Abstract
Objective/Background
Although shoulder pain is a problem in up to 86% of persons with a spinal cord injury (SCI), so far, no studies have empirically identified longitudinal patterns (trajectories) of musculoskeletal shoulder pain after SCI. The objective of this study was: (1) to identify distinct trajectories of musculoskeletal shoulder pain in persons with SCI, and (2) to determine possible predictors of these trajectories.
Design/Methods
Multicenter, prospective cohort study in 225 newly injured persons with SCI.
Outcome Measure
Shoulder pain was assessed on five occasions up to 5 years after discharge. Latent class growth mixture modeling was used to identify the distinct shoulder pain trajectories.
Results
Three distinct shoulder pain trajectories were identified: (1) a “No or Low pain” trajectory (64%), (2) a “High pain” (30%) trajectory, and (3) a trajectory with a “Decrease of pain” (6%). Compared with the “No or Low pain” pain trajectory, the “High pain” trajectory consisted of more persons with tetraplegia, shoulder pain before injury, limited shoulder range of motion (ROM), lower manual muscle test scores, or more spasticity at t1. Multiple logistic regression analysis showed two significant predictors for the “High pain” trajectory (as compared with the “No or Low pain” trajectory): having a tetraplegia (odds ratio (OR) = 3.2; P = 0.002) and having limited shoulder ROM (OR = 2.8; P = 0.007).
Conclusion
Shoulder pain in people with SCI follows distinct trajectories. At risk for belonging to the “High pain” trajectory are persons with tetraplegia and those with a limited shoulder ROM at start of active rehabilitation.
Keywords: Longitudinal studies, Prospective studies, Shoulder, Spinal cord injuries, Upper extremity
Introduction
Although shoulder pain is a problem in up to 67% of persons with a spinal cord injury (SCI), so far, no studies have empirically identified longitudinal patterns (trajectories) of musculoskeletal shoulder pain after SCI.1–5 Many people with SCI depend on their arms for mobility and several activities of daily living, such as transferring from the wheelchair to the car. Therefore, they are at higher risk for problems associated with overuse of the shoulder compared with those without SCI.6,7 Of those persons with SCI and shoulder pain, 86% of the persons also report limitations in daily activities1–5 and reported limitations in participation. For example, in 84% of persons with SCI, shoulder pain leads to a limitation in sport and leisure activities.2,8 Furthermore, shoulder pain is associated with lower perceived health,9 lower quality of life,10,11 and increased use of assistive devices.12
Previous research has found predictors of musculoskeletal shoulder pain to be: older age, longer time since injury (TSI), higher body mass index (BMI), lesion level (tetraplegia), muscle strength (inversely related), shoulder range of motion (ROM), and functional outcome (inversely related).11,13–16 However, most of these findings are based on cross-sectional studies of persons with chronic SCI.17 Only a few studies on shoulder problems in SCI had a prospective longitudinal design.5,11,18 These studies model the group mean scores of shoulder pain over time which, although useful, may hide distinct patterns of change in shoulder pain after SCI (trajectories). Trajectory analysis models patterns of change over time in the dependent variable and identifies distinct subgroups within the population. Understanding the distinct trajectories of shoulder pain and their determinants offers insight into the development of shoulder pain and in the possible risk factors for chronic shoulder pain, which is prerequisite for early intervention. To our knowledge, there are no studies that empirically identified trajectories of shoulder pain after SCI.
The current study is an extension of an earlier prospective cohort study that addressed shoulder pain in persons with SCI.5 The objective of the current study was (1) to identify distinct trajectories of shoulder pain in the period between the start of active SCI inpatient rehabilitation and 5 years after discharge, and (2) to find determinants of these trajectories.
We hypothesized that four trajectories of shoulder pain would be identified: a stable high, a stable low, a decrease and an increase trajectory. We hypothesized that TSI, presence of shoulder pain before SCI, age, gender, lesion characteristics, physical characteristics (BMI, manual muscle strength (MMT)), spasticity of the elbow flexors and/or extensors, and limitation in shoulder ROM at the start of active rehabilitation would be determinants of shoulder pain trajectory.
Methods
The manuscript used the checklist for cohort studies as provided by the STROBE-statement (Strengthening the Reporting of OBservational studies in Epidemiology) (http://www.strobe-statement.org).
Participants
Participants with a recently acquired SCI (n = 225) were included in the longitudinal Dutch study “Physical strain, work capacity, and mechanisms of restoration of mobility in the rehabilitation of individuals with spinal cord injury”.19 Participants were admitted to inpatient rehabilitation in one of the eight Dutch rehabilitation centers with a specialized SCI department. Inclusion criteria of the study were: (i) a recently acquired SCI; (ii) age between 18 and 65 years; (iii) grades A, B, C, or D on the American Spinal Injury Association (ASIA) Impairment Scale (AIS); (iv) expected permanent wheelchair dependency for long distances and; (v) having completed a minimum of one outcome assessment on shoulder pain.20 Participants were excluded if they had a SCI due to a malignant tumor, a progressive disease, psychiatric problems, or insufficient command of the Dutch language (necessary for understanding the goal of the study and test instructions).
The research protocol was approved by the Medical Ethics Committees of the SRL/iRv and University Medical Center Utrecht. All persons gave written informed consent to participate in the study.
Study design
A multicenter, prospective cohort study was conducted with measurements taken at the time when a participant was able to sit four or more hours in the wheelchair (t1), 3 months later (t2), at discharge from inpatient rehabilitation (t3), and 1 and 5 years after inpatient rehabilitation (t1 and t5, respectively).
Instruments
All clinical measurements were assessed by trained physicians and research assistants.
Shoulder pain
On all five test occasions (t1–t5), the participants were asked, in a standardized questionnaire, whether they experienced pain on the joints or muscles of both shoulders (since SCI at t1, since last measurement time at t2, t3, t4, and t5). The question to the participants was formulated as follows: “Did you experience pain to your joints or muscles since your spinal cord injury?”. If the question was answered positive, patients were asked to rate the severity of shoulder pain. Severity of musculoskeletal pain was measured for both shoulders on a scale of 0–5 (0 = no pain, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, and 5 = very severe).5
The questionnaire tried to distinguish musculoskeletal pain from neuropathic pain as best as possible by also asking the character of the pain (i.e. pain related to movement in musculoskeletal pain vs. other sensations of pain (e.g. itching or blunt) in neuropathic pain).21
Furthermore, participants were asked at the start of active rehabilitation whether they had suffered shoulder pain before SCI. In the analysis, a patient was considered to have shoulder pain if he/she suffered pain in at least one shoulder. In cases where both shoulders were affected, only the shoulder with the highest pain score was included in the analysis.
