SUMMARY
The human FOXP3 molecule is an oligomeric transcriptional factor able to mediate activities that characterize T regulatory cells, a class of lymphocytes central to the regulation of immune responses. The activity of FOXP3 is regulated at the post-translational level, in part by two histone acetyltransferases (HAT), TIP60 and p300. TIP60 and p300 work cooperatively to regulate FOXP3 activity. Initially p300 and TIP60 interactions lead to the activation of TIP60 and facilitate acetylation of K327 of TIP60, which functions as a molecular switch to allow TIP60 to change binding partners. Subsequently, p300 is released from this complex and TIP60 interacts with and acetylates FOXP3. Maximal induction of FOXP3 activities is observed when both p300 and TIP60 are able to undergo cooperative interactions. Conditional knockout of TIP60 in Treg cells significantly decreases the Treg population in the peripheral immune organs, leading to a scurfy-like fatal autoimmune disease.
INTRODUCTION
FOXP3 plays an important role in the regulation of Treg function. (Fontenot et al., 2003; Hori et al., 2003; Li and Greene, 2007). Acetylation, a process catalyzed by opposing actions of histone acetyltransferases (HAT) and histone deacetylases (HDAC), is one of the set of post-translational modifications that regulates the stability and transcriptional activity of FOXP3. HATs and HDACs were first identified as enzymes responsible for histone acetylation, but were later found to promote acetylation of many substrates other than histone(Li et al., 2007; Tao et al., 2007; van Loosdregt et al., 2010; Xiao et al., 2010; Zhang et al., 2012).
Based on sequence homology, HATs can be divided into three major categories, the Gcn5/PCAF family, the p300/CBP family, and the MYST family (Yang, 2004). Two HATs, TIP60 a member of the MYST family, and p300 of the p300/CBP family, have been reported to promote FOXP3 acetylation (Li et al., 2007; Liu et al., 2013; van Loosdregt et al., 2010). TIP60 interacts with the N terminal domain of FOXP3 and is required for the increased repressive transcriptional activity of FOXP3. Acetylation of Lysine (K) 8 of FOXP3 promoted by TIP60 is important to the increased activity of FOXP3, because a HAT deficient TIP60 mutant is not able to enhance pFOXP3 suppressive activity (Li et al., 2007). p300 has been suggested to have a similar effect in promoting the repressive transcriptional activity of FOXP3 by increasing the stability of certain pools of FOXP3 (van Loosdregt et al., 2010). As in the case of many other proteins, the stability of FOXP3 is regulated by ubiquitination which leads to proteosome mediated protein degradation. The p300 moiety increases the acetylation level of FOXP3 which then decreases the ubiquitination level of FOXP3, preventing its degradation (van Loosdregt et al., 2011).
In a comparable manner to regulation of the activity of many kinases by phosphorylation, the acetyl-transferase activities of certain HATs are also regulated through acetylation catalyzed either by itself or by other HATs. Auto-acetylation of TIP60 can be induced by diverse signals such as UV irradiation of cells. This type of injury and its signals increases TIP60 HAT activity. Deacetylation of TIP60 by SIRT1 decreases its HAT activity and maintains levels of TIP60 proteins (Wang and Chen, 2010; Yamagata and Kitabayashi, 2009). Similarly, auto-acetylation is also important for the function of p300. Auto-acetylation of an inhibitory loop in p300 is thought to be required to activate the HAT activity of p300 and increase substrate accessibility (Thompson et al., 2004). p300 may further promote the acetylation of TIP60(Col et al., 2005). Therefore a complicated set of interactions occurs between different HATs and is required for regulation of acetyltransferase activities.
TIP60 and p300 have been identified previously as HATs that individually influence the activity of FOXP3 (Li et al., 2007; Liu et al., 2013; van Loosdregt et al., 2010). Since acetylation is critical to the function of FOXP3, understanding the separate and combine roles of these HAT in the regulation of FOXP3 is important to understand the molecular mechanisms involved in regulation of Treg cells.
Our studies indicate that p300 interactions with Tip60 promotes TIP60 auto-acetylation, which we have defined as important to maintain the stability of the TIP60 protein. p300 interaction with Tip60 also critically promotes a specific modification which acts as a switch to govern TIP60's interaction with its substrates. TIP60 in turn promotes p300 acetylation which is critical for HAT activity of p300. Thus these two enzymes promote the acetylation level and HAT activities of each other, which promotes a synergistic effect on FOXP3 acetylation, and increases the repressive transcriptional activity of FOXP3. We have also unexpectedly discovered a dominant role for TIP60 in maintenance of peripheral Treg survival and function. Selective loss of TIP60 in FOXP3 expressing Treg cells can lead to significant peripheral deficits of suppressive activity that lead to catastrophic scurfy like disease.
RESULTS
TIP60 and p300 promote FOXP3 acetylation cooperatively in a HAT-dependent manner
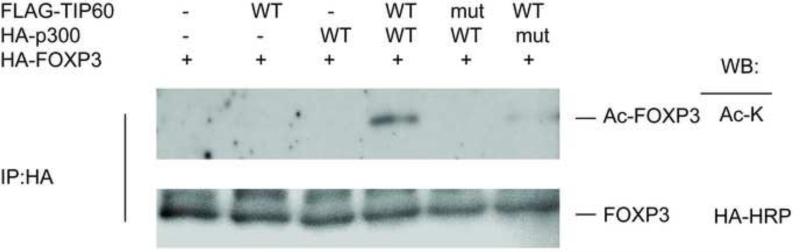
Both TIP60 and p300 have been shown to promote the acetylation of FOXP3. We sought to understand if these enzymes act in a cooperative manner. To investigate this cooperative interplay, 293T cells were co-transfected with FOXP3, TIP60, and p300. The acetylation of FOXP3 was then determined in the presence of TIP60 and p300. Figure 1 shows that strong acetylation pattern of FOXP3 is observed when both TIP60 and p300 are present. In the absence of either enzyme, however, acetylation of FOXP3 is weak. These studies indicate that synergistic interactions occur between TIP60 and p300 in dominant acetylation of FOXP3.
