA-IR | 0.9±0.2 | 1.3±0.S | 1.3±0.3 | 3.5±0.5 | 33.5±7.0 | 14.2±3.7* |
P<0.01 compared to saline treatment within genotype. Serum was collected after a 4 h fast.
While the MC4R has been studied extensively using a combination of mouse genetics and pharmacological tools, far less is known about the functions of MC3Rs expressed in the brain. MC3Rs are expressed in hypothalamic and limbic structures of the central nervous system controlling ingestive behaviors and metabolic homeostasis.96 The endogenous agonist α-melanocyte-stimulating hormone (α-MSH) exhibits affinity for both receptors.96,97 Another form of MSH (γ-) exhibits selectivity for the MC3R, suggesting that there may be differential regulation of the two receptors in situations where activity of proopiomelanocortin (POMC) neurons that release the endogenous ligands is increased.96 A second member of the agouti family, agouti-related peptide (AgRP), is expressed in the brain and binds with high affinity to MC3Rs and MC4Rs98,99 suggesting parallel regulation in situations where AgRP expression is upregulated. The additive obesity phenotype observed in double Mc3r−/−.Mc4r−/− mice suggests that the actions of each receptor affecting body weight involve independent pathways.87
While the analysis of Mc3r knockout mice suggests functions that impact on adiposity,32,62,63,86-90 how and where this receptor affects these processes have remained a mystery. Studies analyzing the response of melanocortin receptor knockout mice indicate that MC3Rs are not required for regulating satiety or neuroendocrine and autonomic systems governing metabolism in the periphery.30,87,100,101 The link between mutations in the human MC3R gene and obesity has also not been well established when compared to MC4R mutations. These findings may explain in part the lack of enthusiasm shown by the obesity field for investigating this receptor. However, recent observations by our laboratory and others suggest that this receptor may have a critical role in metabolic homeostasis in certain conditions. Specifically, while loss of MC3Rs has minor effects in “nonobesogenic” conditions, the actions of the receptor appear to be critical for metabolic flexibility when mice are challenged with high fat diets. These receptors are also required for both behavioral and metabolic flexibility during times when food availability occurs outside of the normal circadian rhythm. These actions may be related to the regulation of circadian oscillators and the synchronization of circadian rhythms with nutrient availability.32-34,91,92 Moreover, a recent study by another laboratory reported a critical role for this receptor in the adaptation to fasting, while also suggesting a possible mechanism (mild hyper-corticosteronemia) in explaining the obese phenotype.35
3. THE CENTRAL NERVOUS MELANOCORTIN SYSTEM
3.1. Multiple ligands with biased agonist and competitive antagonist properties
The melanocortin system is comprised of six known ligands that bind with high affinity to five receptors.6,102 The cDNA encoding POMC was one of the first to be identified and sequenced in the late 1970s.103-105 POMC is a precursor polypeptide of 241 amino acids that is processed post-translationally into several peptides including the melanocortins, (β-endorphin, and (β- and γ-lipotropin. The peptides originally considered to function as melanocortin receptor agonists are adrenocorticotrophic hormone (ACTH) and the three isoforms of MSH (α, β and γ). ACTH is produced by corticotroph cells of the anterior pituitary, which express POMC. In the central nervous system, POMC is processed to produce α-MSH, γ-MSH and (in humans, but not rodents) (β-MSH.
The melanocortin system is unique in that soon after the receptors were cloned, high affinity ligands were described with both agonist and antagonist properties. ASIP and AgRP display high affinity for specific melanocortin receptors and were originally reported to function as competitive antagonists98,53,106-108 against the MSH peptides classically considered to have agonist functions. ASIP was the first to be identified by positional cloning and is expressed in hair follicles and regulates pigmentation by modulating activity of the MC1R.51-53 Ectopic expression of ASIP due to a spontaneous insertion mutation in the promoter region (e.g., the lethal yellow (AY/a) mouse) or transgenic expression using a human beta actin promoter produces mice with a yellow coat color. In addition, antagonism of neural MC4Rs results in a hyperphagic obesity syndrome.54,109,110
The second member of the agouti family of ligands to be identified (AgRP) functions as an antagonist in cell–based assays using MC3R and MC4R.98,99,108,111,112 Central administration of AgRP in rodents has the opposite effect to that observed when α-MSH is administered, increasing food intake and altering metabolism to favor deposition of adipose mass.113 Structurally, AgRP is a 132 residue protein composed of two domains. Residues 1–82 (the N–terminus) are removed by prohormone convertases, while a second domain comprising residues 83–132 is sufficient to induce increased food intake113 when administered intracerebroventricularly and is the dominant form found in the hypothalamus.114
Further investigation has indicated that ASIP and AgRP have functions beyond acting as competitive antagonists. MC4Rs display high basal levels of adenylyl cyclase (AC) activity resulting in high cAMP levels that can be reduced in the presence of AgRP, suggesting “inverse agonist” properties.115,116 ASIP functions as an MC1R inverse agonist.117 Mouse genetics also supports the hypothesis that the high level of constitutive activity of melanocortin receptors is physiologically significant. Mice lacking all neural MSH respond to centrally administered AgRP, displaying reduced oxygen consumption, increased respiratory exchange ratio (RER, an indicator of whole body substrate preference), and increased food intake.118 This observation suggests actions of AgRP at MC3R and MC4R in the central nervous system independent of competitive displacement of MSH. The black coat color phenotype of Pomc−/− mice backcrossed onto the C57BL/6J (a/a) background also suggests that the stimulation of eumelanin (black/brown) pigmentation by MC1R is not dependent on α-MSH.119 Finally, an examination of the AY allele crossed onto mouse lines with varying coat colors suggested that antagonism of MC1R by ASIP is not the equivalent to loss of receptor function106; Yang et al. interpreted these results as indicating that ASIP could function as a potential MC1R agonist, stimulating signaling through intracellular pathways other than Gαs coupling.
One of the first mechanisms proposed for explaining the high level of constitutive activity of the MC4R involved the long N-terminal extracellular loop functioning as a “tethered” intramolecular ligand.120 More recently, MSH and AgRP have been suggested to function as biased agonists that regulate the association of MC4R with distinct heterotrimeric G-protein signaling pathways.121 Activation of MC4R by α-MSH stimulates accumulation of cAMP through increasing coupling with Gαs. In contrast, AgRP stimulates the coupling of MC4R expressed in a hypothalamic neuronal cell line (GT1-7) to the Gαi/o subunit, thereby inhibiting adenylate cyclase activity and reducing cellular cAMP levels.
In addition to the melanocortins, “nonclassical” melanocortin ligands were recently identified during a genetic analysis of the K locus controlling pigmentation in dogs.122 The product of the K locus was identified as β-defensin103, which regulates coat color by acting as a high affinity ligand for the MC1R. Interestingly, β-defensin103 also binds with low affinity to MC4Rs. The physiological significance of the interaction between defensins and the melanocortin system, beyond influencing coat color variation, remains to be explored and will not be further addressed in this review. Another recent topic in the melanocortin field that will not be discussed in depth here is heterodimerization of melanocortin receptors with other G protein coupled receptors (GPCR) involved in metabolic homeostasis. For example, MC3Rs form heterodimers with ghrelin receptors (GHSR).123,124 Heterodimers broaden the selection of ligands that can regulate receptor activity while also modulating post-receptor signaling pathways.