Lesion characteristics
Level and completeness of SCI were recorded according to the International Standards for Neurological Classification of SCI.22 Tetraplegia was defined as a neurological level of SCI above the T1 segment. SCI was defined as motor complete when participants met the criteria of the International Standards for Neurological Classification of SCI AIS A or B.
Demographics
Age and sex were recorded in the study at the start of active rehabilitation.
Time since injury
For all participants, TSI was determined as the time between the occurrence of SCI and the first measurement time (t1) (noted in months).
Shoulder ROM
Following a standardized protocol, passive ROM of both shoulders were measured using goniometry in the sitting position for flexion, external rotation, and abduction.23 Patients were measured in their own wheelchair and were instructed to sit as upright as possible. For each patient, measurement of ROM was performed at all measurement times by the same trained health professional.
Normal ROM was defined as: 180° for shoulder flexion, 60° for external rotation, and 90° for glenohumeral abduction.24 A decrease in ROM of 10° or more in one of the movements was considered to be an impaired ROM. This cut-off point was chosen by experts working in the field of SCI.
Manual muscle strength
To assess the strength of five muscle groups of the upper extremities, standardized MMT25 was performed for the elbow flexors and extensors, internal and external shoulder rotators, and shoulder abductors. The MMT for each muscle group was performed in a standardized sitting position. However if, the MRC score of shoulder external rotation and internal rotation was scored as MRC grade 3, or for shoulder abduction grades 0, 1, or 2, patients were retested in a supine position.25 Muscle force was assessed by the research assistant on a scale of 0–5 as follows: (0) no muscle contraction, (1) palpable or visible muscle contraction, (2) active movement through full ROM with gravity eliminated, (3) active movement through full ROM against gravity, (4) active movement through full ROM against resistance, and (5) normal muscular strength. The muscle group scores of the right and left upper extremities were added together to obtain an overall MMT score, ranging from 0 to 50.
Obesity
A BMI (body mass (kg)/height (m2)) of greater than 22 was used to define overweight or obese; this was defined using the cut-offs for SCI suggested by Laughton and Powley.26 Mass (in kg) was measured on a wheelchair scale with the patient sitting in the wheelchair or with a weighting lift scale. In the first case, the wheelchair and orthotics of the patient were weighed separately and the mass was subtracted from the first measurement to obtain body mass. Body height was defined in meters according to self-report by participants at t1.27
Spasticity of upper elbow flexors/extensors
The presence of spasticity of the elbow flexors and extensors of both arms was determined in participants with tetraplegia. Spasticity was defined, based on the definition of Lance,28 as a velocity-dependent increase in muscle tone combined with exaggerated reflexes, through a direct standardized examination (1: catch; 2: clonus <5 beats; 3: clonus ≥5 beats). In the analyses, we used a dichotomous variable of spasticity (0 = no spasticity or grade 1, 1 = grades 2 and 3).
Statistical analyses
Respondent characteristics
Descriptive data are displayed as means, standard deviations (SDs), and range or interquartile range (IQR). To identify significant differences between the trajectories (demographics, lesion characteristics, and physical characteristics), cross-tabulations with chi-square tests were performed for nominal data and one-way analysis of variance for numerical data. Non-parametric statistical analyses were used for data that were not normally distributed.
Identifying trajectories
Distinct trajectories of shoulder pain were determined by fitting latent class growth mixture modeling (LCGMM)29,30 to the data, using Mplus software.31 LCGMM are contemporary statistical techniques based on regression and structural equation models32 and aim to capture heterogeneity in the course of shoulder pain in k number of subgroups (or classes), each with a unique trajectory. Each subgroup has its own growth parameters (e.g. intercept, slope, and variance) and characteristics. In LCGMM, missing data are handled by the expectation-maximization algorithm (EM algorithm) when they are missing at random. This means that the analysis makes full use of all available data, thereby preventing the inclusion of only those patients that have data on all data points available. This method of data analysis is common in settings where longitudinal data are available; many studies have shown that when the data are missing at random (meaning that missing data are assumed to be unrelated to the outcome variable or that dropout at each occasion is assumed to be conditionally independent of current and future responses on the particular outcome variable, in this study shoulder pain), bias in parameter estimates is avoided. Although the missing data assumptions are difficult to test, we have compared the patients with full availability of the data to on the relevant variable (shoulder pain).33
To determine the optimal number of trajectories, a common forward procedure was conducted where models with varying number of classes and parameters are assessed and compared.34 To guide the choice for the optimal model, the Bayesian Information Criterion (BIC)35 and the bootstrapped likelihood ratio test (BLRT),36 two commonly used indices,37 were used to assess model fit. A lower BIC value indicates a better fitting model, while a significant P value for the BLRT favors the model with k classes over the model with k − 1 classes. Besides the model fit indices, high posterior probabilities (high probabilities imply distinctive classes) and clinical relevance were taken into account in the modeling process (rejecting clinically uninterpretable classes).37,38 Once the choice for the optimal model was made, participants were assigned to the trajectory to which they had the highest probability of belonging.39
Additional sensitivity analyses were conducted by re-running LCGMM using only the data of the patients for which data on shoulder pain were available for all time points.
Predictors of shoulder pain trajectories
Logistic regression models were used to determine which predictors (i.e. TSI, age, gender, lesion characteristics (level and completeness), physical characteristics (presence of shoulder pain before SCI), BMI, MMT, spasticity of the elbow flexors and/or extensors, and limitation in shoulder ROM) could discriminate between the trajectories. First, bivariate logistic regression analyses were conducted to determine which predictors should be included in the multiple logistic regression analyses by using the selection criterion of a P value less than 0.10. All selected predictors at t1 were then simultaneously entered into the model and backward elimination was used, leading to a final multivariable logistic regression model including only significant predictors.
SPSS statistical program for Windows (SPSS version 16.0, 2007, SPSS Inc., Chicago, IL, USA) was used for testing the group differences and performing regression analyses.
