Abstract
Tumours have two main ways to develop a vasculature: by angiogenesis, the sprouting of endothelial cells from nearby blood vessels, and vasculogenesis, the formation of blood vessels from circulating cells. Because tumour irradiation abrogates local angiogenesis, the tumour must rely on the vasculogenesis pathway for regrowth after irradiation. Tumour irradiation produces a marked influx of CD11b+ myeloid cells (macrophages) into the tumours, and these are crucial to the formation of blood vessels in the tumours after irradiation and for the recurrence of the tumours. This process is driven by increased tumour hypoxia, which increases levels of HIF-1 (hypoxia-inducible factor 1), which in turn upregulates SDF-1 (stromal cell-derived factor 1 or CXCL12), the main driver of the vasculogenesis pathway. Inhibition of HIF-1 or of its downstream target SDF-1 prevents the radiation-induced influx of the CD11b+ myeloid cells and delays or prevents the tumours from recurring following irradiation. Others and we have shown that with a variety of tumours in both mice and rats, the inhibition of the SDF-1/CXCR4 pathway delays or prevents the recurrence of implanted or autochthonous tumours following irradiation or following treatment with vascular disrupting agents or some chemotherapeutic drugs such as paclitaxel. In addition to the recruited macrophages, endothelial progenitor cells (EPCs) are also recruited to the irradiated tumours, a process also driven by SDF-1. Together, the recruited proangiogenic macrophages and the EPCs reform the tumour vasculature and allow the tumour to regrow following irradiation. This is a new paradigm with major implications for the treatment of solid tumours by radiotherapy.
TUMOURS HAVE TWO MAIN WAYS TO DEVELOP A VASCULATURE: ANGIOGENESIS AND VASCULOGENESIS
Judah Folkman1 in 1971 published the then revolutionary claim that tumours could not grow without new blood vessels, they secreted a factor (tumour angiogenic factor) that stimulates neovascularization and, if this process of angiogenesis could be stopped, the tumour would cease to grow. This led to the huge field of anti-angiogenesis therapy for tumours, and today there are six anti-angiogenic drugs approved for clinical use (the mAb bevacizumab and the receptor tyrosine kinase inhibitors sunitinib, sorafenib, pazopanib, vandetanib and axitinib) and many more in advanced clinical testing.2 Those who have followed the clinical experience from the many thousands of cancer patients who have been treated with these drugs would summarise the results obtained as follows:
The drugs produce a benefit only when combined with cytotoxic therapy and not when used alone.
Several randomized studies have shown an improved overall survival (OS), but more often an improved progression-free survival when the anti-angiogenic drug [typically anti-vascular endothelial growth factor (VEGF)] is combined with standard therapy.
Several randomized trials have shown no benefit of the addition of an anti-angiogenic drug to the standard therapy, and even to those showing a benefit the increase in OS has been quite modest (2–4 months).