3.2. Neurons expressing the endogenous ligands in the central nervous system: “First order” neurons acting as sensors of metabolic status and vigilance
Neurons expressing the endogenous melanocortin ligands have been observed in two brain regions.6 Neurons expressing POMC or AgRP are located in the arcuate nucleus of the hypothalamus, sending projections to similar terminal fields in the forebrain, although AgRP neurons may not send projections to the brain stem.125,126 A smaller population of POMC-expressing neurons is also located in the nucleus of the solitary tract in the brain stem. Deletion of Pomc gene in mice results in adrenal insufficiency due to the absence of ACTH and severe obesity.127 Rescuing Pomc expression in the pituitary exacerbates the obese phenotype by restoring glucocorticoid levels.128 Homozygous carriers of null mutations in the human POMC gene have also been identified who display the same characteristics of morbid obesity and adrenal insufficiency.129 Interestingly, heterozygous carriers of null mutations in the human POMC gene also exhibit increased propensity for obesity.130
As mentioned before, some of the neurons that release the endogenous ligands for the melanocortin receptors are important central targets for leptin; actions involving the central nervous melanocortin system at least partially mediate the effects of leptin on appetite and metabolism.131-135 However, these neurons are probably heterogeneous with respect to the kinds of receptors expressed, and integrate multiple inputs from signals of metabolic state including ghrelin, 36-138 insulin,29,139-142 orexin,143 serotonin,66,144-147 and glucose148,149 (Fig. 4.1). Studies examining hypothalamic neural activity in rats subjected to fasting-refeeding paradigms suggest that these inputs rapidly regulate the activity of arcuate POMC and AgRP neurons.150-152 In fasted conditions, AgRP neurons are “active” while the activity of POMC neurons is suppressed. In rats allowed access to food for 2 hours, an increase in Fos activity is observed 60 and 90 min after the initiation of feeding in ARC neurons positive for α-MSH staining with a return to normal levels at 180 min. These neurons may therefore integrate signals of long-term energy balance (i.e., leptin and perhaps insulin) with acute meal-related signals.
4. CLONING OF THE MELANOCORTIN RECEPTORS
As key regulators of many biochemical cascades, GPCRs are the largest and most diverse class of cell surface receptors in the human genome and the richest source of drug targets for the pharmaceutical industry. It is estimated that GPCRs are the targets of ~27% of all Food and Drug Administration approved drugs.153 GPCRs have evolved to respond to a large palette of extracellular signals such as neurotransmitters, hormones, peptides, lipids, biogenic amines, and metabolites. They control and regulate many physiological responses such as cell proliferation, endocrine hormone secretion, learning and memory processes, heart rate, and energy homeostasis.154 These receptors share a common molecular structure comprising seven hydrophobic transmembrane spanning domains, with an extracellular N-terminus and an intracellular C-terminus linked by three extracellular and three intracellular domains.151 Based on sequence similarity in the seven transmembrane domains, these receptors have been divided into at least six classes/families: rhodopsin-like receptors (Family A), secretin-like receptors (Family B), metabotropic glutamate receptors (Family C), fungal pheromone receptors (Family D), cyclic AMP receptors (Family E), and fiizzled/smoothened receptors.
The first melanocortin receptor (MC1R) was identified using a cDNA library prepared from a human melanoma cell line that exhibited very high levels of MSH binding; the sequence data obtained was used to isolate a second melanocortin receptor (MC2R), which turned out to be the receptor for ACTH.155 Another three receptors were subsequently identified by several laboratories, and were classified as MC3R, MC4R, and MC5R in the order of their discovery.96,156-164 Melanocortin receptors belong to the rhodopsin-like family, which is the largest and most intensively studied family of GPCR.153 The melanocortin receptors were initially reported to be coupled to Gs, with stimulation by agonists leading to increased accumulation of cAMP levels through activation of specific adenylate cyclase isoforms.165,166
Of the five melanocortin receptors identified, two (MC3R and MC4R) are expressed in areas of the central nervous system linked to expression of complex behaviors related to feeding and the regulation of metabolic activity. MC4Rs are widely expressed in the brain (~150 nuclei) including the cortex, thalamus, hypothalamus, brainstem, and spinal cord.55,167 MC4Rs are distributed in many sites involved in autonomic and endocrine function such as the PVN of the hypothalamus, the dorsal motor nucleus of the vagus, and the raphe, consistent with the role of this receptor in feeding and autonomic regulation.65,168,169 In contrast, Mc3r expression measured by in situ hybridization was reported in approximately 35 nuclei that are predominandy in hypothalamic and limbic structures; high levels of expression are observed in the ventromedial hypothalamus (VMH), medial habenula, ventral tegmental area, and central linear nucleus of the raphe.96 Mc3r expression has also been reported in peripheral tissues including the immune system, kidney, and areas of the stomach, duodenum, and pancreas.158
5. MELANOCORTIN-3 RECEPTORS IN METABOLIC HOMEOSTASIS
5.1. Mutations in the human population
A clear role of the endogenous ligands for the melanocortin receptors in energy balance was reported by Krude et al. in 1998, who provided the first description of humans congenitally lacking POMC gene products.129 Patients with POMC deficiency developed severe early onset-obesity, hyperphagia, and hypocortisolemia secondary to ACTH deficiency. Homozygous carriers of null mutations in the POMC gene therefore require glucocorticoid supplementation.170 Obesity in POMC deficiency is due to loss of agonists regulating MC3R and MC4R, two central receptors known to play an important role in the hypothalamic leptin–melanocortin pathway of body weight regulation. In 2006, Farooqi et al. reported that loss of one copy of POMC gene (haploinsufficiency) is sufficient to increase risk of obesity in humans.130 Humans heterozygous for POMC null mutation have a body weight toward the upper end of the normal range or are overweight.130,171 Analysis of mice where one copy of the Pomc gene has been deleted also indicates a gene-dosage effect, with increased propensity for diet-induced obesity.172 Analysis of POMC mutations affecting specific melanocortin peptides also provided considerable insights in understanding the relative importance of particular POMC-derived melanocortin ligands in the control of energy balance. Human genetic studies were particularly important to determine the physiological role of β-MSH, a peptide that is not endogenously present in rodents. In 2006, two studies reporting heterozygous missense mutations in the region of POMC encoding (β-MSH have suggested a role of this peptide in the hypothalamic control of body weight in humans.173,174
Contrasting with these rare monogenic forms of obesity, mutations located in the MC4R gene have been shown to be a frequent cause of an early-onset dominant form of obesity. Defects in MC4R signaling account for approximately 1–6 % morbidly obese individuals across diverse ethnic groups.175-178 Human MC4R deficiency was reported to affect 4% and 5.8% of severely obese French and British populations, respectively.59,175,179 However, other studies reported a lower incidence of MC4R mutations in their respective obese populations. The prevalence of MC4R mutations was particularly low in Asian and Scandinavian populations.178,180 Observation of phenotypes of humans with deficits in MC4R signaling reveal physiological roles of this receptor in the regulation of appetite, body weight, bone mineral content, and cardiovascular function.59,175,176 Subjects with MC4R deficiency exhibit an obese phenotype characterized by an increase in both fat mass and lean mass from early age.59 Besides obesity, clinical features of MC4R deficiency include also hyperphagia, which appears in the first year of life, increased bone mineral density and accelerated linear growth probably due to the disproportionate early hyperinsulinemia.59
In contrast to MC4R mutations, the relevance of MC3R mutations to human obesity is less clear and a pathophysiological role of MC3R in human obesity is still debated. To determine whether variations in the MC3R gene are associated with obesity, large-scale screening studies were conducted on normal and obese subject cohorts. In 2000, Li et al. reported several sequence variants in the coding and 5′ flanking regions of the MC3R gene but no significant associations of MC3R variants with obesity have been detected.181 This absence of association between MC3R variants and obesity was also observed by other groups. Investigating the prevalence and function of MC3R and MC4R mutations in two cohorts of North American severely obese and nonobese subjects, Calton et al. confirmed that MC4R mutations are a significant cause of severe obesity in North American adults whereas MC3R mutations are not associated with severe obesity.182 Recendy, an European study conducted by French and Italian investigators reported that rare MC3R mutations with in vitro functional consequences are associated with human obesity.183 Collectively these studies indicate that the role of MC3R in regulating body weight is not straightforward, and associations of MC3R variants with human obesity are dependent on the ethnicity and the impact of the mutation on receptor functionality. MC3R variants have also been associated with subtle changes in onset of weight gain, hyperleptinemia, and hyperinsulinemia.184,185
5.2. Targeted deletion of the mouse Mc3r gene
The first reports of the phenotype of mice in which the Mc3r gene was targeted came in 2000 from Roger Cone’s laboratory at The Vollum Institute and a group led by Lex Van der Ploeg at Merck.86,87 Amgen also produced a Mc3r knockout mouse strain that was used in a study reported by a third group at the Pennington Biomedical Research Center.90 The phenotype of all three strains is very similar, with Mc3r−/− mice displaying a moderate obesity syndrome characterized by increased adipose mass and reduced lean mass.86,87 Chow-fed Mc3r−/− mice are hyperinsulinemic suggesting an insulin-resistant phenotype commensurate with a mild increase in adiposity. The hyperinsulinemia in chow-fed conditions that were observed in Mc3r−/− mice is modest when compared to mice lacking Mc4r.62,63,69 While the phenotype in the chow-fed condition is modest, Mc3r−/− mice display accelerated obesity when fed high-fat diets.86,87 In these conditions, adiposity, hyperinsulinemia, and impaired glucose tolerance are comparable to that associated with loss of Mc4r when using mice backcrossed onto the B6 background.66
The results from most of these studies suggest that obesity in Mcr−/− mice does not involve hyperphagia, with altered nutrient partitioning owing to reduced fat oxidation as one possible mechanism.63 In one study performed by our laboratory where food intake was measured using an automated system (CLAMS unit), there was moderate hyperphagia during the daytime when Mc3r−/− mice were first fed a high-fat diet.63 However, the increased adiposity in Mc3r−/− mice in chow-fed conditions is subtle and appears to be stable at least to 6–11 months of age.87,93 It is therefore unlikely that marked imbalances between energy intake and expenditure occur in this model. The differences in weight gain observed when Mc3r−/− and wild type mice are fed a high-fat diet can be more pronounced. However, the differences in energy balance are still technically hard to quantify using indirect calorimetry.11 Our observations suggest that a subtle imbalance between fat intake and oxidation may be a significant factor in the accelerated diet-induced obesity.63 Fatty acid oxidation and citrate synthase activity are reduced in skeletal muscle of female Mc3r−/− mice, suggesting reduced mitochondrial activity.62 The physiological significance and mechanisms explaining the differences in mitochondrial activity have, however, not been further explored.
