Abstract
Objective
To study the effect of 14 weeks of electromyostimulation (EMS) training (47 minutes/day, 5 days/week) on both muscle and bone loss prevention in persons with recent, complete spinal cord injury (SCI).
Design
Prospective, experimental, controlled, single-blind randomized trial with external blind evaluation by third parties.
Methods
Eight men with recent SCI (8 weeks from injury; ASIA Impairment Scale (AIS) “A”) were randomized into the intervention or the control groups. Cross-sectional area of the quadriceps femoris (QF) muscle was quantified using magnetic resonance imaging. Bone mineral density changes were assessed with a dual-energy X-ray absorptiometry. Several bone biomarkers (i.e. total testosterone, cortisol, growth hormone, insulin-growth factor I, osteocalcin, serum type I collagen C-telopeptide), lipid, and lipoprotein profiles were quantified. A standard oral glucose tolerance test was performed before and after the 14-week training. All analyses were conducted at the beginning and after the intervention.
Results
The intervention group showed a significant increase in QF muscle size when compared with the control group. Bone losses were similar in both groups. Basal levels of bone biomarkers did not change over time. Changes in lipid and lipoprotein were similar in both groups. Glucose and insulin peaks moved forward after the training in the intervention group.
Conclusions
This study indicates that skeletal muscle of patients with complete SCI retains the ability to grow in response to a longitudinal EMS training, while bone does not respond to similar external stimulus. Increases in muscle mass might have induced improvements in whole body insulin-induced glucose uptake.
Keywords: Bone density, Biological markers, Electric stimulation, Skeletal muscle, Spinal cord injuries, Paraplegia, Tetraplegia
Introduction
Muscle atrophy1–3 and bone loss4–6 are two immediate consequences of spinal cord injury (SCI). Those changes occur rapidly after injury and are associated with several complications. In brief, skeletal muscle atrophy is associated with reduced vascularisation, basal metabolism, cardiac output, oxygen consumption, increased intramuscular fats, and impaired glucose tolerance.2,7,8 Simultaneously, bone demineralization leading to osteoporosis increases the risk of fracture after minor trauma in subjects with SCI.5 As soon as 5 days after SCI, changes in gene and mRNA expression provide evidence of muscle atrophy in humans,9 whereas the use of bone biochemical markers reveals that bone resorption continuously increases from the first weeks post-injury.10 It has been postulated that an interruption of the autonomic nervous system influences lipid metabolism and serum lipid levels.11
Weight-bearing, electromyostimulation (EMS), pulsed ultrasound, vibratory therapies, pulsed electromagnetic fields, or physical exercise are among the proposed non-pharmacological interventions used to reduce the negative effects observed shortly after SCI.12 Shortly after SCI occurs, EMS was shown to be effective preventing muscle atrophy,13,14 whereas benefits are more controversial in the treatment of osteoporosis resulting from neurological damage.15–20
An early initiation of the EMS program under supervision of a physician following SCI has been postulated to maximize muscle benefits.13 Similarly, the extensive destruction of the trabecular elements in patients with long-term SCI limits the available surface area for bone remodeling, limiting the potential for adaptation to mechanical loading interventions. The plastic potential of bone tissue after SCI diminishes with time, suggesting that mechanical loading interventions have the greatest chance for success if they are administered soon after SCI.17 Since the EMS effects on both muscle content and bone mineral density (BMD) have not yet been studied simultaneously, the purpose of this pilot study was to conduct a comprehensive analysis of muscle and bone adaptations to a 14-week electrical stimulation training in persons with recent SCI. We made a hypothesis that the EMS intervention would significantly reduce the negative changes observed after SCI on muscle mass and bone (i.e. increase formation and/or reduce loss). Since the predominant peripheral tissue responsible for insulin-induced glucose uptake is muscle,21 it was also hypothesized that an intervention counteracting muscle atrophy would improve glucose metabolism. Being physically active after SCI improved lipid and lipoprotein profiles22; thus, any additional demand to the skeletal muscle system is expected to improve those markers.
Methods
Inclusion/exclusion criteria
Inclusion criteria for subjects with SCI were (1) male gender; (2) complete motor post-traumatic SCI between T4–T12 with total and sensory loss, corresponding to stage A according to ASIA Impairment Scale after a neurophysiological examination (including sural nerve neurography, electromyography, transcranial magnetic stimulation and evoked response sensitive) occurred less than 8 weeks before the study; (3) age between 18 and 55 years old; (4) voluntary and informed participation in the study.
Exclusion criteria for SCI subjects were (1) use of drugs affecting bone and/or muscle metabolism (corticoids, thyroid hormones, etc.); (2) serious cognitive, muscular, or dermatological affectation preventing the use of EMS; (3) any oncological, rheumatic, neurological, endocrine, or any other disease being a bias for the study.
Study design and overall protocol
This was a single-blind, parallel-group study conducted in the National Hospital for Paraplegics (Toledo, Spain). All subjects gave written informed consent to participate. Thereafter, they were allocated to the treatment (EMS) or the control group (CON), according to the random number table. This study received ethical approval by the Regional Clinical Research Ethics Committee (Virgen de la Salud Park Hospital, Toledo, Spain) and complied with the Declaration of Helsinki.
Since electrically evoked isometric muscle contractions were relatively mild throughout the study, subjects were blind to the group they belonged, but not the physiotherapist that applied the electrical stimulation to them. In order to prevent any muscle injury during testing, subjects of both groups received a light electrical stimulation muscle conditioning period from the moment they entered the study (i.e. weeks 4 to 7 after SCI) until the pre-training measurements (i.e. week 8 after SCI). Post-training measurements were conducted at week 23 after SCI (i.e. 14 weeks of trainings). Patients were normally discharged from Hospital 6 months after SCI, and during their period in our facility, were on a balanced diet.