Results
Respondent characteristics
At t1, 225 persons with a newly acquired SCI were included in the study. After 3 months (t2) 155 persons participated, at discharge (t3) 198, 1 year after discharge (t4) 156, and 131 persons participated 5 years after discharge (t5). The lower number of participants in the second measurement is due to the measurement of 3 months after SCI (t2) not being recorded for those participants with a short duration of inpatient rehabilitation and were instead directly included in the measurement at discharge (t3). At 5 years after discharge (t5) 30 persons had died, 10 could not be contacted, 17 declined to participate in the study anymore, 5 had moved, and the other 1 dropped out for other reasons.
Participants' characteristics at t1 are displayed in Table 1. The median time from injury until admission to the rehabilitation center was 32 days (IQR 19–54 days). The median time between the onset of SCI and first assessment was 75 days (IQR 52–115 days). Median duration of rehabilitation in the study population was 225 days (IQR 156–328 days), for persons with a paraplegia median length of stay was 194 (IQR 148–279 days), and for persons with a tetraplegia 293 days (IQR 192–407 days). All 44 persons with a level of SCI C5 or higher had preserved sensory function above, at, or below the neurological level of injury.
Table 1.
Descriptive characteristics of participants at t1
| Characteristics | Participants (n = 225) |
|
|---|---|---|
| N | % | |
| Male | 168 | 74.7 |
| Type of injury | ||
| Tetraplegia | 91 | 40.4 |
| Motor complete (AIS A or B) | 152 | 67.6 |
| Neurological level | ||
| C1–C4 | 21 | 9.3 |
| C5–Th1 | 50 | 22.2 |
| Th2–Th6 | 48 | 21.3 |
| Th7–Th12 | 58 | 25.7 |
| L1–S4-5 | 48 | 21.3 |
| Presence of shoulder pain before SCI (years/n) | 21 | 9.3 |
| Presence of any shoulder pain (years/n) | 79 | 43.1 |
| Presence of bilateral shoulder pain (years/n) | 55 | 24.4 |
| Presence of limitation in shoulder ROM > 10° (years/n) | 80 | 35.6 |
| Age (years) (mean, range) | 40.7 (18–66) | |
| BMI (kg/m2) (mean, range) | 22.9 (15.5–35.6) | |
| MMT sum score (mean, range) | 42 (0–50) | |
| Spasticity of elbow flexors or extensors score (mean, range) | 0.25(0–5) | |
Values are n (%), or as otherwise indicated.
AIS, ASIA impairment scale; ROM, range of motion; BMI, body mass index; TSI, time since injury; MMT, manual muscle testing.
Identifying trajectories
In the current study, a prevalence of musculoskeletal shoulder pain in the total group was found to be 43% at start of active rehabilitation (n = 225), 50% 3 months later, 40% at discharge, 34% 1 year after discharge, and 42% 5 years after discharge. Shoulder pain trajectories were identified using LCGMM. Table 2 shows that a model with three shoulder pain trajectories best represented the data (i.e. having the lowest BIC number and a significant P value of BLRT).
Table 2.
Criteria for selecting the number of trajectories
| Number of trajectories | Bayesian Information Criterion | Bootstrapped likelihood ratio test | Mean posterior probabilities | Entropy |
|---|---|---|---|---|
| 1 | 1992.61 | Not applicable | 1 | Not applicable |
| 2 | 1864.121 | P < 0.001 | 0.914 | 0.71 |
| 3 | 1863.248 | P < 0.001 | 0.853 | 0.79 |
| 4 | 1864.09 | P < 0.001 | 0.79 | 0.69 |
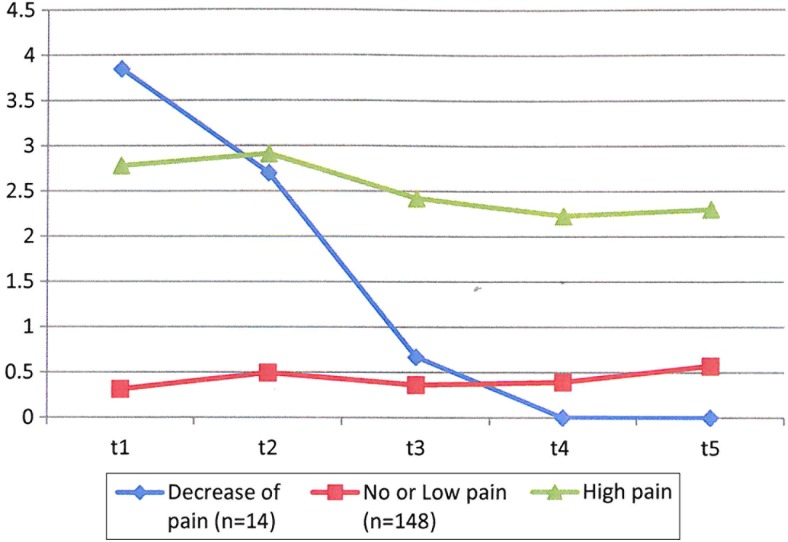
The three trajectories found are: (i) “No or Low pain” (n = 148, 64%), (ii) “High pain” (n = 63, 30%), and (iii) a “Decrease of pain”, for which pain decreased over time (n = 14, 6%) (Fig. 1).
Figure 1.
Three estimated trajectories in shoulder pain between the start of active rehabilitation and 5 years after discharge from inpatient rehabilitation (n = 225).
Additional sensitivity analyses re-running latent class growth mixture on the data of the patients who have data at every time points for shoulder pain (total n = 87 patients) yielded similar trajectories, a “No or Low pain” trajectory (n = 57 patients; 65%), a “High pain” trajectory (n = 27 patients; 31%), and a “Decrease of pain” trajectory (n = 3 patients; 4%).
Table 3 gives a descriptive overview of the course of shoulder pain (number and percentage of persons having shoulder pain) of the total group as used in LCGMM and of the three trajectories with their shoulder pain scores (median, SD) at each measurement time (with their actual n).
Table 3.