Another potential factor underlying the obese phenotype of Mc3r−/− mice is reduced physical activity. Mc3r−/− mice can exhibit reduced locomotor behavior in the home-cage and reduced wheel running in the dark phase.32,63,86,87,186 Reduced physical activity-based or “nonresting” energy expenditure may therefore play a role in the obese phenotype observed in Mc3r−/− mice.32,62 What has yet to be determined is whether reduced locomotor activity is a behavioral disorder (i.e., reduced frequency in the initiation of movement that might represent an adaptation to negative energy balance) or a result of deficits in skeletal muscle oxidative metabolism suggested by measurements of fatty acid oxidation and citrate synthase activity.62
A very recent article from Roger Cone’s laboratory suggests the interesting hypothesis that MC3Rs are required for adaptation to fasting.35 The increase in adipose tissue lipolysis, accumulation of triglycerides in the liver, and increased activity of the hypothalamo–pituatary adrenal axis is compromised in Mc3r−/− mice. A modest increase in corticosterone in the morning when corticosterone levels are normally low was also proposed as a mechanism for explaining the increased fat mass and reduced lean mass observed in this model. These are important findings, as they are the first to define a real “stimulus-response” function for this receptor. While the site of action and mode of regulation remain to be determined, it is nevertheless interesting to note that the actions of this receptor appear to be dominant in times when AgRP expression is stimulated and POMC activity reduced. Whether this implies that AgRP is acting as a form of “agonist” to regulate the fasting response via modulation of hypothalamic MC3Rs or altered activity of arcuate MC3R/GHSR heterodimers in fasted conditions are involved remains to be determined.
5.3. Response of melanocortin receptor knockout mice to melanocortin analogs
Knockout mice provide an important biological tool to investigate the specificity of action when compounds with high selectivity are not available. They are also a useful tool for investigating “off target” responses in highly selective compounds. Several groups have used Mc3r−/− and Mc4r−/− mice to dissect the role of each receptor in mediating the response to melanocortin analogs, examining for loss of specific responses. In general, the outcomes from these studies have been inconsistent and, in most cases, have not been that informative with respect to defining the actions of MC3Rs.
Marsh et al. reported that Mc4r−/− mice do not respond to centrally administered MTII, a potent nonselective analog, indicating that the inhibitory effect of melanocortin agonists is dependent on functional MC4Rs and that MC3Rs cannot compensate.30 The group at Merck also reported that Mc4r−/− mice do not exhibit reduced food intake and increased oxygen consumption in response to peripherally administered MTII. MC4Rs may therefore be required for regulating both aspects of the energy balance equation in situations where energy balance is positive. MC3Rs are also not able to compensate in the absence of MC4Rs. Consistent with this hypothesis, in an experiment designed to examine the response of the melanocortin receptor mutant mice in a more physiological setting, the increase in oxygen consumption associated with hyperphagia is not observed in Mc4r−/− mice, but is observed in Mc3r−/− mice.60,64 Again, however, this result needs to be interpreted with caution. It could indicate different responses of secondary neurons expressing either receptor following activation of POMC neurons in response to overfeeding, which has been observed in rats.187 However, another plausible interpretation would be a “pre-existing” metabolic condition (e.g., reduced sympathetic tone of Mc4r−/− mice) that alters the response of peripheral tissues to dietary modifications.
We have also investigated the response of Mc3r−/− and Mc4r−/− mice to melanocortin receptor agonists,93 and their response to compounds targeting serotonin receptors expressed by POMC and AgRP neurons.66,146 The melanocortin analogs provided by Biomeasure (BIM-22493, BIM-22511) are potent (EC50 ranging from <1 to 10 nM) agonists for the human MC1R, MC3R, and MC4R, but with low activity at the human MC5R.93 BIM-22493 is effective when administered by intraperitoneal injection at inhibiting feeding in wild type and Mc3r−/− mice, but not in Mc4r−/− mice following an overnight fast. A single injection of BIM-22493 also acutely improved glucose tolerance in obese leptin-deficient (Lepoh/Lepoh) mice, indicating that activation of melanocortin receptors rapidly improves glucose disposal. Studies analyzing melanocortin receptor knockout mice treated with serotonin receptor agonists that improve insulin action through regulating POMC neurons suggest that stimulation of MC4Rs on preganglionic autonomic neurons is the most probable “central” mechanism mediating this response.65,66,188
We also compared the response of wild type, Mc3r−/− and Mc4r−/− mice to BIM-22511 administered by osmotic mini-pump over 2 weeks (Fig. 4.2, Table 4.1). Weight loss was observed in wild type and Mc3r−/− mice, but was not observed in Mc4r−/− mice. Weight loss associated with administration of melanocortin receptor agonists is therefore dependent on functional MC4Rs. Surprisingly there was no significant effect on cumulative food intake in wild type or Mc3r−/− mice. However, Mc4r−/− mice exhibited a modest increase in food intake. Why no significant effect on food intake was observed in wild type mice is not clear, but could be due to the relatively lean phenotype of the wild type and Mc3r−/− mice and modest changes in body weight. This study also used female mice of the B6 background. It would be interesting to repeat these studies with male mice where body weight and adiposity are increased and matched between groups using a high fat diet, as was done for the studies examining the role of melanocortin receptors in response to serotonin receptor agonists.66
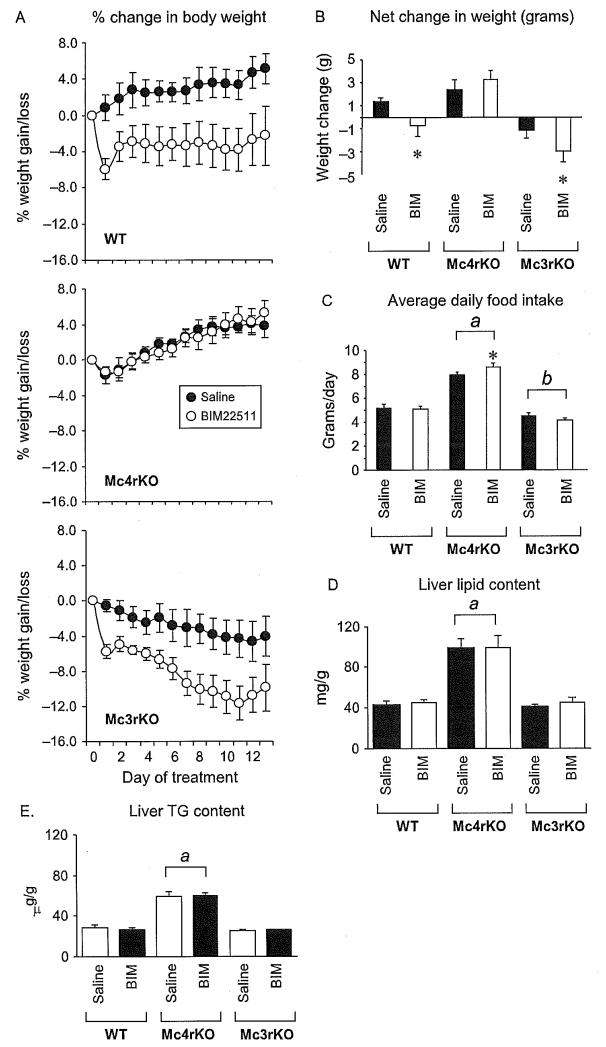
Figure 4.2.