Electrical stimulation instrumentation and training protocol
The EMS training was performed bilaterally using a commercially available stimulator (T-ONE MEDI PRO, Electromnedical Mediterranea, S.L., Spain). Surface electrodes (DORMO-TENS, Telic, S. A., Barcelona, Spain) were placed as previously reported.22 In short, one electrode was located on the rectus femoris, another on the vastus medialis, and a third one on the vastus lateralis. The last two electrodes were connected to the first one in order to differentiate the received intensity of each vastus. The stimulation pattern for the EMS group consisted of 200-μs pulse duration as suggested by Bax et al.23 at 30 Hz based on the time–intensity curve and maximum current amplitude of 140 mA. Current amplitude was progressively adjusted in the intervention group during the protocol to elicit the similar isometric muscle contraction levels during the 14-week protocol. For the CON group, all patterns were the same with the exception of current amplitude, which was 0 mA. One session consisted on 80 muscle contractions during 47 minutes divided in 10 contractions sets with a 60-second rest between sets. Every two sets, knee angle was changed in order to workout throughout all knee angles (i.e. 10°, 35°, 60°, and 85°, corresponding 0° to full extension). The selection of this protocol was based on the positive acute adaptation, which was proved to induce on bone biomarkers in recent spinal cord injured persons.24 The EMS training program was performed 5 days a week for 14 weeks by the same physiotherapist for a given subject. Sessions took place in the morning or in the afternoon depending on participant's availability.
Outcome measures
The muscle cross-sectional area (CSA) of right and left quadriceps femoris was assessed by using magnetic resonance imaging (3Tesla, Magnetom Trio; Siemens AG, Erlangen, Germany) at our Hospital facility as previously reported.24 Briefly, 18 T1 2D axial scans of the thigh were obtained perpendicularly to the femur diaphysis. Each scan was separated 1/18 of the distance between greater trochanter and the lateral condyle of femur on a coronal plane using a 500-mm localizer. This localizer was used to relocate scans after the 14-week intervention. Great care was taken to reproduce the same individual femur length each time using the appropriate anatomical landmarks. All measurements were performed blindly by the same technician. Fourteen scans from 3/18 (close to greater trochanter) to 16/18 were ported to a personal computer to manually measure muscle size by using the ImageJTM program (version 1.40, freely available java-based public-domain image processing and analysis program developed at the National Institutes of Health). Quadriceps femoris CSA was analyzed considering the average of the 14 scans and each individual scan. CSA analysis reproducibility and reliability were verified by blind analysis of duplicate 12 scans (average coefficient of variation: 4.97% and intra-class correlation coefficient 95% lower and upper confident intervals: 0.99).
Dual-energy X-ray absorptiometry (DEXA; Hologic Explorer/W, Waltham, MA) was used before and after the 14-week intervention to measure BMD (g/cm2) of both legs, at the lumbar area and both hips considering the whole hip, the femoral neck, the trochanteric and intertrochanteric areas, and Ward's triangle as previously reported.25,26 All scanning and analyses were performed blindly by the same technician following the operational manual provided by Hologic to users. Prior any testing, quality control for DEXA was checked by scanning a lumbar spine phantom consisting of calcium hydroxyapatite embedded in a cube of thermoplastic resin (DPA/QDR-1; Hologic x-caliber anthropometrical spine phantom). The scanning procedure lasted 30 minutes. Coefficient of variation was 1% for total BMD. Least significant change (LSC) for whole hip was 0.00963 g/cm2, while LSC for lumbar spine was 0.0806 g/cm2. Due to technical problems, only leg BMD was available for one of the subjects.
Fasting blood samples were drawn at basal conditions (8:00 a.m.) before and after the 14-week electrical stimulation training. All samples were collected into dry glass tubes and centrifuged at 3500 rpm for 7 minutes at 20°C and stored at 4°C until analysis. Serum total testosterone was measured by chemiluminescent microparticle immunoassay using ARCHITECT i2000SR (Abbott Laboratories S.A, Madrid, Spain). The sensitivity of the testosterone assay was 0.08 ng/ml, and the intra-assay coefficient of variation was 4.5%. Growth hormone (GH) and insulin-like growth factor-1 (IGF-I) were assessed by radioimmunoassay (Immunotech S.A.S., France). The intra- and inter-assay coefficients of variation for GH were 1.5 and 14.0%, respectively. The intra- and inter-assay coefficients of variation for IGF-I were 3.9 and 7.7%, respectively. Serum cortisol was measured by microparticle enzyme immunoassay using ARCHITECT c4000 (Abbott Laboratories S.A, Madrid, Spain). The sensitivity of the cortisol assay was < 1 µg/dl, and the intra-assay coefficient of variation was ≤10%. Serum osteocalcin was kept in ice after taken, immediately centrifuged, and stored at −20°C until analyzed by radioimmunoassay using a Diasource kit (DIAsource ImmunoAssays S.A., Barcelona, Spain). The intra-assay coefficient of variation for osteocalcin was 7.2%. Serum type I collagen C-telopeptide (CTx) was measured by electrochemiluminescence using the E 170 module for MODULAR ANALYTICS (Roche Diagnostics, S.L., Madrid, Spain). The intra-assay coefficient of variation for CTx was 9.6%. Total cholesterol was assayed enzymatically (LX20 PRO Beckman Coulter; Diamond Diagnostics, Fullerton, USA). High-density lipoprotein (HDL) was assayed following elimination of other particles and reaction with cholesterol esterase (LX20 PRO Beckman Coulter; Diamond Diagnostics, USA). Low density lipoprotein (LDL) was estimated using the Friedewald equation. Plasma triglycerides were measured enzymatically (LX20 PRO Beckman Coulter; Diamond Diagnostics, USA). The testosterone/cortisol, osteocalcin/CTx, TC/HDL-C and LDL-C/HDL-C ratios were calculated. A standard 75-g oral glucose tolerance test (OGTT) was administered before and after the 14-week training. Blood samples were drawn at fasting and up to 120 minutes later following OGTT with 30 minutes intervals. Glucose was assayed by glucose oxidase (LX20 PRO Beckman Coulter; Diamond Diagnostics, USA). Insulin was measured by electrochemiluminescence using the E 170 module for MODULAR ANALYTICS (Roche Diagnostics, S.L., Madrid, Spain).