The three shoulder pain trajectories, number (%) of participants with pain at the measurement times, and shoulder pain scores (mean, SD); range of pain score 0–5
| n* | Start active rehabilitation (n = 225) | Three months after start rehabilitation (n = 155) | Discharge (n = 198) | One year after rehabilitation (n = 156) | Five years after rehabilitation (n = 131) | ||
|---|---|---|---|---|---|---|---|
| Total group | 225 | N with pain (%) | 97 (43) | 77 (50) | 78 (40) | 53 (34) | 59 (42) |
| High pain | 63 (28) | N with pain (%) | 26 (41) | 23 (73) | 25 (78) | 22 (49) | 27 (51) |
| Mean pain score | 2.78 (1.32) | 2.92 (1.37) | 2.23 (1.45) | 2.15 (1.55) | 2.18 (1.55) | ||
| Decrease of pain | 14 (6) | N with pain (%) | 14 (100) | 8 (57) | 4 (29) | 0 | 0 |
| Mean pain score | 3.86 (0.36) | 2.36 (1.75) | 0.46 (0.87) | 0 | 0 | ||
| No or low pain | 148 (66) | N with pain (%) | 57 (39) | 46 (16) | 49 (17) | 31 (14) | 32 (18) |
| Mean pain score | 0.25 (0.66) | 0.42 (0.91) | 0.32 (0.80) | 0.35 (0.84) | 0.55 (1.03) |
*Total n used in LCGMM.
Predictors of shoulder pain trajectories
The “High pain” trajectory, compared with the “No or Low pain” trajectory, was characterized by having more persons with tetraplegia, more persons with shoulder pain before the SCI, a limited shoulder ROM, lower MMT, and more spasticity at t1. The group with the “High pain” trajectory compared with the “Decrease of pain” trajectory showed to be more often obese, have a slightly higher MMT score, and suffer from more severe spasticity at t1. The “Decrease of pain” trajectory compared with the “No or Low pain” trajectory was characterized by having fewer persons with a paraplegia, more persons with shoulder pain before SCI, fewer obsess persons, more shoulder ROM limitations, and a lower MMT at t1. The P values are displayed in the Table 4.
Table 4.
Characteristics per shoulder pain trajectory measured at start of active rehabilitation (t1) (N = 225) and significant differences between trajectories (described in n and % per trajectory)
| Descriptives |
P values of differences between trajectories |
|||||
|---|---|---|---|---|---|---|
| High pain (n = 63) | Decrease of pain (n = 14) | No or low pain (n = 148) | High pain vs. decrease of pain | High pain vs. no or low pain | Decrease of pain vs. no or low pain | |
| Demographics | ||||||
| Male | 44 (70%) | 13 (93%) | 111(74%) | 0.067 | 0.270 | 0.114 |
| Female | 19 (30%) | 1(7%) | 37(26%) | |||
| Age (years) | 40.4 (13.7) | 32.6 (12.0) | 41.7(14.4) | 0.195 | 1.00 | 0.068 |
| TSI (days) | 107 (72) | 100(40) | 92(75) | 0.516 | 0.086 | 0.033 |
| Lesion characteristics | ||||||
| Paraplegia | 21 (33%) | 3 (21%) | 110 (74%) | 0.298 | <0.001 | <0.001 |
| Tetraplegia | 42 (67%) | 11 (79%) | 38 (26%) | |||
| Complete | 45 (71%) | 11 (79%) | 96 (65%) | 0.430 | 0.283 | 0.267 |
| Incomplete | 18 (29%) | 3 (21%) | 49 (35%) | |||
| Physical characteristics | ||||||
| Presence of shoulder pain before SCI | 10 (16%) | 2 (14%) | 9 (6%) | 0.287 | <0.001 | <0.001 |
| Presence of obesity (BMI ≥ 22) | 34 (50%) | 2 (14%) | 86 (58%) | 0.012 | 0.219 | 0.002 |
| Presence of limitation in shoulder ROM of >10° | 41 (60%) | 8 (57%) | 31 (21%) | 0.547 | <0.001 | 0.004 |
| MMT score (range 0–50) | 36.6 (13.1) | 33.3 (10.3) | 45.4 (9.4) | 0.011 | <0.001 | <0.001 |
| Spasticity of elbow flexors or extensors score (range 0–3) | 0.54 (1.18) | 0.33 (0.65) | 0.10 (0.47) | <0.001 | <0.001 | 0.982 |
For sex, lesions level, completeness of SCI, the presence of shoulder pain before SCI, the presence of obesity, the presence of shoulder ROM limitation, the values are n (and %) per trajectory. For age, TSI, MMT, and spasticity, the mean (and SD) is described. All variables were measured at the start of active rehabilitation. Significance was set at P < 0.001.
TSI, time since injury; BMI, body mass index; ROM, range of motion; MMT, manual muscle testing.
The results of the multiple backward logistic regression analyses show that lesion level and a presence or absence of a shoulder ROM limitation at t1 distinguishes between the “High pain” and “No or Low pain” trajectories (Nagelkerke's R2: 0.243). Persons with tetraplegia (odds ratio (OR) = 2.8) and those with a limited shoulder ROM (OR = 3.6) were more likely to belong to the “High pain” trajectory. The results of the multiple backward regression analyses, including beta, standard error, 95% confidence interval, and P values are displayed in Table 5.
Table 5.
Outcome of multiple logistic regression analyses
| Outcome of logistic regression analyses |
|||||
|---|---|---|---|---|---|
| B | SE | OR | 95% CI | P | |
| Constant | −0.481 | 0.359 | |||
| Lesion characteristics | |||||
| Lesion level (paraplegia) | −1.163 | 0.382 | 0.312 | −1.912/ − 0.414 | 0.002 |
| Physical characteristics | |||||
| Presence of limitation in shoulder ROM of >10° | 1.037 | 0.382 | 2.820 | 0.228/1.786 | 0.007 |
SE, standard error; CI, confidence interval.
Because the “Decrease of pain” trajectory was only comprised of 14 persons, no multiple statistical analyses were performed on this group.
Discussion
The current study is, to our knowledge, the largest study in which shoulder pain in persons with SCI was examined over a time period from start of active rehabilitation until 5 years after discharge. It is also the first study that identified distinct trajectories of musculoskeletal shoulder pain in SCI using LCGMM, thus giving more insight into subgroup patterns on shoulder pain in time.
Limitations
In the study, persons with SCI between 18 and 65 years and with expected permanent wheelchair dependency admitted to a rehabilitation center were included. Persons that were mainly walking (with or without aids) or expected to do so were not included in the study. This influences the representativeness of the population and thereby the degree to which the results of our study can be generalized to the whole population of persons with SCI (e.g. persons that are able to walk).