Figure 1. Cooperative effect of TIP60 and p300 on FOXP3 acetylation.
293T cells are transfected with HA-FOXP3, FLAG-TIP60, FLAG-p300, or HAT deficient FLAG-TIP60 mutant or FLAG-p300 mutant as indicated. 24 h after transfection, cell lysates were collected and immunoprecipitated with anti-HA agarose, followed by blotting with anti-acetylated lysine antibody or anti-HA antibody.
We next evaluated the contributory role of intrinsic TIP60 HAT activity for the co-activation of p300 and other transcription factors (Korzus et al., 1998; Senf et al., 2011). The acetylation of FOXP3 was tested using HAT-deficient TIP60 (Q377E/G380E) and p300 (F1504A)(Ikura et al., 2000; Ito et al., 2001) species. While the acetylation of FOXP3 was strong in the presence of both wild type TIP60 and wild type p300, no acetylation was observed when either of the HAT-deficient mutants was present (Figure 1). These studies indicate that the cooperative effects that occur between TIP60 and p300 are HAT-dependent with respect to acetylation of FOXP3.
p300 acetylation promoted by TIP60 requires HAT activity of TIP60
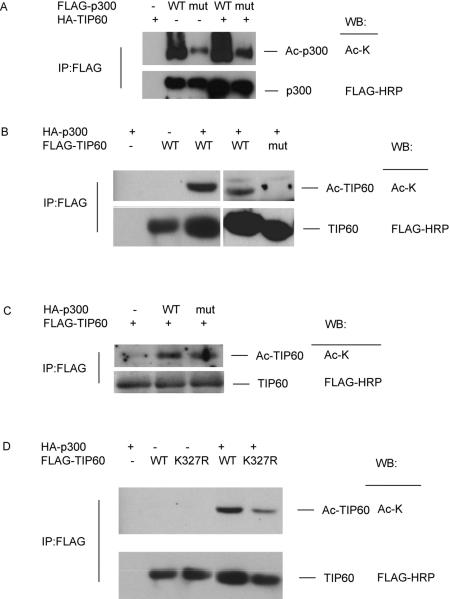
Since TIP60 and p300 work synergistically to promote the acetylation of FOXP3, we investigated how each enzyme is affected by the activity of the other. To study this, the acetylation level of p300 was tested in both the presence and absence of TIP60. As shown in Figure 2A, the presence of TIP60 led to increased acetylation of p300. Surprisingly this modification effect was even more significant for the HAT-deficient mutant of p300. Since the p300F1504A mutant lacks the HAT activity required for auto-acetylation, the increased level of acetylation of p300 must be induced by the HAT activity of TIP60. In addition the acetylation of p300 may affect how it binds to other proteins (Thompson et al., 2004). Therefore, we examined the interaction between TIP60 and p300, and unexpectedly discovered reduced interactions between the HAT-deficient p300 mutant and TIP60 (Supplementary Figure 1). That is acetylation activity of p300 correlates with its ability to interact with TIP60.
Figure 2. p300 and TIP60 promote the acetylation of each other.
(A) TIP60 promotes the acetylation of p300. 293T cells were transfected with HA-TIP60 and wild type or HAT deficient FLAG-p300 as indicated. 24 h after transfection, cell lysates were immunoprecipitated with anti-FLAG agarose and blotted with anti-acetylated lysine or anti-FLAG HRP. (B-D) 293T cells were transfected with HA-p300 and wild type or mutated FLAG-TIP60 as indicated. 24 h after transfection, cell lysates were immunoprecipitated with anti-FLAG agarose and blotted with anti-acetylated lysine antibody or anti-FLAG HRP. (B) p300 promotes the acetylation level of WT TIP60, but not HAT deficient TIP60 mutant. (C) HAT deficient p300 mutant has the same effect in promoting TIP60 acetylation. (D) TIP60 K327R mutation decreases TIP60 acetylation.
TIP60 auto-acetylation at K327 is promoted by its interaction with p300
It was unclear whether the HAT activity of both enzymes is required for p300 to promote TIP60 acetylation. Therefore, HAT-deficient mutants of TIP60 and p300 were employed to resolve this issue. Although wild type p300 significantly increased the acetylation of wild type TIP60, it had no effect on the TIP60 mutant (Figure 2B), indicating that TIP60 relies on its own intrinsic auto-acetylation even in the presence of p300. The p300 mutant, on the other hand, showed comparable activity to that of wild type p300 to enhance TIP60 acetylation (Figure 2C). Thus, unlike TIP60, the HAT activity of p300 does not play a determinant role in promoting TIP60 acetylation.
K327 of TIP60, has been identified as a strictly conserved lysine site among the MYST family proteins and moreover this residue can be auto-acetylated (Peng et al., 2012; Wang and Chen, 2010; Yang et al., 2012). We explored the possibility that auto-acetylation at K327 is promoted by p300 collisions as well. In the absence of p300, auto-acetylation of TIP60 is totally abolished by the K327R mutation, indicating that K327 is the major auto-acetylation site in TIP60 (Supplementary Figure 2). Similarly, in the presence of p300, the acetylation of TIP60 was significantly reduced when K327 was substituted with arginine (Figure 2D), indicating that p300 physical interactions with Tip60 actually promotes the auto-acetylation of TIP60 at K327. Acetylation of TIP60 (in particular autoacetylation) is known to be important for supporting the total HAT activity of TIP60(Yang, 2004). Therefore, K327 acetylation promoted by p300 also regulates the activity of TIP60.
However, unexpectedly mutating K327 in TIP60 only slightly decreased the cooperative effect of TIP60 and p300 in promoting Foxp3 acetylation. Our studies thus distinguish the consequences of two discreet mutations. Unlike the Q377E/G380E mutation of TIP60 which limits HAT activity and FOXP3 modification , the TIP60 K327 residue is not critical for the cooperative effects of TIP60 and p300 that leads to cooperative FOXP3 acetylation (Supplementary Figure 3).