Statistical analysis
Changes over time were determined using a Wilcoxon test for two related samples. Group differences were assessed by the Mann–Whitney U test for two-independent samples. A Friedman test with Bonferroni post hoc was applied to test any changes over time in glucose and insulin level. In this case, only intervention group results were analyzed due to the low n of the control group. All data are presented as mean ± standard deviation. In the OGTT, due to the small sample size in the control group for this analysis, no statistical comparisons were conducted for this group, and individual data from the study are then described in lieu of statistical measurements. An alpha level of P < 0.05 was considered statistically significant. Statistical analysis was done with SPSS v. 17.0 (SPSS Inc., Chicago, IL, USA). Given the relatively small sample size, Cohen's delta effect sizes were calculated to compare baseline scores to post-activity scores within condition and to compare change scores between conditions for each outcome; relevant medium (≥0.50) or large (≥0.80) effect sizes are discussed.30 Effect size measurements were conducted using Cliff's Delta Calculator (free download from the website of the Institute for Psychological Research at Universidad del Salvador; http://www.iipus.com).
Results
Participants
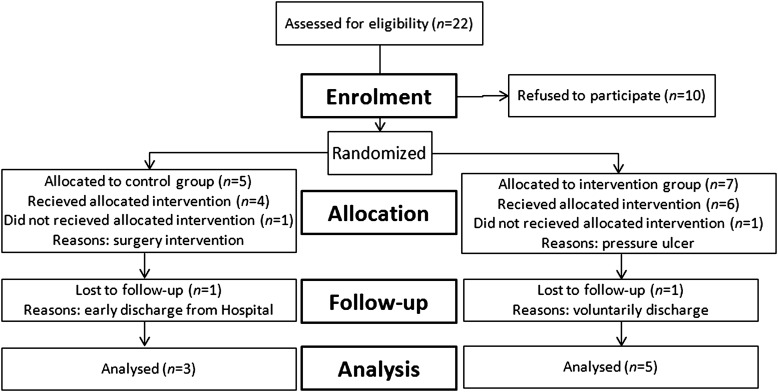
Twenty-two candidates entered our hospital facility during the study period (March 2009–October 2011) as illustrated in Fig. 1. Ten men with paraplegia caused by a recent (<8 weeks) complete upper motor neuron lesion (T4–T12) agreed to participate in this study. Two of them withdrew during the protocol for reasons not related to the study. Thus, eight subjects completed the 14-week study. Participants were randomly divided into intervention (n = 5) and control groups (n = 3). There were no significant differences (P > 0.05) across training groups in subject age, height, weight, body mass index, and time after injury (Table 1).
Figure 1.
CONSORT diagram summarizing numbers of subjects recruited and randomized to each of the two study groups.
Table 1.
Descriptive data of participants
| Group | Age (years) | Height (m) | Weight (kg) | Body mass index (kg/m2) | Weeks post-injury |
|---|---|---|---|---|---|
| ES | 41.7 ± 12 | 1.76 ± 0.1 | 75 ± 9.3 | 24.3 ± 2.7 | 5.5 ± 1.1 |
| CON | 36 ± 13.6 | 1.78 ± 0.1 | 84 ± 21.3 | 26.5 ± 4.9 | 5.8 ± 1.7 |
Values expressed as mean ± SD.
ES, intervention group; CON, control group. n.s.
Muscle size
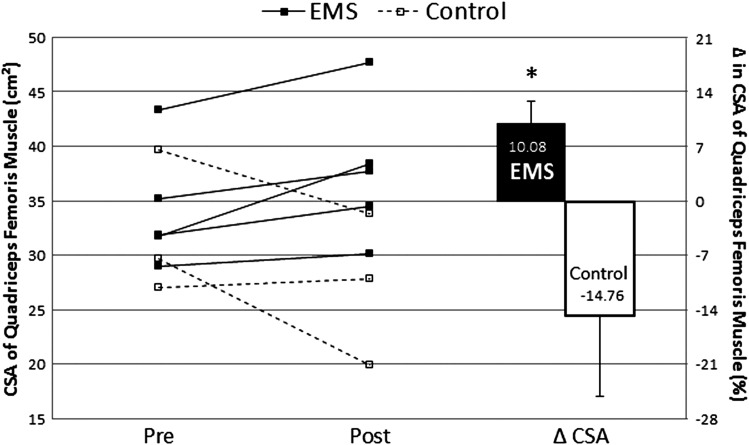
Average CSA was not significantly different (P = 0.228) between experimental groups at the beginning of the treatment. After 14 weeks of training, the percentage of change of average CSA from pre- to post-intervention was significantly higher in the EMS (U = 0, P < 0,05; δ = 1) (Fig. 2). In the intervention group, the percentage of change of average CSA from pre- to post-intervention was 10.08% (range: 3.95 to 20.85%), whereas in the control group it was −14.76 (range: 3.34 to −32.84%).
Figure 2.
Individual values (left side of the panel) and mean percentage of change of average quadriceps femoris CSA (mean ± SD). *P < 0.05 intervention group (EMS) vs. control group.