The assessment of pain in persons with SCI is difficult. It is difficult to distinguish between neuropathic pain from musculoskeletal pain, especially among persons with a level of SCI of C5 and higher. Shoulder pain typically presents in dermatome C5. In 21 cases the sensory level of injury was diagnosed at t1 at C4 or higher. All persons with a sensory level of C4 or higher had preserved sensation above, at or below the neurological level. In the questionnaire we tried to distinguish neuropathic pain from musculoskeletal pain by asking the character of pain. However, we cannot completely rule out that some persons could not clearly distinguish between neuropathic pain and musculoskeletal pain.
Body height was defined in meters according to self-report by participants at t1. One could argue that self-reported height might not be the best way to record height. The study of Froehlich-Grobe et al. concluded: “Recumbent length yields the most accurate height estimate for wheelchair users. However, when logistical and practical considerations pose difficulties for obtaining this measure, height estimates based on knee height and self-report may provide reasonable alternatives”.27 For our study hypothesis, we felt self-reported height was acceptable.
Although we had the largest SCI cohort population to study shoulder pain to-date, some persons were lost to follow-up. For the LCGMM, missing data were handled according to the EM algorithm. Although statistically sound, this algorithm assumes data to be missing at random. This assumption is unfortunately difficult to test and we therefore cannot rule out that the group lost to follow is “not random” and could have influenced our outcomes. However, a sensitivity analysis found no clear indications that this was the case.
Identifying trajectories
Based on data of 225 persons with SCI, three distinct musculoskeletal shoulder pain trajectories were identified in the period between the start of active SCI rehabilitation and 5 years after discharge: a “High pain” trajectory, a “No or Low pain” trajectory, and a “Decrease of pain” trajectory. We hypothesized that we would also identify a fourth trajectory with an increase of shoulder pain. Both the “High pain” and “No or Low pain” trajectory showed a slight tendency for increase in musculoskeletal shoulder pain between 1 and 5 years after discharge (t4 and t5, respectively), but no distinct “Increase” trajectory could be identified. We assume that the follow-up time of 5 years after discharge is too short to show pain problems in the shoulders due to overuse, especially in paraplegics, which might occur later.
In the current study, a prevalence of musculoskeletal shoulder pain in the total group was found to be 43% at start of active rehabilitation, 50% 3 months later, 40% at discharge, 34% 1 year after discharge, and 42% 5 years after discharge. This is lower than prevalence of shoulder pain in the literature on persons with chronic SCI, which is found to be between 60 and 89%.2,4,12,14,15 Unfortunately, these studies mostly do have a cross-sectional study design, use a different definition of shoulder pain (distinguish musculoskeletal and neuropathic pain), and use different outcome measures and/or include different populations and TSI. Therefore, comparison with prevalence of shoulder pain in our study should be interpreted with caution.
One prospective cohort study by Salisbury et al.,11,18 was conducted in 41 persons with a tetraplegia during first inpatient rehabilitation with a follow-up after 2 and 4 years. They showed that shoulder pain was present during inpatient rehabilitation in 85% of the patients. In our study the “High” shoulder pain trajectory showed similar prevalence (90% at t1, 73% at t2, and 78% at t3). After 4 years, Salisbury et al. found a shoulder pain prevalence of 70%, which is higher compared with what was found after 5 years in our study population (51%). The higher percentage compared with our study is probably due to the fact that Salisbury et al.11,18 included only persons with a tetraplegia (n = 41), while one-third of our “High” group consisted of persons with paraplegia.
Predictors of shoulder pain trajectories
Although significant differences between the three trajectories exist by group characteristics using bivariate analysis (Table 4), based on multivariable logistic regression our current study identified two significant predictors of belonging to the “High pain” trajectory (as compared with the “No or Low pain” trajectory): (i) having a tetraplegia and (ii) having a limited shoulder ROM.
The other included factors that were expected to be possible predictors for belonging to a distinct (High pain and No or Low pain) trajectory (age, TSI, completeness of the injury, presence of shoulder pain before SCI, obesity, and spasticity) were, not revealed as significant in the final multiple logistic regression analyses. In the literature different variables associated with shoulder pain in SCI have been described. In recently published studies older age,18 longer TSI, higher BMI,5,13,14 lesion level (tetraplegia),5 muscle strength (inversely related)5 longer duration of bed rest,18 and functional outcome (inversely related)5,15 were related to higher shoulder pain scores. However, as aforementioned, most of above-described findings are based on studies in persons with chronic SCI using a cross-sectional design, and should therefore be interpreted with caution.
The “Decrease of pain” trajectory only existed of 14 persons, and was therefore was not included in the multiple analyses. In the bivariate analysis, the “Decrease of pain” trajectory was not to be significantly different from the “High pain” trajectory with regard to level of injury and shoulder ROM, but were significant more obese, had a lower MMT score, and suffer more frequent from spasticity.
Although we hypothesized an “Increase of pain” trajectory, we did not find it in the analysis; this is probably due to the limited follow-up time of the study. The “High pain” shoulder pain trajectory consists of mainly persons with a tetraplegia. People with a SCI might develop overuse issues in the shoulders and shoulder pain at a later stage, especially persons with a paraplegia. In the current study, we did not study the causes of shoulder pain by clinical exam or radio diagnostics. Adding clinical and radiodiagnostic examinations to future studies would give us better insight on the potential different patho-physiological mechanisms of shoulder problems among persons with tetraplegia and paraplegia.
Future directions
Although our results suggest a likely causal relationship, one should test our findings with other datasets of persons with SCI to confirm this relationship.
Larger studies are needed to be able to show relevant associations of, for example, lesion level within the paraplegic group (high paraplegics vs. low paraplegics), and to study the role of posture and trunk stability on the development of shoulder pain.
The duration of the current study was up to 5 years after inpatient rehabilitation. Two trajectories show a tendency of increase from 1 to 5 years after SCI. To show whether this increase is relevant, and might retrieve a fourth “Increase of pain” trajectory a follow-up measurement at, for example, 10 years is needed. Adding clinical testing, radio diagnostics and kinematics would be a key to understanding the mechanism of shoulder pain in the various trajectories. Studies should also include the assessment of postural control. Persons with SCI have shown to make use of non-postural muscles to maintain their sitting balance.40–44 Whether these adaptations in postural control are associated with the development of shoulder pain so far has not been studied.
It was beyond the scope of this study to include all interventions for the reduction of shoulder pain. Assessment of interventions is needed in order to open the “black box”. Current initiatives such as the SCIRehab project45–49 and the Spinal Cord Injury-International Classification System50–54 have now provided us with the possibility to open this “black box”, at least to some extent.