Autoacetylation of TIP60 promoted by p300 increases TIP60 stability through inhibiting TIP60 ubiquitination
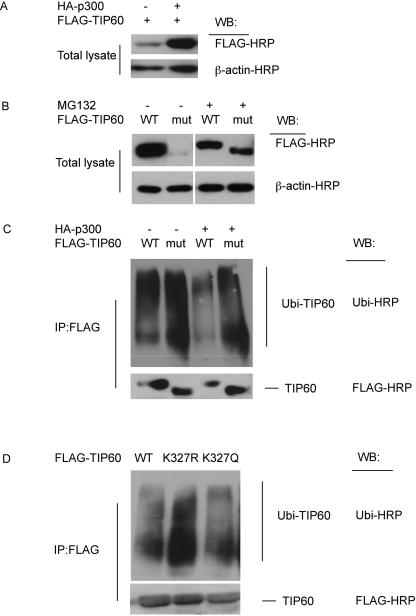
Post-translational acetylation is important for protein stability since it prevents protein degradation mediated by ubiquitination(Caron et al., 2005). To investigate whether p300 regulates TIP60 in a similar manner, the expression level of TIP60 was examined. Expression of TIP60 was found to be increased, (Figure 3A), in the presence of p300. To define the correlation between acetylation and protein stability, the HAT-deficient mutant of TIP60 was studied. As shown in Figure 3B, expression of the TIP60 mutant was significantly reduced compared to that of wild type TIP60, indicating the crucial role acetylation plays on the stability of the TIP60 protein. Only in the presence of MG132, a chemical used to inhibit proteasome dependent protein degradation, was the expression level of the TIP60 mutant restored (Fig 3B).
Figure 3. TIP60 acetylation increases TIP60 stability through inhibiting TIP60 ubiquitination.
(A) Expression level of TIP60 is significantly increased in the presence of p300. 293T cells were transfected with FLAG-TIP60 alone, or cotransfected with FLAG-TIP60 and HA-p300. 24 h after transfection, cell lysates were collected and blot with anti-FLAG HRP or anti-actin HRP. (B) Expression level of TIP60 HAT deficient mutant can be increased by MG132 treatment. 293T cells were transfected with wild type or HAT deficient FLAG-TIP60 mutant. 24 h after transfection, cells were treated with 2 μM MG132 for 16 h. Cell lysates were then collected and blotted with anti-FLAG HRP or anti-actin HRP. (C and D) TIP60 ubiquitination is correlated with TIP60 acetylation. 293T cells were cotransfected with HA-p300 and wild type or HAT deficient FLAG-TIP60 mutant (C), or transfected with wild type FLAG-TIP60 or FLAG-TIP60 K327 mutants (D). 24 h after transfection, cells were treated with 2 μM MG132 to prevent proteasome dependent degradation of ubiquitinated TIP60. Cell lysates were immunoprecipitated with anti-FLAG HRP, and blotted with anti-ubiquitin HRP or anti-FLAG HRP.
We further investigated the ubiquitination patterns of TIP60. Ubiquitination of wild type TIP60 was much lower than that of the HAT-deficient mutant (Fig 3C). In addition, the presence of p300 further decreased ubiquitination of wild type TIP60, due to increased acetylation promoted by p300. Since p300 promotes the auto-acetylation of TIP60 at K327, we also studied the role of K327 in regulating the ubiquitination of TIP60. When we substituted Lysine with Arginine, the K327R TIP60 mutant showed reduced auto-acetylation (not shown) but increased ubiquitination as compared with wild type TIP60 (Figure 3D).
It is notable that a decrease in ubiquitination occurred when K327 was mutated to glutamine, a modification which mimics acetylation at this site. These results indicate that K327 is not an ubiquitination site itself but rather that the acetylation of this site promotes a conformational change of TIP60 to prevent ubiquitination of the protein. Together, our data indicates that the auto-acetylation of TIP60 on K327, which can be enhanced by p300, limits the protein from ubiquitination-mediated proteasomal degradation by a mechanism that apparently relates to large scale changes in protein conformation.
Acetylation regulates the interaction of TIP60 with p300 and FOXP3
We extended these studies to examine if autoacetylation of K327 of the TIP60 acetyltransferase promoted substrate switching. Acetylation may regulate protein function by altering protein-protein interactions and acetylation of hMOF at K274 changes the spatial orientation of that particular lysine, altering the interactions of hMOF with certain of its substrates (Yuan et al., 2012). Further auto-acetylation of TIP60 may dissociate the TIP60 oligomer, resulting in the activation of TIP60. Autoacetylation driven dissociation may further lead to an improved accessibility for its substrates (Wang and Chen, 2010). We examined the interactions of TIP60 with either p300 or FOXP3.
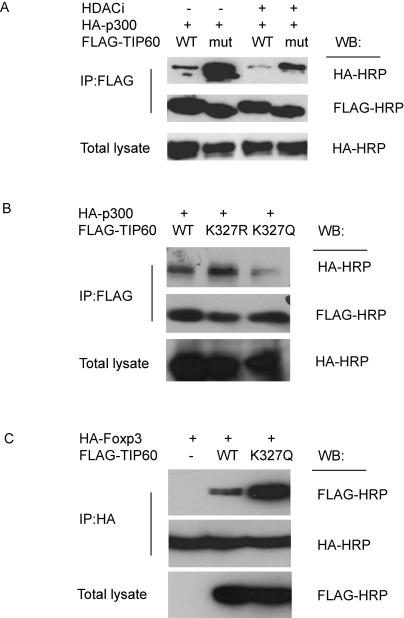
Surprisingly, our results showed that wild type TIP60 interacted much more weakly with p300 than the HAT-deficient mutant of TIP60 (Figure 4A). This interaction was further decreased by HDACi treatment that prevents the deacetylation of TIP60. Together these studies identifies that auto-acetylation lessens the interactions of TIP60 with other proteins such as p300.