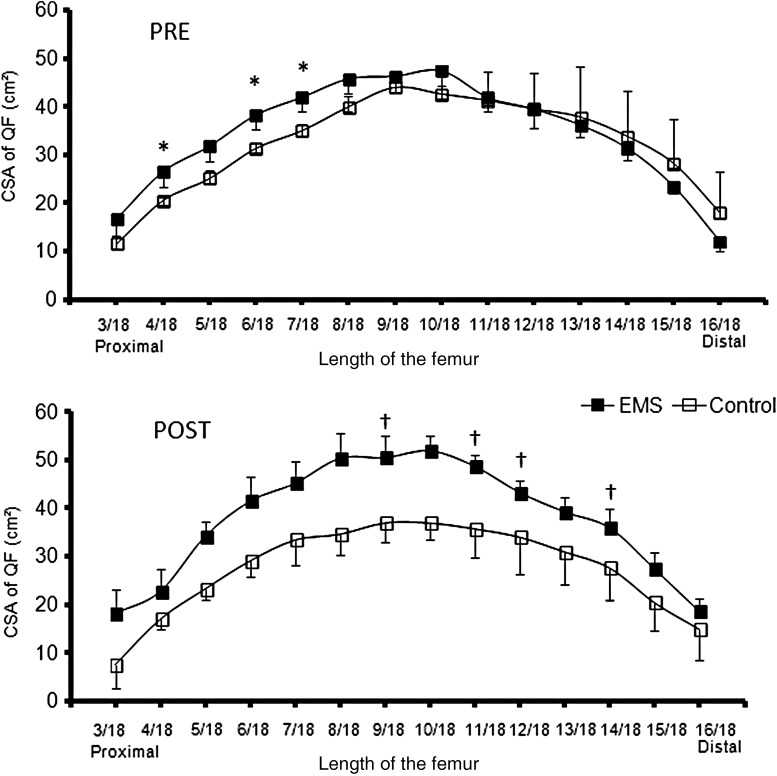
When comparing the sections of the quadriceps femoris (3/18 to 16/18 of femur's length), at pre-measurements the EMS group showed greater values than the CON in the sections 4/18 (U = 1, P < 0.05; δ = 0.866), 6/18 (U = 0, P < 0.05; δ = 1) and 7/18 (U = 1, P < 0.05; δ = 0.866) (Fig. 3). The comparison of the percentage of change from pre to post in each section of the quadriceps femoris revealed a greater increase in muscle CSA for the EMS in the medial to distal portion of the thigh, more specifically in the cuts 9/18 (P < 0.05; δ = 0.866), 11/18 (P < 0.05; δ = 1), 12/18 (P < 0.05; δ = 0.866), and 14/18 (P < 0.05; δ = 0.866) (Table 2).
Figure 3.
Quadriceps femoris CSA (mean ± SD) for length of femur 3/18 to 16/18 before (Pre) and after (Post) electrical-stimulation training for the intervention (EMS, filled boxes) and control group (empty boxes). *P < 0.05 intervention vs. control values at Pre. †P < 0.05 intervention vs. control percentage of change from PRE to POST.
Table 2.
Percentage of change in CSA (cm2) from pre to post-intervention in the intervention (ES) and control group
| Section | Δ Intervention (n = 5) | Δ Control (n = 3) | U Δ ES – Δ Control |
|---|---|---|---|
| 3/18 | 9.9 (35.3) | −34.1 (42.7) | 2 |
| 4/18 | −13.5 (32.2) | −18.2 (14.2) | 5 |
| 5/18 | 10.1 (25.8) | −8.2 (6.0) | 4 |
| 6/18 | 8.6 (23.2) | −7.3 (19.2) | 3 |
| 7/18 | 7.3 (16.1) | −3.9 (29.6) | 6 |
| 8/18 | 9.0 (10.6) | −13.2 (20.7) | 2 |
| 9/18 | 8.6 (7.8)* | −15.2 (20.3) | 1 |
| 10/18 | 10.1 (10.9) | −13.1 (14.1) | 2 |
| 11/18 | 17.4 (11.8)* | −13.2 (15.4) | 0 |
| 12/18 | 11.7 (12.3)* | −14.2 (16.6) | 1 |
| 13/18 | 9.1 (13.5) | −14.9 (19.1) | 2 |
| 14/18 | 15.0 (19.1)* | −15.6 (20.8) | 1 |
| 15/18 | 18.4 (33.6) | −23.0 (27.0) | 2 |
| 16/18 | 102.3 (170.0) | −11.9 (29.5) | 2 |
Values expressed as mean (±SD).
*P < 0.05 compared to control values.
Bone density
Before treatment there were no differences in BMD or T-scores between intervention and control groups for any measured region with the exception of the lumbar area in which the control group had higher values than the intervention group (P < 0.05). The percentages of change in BMD from pre- to post-intervention were not significantly different between the two groups (P > 0.05) (Table 3).
Table 3.
BMD (g/cm2) and T-score at pre-intervention (pre), post-intervention (post) and % of change from pre to post in the intervention (ES) and control groups
| Region BMD | Intervention (n = 5) |
Control (n = 3) |
U Pre GE – pre–control | U Δ GE – Δ Control | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ (%) | Pre | Post | Δ (%) | |||
| Leg | 1.19 (0.12) | 1.16 (0.08) | −2.92 (5.14) | 1.44 (0.17) | 1.38 (0.04) | −3.34 (8.58) | 2 | 7 |
| Femoral neck† | 0.79 (0.14) | 0.72 (0.08) | −7.56 (7.24) | 0.96 (0.15) | 0.88 (0.16) | −8.34 (2.48) | 1 | 5 |
| Trochanteric† | 0.70 (0.14) | 0.62 (0.09) | −9.94 (6.86) | 0.83 (0.01) | 0.77 (0.07) | −8.12 (7.75) | 2 | 5 |
| Intertrochanteric† | 1.05 (0.19) | 0.98 (0.13) | −5.86 (5.01) | 1.22 (0.15) | 1.21 (0.09) | −0.95 (8.28) | 2 | 4 |
| Ward's triangle† | 0.65 (0.16) | 0.59 (0.09) | −6.47 (13.68) | 0.86 (0.29) | 0.79 (0.27) | −7.74 (1.01) | 2 | 2 |
| Whole hip† | 0.92 (0.16) | 0.85 (0.11) | −6.99 (5.19) | 1.08 (0.03) | 1.07 (0.04) | −0.42 (6.23) | 2 | 1 |
| Lumbar area† | 0.91 (0.07)* | 0.95 (0.11) | 3.47 (5.54) | 1.23 (0.1) | 1.26 (0.15) | 2.08 (3.79) | 0 | 4 |
| T-score | Pre | Post | U Pre GE – Pre Control | Pre | Post | U Pre–post GE – pre–post control | ||
| Femoral neck† | −1.55 (1.49) | −2.31 (0.83) | 2 | 0.28 (1.67) | −0.48 (1.87) | 4.5 | ||
| Trochanteric† | −0.73 (1.67) | −1.79 (1.10) | 2 | 0.68 (0.04) | 0.7 (0.5) | 1 | ||
| Intertrochanteric† | −1.02 (1.67) | −1.78 (1.03) | 2.5 | 0.18 (0.18) | 0.08 (0.81) | 3 | ||
| Ward's triangle† | −1.51 (1.48) | −2.08 (0.95) | 3 | 0.9 (3.18) | 0.25 (3.04) | 2 | ||
| Whole hip† | −0.97 (1.66) | −1.8 (1.04) | 2 | 0.48 (0.39) | 0.3 (0.5) | 3 | ||
| Lumbar area† | −1.56 (0.5)* | −1.36 (0.94) | 0 | 1.3 (0.85) | 1.55 (1.34) | 5 | ||
Values are expressed as mean (±SD).