Future intervention studies for treatment and/or prevention should include a large, homogeneous study population, should have a long duration of follow-up time, and should include, if possible, a control group. Interesting would be to study the effects of earlier shoulder mobilization starting early after SCI by specialized physiotherapists paying attention not only on shoulder external rotation and abduction, but also to preserve shoulder flexion by, for example, scapula stabilization and mobilization55 and balanced muscle training.
In summary, the results of the current study and (lack of) available evidence show that there is a need for longitudinal studies with longer follow-up time, comprehensively studying the course over time of shoulder pain and studying the effect of interventions, such as early mobilization and early muscle strength training, on shoulder pain in persons with SCI.
Clinical implications
Our findings show that shoulder pain is a frequent problem, even in patients who have a SCI for “only” approximately 5 years. Health professionals should be aware of the increased risk of belonging to the “High pain” trajectory in persons with tetraplegia and those with a limited shoulder ROM. In a former study, in the same cohort, on shoulder ROM limitations in persons with SCI, it was shown that especially shoulder flexion was limited.16 Prevention of shoulder problems should be a main goal of rehabilitation. Using guidelines such as the guideline for “Preservation of Upper Limb Function Following Spinal Cord Injury” or the “Guidelines for the prescription of a seated wheelchair or mobility scooter for people with a traumatic brain injury or spinal cord injury” (Download: http://www.enable.health.nsw.gov.au/publications or LTCSA; http://www.lifetimecare.nsw.gov.au/Resources.aspx) could be helpful in structuring treatment and preventive interventions of shoulder problems in persons with SCI.56 Furthermore, wheelchair propulsion has been shown to be straining and to place a high load on the shoulder,57–61 thereby increasing the risk of structural changes and the development of shoulder pain. Alternative propulsion modes, such as hand cycling for mobility and exercise, instead of hand rim wheelchair propulsion, should be considered by clinicians at an early stage.57,62,63
Conclusions
This study confirmed that shoulder pain is a problem in the SCI population during, and after inpatient rehabilitation, with a prevalence of 43% 5 years after discharge from inpatient rehabilitation.
In the current study, we unraveled some of the complexity of musculoskeletal shoulder pain, showing different trajectories of shoulder pain and their predictors on basis of a longitudinal dataset.
Three distinct musculoskeletal shoulder pain trajectories in persons with acute SCI exist; a “High pain” trajectory, a “Decrease of pain” trajectory, and a “No or Low pain” trajectory.
Having a tetraplegia and having a limited shoulder ROM at the beginning of active rehabilitation increases the risk of belonging to the “High pain” trajectory, and therefore special attention should be paid to these persons. Monitoring shoulder pain at the start of active rehabilitation might allow identification of persons at risk for poor long-term outcomes.
Statement of funding sources
This study was supported by the Dutch Health Research and Development Council, ZonMw Rehabilitation program, grant nos. 1435.0003 and 1435.0025.
Conflict of interest statement
Each author certifies that he or she has no commercial association that might pose a conflict of interest in connection with the submitted article.
Ethical review committee statement
The study was approved by the local Medical Ethics Committees of SRL/iRv and University Medical Center Utrecht and is in accordance with the ethical standards in the 1975 (as revised in 2000) Declaration of Helsinki. All persons gave written informed consent to participate in the study.
Acknowledgments
A special thanks is given to the participating Dutch rehabilitation centers especially the research assistants and physicians in these centers who collected the data: Rehabilitation Center De Hoogstraat (Utrecht), Reade, Centre for Rehabilitation and Rheumatology (Amsterdam), Rehabilitation Center Het Roessingh (Enschede), Adelante (Hoensbroek), Rehabilitation Center Sint Maartenskliniek (Nijmegen), Center for Rehabilitation – Location Beatrixoord (Haren), Heliomare (Wijk aan Zee), and Rijndam Rehabilitation Center (Rotterdam).Furthermore, we like to thank Jonviea Chamberlain for editing the manuscript. A part of the manuscript was presented at the joint meeting of the 51th ISCoS Annual Scientific Meeting.
References
- 1.Akbar M, Balean G, Brunner M, Seyler TM, Bruckner T, Munzinger J, et al. . Prevalence of rotator cuff tear in paraplegic patients compared with controls. J Bone Joint Surg Am 2010;92(1):23–30 [DOI] [PubMed] [Google Scholar]
- 2.Alm M, Saraste H, Norrbrink C. Shoulder pain in persons with thoracic spinal cord injury: prevalence and characteristics. J Rehabil Med 2008;40(4):277–83 [DOI] [PubMed] [Google Scholar]
- 3.Hastings J, Goldstein B. Paraplegia and the shoulder. Phys Med Rehabil Clin N Am 2004;15(3):vii, 699–718 [DOI] [PubMed] [Google Scholar]
- 4.Medina GI, Nascimento FB, Rimkus CM, Zoppi Filho A, Cliquet A, Jr. Clinical and radiographic evaluation of the shoulder of spinal cord injured patients undergoing rehabilitation program. Spinal Cord 2011;49(10):1055–61 [DOI] [PubMed] [Google Scholar]
- 5.van Drongelen S, de Groot S, Veeger HE, Angenot EL, Dallmeijer AJ, Post MW, et al. . Upper extremity musculoskeletal pain during and after rehabilitation in wheelchair-using persons with a spinal cord injury. Spinal Cord 2006;44(3):152–9 [DOI] [PubMed] [Google Scholar]
- 6.Harvey LA, Herbert RD. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord 2002;40(1):1–9 [DOI] [PubMed] [Google Scholar]