Figure 4. Interaction of TIP60 with its substrates is regulated by the acetylation status of TIP60.
(A) Reverse correlation between TIP60 acetylation and interaction of Tip60 and p300. 293T cells were transfected with HA-p300, wild type or HAT deficient FLAG-TIP60. 24 h after transfection, cells were treated with HDACi 400 nM TSA and 10 mM NAD for 16h. Cell lysate were then immunoprecipitated with anti-FLAG agarose and blotted with anti-HA HRP or anti-FLAG HRP. (B) Acetylation status at TIP60 K327 regulates the interaction of TIP60 and p300. 293T cells were transfected with HA-p300 and wild type FLAG-TIP60 or FLAG-TIP60 K327 mutants. 24 h after transfection, cell lysate were immunoprecipitated with anti-FLAG agarose and blotted with anti-HA HRP or anti-FLAG HRP. (C) TIP60 acetylation at K327 increases the interaction of TIP60 and FOXP3. 293T cells were co-transfected with HA-FOXP3 and wild type FLAG-TIP60 or FLAG-TIP60 K327Q mutant. 24 h after transfection, cell lysate were immunoprecipitated with anti-HA agarose and blotted with anti-FLAG HRP or anti-HA HRP.
To determine if acetylation of the K327 residue of TIP60 itself influences the interactions of TIP60 with other proteins, we first investigated the interactions between p300 and the K327 mutants of TIP60 (Figure 4B). The K327R mutant of TIP60, which is less acetylated than the wild type species, interacted strongly with p300, while the K327Q mutant, a residue mutation that mimics the acetylated status of wild type K327, was found to interact only weakly with p300. Thereafter, we investigated whether the interactions between FOXP3 and TIP60 are altered by the acetylation of TIP60. Since the auto-acetylation of TIP60 is weak in the absence of p300, the K327Q mutant was used to mimic the acetylated TIP60. In contrast to the interaction of TIP60 and p300, a much stronger interaction with FOXP3 was observed for the TIP60 K327Q mutant as compared with the wild type TIP60 (Figure 4C). In vitro pull down assays using purified TIP60 and Foxp3 fragments also verified that TIP70 K327R mutants has stronger interaction with Foxp3 (Supplementary Figure 4A). The same results were also observed when other substrates of TIP60 are used, such as p53 or HDAC7 (Supplementary Figure 4B and C). Collectively, our data indicate that the acetylation of the single K327 residue plays a pivotal role in the regulation of the interactions of TIP60 with other proteins and provides a biochemical explanation for increased interactions with FOXP3 after substrate switching conformations are induced.
Transcriptional activity of FOXP3 is promoted by TIP60 and p300 cooperative interactions
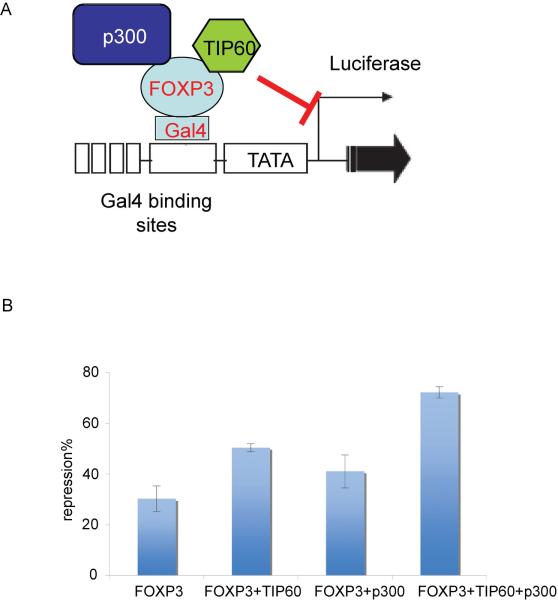
As mentioned TIP60 and p300 can cooperatively increase the acetylation of FOXP3. We examined if cooperative interactions between TIP60 and p300 that affect FOXP3 acetylation correlate with the change in the transcriptional activity of FOXP3. A facile transcriptional repression assay has been established to determine the effect of FOXP3, using a Gal4-FOXP3 fusion protein, on the expression of the firefly luciferase reporter gene driven by a promoter region containing five Gal4-binding sites(Li et al., 2007). This system was used to evaluate the cooperative effect of TIP60 and p300 on the transcriptional activity of FOXP3 (Figure 5A). The MSVβgal vector with a constitutive expression of β-galactosidase was used as a control for transfection. As shown in Figure 5B, FOXP3 alone repressed transcription of the luciferase reporter gene. Such repression was slightly enhanced in the presence of either TIP60 or p300. When both enzymes were present, however, there was a significant increase in the repressive transcriptional activity of FOXP3, indicating that TIP60 and p300 function cooperatively to increase the transcriptional activity of FOXP3.
Figure 5. p300 and TIP60 increase the repressive transcriptional activity of FOXP3 synergistically.
(A) Schematic model of FOXP3 binding to luciferase reporter construct used in luciferase assay. (B) Synergistic effect of TIP60 and p300 on the repressive transcriptional activity of FOXP3. 293T cells were transfected with pBIND (empty vector), pBIND-FOXP3, FLAG-TIP60, FLAG-p300, pG5-Luc luciferase reporter and the control MSV-gal plasmid as indicated, The luciferase activity of the reporter gene was normalized with β-Gal activity. The error bars indicate the SD value.