Δ (%), Percentage of change in BMD.
*Compared to control P < 0.05.
†n = 2 in control group.
Blood markers
There were no significant differences in pre-training values of any bone biomarker (Table 4). Regarding lipids and lipoproteins, LDL, total cholesterol/HDL ratio, and LDL/HDL ratio were significantly higher in the CON group at pre-training measurements. After 14 weeks of training, EMS and CON groups had the same percentage of change in all serum markers (P > 0.05).
Table 4.
Serum markers before (pre) and after (post) 14 weeks of electro-stimulation training (media ± SD) in the intervention (ES) and control groups
| Intervention |
Control |
U pre EMS – pre control | U Δ EMS – Δ control | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Δ from pre | n | Pre | Δ from pre | n | |||
| T (ng/ml) | 5.59 ± 2.1 | −3.1 ± 25 | 5 | 4.68 ± 1.8 | 9.8 ± 26.6 | 3 | 4 | 5 |
| C (ug/dl) | 13.5 ± 3.6 | −21.4 ± 22.2 | 4 | 10.13 ± 4.5 | 20.6 ± 38 | 2 | 4 | 1 |
| Ratio T/C | 0.46 ± 0.3 | 40.5 ± 43.3 | 4 | 0.48 ± 0.1 | 0.9 ± 8.6 | 2 | 6 | 2 |
| GH (ng/ml) | 0.34 ± 0.3 | 28.9 ± 64.6 | 5 | 0.2 ± 0.0 | 16.7 ± 28.9 | 3 | 6 | 7 |
| IGF-I (ng/ml) | 179.8 ± 75.7 | 4.3 ± 81.4 | 5 | 136 ± 58.1 | 33.9 ± 31.9 | 3 | 4 | 4 |
| O (ng/ml) | 10.64 ± 5.5 | 51.5 ± 50.5 | 5 | 6.63 ± 2.5 | 27.7 ± 57 | 3 | 4 | 5 |
| CTx (ng/ml) | 1.26 ± 0.6 | −26.4 ± 38 | 5 | 0.93 ± 0.1 | −28.1 ± 26 | 3 | 6 | 7 |
| Ratio O/CTx | 8.63 ± 4.0 | 122.5 ± 53.1 | 5 | 7.29 ± 3.3 | 84.6 ± 92.1 | 3 | 7 | 4 |
| TC (mg/dl) | 214.4 ± 24.6 | −14.84 ± 10.4 | 5 | 236.0 ± 16.7 | −11.18 ± 21.9 | 3 | 3 | 7 |
| LDL (mg/dl) | 147.6 ± 15.2* | −18.71 ± 7.4 | 4 | 176.0 ± 5.6 | −14.49 ± 24.3 | 3 | 0 | 5 |
| HDL (mg/dl) | 38.6 ± 8.7 | −10.21 ± 11.1 | 4 | 31.0 ± 12 | 17.12 ± 33.3 | 3 | 5 | 3 |
| TG (mg/dl) | 141.0 ± 35.3 | −16.76 ± 30.1 | 5 | 145.0 ± 17.6 | −11.59 ± 30.3 | 3 | 6 | 6 |
| Ratio CT/HDL | 5.71 ± 1.1* | −8.19 ± 5.26 | 4 | 8.35 ± 3 | −18.67 ± 31.6 | 3 | 1 | 5 |
| Ratio LDL/HDL | 3.94 ± 0.8* | −8.83 ± 9.92 | 4 | 6.34 ± 2.7 | −20.82 ± 34.9 | 3 | 1 | 6 |
Δ, change; EMS, intervention group; T, total serum testosterone; GH, growth hormone; IGF-I, insulin growth factor-I; C, serum cortisol; O, serum osteocalcin; CTx, serum type I collagen C-telopeptide; TC, total cholesterol; TG, triglycerides.
*P < 0.05 compared to control values.