Therefore, some 40 years after Folkman proposed the concept of anti-angiogenic therapy, we have to conclude that the strategy has had some success but not to the extent that was originally hoped for. This is perhaps not surprising—we have become accustomed in this age of molecularly targeted therapy to the development of rapid resistance to the targeted therapy. However, it was reasonable, as was pointed out early in the quest for anti-angiogenic agents, that as the target tissues were normal [endothelial cells (ECs) which, unlike tumour cells are genetically stable], it was much less likely that mutations leading to resistance would develop. However, there are other ways that tumours could become resistant to anti-angiogenic therapy, and one was highlighted by the seminal work from Jeff Isner's laboratory in 1997. In this study, Asahara et al3 isolated putative endothelial cell progenitors from human peripheral blood and showed that in animal models of ischaemia these cells incorporated into the sites of active angiogenesis. This finding ushered in the birth of the therapy to reverse vascular damage (such as in myocardial infarction) using EPCs. However, it is important also for cancer therapy and means that, in addition to angiogenesis from the sprouting of local vessels, tumours also can obtain blood supply from circulating cells, a process known as vasculogenesis (Figure 1). However, vasculogenesis is largely overlooked, with 98% of recent articles on tumour blood vessel formation being on angiogenesis, and the reason is clear; under normal circumstances, the dominant way by which tumours obtain their vasculature is through angiogenesis. So, vasculogenesis can be regarded as a “backup” pathway if angiogenesis is inhibited. Thus, is this pathway the reason for the modest benefit of anti-angiogenic therapy? This is yet to be established, although there is evidence that the influx of CD11b+ myeloid cells (which are the key to the vasculogenesis pathway) can be responsible for the resistance to anti-VEGF therapy.4,5
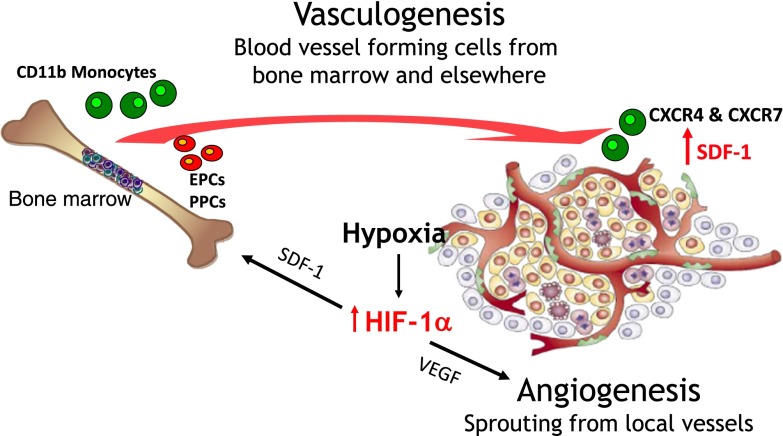
Figure 1.
Cartoon of the two main ways for tumours to develop a functioning vasculature. Also shown are the two principal cytokines governing these pathways: vascular endothelial growth factor (VEGF) for angiogenesis and stromal cell-derived factor 1 (SDF-1; CXCL12) for vasculogenesis. Tumour hypoxia through its upregulation of levels of the transcription factor HIF-1 (hypoxia inducible factor 1) is the main driver of both processes. EPC, endothelial progenitor cell; PPC, pericyte progenitor cell.
INHIBITION OF VASCULOGENESIS SENSITIZES TUMOURS TO IRRADIATION
As indicated above, the vasculogenesis pathway is only a minor player in the development of vasculature in tumours under normal circumstances. However, if the primary pathway of angiogenesis is blocked, then vasculogenesis may become of prime importance. Sometime ago, we showed that irradiation of tumours at doses comparable to those delivered in radiotherapy completely abrogated local tumour angiogenesis.6 Although the mechanism of this is not absolutely clear, it is likely to be a consequence of the killing of the ECs in the irradiation field. This is likely to be a tumour-specific phenomenon, as the tumour ECs are dividing and therefore will die a mitotically linked death, whereas the normal ECs are largely non-proliferating. It follows, therefore, that the principal way for tumours to regrow after radiotherapy is by regrowth of the vasculature from circulating cells and that, if the vasculogenesis pathway could be blocked, then tumours would become more sensitive to irradiation.
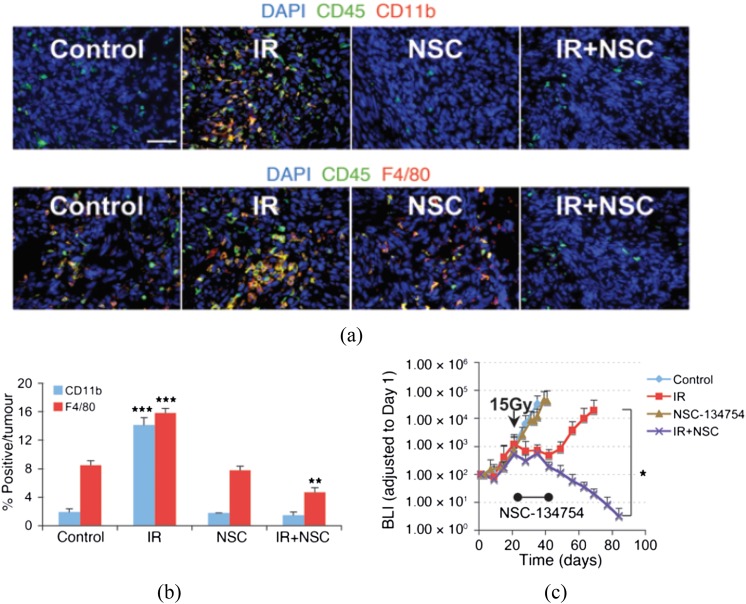