Response of WT, Mc3rKO, and Mc4rKO treated with BIM-22511 for 14 days. (A) Change in body weight as a percent of initial weight in WT (top panel), Mc4rKO (middle panel), or Mc3rKO (bottom panel) treated with BIM-22511 (solid circles) or saline (open circles). Note the absence of a response in Mc4rKO. (B) Net weight gain or loss in mice treated with saline or BIM-22511. The effects of BIM-22511 on body weight are dependent on MC4R activation. (C) Average daily food intake of mice treated with saline or BIM-22511. Mc4rKO were hyperphagic, while Mc3rKO exhibited reduced food intake relative to WT (a, P<0.001 vs. WT, Mc3rKO; b, P< 0.05 vs. WT). Note that the bars with letters indicate within genotype comparisons (i.e., LSM of saline and BIM treatment groups combined for Mc4rKO, compared to LSM of saline and BIM treatment groups for WT). Repeated measures ANOVA indicated a significant increase in Mc4rKO treated with BIM-22511. *P< 0.01 vs. saline within treatment. (D, E) Mc4rKO exhibit hepatic steatosis (elevated liver lipid and triglyceride) relative to WT and Mc3rKO. There was no significant effect of treatment with BIM-22511 on hepatic lipid content, irrespective of genotype. Reproduced with permission from Kumar et al., 2009.93
Another interesting observation made during this experiment was the reduction in fasting insulin and glucose observed in Mc4r−/− mice treated with BIM-22511 for 2 weeks. The reduction (50%) was similar in magnitude to that observed in male diet-induced obese mice, but is clearly not dependent on weight loss. This outcome suggests that some of the effects of melanocortin agonists to improve insulin sensitivity are independent of MC4Rs. The improved insulin sensitivity is probably not hepatic, as the severe fatty liver phenotype and increased expression of insulin-responsive genes associated with obesity was not improved by the treatment. This may suggest a specific role for the functions of MC4Rs in maintaining a healthy liver, or that weight loss is important for improving hepatic steatosis.
The agonist used for this study has very low activity at the MC5R, suggesting that these effects involved MC3Rs (and/or perhaps MClRs). This effect could be a peripheral response, as stimulation of central MC3Rs in the studies using serotonin agonists was not effective.66 Future studies exploring whether MC3R agonists can improve insulin sensitivity and the tissues involved are clearly needed. However, a role for MC3Rs in regulating systems that impact on insulin action is not without precedent. Mc3r−/− mice fed a high-salt diet develop hypertension and insulin resistance. As discussed in the next section, we have observed what appears to be extrahepatic insulin resistance in Mc3r−/− mice subjected to restricted feeding (RF) protocols where food is available in the daytime.92
It is important to note, however, that there are discrepancies in the literature. For example, there have been reports indicating differences in the response of melanocortin receptor knockout mice to MTII. Marsh et al. observed that AgRP retains an ability to stimulate food intake in Mc4r−/− mice when administered icv.,189 although another group only observed a tendency for response when AgRP was administered over 3 days.190 One interpretation of these results is that the “agonist” properties of AgRP at the MC3R can have a modest effect to increase food intake. The basis for this interpretation is that no decrease in food intake has been observed in most studies where MC3Rs are stimulated either directly or indirectly30,66,93,100,146; simple competitive antagonism by AgRP to inhibit the actions of the α- and γ-MSH at this receptor therefore seems an unlikely explanation for the increase in appetite.
We have observed that PG932, a derivative of SHU9119 (the first functional MC3/4R antagonist reported to increase food intake when administered icv.54) and also an MC3/4R antagonist, stimulates food intake when administered peripherally, and that this action is dependent on MC4Rs.191 Navarro et al. observed what appeared to be a stimulatory effect of MTII administered icv. to Mc4r−/− mice, while the inhibitory effects of peripherally administered MTII were retained.192 On the other hand, 194 observed an attenuation of the response of Mc4r−/− (and Mc3r−/−) mice to centrally administered MTII, with a complete loss of responsiveness in double Mc3 r− /−. Mc4r−/− mice.
It is really not clear how we can reconcile these differences in the response of knockout mice to what is considered the “gold standard” melanocortin agonist used to investigate the behavioral and metabolic responses in rodents (MTII). Of course, technical and environmental variation between laboratory settings could be a factor. However, it is also clear that our understanding of the knowledge of melanocortin receptor signaling and pharmacology remains incomplete. There is a clear need for the development of highly selective compounds with clearly defined agonist/antagonist properties at the MC3R and MC4R. These compounds should also be characterized using mouse receptors, as there can be species specificity in activity.193 The pharmacology is further complicated by the issue of biased agonism (i.e., ligands of the same receptor that can stabilize distinct receptor conformations and preferentially modulate one signaling pathway to the exclusion of others) and by the heterodimers formed between the MC3R and MC4Rs with other GPCRs that are involved in the regulation of ingestive behaviors and metabolism.
5.4. MC3Rs and the entrainment of rhythms anticipating food presentation: Mc3r−/− mice as a form of circadian mutant with reduced sensitivity to caloric cues?
Over the last 5 years, one focus of our laboratory has been to investigate whether MC3Rs are modulators of feeding-related inputs into neurons governing the development of food anticipatory activity (FAA).32-34,91,92,195 In the circadian research field, scheduled feeding protocols are frequently used to entrain food-seeking behavior in rodents. Indeed, restricting nutrient availability to defined periods at 24 h intervals induces rhythms of locomotor activity and increased wakefulness that anticipate food presentation.196 In most life forms, the synchronization of rhythms in activity, metabolism, and ingestive behaviors is essential for adaptation to cyclical environmental changes. The ability to predict food intake is an important adaptive feature for surviving competitive environments where nutrients may be limited while also having an important role in the daily maintenance of metabolic homeostasis.197
Biological clocks play an important but sill poorly understood role in synchronizing rhythms with caloric intake. The “molecular clock” machinery is broadly expressed in the CNS and in numerous peripheral tissues.198,199 The core clock machinery responsible for these rhythms is composed of nuclear transcription factors (Bmal1, Clock, Npas2, RORα) and transcription repressors (Per1/2, Cry1/2, Rev-erbα) that establish rhythms of approximately 24 h.195,200 These transcription factors affect the activity of a large proportion of the transcriptome (approximately 3000 genes) by direct or indirect mechanisms. In mammals, many aspects of behavior and physiology including sleep/wake cycles, locomotor activity, blood pressure, body temperature, hormone secretion, and metabolic pathways exhibit daily rhythmicity under the control of circadian clock.195,200 Presented first as simple timekeepers, clocks oscillators are now recognized to be essential in metabolic processes and the maintenance of energy homeostasis. Emerging evidence indicates that disturbances of circadian rhythms because of either mutations in clock genes201,202 or lifestyle modifications203,20 increase the risk of developing a metabolic syndrome.