Carbohydrate metabolism
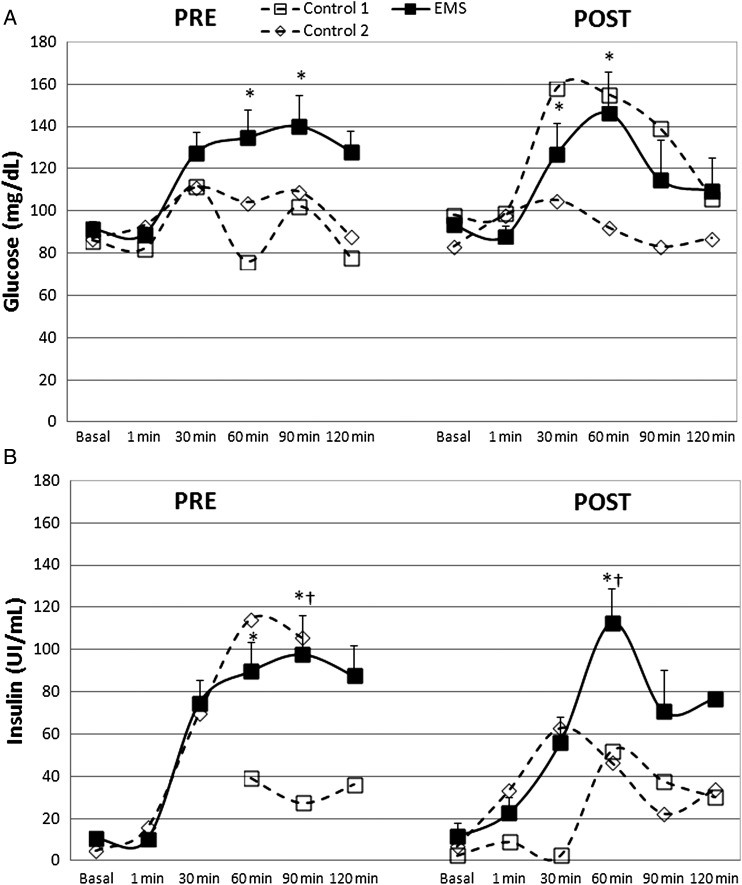
Blood glucose concentration significantly changed in the EMS group at the beginning of the protocol (χ2(5) = 17.914, P = 0.003). Further multiple comparison test showed significant increases at 60 and 90 minutes samples when compared to 1 minute (P < 0.05) (Fig. 4A). After the 14 weeks of electro-stimulation, glucose concentration significantly increased (χ2(5) = 18.486, P = 0.002) at moments 30 and 60 minutes when compared to 1 minute (P < 0.05). Blood insulin significantly changed in the EMS group during the pre-measurements (χ2(5) = 18.600, P = 0.002). Further multiple comparison test revealed significant increases at 60 and 90 minutes when compared to 1 minute (P < 0.05) (Fig. 4B). After 14 weeks of electro-stimulation, insulin concentrations were significantly changed (χ2(5) = 14.257, P = 0.014). Multiple comparison test showed significant increases at 60 minutes when compared to basal and 1-minute samples (P < 0.05).
Figure 4.
Blood glucose (A) and insulin (B) (mean ± SE) after oral administration of 75 g of glucose in the intervention group (EMS); and individual values of controls before (Pre) and after (Post) 14 weeks of electrical-stimulation. †P < 0.05 compared to basal; *P < 0.05 compared to 0 minute.
Discussion
To our knowledge, this is the first study comparing the effects of EMS on both muscle size and BMD in individuals with recent ASIA A SCI. The goal of this study was to investigate bone and muscle adaptations to an electrical stimulation treatment shortly after SCI occurs. The previous literature on electrical stimulation training in recent spinal cord injured persons showed improvements in muscle content.13,14 The intensity of the evoked contraction proved to be an important factor in an electrical stimulation training program in SCI,27 which is true for patients with either recent13 or chronic SCI.28 The present protocol was proved to acutely induce positive alterations in serum markers of bone turnover in persons with recent SCI24; therefore, the selected evoked contraction intensity was thought to be sufficient to elicit positive changes in bone metabolism longitudinally.
Muscle atrophy occurs rapidly after SCI. This atrophy has been related to both denervation and disuse.29 Six weeks after trauma, muscle fiber size is about 60% of those of able-bodied controls,1 with a further 32% decrease at 24 weeks after SCI. Muscle size increased in all participants of the intervention group from 8 weeks to 22 weeks after SCI an average of 10.08%, while the control group showed a mean decrease of 14.76%. The differences between groups were both significant and large after Cohen's proposed convention for some effect size estimates.30 It has been previously proposed that skeletal muscle exposed to minimal activity is more apt to grow in response to stimulus compared with muscle exposed to normal weight bearing.31 Nonetheless, the CSA of quadriceps femoris in the group exposed to electrical stimulation remained smaller (about 51 cm2 at its greatest site) than that observed in non-trained healthy adults measured by the same technique (about 80 cm2 at its greatest site).32 Thus, there is still a large room of improvement to achieve healthy muscle content values. Whether a different training intervention, in terms of length and/or intensity, induced further muscle increases or improvements had already reached a plateau, is unknown. Future studies including muscle biopsies might help to elucidate any change on muscle fiber typing, size, or fatigability.
Previous anti-osteoporosis studies using electrical stimulation in persons with recent SCI demonstrate contradictory results.15–20 A possible reason for this conflict may be the location of measured sites, since bone mineral adaptations are limited to the bone area under mechanical stress.33 The study by Groah et al.18 showed positive effects on BMD in the distal femur and proximal tibia, whereas lumbar and hip measurements did not show any benefits from quadriceps electrical stimulation. Shield and Dudley-Javorosky19 reported positive adaptation on the distal tibiae after plantar flexor muscle stimulation. In the study by Clark et al.15 quadriceps femoris and tibialis anterior muscles were electrically stimulated, and BMD on proximal tibia or distal femur was not measured due to an acknowledged limitation of the DEXA software, while proximal femur did not show any positive adaptation to training. Finally, the study by Eser et al.16 stimulated quadriceps, gluteal and hamstring muscles, recording no effects on BMD on the tibial diaphysis. This study did not show any attenuation of bone loss in the measured sites (i.e. proximal femur, lumbar, and hip) after electrical stimulation, as previously reported from those places.15,18 Since muscle adaptations were maximal in the middle to distal portion of the thigh, it remains unknown whether any bone adaptations occurred at a different portion of the femur.