- 7.Luime JJ, Koes BW, Hendriksen IJ, Burdorf A, Verhagen AP, Miedema HS, et al. . Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol 2004;33(2):73–81 [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez DD, Thompson L, Kemp B, Mulroy SJ. The relationship of shoulder pain intensity to quality of life, physical activity, and community participation in persons with paraplegia. J Spinal Cord Med 2007;30(3):251–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballinger DA, Rintala DH, Hart KA. The relation of shoulder pain and range-of-motion problems to functional limitations, disability, and perceived health of men with spinal cord injury: a multifaceted longitudinal study. Arch Phys Med Rehabil 2000;81(12):1575–81 [DOI] [PubMed] [Google Scholar]
- 10.Kemp BJ, Bateham AL, Mulroy SJ, Thompson L, Adkins RH, Kahan JS. Effects of reduction in shoulder pain on quality of life and community activities among people living long-term with SCI paraplegia: a randomized control trial. J Spinal Cord Med 2011;34(3):278–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salisbury SK, Nitz J, Souvlis T. Shoulder pain following tetraplegia: a follow-up study 2–4 years after injury. Spinal Cord 2006;44(12):723–8 [DOI] [PubMed] [Google Scholar]
- 12.Jain NB, Higgins LD, Katz JN, Garshick E. Association of shoulder pain with the use of mobility devices in persons with chronic spinal cord injury. PM R 2010;2(10):896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boninger ML, Towers JD, Cooper RA, Dicianno BE, Munin MC. Shoulder imaging abnormalities in individuals with paraplegia. J Rehabil Res Dev 2001;38(4):401–8 [PubMed] [Google Scholar]
- 14.Brose SW, Boninger ML, Fullerton B, McCann T, Collinger JL, Impink BG, et al. . Shoulder ultrasound abnormalities, physical examination findings, and pain in manual wheelchair users with spinal cord injury. Arch Phys Med Rehabil 2008;89(11):2086–93 [DOI] [PubMed] [Google Scholar]
- 15.Curtis KA, Drysdale GA, Lanza RD, Kolber M, Vitolo RS, West R. Shoulder pain in wheelchair users with tetraplegia and paraplegia. Arch Phys Med Rehabil 1999;80(4):453–7 [DOI] [PubMed] [Google Scholar]
- 16.Eriks-Hoogland IE, de Groot S, Post MW, van der Woude LH. Passive shoulder range of motion impairment in spinal cord injury during and one year after rehabilitation. J Rehabil Med 2009;41(6):438–44 [DOI] [PubMed] [Google Scholar]
- 17.Figoni SF Overuse shoulder problems after spinal cord injury: a conceptual model of risk and protective factors. Clin Kinesiol 2009;63(2):12–22 [Google Scholar]
- 18.Salisbury SK, Choy NL, Nitz J. Shoulder pain, range of motion, and functional motor skills after acute tetraplegia. Arch Phys Med Rehabil 2003;84(10):1480–5 [DOI] [PubMed] [Google Scholar]
- 19.de Groot S, Dallmeijer AJ, Post MW, van Asbeck FW, Nene AV, Angenot EL, et al. . Demographics of the Dutch multicenter prospective cohort study “Restoration of mobility in spinal cord injury rehabilitation”. Spinal Cord 2006;44(11):668–75 [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen CM, Post MW, Hoekstra T, van der Woude LH, de Groot S, Snoek GJ, et al. . Trajectories in the course of life satisfaction after spinal cord injury: identification and predictors. Arch Phys Med Rehabil 2011;92(2):207–13 [DOI] [PubMed] [Google Scholar]
- 21.Widerstrom-Noga E, Biering-Sorensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, et al. . The international spinal cord injury pain basic data set. Spinal Cord 2008;46(12):818–23 [DOI] [PubMed] [Google Scholar]
- 22.Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, et al. . International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997;35(5):266–74 [DOI] [PubMed] [Google Scholar]
- 23.Sabari JS, Maltzev I, Lubarsky D, Liszkay E, Homel P. Goniometric assessment of shoulder range of motion: comparison of testing in supine and sitting positions. Arch Phys Med Rehabil 1998;79(6):647–51 [DOI] [PubMed] [Google Scholar]
- 24.Riddle DL, Rothstein JM, Lamb RL. Goniometric reliability in a clinical setting. Shoulder measurements. Phys Ther 1987;67(5):668–73 [DOI] [PubMed] [Google Scholar]
- 25.Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Spieren. 3rd ed Houten: Bohn Staflue van Loghum; 2008 [Google Scholar]
- 26.Laughton W, Powley TL. Bipiperidyl mustard produces brain lesions and obesity in the rat. Brain Res 1981;221(2):415–20 [DOI] [PubMed] [Google Scholar]
- 27.Froehlich-Grobe K, Nary DE, Van Sciver A, Lee J, Little TD. Measuring height without a stadiometer: empirical investigation of four height estimates among wheelchair users. Am J Phys Med Rehabil 2011;90(8):658–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lance JW The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980;30(12):1303–13 [DOI] [PubMed] [Google Scholar]
- 29.Muthén B Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. Newbury Park, CA: Sage Publications; 2004 [Google Scholar]
- 30.Muthén B The potential of growth mixture modelling. Infant Child Dev 2006;(15):623–5 [Google Scholar]
- 31.Muthén B, Muthén L.. Mplus 6.12, 2011 [Google Scholar]
- 32.Bollen K Structural equations with latent variables. New York: Wiley; 1989 [Google Scholar]
- 33.Potthoff RF, Tudor GE, Pieper KS, Hasselblad V.. Can one assess whether missing data are missing at random in medical studies? Stat Methods Med Res 2006;15(3):213–34 [DOI] [PubMed] [Google Scholar]
- 34.Hoekstra T, Barbosa-Leiker C, Koppes L, Twisk J. Developmental trajectories of body mass index throughout the life course: an application of latent class growth (mixture) modelling. Longit Life Course Stud 2011;2(3):319–30 [Google Scholar]
- 35.Schwarz G Estimating the dimension of a model. Ann Stat 1978;6:461–4 [Google Scholar]
- 36.McLachlan G, Peel D.. Finite mixture models. New York: Wiley; 2000 [Google Scholar]
- 37.Nylund K, Asparouhov T, Muthén B. Deciding on the number of classes in Latent Class Analysis and Growth Mixture Modelling: a Monte Carlo simulation study. Struct Equ Model 2007;14:535–69 [Google Scholar]