Effect of p300 and TIP60 in the development of Treg cells
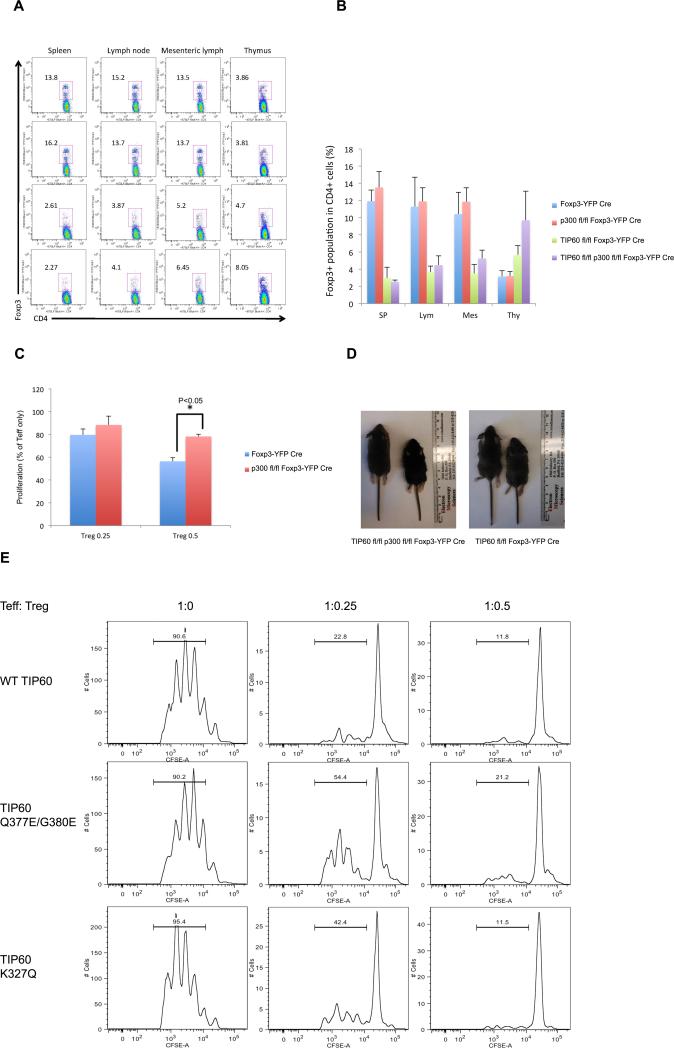
To define the importance of TIP60 and p300 in regulating Treg function in vivo, we conditionally deleted p300 or TIP60 in Treg cells by crossing p300fl/fl or TIP60fl/fl mice with Foxp3YFP-Cre mice. The resultant p300fl/fl Foxp3YFP-Cre mice were further crossed with TIP60fl/fl mice to generate p300fl/fl TIP60fl/fl Foxp3YFP-Cre mice which represent double conditional knockouts of p300 and TIP60 in Treg cells.
p300fl/fl Foxp3YFP-Cre mice developed normally until 8 weeks of age with a normal population of Treg cells in thymus, lymph node and spleen (Figure 6A and B). The size of p300fl/fl Foxp3YFP-Cre mice is also similar to the littermate control without Foxp3-Cre gene (data not shown). Although p300 is important for the stability of the Foxp3 protein, suppressive assays using Treg cells from p300fl/fl Foxp3YFP-Cre mice reveals that p300 has a modest effect on the suppressive function of Treg cells (Figure 6C). In accordance with this, p300fl/fl Foxp3YFP-Cre mice have larger spleen and lymph nodes compared with Foxp3YFP-Cre mice, but the size of lymph nodes from p300fl/fl Foxp3YFP-Cre is smaller than that from Tip60fl/fl Foxp3YFP-Cre or p300fl/fl TIP60fl/fl Foxp3YFP-Cre mice (Supplementary Figure 5A). H&E staining of liver and lung sections also shows modest inflammation in these sections (Supplementary Figure 5B). p300 therefore plays a role in regulating the development and function of Treg cells, but it is not absolutely required to prevent the development of fatal autoimmune diseases.
Figure 6. TIP60 plays a major role in the maintenance of peripheral Treg cells.
(A) Representative dot plots of Treg populations in thymus, lymph node, mesenteric lymph node, and spleen from Foxp3YFP-Cre, p300fl/fl Foxp3YFP-Cre, Tip60fl/fl Foxp3YFP-Cre and p300fl/fl TIP60fl/fl Foxp3YFP-Cre mice. (B) The averge percentage of CD4+ T cells expressing Foxp3 in thymus, lymph node, mesenteric lymph node, and spleen. The percentages shown are the mean values from three individual mice. The error bars indicate the SD value. (C) Suppressive function of Treg cells from p300fl/fl Foxp3YFP-Cre mice. (D) Size of TIP60 conditional knockout mouse. Left is littermate without Foxp3-cre gene. (E) CD4 + T cells were transduced with Foxp3 and wild type TIP60 or TIP60 mutant. Transduced T cells were then collected and subject to suppressive assay at the indicated Treg and Teff ratio.
In contrast to p300fl/fl Foxp3YFP-Cre mice, Tip60fl/fl Foxp3YFP-Cre and p300fl/fl TIP60fl/fl Foxp3YFP-Cre mice developed severe weight loss, dermatitis, and splenomegaly from 2 weeks old and die at an early age (Figure 6D and Supplementary Figure 5). Treg cells from these mice were analyzed to investigate the role of TIP60 in regulating Treg function. Surprisingly TIP60 knockout in Foxp3 expressing cells greatly decreased the Treg populations in both the spleen and lymph node (Figure 6A and B), indicating an indispensible role of TIP60 in the peripheral development and function of Treg cells. Unexpectedly, the Treg population in the thymus is increased by TIP60 knockout, or in the TIP60 and p300 double knockout, indicating that TIP60 is differentially required for the development of Treg cells in thymus or in periphery. TIP60 knockout in Treg cells might cause a defect in the exit of Treg cells from thymus, and TIP60 influenced functionalities are important in peripheral aspects of Treg cell biology.
The suppressive function of Tregs from Tip60fl/fl Foxp3YFP-Cre and p300fl/fl TIP60fl/fl Foxp3YFP-Cre mice could not be characterized due to the paucity of Treg cells in spleen and lymph node. CD4+ T cells acquire suppressive function when they are transduced with Foxp3.