Is there any evidence for this? Many years ago, Stephens et al7 noted that the irradiated tumours had higher levels of macrophages than the tumours prior to irradiation. More recently, we,6,8 and others,9 have seen a similar effect: a large increase in CD11b+ myeloid cells, the precursors of tumour-associated macrophages (TAMs), in transplanted tumours in mice after irradiation. To determine whether this phenomenon also applies to human tumours, we obtained 12 pairs of glioblastomas from patients prior to treatment and following recurrence. In 10/12 of these pairs, there was an approximate 10-fold increase in CD11b+ cells in the tumours following treatment.8 Do these macrophages play a role in protecting the tumour from irradiation? To address this question, we reasoned, based on data from normal tissues,10 that this influx of macrophages might be produced by higher tumour levels of the chemokine SDF-1 (stromal cell-derived factor 1 or CXCL12) in the irradiated tumour by an increase in the transcription factor HIF-1 (hypoxia inducible factor 1) produced by increased tumour hypoxia subsequent to irradiation. Consistent with the hypothesis that the increase in TAMs after irradiation was due to increased tumour HIF-1 levels, we showed in an intracranial U251 glioblastoma multiforme (GBM) model in mice that HIF-1 levels rose in tumours 2–3 weeks following single-dose irradiation, as a consequence of increased tumour hypoxia,8 and that when we treated mice with the HIF inhibitor NSC 134754, developed by Chau et al,11 the radiation-induced influx of macrophages was totally abolished (Figure 2a,b).
Figure 2.
Myeloid cells (macrophages) are recruited into irradiated tumours, and inhibition of HIF (hypoxia inducible factor) abrogates this influx and prevents tumour recurrence. (a) immunohistochemistry (IHC) staining for leukocyte (CD45) and monocyte (CD11b, top row) or macrophage (F4/80, bottom row) infiltration into i.c. (intracranial) control or 12-Gy treated tumours. Tumours were harvested on the day of irradiation for controls and 17 days after irradiation in treatment groups. Scale bar: 50 μm. (b) Quantification of CD11b+ and F4/80+ cell influx in tumours. Error bars indicate standard deviation. **p < 0.01, ***p < 0.001 versus control. (c) Growth curves (by bioluminescence imaging, BLI) of U251 tumours growing in the brains of nude mice and given 15 Gy with or without treatment of the mice with the HIF inhibitor NSC 134754 for 21 days started immediately after irradiation. *p < 0.05. Adapted from Kioi et al8 with permission. DAPI, 4′,6-diamidino-2-phenylindole; IR, irradiation.
What is the effect of the HIF inhibitor on the response of tumours to irradiation? This was a key finding of our study: application of the HIF inhibitor after irradiation prevented the regrowth of irradiated tumours (Figure 2c). This result is also consistent with the work of Williams et al,12 who have shown that HIF deficient tumours are more sensitive to irradiation. These data therefore suggest that the influx of myeloid cells into the tumours after irradiation is important for the recurrence of tumours, a conclusion supported by our data that treatment of mice after irradiation with carrageenan, an agent that depletes macrophages, also sensitized the U251 GBM to irradiation.8 This inhibition of HIF did not affect the growth of the unirradiated tumours, consistent with the concept that inhibition of the vasculogenesis pathway should have little or no effect on unirradiated tumours, as their vasculature is largely supplied by angiogenesis.