The master clock that synchronizes daily rhythms with photoperiod resides in the suprachiasmatic nucleus (SCN) of the hypothalamus. The master clock is able to maintain rhythms even in absence of photic cues. Outside the SCN, other hypothalamic structures have been implicated for maintaining expression of rhythms especially during periods of nutrient insufficiency.205,206 Clocks residing outside the SCN are rapidly entrained by feeding-related cues that represent the dominant “zeitgeber” or time giver. These clocks in turn send outputs that regulate many enzymatic processes related to energy homeostasis. Because of their properties to sense and rapidly respond to nutritional cues, these oscillator centers located outside of the SCN have been suggested to constitute the Food Entrainable Oscillator (FEO).207,208 The entrainment to feeding has been proposed to involve clocks distributed in the brain and in peripheral tissues suggesting the existence of several FEO loci. In peripheral tissues, clock oscillators can be rapidly entrained by hormonal signals and restricting feeding.198 In the liver, clock gene expression is entrained within 2 h even before apparition of FAA suggesting that peripheral oscillators are directly responsive to cellular energy status.209
To determine whether MC3Rs regulate inputs into systems governing the expression of rhythms anticipating nutrient intake, wild type and Mc3r knockout mice were subjected to a RF protocol. After acclimation to housing conditions where activity can be monitored using running wheels33,34,92 or infrared beam breaks,91 access to food was limited to defined intervals with a period length of 24 h. Studies using two models of Mc3r deficiency (the original Mc3r−/− mouse86 backcrossed onto the B6 background and the Mc3rTB/TB mouse32 developed by our laboratory discussed later in this review) where running wheels were used to measure FAA yielded similar results. They suggest that MC3Rs are necessary for the coordinated development of anticipatory activity and increased wakefulness in response to RF.32,34 Wild type mice subjected to the RF rapidly respond by increasing their activity and vigilance 2h prior food presentation, while Mc3r−/− mice exhibit a significant attenuation of FAA. A similar outcome was observed when Mc3r−/− mice housed in constant dark were subjected to the RF protocol.33
We have also observed abnormal rhythmic expression of clock genes in the cortex of Mc3r−/− mice in ad libitum and RF conditions.32-34 MC3Rs may thus have a critical role in maintaining the activity of clocks distributed throughout the central nervous system. This is an important observation as clocks have been implicated in a range of behavioral disorders including bipolar disorder, depression, mania, sensitivity to drugs, and the formation and consolidation of memories.210
A recent paper published in PLoS One examined the phenotype of double Lepoh/Lepob. Mc3r−/− mice, and suggested that MC3Rs are not essential for FAA.211 The authors reported an inhibitory effect of leptin on FAA, as anticipated based on previous studies using rats with mutations in the leptin receptor.212 Lepob/Lepob. Mc3r−/− mice actually displayed increased FAA while the inhibitory effects of leptin were preserved. While this result appears at face value to discount a role for MC3Rs in modulating signals governing FAA, these results should be viewed with caution. Bouret and Simerly observed that leptin plays a critical role as a trophic factor in the development of the hypothalamus, stimulating the growth of projections from the arcuate nucleus to other areas (e.g., dorsomedial hypothalamus and PVN).213 Lepob/Lepob mice display marked and permanent disruptions in neural projections from the arcuate. The central nervous melanocortin system is therefore likely also immature in this model, and the results from studies using it are open to debate (including results of our own experiments examining the response of Lepob /Lepob. Mc4r−/− mice to leptin135)
MC3Rs are also highly expressed in hypothalamic regions that are critically involved in metabolic homeostasis (e.g., the arcuate and ventromedial nuclei of the hypothalamus96). While some studies have reported the importance of clock genes in the expression of food anticipatory behavior, others have questioned this role arguing that the absence of FAA observed in clock mutants is more a consequence of a lethargic and moribund state due to the severe RF paradigm.214,215 We therefore investigated whether Mc3r− /− mice would exhibit evidence of metabolic distress during RF.92 While Mc3r−/− mice fed ad libitum exhibited normal glycemia and insulinemia throughout the circadian day, remarkably, they developed a state of insulin resistance and dyslipidemia during RF despite the weight loss promoted by the RF. They also exhibited hyperglycemia, hyperinsulinemia, and glucose intolerance. The expression of genes involved in lipogenesis in the liver was increased as were serum triglycerides. The expression of insulin-responsive lipogenic enzymes correlated with the degree of hyperinsulinism, while the expression of genes involved in gluconeogenesis was reduced. This combination suggests a “mixed insulin resistant” phenotype, where the liver is responding normally to insulin while other tissues responsible for glucose clearance such as skeletal muscle are probably insulin resistant. In contrast, obese Mc4r−/− and AV /a mice responded to RF by exhibiting the anticipated weight loss and improvements in glucose clearance.92 Mc3r−/− mice thus display a unique combination of physical characteristics when subjected to daytime feeding, exhibiting deteriorating metabolic homeostasis while losing weight.
Mc3r−/− mice also exhibit abnormal rhythmicity in the expression of clock genes in the liver, and abnormal rhythmicity of metabolic oscillators implicated in glucose and lipid metabolism during the RF.33,92 While this could indicate a role for MC3R to influence rhythms of the liver clock, it is possible that this result is due to feedback/inhibitory effects of abnormalities in metabolic homeostasis that develop in Mc3r−/− mice during RF.
5.5. Cell-specific restoration of Mc3r expression in LoxTB Mc3r mice: Potential for new insights into the biology of MC3Rs
Two factors have hindered progress in the research of MC3R function. One important problem that is beyond the focus of this article is a scarcity of compounds with specific agonist and antagonist properties. Another issue has been the lack of transgenic approaches using more sophisticated techniques beyond the global knockout approach originally employed to examine MC3R function. To address this issue, we have developed the LoxTB Mc3r mouse using a similar strategy used in the genetic dissection of Mc4r activity.70 A “transcriptional block” flanked by LoxP site (LoxTB) was inserted into the 5′ UTR of the Mc3r gene, and various Cre-expressing transgenic strains were used to remove the LoxTB sequence and restore expression.32
Homozygous carriers of the LoxTB Mc3r allele exhibit the phenotype observed in all of the previously developed Mc3r knockout strains, with reduced lean mass and increased fat mass and normal food intake in chow-fed conditions. They also exhibit the accelerated diet-induced obese phenotype. Crossing LoxTB Mc3r mouse with Eiia-Cre mice to remove the LoxTB sequence in early embryogenesis rescued the phenotype. We then used two Cre-expressing lines to answer two questions: the first was to use the Nestin-Cre transgene to investigate whether restoring central MC3R expression would rescue the obese phenotype as observed in the LoxTB Mc4r mouse.70 The second was to examine the function of MC3R expressed in the VMH. The rationale for focusing on the VMH was based on previous studies of expression of the Mc3r gene in the rodent brain, and on the abundance of data suggesting functions of VMH neurons in metabolic homeostasis. The dorsomedial VMH (dmVMH) exhibits high levels of Mc3r expression relative to other hypothalamic and limbic structures.96 The VMH is also known to have a role in the regulation of outputs governing glucose homeostasis and that impact on obesity.216 When the project began, lesioning experiments had suggested a role for the VMH in the expression of FAA.205,217 More defined studies had suggested that the dmVMH may have an inhibitory role in the expression of FAA,206 while others have questioned whether any of the hypothalamic nuclei targeted using the lesion approach are required for FAA.207,218
The first surprising outcome from these studies was the failure of the Nestin-Cre transgenic to restore a normal body weight phenotype. Expression of MC3R in the brain attenuated the obese phenotype in chow-fed conditions, while having no affect on the obese phenotype when mice were fed a high-fat diet.32 While this experiment is being repeated, the results nevertheless are startling in suggesting that the functions of peripheral MC3R have some role in the defense of body weight when dietary fat intake is elevated. Ongoing studies are further exploring the phenotype of NesCre. LoxTB Mc3r mice in an attempt to identify the role of peripheral MC3R. We are also investigating whether the expression of FAA is restored in NesCre.LoxTB Mc3r mice. While speculative, one possible outcome is that the regulation of circadian-related behaviors will involve “central” MC3R, while the accelerated diet-induced obese phenotype is due to loss of peripheral receptors that impact on metabolic homeostasis.