Therefore, to investigate the role TIP60 in regulating thee suppressive function of Treg cells, CD4+ naïve T cells were transduced with both Foxp3 and wild type TIP60 or TIP60 mutants, then the suppressive function of these transduced cells were investigated. As expected and as reported previously, TIP60 HAT deficient mutants (Q377E/G380E) show reduced suppressive function compared to wild type TIP60 (Figure 6D). However, we note that the TIP60 K327Q mutant transduced T cells yield intermediate suppression. Structurally, while K327Q mimics the conformation of acetylated 327, it does not permit the entire set of functions promoted by acetylation of that specific residue in TIP60. Complete functionality may require further conformational changes after acetylation that induce more consistent substrate switching and binding.
Overall these data indicate that is the TIP60 HAT that is indispensible for the development and function of a population of peripheral Treg cells.
DISCUSSION
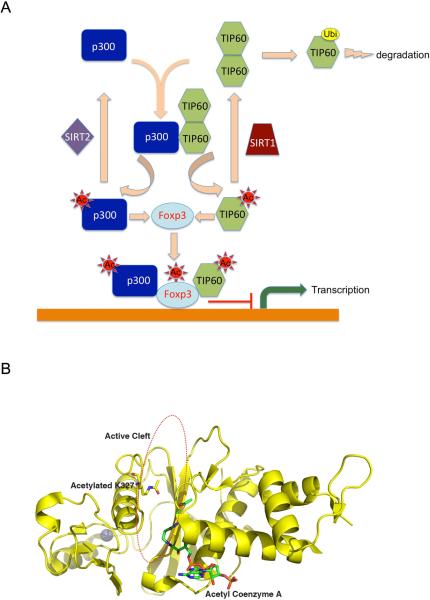
The acetylation of lysine residues represents an important post-translational modification to modify the activity of transcriptional factors. During our studies of FOXP3 complexes, we observed that two important HATs, TIP60 and p300, interact cooperatively to promote the acetylation of FOXP3. Based on these findings, we also investigated the molecular mechanisms responsible for this cooperative effort. Our results indicate that the acetylation of TIP60 and p300 triggers a specific residue defined “switch” that is responsible for controlling the TIP60 protein stability, HAT activity, and substrate interactions. Acetylation of K327 is proposed to alter conformation to promote changes in substrate interactions. We have developed a scheme that illustrates this complex process and also created a structural model to identify the atomic features of Tip60 that appear relevant (see Figures 7a and b.)
Figure 7. Schematic model of the cooperation between TIP60 and p300 on the regulation of FOXP3.
(A) Cooperative actions of TIP60 and p300. TIP60 and p300 promote the acetylation of each other, and their deacetylation is catalyzed by SIRT1 and SIRT2 respectively. De-acetylated TIP60 is ubiquitinated and then degraded in a proteasome dependent manner, while acetylated TIP60 disassociates with p300 and form stable complex with FOXP3. Together with p300, TIP60 promotes the acetylation of FOXP3 and the repressive transcriptional activity of FOXP3 is maximized by the synergistic effect of TIP60 and p300. (B) Structural model of acetylated TIP60 indicating that acetylation of K327 would favor FOXP3 binding to the cleft.
Our results also indicate that the cooperative interactions between TIP60 and p300 lead to a complex mode of regulation for FOXP3. Acetylation of the p300 molecule increases the enzyme's HAT activity (Thompson et al., 2004). TIP60 regulates FOXP3 indirectly by mediating the acetylation of p300. With increased acetylation, p300 can then acetylate FOXP3 at K249 and K251, forcing an atomically definable structural change in FOXP3's dimers (Song et al., 2012). At the same time, p300 regulates TIP60 modifications as well. p300 interacts with the zinc-finger region of TIP60 to promote its acetylation(Col et al., 2005). Our studies now identify molecular details of the process by which p300 regulates TIP60. Using the HAT-deficient mutant of TIP60 in the presence of p300, we demonstrated that intrinsic auto-acetylation processes are responsible for the increased acetylation of TIP60 that results from this interaction.
There are three different paths by which the acetylation process regulates TIP60. First, acetylation of K327 of TIP60 is important for the HAT activity of TIP60. Therefore, K327 acetylation promoted by p300 interactions enhance the HAT activity of TIP60. Second, acetylation increases the stability of TIP60 by inhibiting ubiquitination, thus preventing proteasome-dependent degradation. When compared to the HAT-deficient mutant of TIP60, wild type TIP60 showed less ubiquitination and therefore, increased expression. Upon interaction with p300, ubiquitination of TIP60 was further decreased. Third, acetylation regulates TIP60's interactions with other proteins. Oligomerization of TIP60 is disrupted by acetylation which can be reversed by SIRT1(Wang and Chen, 2010). In this study, we showed that the acetylation of TIP60 at one residue, K327, prompted a protein conformation change that leads to disassociation from p300 along with the re-association with its substrates such as FOXP3. Of note, our laboratory has recently identified small allosteric molecules that target TIP60 functions. These synthetic allosteric small molecules can promote Treg functionalities (unpublished data) supporting the role of changing TIP60 conformation to alter function,
We now propose a schematic model of how p300 and TIP60 cooperatively regulate FOXP3 functionalities (Figure 7A). To begin with, both p300 and SIRT1 regulate the function of TIP60 by modulating its acetylation. With SIRT1, deacetylation of TIP60 occurs, resulting in the formation of an oligomeric complex. Upon its interaction with p300, acetylation of TIP60 then occurs. Once acetylated, TIP60 dissociates from the oligomer as well as p300 to facilitate its final interaction with FOXP3 .
Although the role of TIP60 and p300 in regulating Foxp3 activity has herein been delineated in vitro, relatively little is known about the roles of these discreet enzymes in the regulating the function of Treg cells. The complex role of p300 includes maintaining Treg/Th17 cell levels by contributing to differentiation events of Th17 cells(Dang et al., 2011). Although p300 has been shown to be important to Foxp3 activity in vitro, a dominant regulatory significance of p300 was not observed in this study. We are aware that we have not excluded other HAT enzymes with similar functions to p300, such as CBP. CBP is highly homologous to p300 and shares many common substrates including FOXP3 (Greene and Yan, unpublished data). Consequently we conclude that it is the redundancy of the p300 type of HAT that accounts for its role in vivo and disabling p300 by itself may not suffice to alter Treg activities.