Another reason for the inconsistency of bone adaptations to electrical stimulation observed in the literature is related to the intensity of the electrically evoked muscle contractions. A study in chronic spinal cord injured persons (i.e. more than 2 years from SCI) showed a direct relationship between the intensity of the stimuli and the osteogenic effects.34 Furthermore, EMS was suggested to be less effective than electrical stimulation cycling on BMD prevention after SCI.20 The present protocol used stimulation patterns that acutely decrease the serum concentration of osteoclastic markers in men with recent SCI (i.e. CTx),24 suggesting a positive effect on bone metabolism. However, the training effects of our study could have been either insufficient to attenuate bone loss longitudinal or encumbered by as-yet-unexplained factors affecting the osteogenic response to mechanical strain.15 Both groups moderately increase their BMD at the lumbar area. Most probably, changes are not related to the EMS intervention, but may be due to weight-loading at the lumbar spine while sitting in a wheelchair.35
Serum markers were selected in order to provide a comprehensive approach of the anabolic/catabolic state of subjects and the bone formation/destruction status. None of the measured serum markers showed significant changes from week 8 to week 22 after SCI in either the intervention or the control group. Serum testosterone is suppressed after SCI, but it returns to normal levels 6 weeks after SCI.36 The study by Maïmoun et al.4 reported testosterone values within normal clinical levels at 13.4 weeks after a thoracic SCI, but below those of able-bodied persons. Another study did not report any change in testosterone levels from week 16 to week 24 after SCI.37 Levels of GH and IGF-I are not altered during the early stages following SCI.4 No data concerning cortisol alterations following SCI were available. Osteocalcin levels in person with recent SCI (16 weeks after trauma) were reported to be higher than those of able-bodied persons,37 but within normal values5,18 as reported herein. There were no reported changes from week 8 to week 24 after SCI in osteocalcin levels.5,37 Levels of bone resorption markers, such as CTx, were reported systematically elevated after SCI.4,5,18,26,37 These markers remained higher for longer than week 24 after SCI.5,37
In spite of the relatively large decreases in cortisol (>20%) and in CTx (>26%) concentrations observed in both groups, together with a noteworthy increase in osteocalcin levels (>50% in the intervention group), our data did show any significant change over time in the measured serum markers partially due to the small sample size. Thus, the measured basal serum biomarkers do not clarify the observed muscle and bone adaptations to training.
Persons with SCI have higher serum triglycerides, total and LDL cholesterol, together with lower serum HDL cholesterol levels compared to healthy populations.21,38,39 Disturbances in the autonomic nervous system and muscle paralysis39 lead to an increased morbidity and mortality from cardiovascular causes after SCI,40 with a 228% incidence of developing cardiovascular diseases in SCI compared to healthy able-bodied controls.41 Sport activity was the most important determinant of changes in total cholesterol, LDL and TC/HDL-C between the 6th and 24th month after SCI, explaining 34, 47, and 29% of the variance, respectively.22 These findings imply that subjects who were physically active showed larger improvements of the risk profiles than sedentary or less active subjects. For that reason, we hypothesized that an increase in skeletal muscle activity (i.e. 5 sessions per week of EMS) will reduce the negative effects of SCI in the lipid and lipoprotein profiles. The present EMS intervention induced positive adaptation in muscle size. However, it was not sufficient to positively affect the lipid and lipoprotein profiles. Other more vigorous physical interventions involving larger muscle mass and challenging the cardiovascular system might be necessary to have an impact on these markers.
Glucose intolerance and insulin resistance are abnormalities associated to SCI.2,41 Glucose intolerance after glucose load is characterized by a higher rise of plasma glucose and slower return to the baseline than does in normal individuals.42 Thus, the fact that peak values of glucose and insulin in the EMS group appeared sooner in time after the 14-week intervention suggests faster glucose absorption and, therefore, an improved carbohydrate metabolism. Individual control values do not show any improvements in plasmatic glucose or insulin after OGTT. However, these results should be carefully taken due to the small sample size. The predominant peripheral tissue responsible for insulin-induced glucose uptake is muscle.21 Thus, a probable etiology of the abnormality in carbohydrate handling noted in subjects with SCI may result from morphological changes and lean tissue atrophy with associated potential receptor and post-receptor perturbations. Bauman et al.21 found that subjects with greater neuronal damage (tetraplegia vs. paraplegia), especially males, showed worse carbohydrates tolerance and greater insulin values. Aksnes et al.43 studied whole body insulin-mediated glucose utilization and isolate glucose transport in denervated muscle in vitro in patients with tetraplegia. They concluded that the 43% reduction in whole body glucose transport was due to reductions in muscle mass together with defective transcapillary diffusion of insulin to the cell surface, hormonal changes, and dyslipidemia. Skeletal muscle showed a remarkable capacity to maintain an intact glucose transport system in spite of denervation and sever morphological changes (i.e. myofiber atrophy, lack of type I fibers, and connective tissue increase). In this study, positive correlations were found in the EMS group between the change in muscle size and the change in basal glucose concentration (r = −0.904, P < 0.05). This may suggest that those who increased more their muscle volume had more improvements in their basal glucose from pre to post.
The positive effects observed on muscle content might be clinically relevant since muscle atrophy after SCI proved to result in other health related problems such as reduced vascularisation, basal metabolism, cardiac output and oxygen consumption; increased intramuscular fats, and impaired glucose tolerance.2,7,8 Thus, it would be advisable for physicians and physiotherapists to longitudinally apply a stimulus capable to counteract those changes to individuals with a recent SCI. The current electrical stimulation protocol would be a plausible option. However, further research aimed to improve both muscle and bone is warranted. In this study age, gender, level of lesion, ASIA score and time post-injury were controlled. However, adequate matching may never be possible in the SCI population due to inter-individual variability with a small number of participants.44 The study length may have resulted in a limitation of the study. It is possible that intervention durations were not long enough for the bone benefits to be gained from EMS.
Conclusion
The results of this pilot study indicate that skeletal muscle is more sensitive than bone to the same external stimuli. Eighty electrically induced isometric contractions per day, 47 minutes/day, 5 days/ week during 14 weeks increased muscle content, whereas bone tissue was not significantly affected. The improvements in carbohydrate metabolism could be related to the improvements in muscle tissue. Results should be confirmed by further investigation on this area of research.