Sf1Cre.LoxTB Mc3r mice exhibited a very modest attenuation of the obese phenotype, while the reduction in lean mass was similar to that observed in LoxTB Mc3r mice. There was evidence for improvements in metabolic homeostasis, with fasting hyperinsulinemia normalized suggesting improved insulin sensitivity. Changes in liver gene expression in LoxTB Mc3r mice suggested altered lipid metabolism, and perhaps fatty liver, were also improved in SflCre.LoxTB Mc3rmice. These observations suggest that the functions of MC3Rs expressed in Sf1(+ve) cells can improve metabolic homeostasis in situations of mild obesity. This effect was not due to altered food intake, while analysis using indirect calorimetry suggested altered metabolism. In this group of mice, a significant reduction in the RER was observed in LoxTB Mc3r mice. This suggests a change in substrate preference with less carbohydrate oxidation and increased fat oxidation. LoxTB Mc3r mice also exhibited a normal resting energy expenditure (REE) and reductions in “nonresting energy expenditure” (NREE), which was calculated by subtracting REE from total daily energy expenditure.
6. CONCLUSIONS AND FUTURE DIRECTIONS
Over the last decade, technologic advances in genetics, pharmacology, and molecular biology have largely improved our understanding of how the melanocortin system controls energy balance. This system is important in integrating many afferent inputs with behavioral and autonomic responses that adjust food intake and energy expenditure in order to maintain energy homeostasis.
Indeed, melanocortin neurons not only regulate food intake by processing acute and long-term signals related to nutrient intake and adipostatic status but also play a role in regulating energy expenditure, via effects on autonomic outflow to a variety of tissues, as well as effects on endocrine axes. Generation of more refined transgenic animal models harboring disruption of MC3Rs in specific brain areas enabled dissection of neuronal subpopulations that control food intake and/or energy expenditure. Studies conducted in our laboratory have participated in determining the role of MC3R in energy homeostasis. Our results indicate a new homeostatic function of this receptor that does not implicate the classical pathway of reduced food intake and increased energy expenditure to maintain energy balance. Indeed, our studies indicate that MC3Rs could be important mediators in transmitting feeding-related inputs into neurons governing the development of anticipatory activity especially during periods of nutrient insufficiency. MC3R expression is important for maintaining glucose homeostasis and rhythmicity of clock genes known to regulate gluconeogenic and lipogenic enzymes as well as rhythms in glucose and lipid metabolism. Although these findings have contributed to a better understanding of the function of MC3R in energy homeostasis, further investigations targeting reactivation of MC3R in specific brain areas will be useful to identify the neuronal sub-populations of MC3Rs involved in the maintenance of energy homeostasis and circadian rhythms.
ACKNOWLEDGMENTS
Research on the physiological roles of the melanocortin receptors by Andrew Butler’s laboratory has been supported by the National Institutes of Health (DK073189), The Pennington Biomedical Research Foundation, and The Scripps Research Institute.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 4.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–73. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 5.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 7.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9(2):117–23. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20(5):203–15. doi: 10.1016/j.tem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermo-genesis. Nature. 2000;404(6778):652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 10.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59(2):323–9. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11(4):263–7. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214(Pt 2):242–53. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 15.Flatt JP. Issues and misconceptions about obesity. Obesity (Silver Spring) 2011;19(4):676–86. doi: 10.1038/oby.2011.7. [DOI] [PubMed] [Google Scholar]
- 16.Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011;4(6):733–45. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52(2):232–8. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- 18.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87(2):221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Bray GA, York DA. The MONA LISA hypothesis in the time of leptin. Recent Prog Horn Res. 1998;53:95–117. [discussion 117-8] [PubMed] [Google Scholar]
- 20.Friedman JM. A war on obesity, not the obese. Science. 2003;299(5608):856–8. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- 21.O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462(7271):307–14. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- 22.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16(10):1097–9. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 24.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 25.Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4(10):569–77. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 26.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–93. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414(6859):34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 28.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–51. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30(7):2472–9. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21(1):119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 31.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 32.Begriche K, Levasseur PR, Zhang J, Rossi J, Skorapa D, Solt LA, et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane g-protein coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J Biol Chem. 2011;286(47):40771–81. doi: 10.1074/jbc.M111.278374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begriche K, Sutton GM, Butler AA. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav. 2011;104(4):546–54. doi: 10.1016/j.physbeh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, et al. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28(48):12946–55. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, et al. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci USA. 2012;109(23):E1489–E1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89(3):973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113(3):414–24. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vong L, Ye C, Yang Z, Choi B, Chuajr S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148(8):3987–97. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 41.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115(12):3484–93. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94(16):8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118(7):2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87(5):2391–4. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 46.Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab. 2010;95(2):851–5. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5(6):e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trevaskis JL, Meyer EA, Galgani JE, Butler AA. Counterintuitive effects of double-heterozygous null melanocortin-4 receptor and leptin genes on diet-induced obesity and insulin resistance in C57BL/6J mice. Endocrinology. 2008;149(1):174–84. doi: 10.1210/en.2007-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yen PM, Chin WW. Molecular mechanisms of dominant negative activity by nuclear hormone receptors. Mot Endocrinol. 1994;8(11):1450–4. doi: 10.1210/mend.8.11.7877614. [DOI] [PubMed] [Google Scholar]
- 51.Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993;7(3):454–67. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 52.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 53.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 54.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 55.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8(10):1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 56.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 57.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20(2):113–4. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 58.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20(2):111–2. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 59.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 60.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4(6):605–11. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 61.Ste Marie L, Miura GI, Marsh DJ, Yagalof FK, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA. 2000;97(22):12339–44. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147(5):2183–96. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27(2):281–90. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145(1):243–52. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- 65.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6(5):398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–88. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108(7):1079–85. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9(6):537–47. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 71.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28(19):4957–66. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7(4):335–6. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 73.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R247–R258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 74.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146(9):3739–47. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 75.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141(4):1332–7. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 76.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18(23):10128–35. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 78.Williams DL, Schwartz MW. Neuroanatomy of body weight control: lessons learned from leptin. J Clin Invest. 2011;121(6):2152–5. doi: 10.1172/JCI58027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong JZ, DeOliveira DB, Halem HA, Taylor JE, Roubert P, Plas P, et al. Novel melanocortin-4 receptor agonists that decrease food intake and body weight. Adv Exp Med Biol. 2009;611:485–6. doi: 10.1007/978-0-387-73657-0_209. [DOI] [PubMed] [Google Scholar]
- 80.Pantel J, Williams SY, Mi D, Sebag J, Corbin JD, Weaver CD, et al. Development of a high throughput screen for allosteric modulators of melanocortin-4 receptor signaling using a real time cAMP assay. Eur J Pharmacol. 2011;660(1):139–47. doi: 10.1016/j.ejphar.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Albert JS, Blomberg N, Breeze AL, Brown AJ, Burrows JN, Edwards PD, et al. An integrated approach to fragment-based lead generation: philosophy, strategy and case studies from AstraZeneca’s drug discovery programmes. Curr Top Med Chem. 2007;7(16):1600–29. doi: 10.2174/156802607782341091. [DOI] [PubMed] [Google Scholar]