By conditionally knocking out TIP60 in Treg cells, we show that TIP60 plays a vital role in some fundamental requirement of Treg cells in the periphery. The decrease of Treg population may result from increased CD4+ T cells numbers due to impaired Treg function and T cell activation. However, Treg is not the population that develops independently of CD4+ cells. The observation that the ratio of Foxp3+ cells is decreased indicates that there is a problem in the generation of Treg population in these mutant mice. We consider that migration or some survival function of Treg cells in the periphery may be reasons that the Tip60fl/fl Foxp3YFP-Cre mice develop fatal autoimmune diseases similar to those seen in scurfy mice.
Our data indicate that TIP60 is an important enzymatic factor that is differentially required for the survival of thymic and peripheral Treg cells. Foxp3 forms dynamic complexes with other proteins under different stimulatory conditions. It is noteworthy that Treg specific elimination of NFAT2 which is part of the Foxp3 complex has been found to limit peripheral Follicular regulatory T cell (TFR) populations possibly due to impaired homing to B cell follicles (Li et al., 2007; Vaeth et al., 2014). We suggest that TIP60 is also present in a vital complex that is specially required for the survival of peripheral Treg cells and whose disruption leads to fatal autoimmune diseases.
Recent studies also indicate nonenzymatic roles of deacetylases (HDAC3) in cellular functions (Sun et al., 2013) whereby physical interactions of such vital complexes guide some functions. In this regard binding of HDAC6, independent of its deacetylase activity may contribute to regulation of TIP60 target genes(Chen et al., 2013). Thus, TIP60 may also possess HAT independent activities that can lead to different functional outcomes. Investigations using mice with dominant negative TIP60 forms that lack HAT activity but retain substrate interaction surfaces needed to form dynamic complexes with other proteins will be informative and are underway.
TIP60 and p300 are members of two important and structurally distinct HAT families that regulate many aspects of cellular function. We have discovered some of the features of how these two distinct family elements cooperate. Our study demonstrates the critical role of TIP60 in maintaining peripheral Treg cells and limiting autoimmune responses, therefore providing a new target for regulating immune responses therapeutically. We have also defined a correlation between auto-acetylation and ubiquitination of TIP60 that provides further mechanistic insight into the regulation of TIP60's activity by other HATs. Since many other transcription factors such as p53 are subject to the regulatory mechanisms of TIP60 and p300 (Ito et al., 2001; Tang et al., 2006), then the acetylation of these factors may also be regulated by the same cooperative interplay of TIP60 and p300 discussed herein. This cooperative and complex interplay may represent defining features of a common regulatory mode of action of distinct HATs and their shared substrates.
EXPERIMENTAL PROCEDURES
Mice
p300 conditional knockout mice were kindly provided by Dr. Paul Brindle (St. Jude Children's Hospital, Memphis, Tennessee). Cre-recombinase-mediated excision was designed to remove exons 3-11, which comprises 71% of Tip60 exon structure including the chromo-, Zinc finger and HAT domains, by recombineering wherein LoxP sites were inserted into introns 2 and 11. Correctly targeted ESCs were injected into C57/Bl6 blastocysts, which after implantation transmitted the targeted allele via germline. Following verification of targeting via Southern blotting and removal of the neomycin-resistance gene, mice were bred to the genotypes used in these experiments (Horst, Fisher, Lough et al., manuscript in preparation). Foxp3YFP-Cre mice were obtained from Jackson Laboratory (Bar Harbor, Maine). All animals were housed and bred in a specific pathogen free animal facility of the University of Pennsylvania. All the experiments were performed following national, state and institutional guidelines. Animal protocols were approved by the University of Pennsylvania Animal Care and Use Committee.
Plasmids and antibodies
The following antibodies were used in our studies: anti-Flag M2-Peroxidase (Sigma-Aldrich), anti-HA-Peroxidase (3F10, Roche), anti-uniquitin-Peroxidase (sc-8017, Santa Cruz), and anti-acetyl-lysine (ICP0381, ImmuneChem). Plasmids expressing the wild type or HAT-deficient TIP60 were constructed as previously described (4). p300 are cloned from pCDNA3.1-p300 (kindly provided by Warner Greene, Addgene plasmid 23252) to pFLAG resulting in pFLAG-p300. pFLAG-TIP60 K274R, K274Q and pFLAG-p300 F1504A are constructed using site-directed mutagenesis (Stratagene) and verified by sequencing.
Cell culture and transfection
293T cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS and antibiotics (1% penicillin/streptomycin, Invitrogen) at 37°C in a humidified incubator with 5% CO2 (v/v). Cells were grown to 80% confluency and transient transfection was carried out using a mixture of 6 μg DNA and 18 μl Fugene6 (Roche) according to manufacturer's instructions. 24 h after transfection, cells were washed twice with PBS and cell lysates were then prepared for western blot analysis.
Immunoprecipitation
Cells were lysed in modified RIPA buffer (20 mM Tris-Cl, pH 7.5, 2 mM EDTA, 420 mM NaCl, 1% NP40). After centrifugation, the soluble fractions were collected and incubated with anti-HA or anti-FLAG agarose (Sigma) overnight at 4°C. The precipitates were then washed three times with modified RIPA buffer and boiled for 5 min in SDS loading buffer. Samples were analyzed by SDS-PAGE, transferred to nitrocellulose membrane (Millipore), and probed with antibodies as indicated. Immonocomplexes were detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
Flow cytometry
Spleen, axillary and inguinal Lymph node, mesenteric lymph node and thymus of 18-21 days old male mice were collected for single cell suspension. Cells were stained with anti-CD4-percp, CD8-AF700 (eBioscience) and subjected to flow cytometry with FACS LSR (BD Biosciences). FACS data were analyzed with FlowJo software (Tree Star,Ashland, OR).