References
- 1.Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol 1999;86(1):350–8 [DOI] [PubMed] [Google Scholar]
- 2.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 2004;42(12):711–6 [DOI] [PubMed] [Google Scholar]
- 3.Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88(4):1310–5 [DOI] [PubMed] [Google Scholar]
- 4.Maïmoun L, Lumbroso S, Paris F, Couret I, Peruchon E, Rouays-Mabit E, et al. The role of androgens or growth factors in the bone resorption process in recent spinal cord injured patients: a cross-sectional study. Spinal Cord 2006;44(12):791–7 [DOI] [PubMed] [Google Scholar]
- 5.Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 1998;83(2):415–22 [DOI] [PubMed] [Google Scholar]
- 6.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 1995;33(11):674–7 [DOI] [PubMed] [Google Scholar]
- 7.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006;29(5):489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr T, Dela F, Handberg A, Biering-Sørensen F, Galbo H, Kjaer M. Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sports Exerc 2001;33(8):1247–52 [DOI] [PubMed] [Google Scholar]
- 9.Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol 2007;579(3):877–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maïmoun L, Fattal C, Micallef J-P, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord 2006;44(4):203–10 [DOI] [PubMed] [Google Scholar]
- 11.Apstein MD, George BC. Serum lipids during the first year following acute spinal cord injury. Metabolism 1998;47(4):367–70 [DOI] [PubMed] [Google Scholar]
- 12.Biering-Sørensen F, Hansen B, Lee BS. Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord 2009;47(7):508–18 [DOI] [PubMed] [Google Scholar]
- 13.Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998;36(7):463–9 [DOI] [PubMed] [Google Scholar]
- 14.Crameri RM, Weston AR, Rutkowski S, Middleton JW, Davis GM, Sutton JR. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol 2000;83(4–5):409–15 [DOI] [PubMed] [Google Scholar]
- 15.Clark JM, Jelbart M, Rischbieth H, Strayer J, Chatterton B, Schultz C, et al. Physiological effects of lower extremity functional electrical stimulation in early spinal cord injury: lack of efficacy to prevent bone loss. Spinal Cord 2007;45(1):78–85 [DOI] [PubMed] [Google Scholar]
- 16.Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stüssi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest 2003;33(5):412–9 [DOI] [PubMed] [Google Scholar]
- 17.Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther 2008;88(3):387–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groah SL, Lichy AM, Libin AV, Ljungberg I. Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PM R 2010;2(12):1080–7 [DOI] [PubMed] [Google Scholar]
- 19.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol 2006;95(4):2380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE. Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal Cord 2011;49(8):917–23 [DOI] [PubMed] [Google Scholar]
- 21.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 1999;37(11):765–71 [DOI] [PubMed] [Google Scholar]
- 22.Dallmeijer AJ, van der Woude LH, van Kamp GJ, Hollander AP. Changes in lipid, lipoprotein and apolipoprotein profiles in persons with spinal cord injuries during the first 2 years post-injury. Spinal Cord 1999;37(2):96–102 [DOI] [PubMed] [Google Scholar]
- 23.Bax L, Staes F, Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomised controlled trials. Sports Med 2005;35(3):191–212 [DOI] [PubMed] [Google Scholar]
- 24.Arija-Blázquez A, Ceruelo-Abajo S, Díaz-Merino MS, Godino-Durán JA, Florensa-Vila J. Time-course response in serum markers of bone turnover to a single-bout of electrical stimulation in patients with recent spinal cord injury. Eur J Appl Physiol 2013;113(1):89–97 [DOI] [PubMed] [Google Scholar]
- 25.Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol Endocrinol Metab 2006;290(6):E1098–103 [DOI] [PubMed] [Google Scholar]
- 26.Maïmoun L, Couret I, Micallef J, Peruchon E, Mariano-Goulart D, Rossi M, et al. Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metab Clin Exp 2002;51(8):958–63 [DOI] [PubMed] [Google Scholar]
- 27.Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve 2004;29(1):104–11 [DOI] [PubMed] [Google Scholar]
- 28.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22 [DOI] [PubMed] [Google Scholar]
- 29.Gordon T, Mao J. Muscle atrophy and procedures for training after spinal cord injury. Phys Ther 1994;74(1):50–60 [DOI] [PubMed] [Google Scholar]
- 30.Cohen J A power primer. Psychol Bull 1992;112(1):155–9 [DOI] [PubMed] [Google Scholar]
- 31.Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol 2004;96(4):1451–8 [DOI] [PubMed] [Google Scholar]
- 32.Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Häkkinen K. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol 2003;89(6):555–63 [DOI] [PubMed] [Google Scholar]
- 33.Bélanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 2000;81(8):1090–8 [DOI] [PubMed] [Google Scholar]
- 34.Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 1996;19(1):61–58 [DOI] [PubMed] [Google Scholar]
- 35.Biering-Sørensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 1988;26(5):293–301 [DOI] [PubMed] [Google Scholar]
- 36.Naftchi NE, Viau AT, Sell GH, Lowman EW. Pituitary-testicular axis dysfunction in spinal cord injury. Arch Phys Med Rehabil 1980;61(9):402–5 [PubMed] [Google Scholar]
- 37.Maïmoun L, Couret I, Mariano-Goulart D, Dupuy AM, Micallef JP, Peruchon E, et al. Changes on osteoprotegerin/RANKL system, bone mineral density, and bone biomechanicals markers in patients with recent spinal cord injury. Calcif Tissue Int 2005;76(6):404–11 [DOI] [PubMed] [Google Scholar]
- 38.Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia 1992;30(10):697–703 [DOI] [PubMed] [Google Scholar]
- 39.Washburn RA, Figoni SF. High density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity. Spinal Cord 1999;37(10):685–95 [DOI] [PubMed] [Google Scholar]
- 40.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007;86(2):142–52 [DOI] [PubMed] [Google Scholar]
- 41.Kocina P Body composition of spinal cord injured adults. Sports Med 1997;23(1):48–60 [DOI] [PubMed] [Google Scholar]
- 42.Ganong WF ed. Review of medical physiology. 21st ed New York: Lange medical books/McGraw-Hill; 2003. Chapter 19 [Google Scholar]
- 43.Aksnes AK, Hjeltnes N, Wahlström EO, Katz A, Zierath JR, Wallberg-Henriksson H. Intact glucose transport in morphologically altered denervated skeletal muscle from quadriplegic patients. Am J Physiol 1996;271(3 Pt 1):E593–600 [DOI] [PubMed] [Google Scholar]
- 44.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 2005;43(11):649–57 [DOI] [PubMed] [Google Scholar]