- 82.Emmerson PJ, Fisher MJ, Yan LZ, Mayer JP. Melanocortin-4 receptor agonists for the treatment of obesity. Curr Top Med Chem. 2007;7(11):1121–30. doi: 10.2174/156802607780906636. [DOI] [PubMed] [Google Scholar]
- 83.Ujjainwalla F, Sebhat IK. Small molecule ligands of the human melanocortin-4 receptor. Curr Top Med Chem. 2007;7(11):1068–84. doi: 10.2174/156802607780906609. [DOI] [PubMed] [Google Scholar]
- 84.Hruby VJ, Cai M, Cain J, Nyberg J, Trivedi D. Design of novel melanocortin receptor ligands: multiple receptors, complex pharmacology, the challenge. Eur J Pharmacol. 2011;660(1):88–93. doi: 10.1016/j.ejphar.2010.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 86.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 87.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 88.Trevaskis JL, Gawronska-Kozak B, Sutton GM, McNeil M, Stephens JM, Smith SR, et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 2007;15(11):2664–72. doi: 10.1038/oby.2007.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148(12):6186–94. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005;19(11):1482–91. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 91.Begriche K, Maston O, Rossi J, Burke L, McDonald P, Heisler LK, et al. Melanocortin-3 receptors are required for metabolic adaptation to food restriction. Genes Brain Behav. 2012;11(3):291–302. doi: 10.1111/j.1601-183X.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R, et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24(3):862–72. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. 2009;30(10):1892–900. doi: 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007;282(5):2862–70. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- 95.Ni XP, Humphreys MH. Abnormal glucose metabolism in hypertensive mice with genetically interrupted gamma-melanocyte stimulating hormone signaling fed a high-sodium diet. Am J Hypertens. 2008;21(12):1284–7. doi: 10.1038/ajh.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90(19):8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adan RA, Cone RD, Burbach JP, Gispen WH. Differential effects of melanocortin peptides on neural melanocortin receptors. Mol Pharmacol. 1994;46(6):1182–90. [PubMed] [Google Scholar]
- 98.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 99.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 100.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9(2):145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 101.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284(3):E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 103.Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-Hpotropin precursor. Nature. 1979;278(5703):423–7. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 104.Kita T, Inoue A, Nakanishi S, Numa S. Purification and characterization of the messenger RNA coding for bovine corticotropin/beta-lipotropin precursor. Eur J Biochem. 1979;93(2):213–20. doi: 10.1111/j.1432-1033.1979.tb12813.x. [DOI] [PubMed] [Google Scholar]
- 105.Roberts JL, Seeburg PH, Shine J, Herbert E, Baxter JD, Goodman HM. Corticotropin and beta-endorphin: construction and analysis of recombinant DNA complementary to mRNA for the common precursor. Proc Natl Acad Sci USA. 1979;76(5):2153–7. doi: 10.1073/pnas.76.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998;12(3):316–30. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, et al. Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol. 1997;11(3):274–80. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 108.Rosenfeld RD, Zeni L, Welcher AA, Narhi LO, Hale C, Marasco J, et al. Biochemical, biophysical, and pharmacological characterization of bacterially expressed human agouti-related protein. Biochemistry. 1998;37(46):16041–52. doi: 10.1021/bi981027m. [DOI] [PubMed] [Google Scholar]
- 109.Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci USA. 1995;92(11):4728–32. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perry WL, Hustad CM, Swing DA, Jenkins NA, Copeland NG. A transgenic mouse assay for agouti protein activity. Genetics. 1995;140(1):267–74. doi: 10.1093/genetics/140.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tota MR, Smith TS, Mao C, MacNeil T, Mosley RT, Van der Ploeg LH, et al. Molecular interaction of Agouti protein and Agouti-related protein with human melanocortin receptors. Biochemistry. 1999;38(3):897–904. doi: 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]
- 112.Yang YK, Thompson DA, Dickinson CJ, Wilken J, Barsh GS, Kent SB, et al. Characterization of Agouti-related protein binding to melanocortin receptors. Mol Endocrinol. 1999;13(1):148–55. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]
- 113.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 114.Creemers JW, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, et al. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147(4):1621–31. doi: 10.1210/en.2005-1373. [DOI] [PubMed] [Google Scholar]
- 115.Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15(1):164–71. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 116.Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regal Pept. 2001;99(1):1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- 117.Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, Eberle AN. Interactions of alpha-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J Recept Signal Tmnsduct Res. 1997;17(1-3):75–98. doi: 10.3109/10799899709036595. [DOI] [PubMed] [Google Scholar]
- 118.Tolle V, Low MJ. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57(1):86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 119.Smart JL, Low MJ. Lack of proopiomelanocortin peptides results in obesity and defective adrenal function but normal melanocyte pigmentation in the murine C57BL/6 genetic background. Ann N Y Acad Sci. 2003;994:202–10. doi: 10.1111/j.1749-6632.2003.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 120.Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, et al. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114(8):1158–64. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buch TR, Heling D, Damni E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284(39):26411–20. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, et al. A b-defensin mutation causes black coat color in domestic dogs. Science. 2007;318(5855):1418–23. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rediger A, Piechowski CL, Habegger K, Gruters A, Krude H, Tschop MH, et al. MC4R dimerization in the paraventricular nucleus and GHSR/MC3R heterodimerization in the arcuate nucleus: is there relevance for body weight regulation. Neuroendocrinology. 2012;95(4):277–88. doi: 10.1159/000334903. [DOI] [PubMed] [Google Scholar]
- 124.Rediger A, Piechowski CL, Yi CX, Tarnow P, Strotmann R, Gruters A, et al. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem. 2011;286(45):39623–31. doi: 10.1074/jbc.M111.287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, et al. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140(3):1408–15. doi: 10.1210/endo.140.3.6544. [DOI] [PubMed] [Google Scholar]
- 127.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5(9):1066–70. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 128.Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116(2):495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Graters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 130.Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, et al. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55(9):2549–53. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- 131.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–92. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 132.Wilson BD, Bagnol D, Kaelin CB, Ollmann MM, Gantz I, Watson SJ, et al. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology. 1999;140(5):2387–97. doi: 10.1210/endo.140.5.6728. [DOI] [PubMed] [Google Scholar]
- 133.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, et al. Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 134.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–85. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Trevaskis JL, Butler AA. Double leptin and melanocortin-4 receptor gene mutations have an additive effect on fat mass and are associated with reduced effects of leptin on weight loss and food intake. Endocrinology. 2005;146(10):4257–65. doi: 10.1210/en.2005-0492. [DOI] [PubMed] [Google Scholar]
- 136.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454(7206):846–51. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 138.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 139.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11(4):286–97. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in AgRP and POMC neurons. Diabetes. 2010;59(2):337–46. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–49. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 142.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22(20):9048–52. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–4. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 144.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582–9. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O’Rahilly S, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149(3):1323–8. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thomton-Jones Z, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239–49. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297(5581):609–11. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 148.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144(4):1331–40. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 149.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 150.Singru PS, Sanchez E, Fekete C, Lechan RM. Importance of melanocortin signaling in refeeding-induced neuronal activation and satiety. Endocrinology. 2007;148(2):638–46. doi: 10.1210/en.2006-1233. [DOI] [PubMed] [Google Scholar]
- 151.Sanchez E, Singru PS, Acharya R, Bodria M, Fekete C, Zavacki AM, et al. Differential effects of refeeding on melanocortin-responsive neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2008;149(9):4329–35. doi: 10.1210/en.2008-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4(4):313–21. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Williams C, Hill SJ. GPCR signaling: understanding the pathway to successful drug discovery. Methods Mol Biol. 2009;552:39–50. doi: 10.1007/978-1-60327-317-6_3. [DOI] [PubMed] [Google Scholar]