Histology
Lung and liver tissues were fixed with 10 % neutral buffered formalin and embedded in paraffin. Sections were deparaffinized and stained with H & E by Cell imaging Core in Abramson Cancer Research Institute.
CD4+CD25+ suppression assays
CD4+ T cells were enriched from splenocytes using MACS separation (Miltenyi), and CD4+CD25-CD45RBhigh Teff cells and CD4+CD5+CD45RBlow Treg cells were separated from CD4+ cells respectively by FACSAria II, yielding a purity of ~ 97% for both cells.
Luciferase assay
Luciferase assays were performed as previously described (4). Cells were transfected in a 12-well plate with pG5-luc, MSV-gal, pBIND-FOXP3, pFLAGTIP60, and pFLAG-p300 as indicated. 24 h after transfection, cells were washed twice with PBS and lyxdc in 100 μl passive lysis buffer for 15 min. Luciferase and β-gal activities were then determined separately using the luciferase assay system and the galactosidase enzyme assay system respectively (Promega).
Supplementary Material
HIGHLIGHTS.
p300 and TIP60 work cooperatively to promote FOXP3 acetylation
TIP60 K327 acetylation allows TIP60 to switch binding partners
p300 conditional knockout in Treg cells leads to minimal changes in Treg functions
TIP60 conditional knockout in Treg cells results in fatal autoimmune disease.
ACKNOWLEDGEMENT
This work was supported by grants from the National Institutes of Health (M.I.G. PO1 AI073489-03), Molecular therapy for CF and genetic diseases Pilot (M.I.G. 5P30DK047757-17) and the Abramson Family Cancer Research Institute (M.I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Caron C, Boyault C, Khochbin S. Regulatory cross-talk between lysine acetylation and ubiquitination: role in the control of protein stability. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27:408–415. doi: 10.1002/bies.20210. [DOI] [PubMed] [Google Scholar]
- Chen PB, Hung JH, Hickman TL, Coles AH, Carey JF, Weng Z, Chu F, Fazzio TG. Hdac6 regulates Tip60-p400 function in stem cells. eLife. 2013;2:e01557. doi: 10.7554/eLife.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col E, Caron C, Chable-Bessia C, Legube G, Gazzeri S, Komatsu Y, Yoshida M, Benkirane M, Trouche D, Khochbin S. HIV-1 Tat targets Tip60 to impair the apoptotic cell response to genotoxic stresses. The EMBO journal. 2005;24:2634–2645. doi: 10.1038/sj.emboj.7600734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, Yao TP. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. The EMBO journal. 2001;20:1331–1340. doi: 10.1093/emboj/20.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Torchia J, Rose DW, Xu L, Kurokawa R, McInerney EM, Mullen TM, Glass CK, Rosenfeld MG. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- Li B, Greene MI. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle. 2007;6:1432–1436. [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC, Kapoor V, Bhatti TR, Akimova T, Singhal S, et al. Inhibition of p300 impairs Foxp3(+) T regulatory cell function and promotes antitumor immunity. Nature medicine. 2013;19:1173–1177. doi: 10.1038/nm.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Ling H, Yuan Z, Fang B, Bloom G, Fukasawa K, Koomen J, Chen J, Lane WS, Seto E. SIRT1 negatively regulates the activities, functions, and protein levels of hMOF and TIP60. Molecular and cellular biology. 2012;32:2823–2836. doi: 10.1128/MCB.00496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf SM, Sandesara PB, Reed SA, Judge AR. p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. American journal of physiology. Cell physiology. 2011;300:C1490–1501. doi: 10.1152/ajpcell.00255.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Li B, Xiao Y, Chen C, Wang Q, Liu Y, Berezov A, Xu C, Gao Y, Li Z, et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell reports. 2012;1:665–675. doi: 10.1016/j.celrep.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, Everett LJ, Nabel CS, Li Y, Selvakumaran V, et al. Deacetylase-Independent Function of HDAC3 in Transcription and Metabolism Requires Nuclear Receptor Corepressor. Molecular cell. 2013;52:769–782. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Molecular cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, et al. Regulation of the p300 HAT domain via a novel activation loop. Nature structural & molecular biology. 2004;11:308–315. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- Vaeth M, Muller G, Stauss D, Dietz L, Klein-Hessling S, Serfling E, Lipp M, Berberich I, Berberich-Siebelt F. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. The Journal of experimental medicine. 2014 doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdregt J, Brunen D, Fleskens V, Pals CE, Lam EW, Coffer PJ. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS One. 2011;6:e19047. doi: 10.1371/journal.pone.0019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. The Journal of biological chemistry. 2010;285:11458–11464. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Li B, Zhou Z, Hancock WW, Zhang H, Greene MI. Histone acetyltransferase mediated regulation of FOXP3 acetylation and Treg function. Current opinion in immunology. 2010;22:583–591. doi: 10.1016/j.coi.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Kitabayashi I. Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX. Biochem Biophys Res Commun. 2009;390:1355–1360. doi: 10.1016/j.bbrc.2009.10.156. [DOI] [PubMed] [Google Scholar]
- Yang C, Wu J, Zheng YG. Function of the active site lysine autoacetylation in Tip60 catalysis. PloS one. 2012;7:e32886. doi: 10.1371/journal.pone.0032886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic acids research. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Rossetto D, Mellert H, Dang W, Srinivasan M, Johnson J, Hodawadekar S, Ding EC, Speicher K, Abshiru N, et al. MYST protein acetyltransferase activity requires active site lysine autoacetylation. The EMBO journal. 2012;31:58–70. doi: 10.1038/emboj.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xiao Y, Zhu Z, Li B, Greene MI. Immune regulation by histone deacetylases: a focus on the alteration of FOXP3 activity. Immunology and cell biology. 2012;90:95–100. doi: 10.1038/icb.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.