However, in terms of a useful therapeutic strategy, inhibition of HIF-1 is unlikely to be useful for the dual reason that there is no specific inhibitor of HIF-1, and inhibition of HIF-1, because it would affect so many downstream targets, would not be the ideal approach. So, what about SDF-1 inhibition? As noted above, studies in normal tissues have shown that the trafficking of progenitor cells to sites of angiogenesis is governed by gradients of SDF-1.10 Since SDF-1 is an HIF-1 target gene and both others and we have shown that SDF-1 levels are increased in irradiated tumours,8,13 it became an obvious target. In the adult, SDF-1 functions as a chemoattractant for lymphocytes and monocytes in vivo,14 human CD34+ progenitors15 and mouse pro-B and pre-B cells.16 This pathway also regulates haematopoietic stem cell homing and engraftment.17–19 SDF-1 (CXCL12) has two receptors: CXCR4 and CXCR7. Human CXCR4 was initially identified as a receptor for SDF-1 by screening chemokine receptor orphan genes for their ability to induce intracellular Ca2+ increases in response to human SDF-1.20–22 The mouse CXCR4 receptor was subsequently found by cloning candidate chemokine receptors and comparing the amino acid sequence to the human cDNA (complementary deoxyribonucleic acid).23 More recently, it has been shown that SDF-1 binds to CXCR7,24 a receptor that does not have the classical response of induction of Ca2+ upon binding by SDF-1 and may function as a decoy receptor to maintain SDF-1 gradients.25
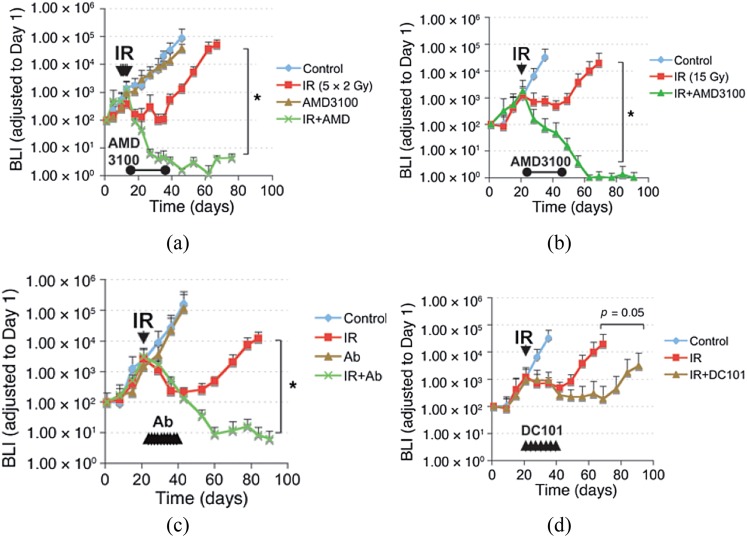
The clinically used drug AMD3100 (Plerixafor, “Mozobil”) is a specific inhibitor of the interaction of SDF-1 with its receptor CXCR4 and is used clinically to mobilize haematopoietic cells from the bone marrow stem. We, therefore, used this inhibitor (obtained from Sigma-Aldrich) post irradiation in a similar protocol to that used with the HIF inhibitor (continuous application for 21 days following irradiation). We found a similar effect as with the HIF inhibitor: namely, inhibition of the recurrence of the tumours following irradiation with no effect on the unirradiated tumours (Figure 3). Importantly, we found a similar effect with both fractionated irradiation (5 × 2 Gy) (Figure 3a) as with a single dose of 15 Gy (Figure 3b). This inhibition of post-irradiation tumour growth by AMD3100 coincided with an effect of the drug on preventing the return of the tumour vasculature after irradiation. To check that this effect on the response of the tumours was in fact the result of inhibition of the SDF-1/CXCR4 pathway, we tested neutralizing antibodies to CXCR4 in the same protocol (application for 21 days following irradiation). We found the same inhibition of the recurrence of the tumours (Figure 3c), demonstrating formally that the effect is due to inhibition of this pathway. To compare the efficacy of the strategy of inhibition of vasculogenesis with that of inhibition of angiogenesis, we treated mice with the U251 intracranial GBM with DC101, an antibody against VEGFR2. Although this also sensitized the tumours to irradiation, the effect was not as great as it was with AMD3100 (Figure 3d). However, this may have overestimated the effect of angiogenesis inhibition alone as VEGF has been reported to also be involved in the homing of circulating mononuclear myeloid cells to angiogenic sites.26 This finding considerably muddies the water in terms of the effect of angiogenesis inhibition by VEGF blockade on the response of tumours to irradiation, as part of the effect may be the result of inhibition of the vasculogenesis pathway.
Figure 3.
Therapeutic effect of blocking the interaction of stromal cell-derived factor 1 (SDF-1) with CXCR4 after whole-brain irradiation. (a) Growth curves of i.c. (intracranial) U251 early tumour model after 5 daily doses of 2 Gy starting on Day 11 after transplantation. *p < 0.05. (b) Growth curves of i.c. U251 advanced tumour model after a single dose of irradiation (15 Gy on Day 22 after transplantation), treated with AMD3100 (21-day infusion). (c) As in (b) but with neutralizing anti-CXCR4 Abs instead of AMD3100, *p < 0.05). (d) Growth curves of U251 i.c. tumour after 15-Gy irradiation, treated with the anti-vascular endothelial growth factor-R antibody DC101. Arrowheads indicate the treatment of DC101 (started immediately after irradiation and maintained for 21 days). Adapted from Kioi et al8 with permission.