- 154.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 155.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–51. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 156.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200(3):1214–20. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 157.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268(20):15174–9. [PubMed] [Google Scholar]
- 158.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268(11):8246–50. [PubMed] [Google Scholar]
- 159.Chhajlani V, Muceniece R, Wikberg JE. Molecular cloning of a novel human melanocortin receptor. Biochem Biophys Res Commun. 1996;;218(2):638. doi: 10.1006/bbrc.1996.0113. [DOI] [PubMed] [Google Scholar]
- 160.Chhajlani V, Muceniece R, Wikberg JE. Molecular cloning of a novel human melanocortin receptor. Biochem Biophys Res Commun. 1993;195(2):866–73. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- 161.Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309(3):417–20. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 162.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200(2):1007–14. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 163.Barrett P, MacDonald A, Helliwell R, Davidson G, Morgan P. Cloning and expression of a new member of the melanocyte-stimulating hormone receptor family. J Mol Endocrinol. 1994;12(2):203–13. doi: 10.1677/jme.0.0120203. [DOI] [PubMed] [Google Scholar]
- 164.Labbe O, Desamaud F, Eggerickx D, Vassart G, Parmentier M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. 1994;33(15):4543–9. doi: 10.1021/bi00181a015. [DOI] [PubMed] [Google Scholar]
- 165.Srinivasan S, Vaisse C, Conklin BR. Engineering the melanocortin-4 receptor to control G(s) signaling in vivo. Ann N Y Acad Sci. 2003;994:225–32. doi: 10.1111/j.1749-6632.2003.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 166.Kim CS, Lee SH, Kim RY, Kim BJ, Li SZ, Lee IH, et al. Identification of domains directing specificity of coupling to G-proteins for the melanocortin MC3 and MC4 receptors. J Biol Chem. 2002;277(35):31310–7. doi: 10.1074/jbc.M112085200. [DOI] [PubMed] [Google Scholar]
- 167.Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol. 2010;681:29–48. doi: 10.1007/978-1-4419-6354-3_3. [DOI] [PubMed] [Google Scholar]
- 168.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor niRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–35. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 169.Yi CX, Kalsbeek A, Tschop MH. Autonomic MC sets the metabolic tone. Cell Metab. 2011;13(2):121–3. doi: 10.1016/j.cmet.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 170.Krade H, Gruters A. Implications of proopiomelanocortin (POMC) mutations in humans: the POMC deficiency syndrome. Trends Endocrinol Metab. 2000;11(1):15–22. doi: 10.1016/s1043-2760(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 171.Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, et al. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88(10):4633–40. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- 172.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci USA. 2004;101(13):4695–700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3(2):135–40. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 174.Biebermann H, Castaneda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, et al. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006;3(2):141–6. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 175.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106(2):253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O’Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12(5):561–74. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- 177.Wang CL, Liang L, Wang HJ, Fu JF, Hebebrand J, Hinney A. Several mutations in the melanocortin 4 receptor gene are associated with obesity in Chinese children and adolescents. J Endocrinol Invest. 2006;29(10):894–8. doi: 10.1007/BF03349193. [DOI] [PubMed] [Google Scholar]
- 178.Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab. 2005;90(1):219–24. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 179.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ohshiro Y, Sanke T, Ueda K, Shimajiri Y, Nakagawa T, Tsunoda K, et al. Molecular scanning for mutations in the melanocortin-4 receptor gene in obese/diabetic Japanese. Ann Hum Genet. 1999;63(Pt 6):483–7. doi: 10.1017/S0003480099007782. [DOI] [PubMed] [Google Scholar]
- 181.Li WD, Joo EJ, Furlong EB, Galvin M, Abel K, Bell CJ, et al. Melanocortin 3 receptor (MC3R) gene variants in extremely obese women. Int J Obes Relat Metab Disord. 2000;24(2):206–10. doi: 10.1038/sj.ijo.0801114. [DOI] [PubMed] [Google Scholar]
- 182.Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, et al. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor imitations with severe adult obesity in a large North American case-control study. Hum Mol Genet. 2009;18(6):1140–7. doi: 10.1093/hmg/ddn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mencarelli M, Dubern B, Alili R, Maestrini S, Benajiba L, Tagliaferri M, et al. Rare melanocortin-3 receptor mutations with in vitro functional consequences are associated with human obesity. Hum Mol Genet. 2011;20(2):392–9. doi: 10.1093/hmg/ddq472. [DOI] [PubMed] [Google Scholar]
- 184.Yiannakouris N, Melistas L, Kontogianni M, Heist K, Mantzoros CS. The Val81 missense mutation of the melanocortin 3 receptor gene, but not the 1908c/T nucleotide polymorphism in lamin A/C gene, is associated with hyperleptinemia and hyperinsulinemia in obese Greek Caucasians. J Endocrinol Invest. 2004;27(8):714–20. doi: 10.1007/BF03347511. [DOI] [PubMed] [Google Scholar]
- 185.Schalin-Jantti C, Valh-Jaakola K, Oksanen L, Martelin E, Laitinen K, Krusius T, et al. Melanocortin-3-receptor gene variants in morbid obesity. Int J Obes Relat Metab Disord. 2003;27(1):70–4. doi: 10.1038/sj.ijo.0802184. [DOI] [PubMed] [Google Scholar]
- 186.Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144(4):1513–23. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- 187.Hagan MM, Rushing PA, Schwartz MW, Yagaloff KA, Burn P, Woods SC, et al. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19(6):2362–7. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010;13(12):1457–9. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999;848(1–2):66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- 190.Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, et al. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002;143(10):3846–53. doi: 10.1210/en.2002-220338. [DOI] [PubMed] [Google Scholar]
- 191.Sutton GM, Josephine Babin M, Gu X, Hruby VJ, Butler AA. A derivative of the melanocortin receptor antagonist SHU9119 (PG932) increases food intake when administered peripherally. Peptides. 2008;29(1):104–11. doi: 10.1016/j.peptides.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Navarro M, Lerma-Cabrera JM, Carvajal F, Lowery EG, Cubero I, Thiele TE. Assessment of Voluntary Ethanol Consumption and the Effects of a Melanocortin (MC) Receptor Agonist on Ethanol Intake in Mutant C57BL/6J Mice Lacking the MC-4 Receptor. Alcohol Clin Exp Res. 2011;35(6):1058–66. doi: 10.1111/j.1530-0277.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Joseph CG, Yao H, Scott JW, Sorensen NB, Marnane RN, Mountjoy KG, et al. Gamma-Melanocyte stimulation hormone (gamma-MSH) truncation studies results in the cautionary note that gamma-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31(12):2304–13. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rowland NE, Schaub JW, Robertson KL, Andreasen A, Haskell-Luevano C. Effect of MTII on food intake and brain c-Fos in melanocortin-3, melanocortin-4, and double MC3 and MC4 receptor knockout mice. Peptides. 2010 Dec;31(12):2314–7. doi: 10.1016/j.peptides.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Begriche K, Sutton GM, Fang J, Butler AA. The role of melanocortin neuronal pathways in circadian biology: a new homeostatic output involving melanocortin-3 receptors? Obes Rev. 2009;10(Suppl. 2):14–24. doi: 10.1111/j.1467-789X.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18(2):171–95. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 197.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121(6):2133–41. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105(39):15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zvonic S, Floyd ZE, Mynatt RL, Gimble JM. Circadian rhythms and the regulation of metabolic tissue function and energy homeostasis. Obesity (Silver Spring) 2007;15(3):539–43. doi: 10.1038/oby.2007.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Scheer FA, Hilton MF, Mantzoros GS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17(11):2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous Oscillator? J Neurosci. 1998;18(10):3843–52. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 207.Davidson AJ. Search for the feeding-entrainable circadian oscillator: a complex proposition. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1524–R1526. doi: 10.1152/ajpregu.00073.2006. [DOI] [PubMed] [Google Scholar]
- 208.Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, et al. Standards of evidence in chronobiology: critical review of a report that restoration of Bmall expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1 −/− mice. J Circadian Rhythms. 2009;7:3. doi: 10.1186/1740-3391-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bass J, Takahashi JS. Circadian rhythms: redox redux. Nature. 2011;469(7331):476–8. doi: 10.1038/469476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Menet JS, Rosbash M. When brain clocks lose track of time: cause or consequence of neuropsychiatric disorders. Curr Opin Neurobiol. 2011;21(6):849–57. doi: 10.1016/j.conb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, Mark AL. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One. 2011;6(8):e23364. doi: 10.1371/journal.pone.0023364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mistlberger RE, Marchant EG. Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat. Physiol Behav. 1999;66(2):329–35. doi: 10.1016/s0031-9384(98)00311-4. [DOI] [PubMed] [Google Scholar]
- 213.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 214.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Misdberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22(6):467–78. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- 215.Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29(7):1447–60. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- 216.Kim KW, Sohn JW, Kohno D, Xu Y, Williams K, Elmquist JK. SF-1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Mol Cell Endocrinol. 2011;336(1–2):219–23. doi: 10.1016/j.mce.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mistlberger RE, Rechtschaffen A. Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol Behav. 1984;33(2):227–35. doi: 10.1016/0031-9384(84)90104-5. [DOI] [PubMed] [Google Scholar]
- 218.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–45. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]