- 38.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Pers Psychol Compass 2008;(2):302–17 [Google Scholar]
- 39.Clark S, Muthén B.. Relating latent class analysis results to variables not included in the analysis, 2012 [Google Scholar]
- 40.Janssen-Potten YJ, Seelen HA, Drukker J, Huson T, Drost MR. The effect of seat tilting on pelvic position, balance control, and compensatory postural muscle use in paraplegic subjects. Arch Phys Med Rehabil 2001;82(10):1393–402 [DOI] [PubMed] [Google Scholar]
- 41.Janssen-Potten YJ, Seelen HA, Drukker J, Spaans F, Drost MR. The effect of footrests on sitting balance in paraplegic subjects. Arch Phys Med Rehabil 2002;83(5):642–8 [DOI] [PubMed] [Google Scholar]
- 42.Seelen HA, Potten YJ, Adam JJ, Drukker J, Spaans F, Huson A. Postural motor programming in paraplegic patients during rehabilitation. Ergonomics 1998;41(3):302–16 [DOI] [PubMed] [Google Scholar]
- 43.Seelen HA, Potten YJ, Huson A, Spaans F, Reulen JP. Impaired balance control in paraplegic subjects. J Electromyogr Kinesiol 1997;7(2):149–60 [DOI] [PubMed] [Google Scholar]
- 44.Seelen HA, Vuurman EF. Compensatory muscle activity for sitting posture during upper extremity task performance in paraplegic persons. Scand J Rehabil Med 1991;23(2):89–96 [PubMed] [Google Scholar]
- 45.Dijkers MP, Whiteneck GG, Gassaway J. CER, PBE, SCIRehab, NIDRR, and other important abbreviations. Arch Phys Med Rehabil 2013;94(4 Suppl):S61–6 [DOI] [PubMed] [Google Scholar]
- 46.Whiteneck G, Gassaway J. The SCIRehab project: what rehabilitation interventions are most strongly associated with positive outcomes after spinal cord injury? J Spinal Cord Med 2012;35(6):482–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteneck G, Gassaway J. SCIRehab: a model for rehabilitation research using comprehensive person, process and outcome data. Disabil Rehabil 2010;32(12):1035–42 [DOI] [PubMed] [Google Scholar]
- 48.Whiteneck G, Gassaway J, Dijkers M, Jha A. New approach to study the contents and outcomes of spinal cord injury rehabilitation: the SCIRehab project. J Spinal Cord Med 2009;32(3):251–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteneck G, Dijkers M, Gassaway J, Lammertse DP. The SCIRehab Project: classification and quantification of spinal cord injury rehabilitation treatments. Preface. J Spinal Cord Med 2009;32(3):249–50 [PMC free article] [PubMed] [Google Scholar]
- 50.van Langeveld SA, Post MW, van Asbeck FW, Gregory M, Halvorsen A, Rijken H, et al. . Comparing content of therapy for people with a spinal cord injury in postacute inpatient rehabilitation in Australia, Norway, and The Netherlands. Phys Ther 2011;91(2):210–24 [DOI] [PubMed] [Google Scholar]
- 51.van Langeveld SA, Post MW, van Asbeck FW, Postma K, Leenders J, Pons K. Feasibility of a classification system for physical therapy, occupational therapy, and sports therapy interventions for mobility and self-care in spinal cord injury rehabilitation. Arch Phys Med Rehabil 2008;89(8):1454–9 [DOI] [PubMed] [Google Scholar]
- 52.van Langeveld SA, Post MW, van Asbeck FW, Postma K, Ten Dam D, Pons K. Development of a classification of physical, occupational, and sports therapy interventions to document mobility and self-care in spinal cord injury rehabilitation. J Neurol Phys Ther 2008;32(1):2–7 [DOI] [PubMed] [Google Scholar]
- 53.van Langeveld SA, Post MW, van Asbeck FW, Ter Horst P, Leenders J, Postma K, et al. . Reliability of a new classification system for mobility and self-care in spinal cord injury rehabilitation: the Spinal Cord Injury-Interventions Classification System. Arch Phys Med Rehabil 2009;90(7):1229–36 [DOI] [PubMed] [Google Scholar]
- 54.van Langeveld SA, Post MW, van Asbeck FW, ter Horst P, Leenders J, Postma K, et al. . Contents of physical therapy, occupational therapy, and sports therapy sessions for patients with a spinal cord injury in three Dutch rehabilitation centres. Disabil Rehabil 2011;33(5):412–22 [DOI] [PubMed] [Google Scholar]
- 55.Pahys JM, Mulcahey MJ, Hutchinson D, Betz RR. Scapular stabilization in patients with spinal cord injury. J Spinal Cord Med 2009;32(4):389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boninger ML, Stripling TE. Preserving upper-limb function in spinal cord injury. Arch Phys Med Rehabil 2007;88(6):817; author reply 8 [DOI] [PubMed] [Google Scholar]
- 57.Arnet U, van Drongelen S, Scheel-Sailer A, van der Woude LH, Veeger DH. Shoulder load during synchronous handcycling and handrim wheelchair propulsion in persons with paraplegia. J Rehabil Med 2012;44(3):222–8 [DOI] [PubMed] [Google Scholar]
- 58.van der Woude LH, Veeger HE, Dallmeijer AJ, Janssen TW, Rozendaal LA. Biomechanics and physiology in active manual wheelchair propulsion. Med Eng Phys 2001;23(10):713–33 [DOI] [PubMed] [Google Scholar]
- 59.Van Drongelen S, Van der Woude LH, Janssen TW, Angenot EL, Chadwick EK, Veeger DH. Mechanical load on the upper extremity during wheelchair activities. Arch Phys Med Rehabil 2005;86(6):1214–20 [DOI] [PubMed] [Google Scholar]
- 60.van Drongelen S, van der Woude LH, Janssen TW, Angenot EL, Chadwick EK, Veeger DH. Glenohumeral contact forces and muscle forces evaluated in wheelchair-related activities of daily living in able-bodied subjects versus subjects with paraplegia and tetraplegia. Arch Phys Med Rehabil 2005;86(7):1434–40 [DOI] [PubMed] [Google Scholar]
- 61.van Drongelen S, van der Woude LH, Veeger HE. Load on the shoulder complex during wheelchair propulsion and weight relief lifting. Clin Biomech (Bristol, Avon) 2011;26(5):452–7 [DOI] [PubMed] [Google Scholar]
- 62.Dallmeijer AJ, Zentgraaff ID, Zijp NI, van der Woude LH. Submaximal physical strain and peak performance in handcycling versus handrim wheelchair propulsion. Spinal Cord 2004;42(2):91–8 [DOI] [PubMed] [Google Scholar]
- 63.van der Woude LH, Dallmeijer AJ, Janssen TW, Veeger D. Alternative modes of manual wheelchair ambulation: an overview. Am J Phys Med Rehabil 2001;80(10):765–77 [DOI] [PubMed] [Google Scholar]