The above data with inhibition of HIF and CXCR4 showed that these agents both prevented the radiation-induced increase of CD11b+ myeloid cells in tumours (largely Tie2-expressing myeloid cells) and sensitized the tumours to irradiation, thereby establishing a correlation between the two. But is this a direct causal effect? To address this, we raised neutralizing monoclonal antibodies against CD11b+ cells and demonstrated that giving these antibodies following irradiation in a different tumour model (the FaDu head and neck human tumour) also produced a substantial radiosensitization of the tumours.27 Taken together, these data show the importance of the influx of bone marrow-derived CD11b+ myeloid cells to tumour recurrence after irradiation and that prevention of this influx by inhibition of the SDF-1/CXCR4 pathway can produce a substantial radiosensitization of tumours. In support of this conclusion, Welford et al28 showed that following treatment with the vascular disrupting agent combretastatin-A-4-phosphate (which produces a rapid vascular collapse and tumour hypoxia), SDF-1 was elevated and there was a rapid accumulation of Tie2-expressing macrophages in the tumours. Furthermore, inhibition of the SDF-1/CXCR4 pathway with AMD3100 or genetically both reduced the Tie2-expressing macrophages in the tumours and enhanced the antitumour efficacy of the treatment.
Are more than CD11b+ myeloid cells involved in tumour regrowth?
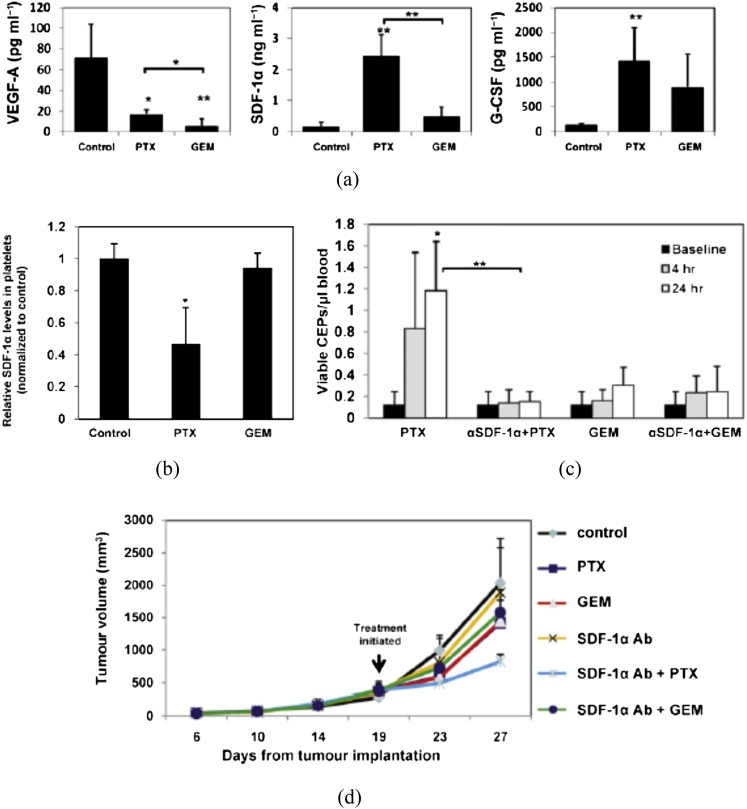
Despite these findings on the importance of influx of bone marrow-derived CD11b+ myeloid cells in tumours post-irradiation to restore the vasculature, it is highly unlikely that the CD11b+ cells themselves become ECs. Indeed, our studies suggest that although these cells are highly pro-angiogenic, they appear to be in close contact with ECs rather than colocalizing with them.6,8 Therefore, what is the source of ECs in the regrowing tumour? The work of Shaked et al29 has shed light on this. These authors initially showed that the vascular disrupting agent OXi-4503, which produces rapid shutdown of tumour blood flow and increased tumour hypoxia,30 produces a rapid spike in EPCs in the blood of tumour-bearing mice and incorporation of these cells into the viable rim of the tumours after therapy. They went on to show that this is a phenomenon that also occurs with some chemotherapeutic drugs, such as paclitaxel, but not others, such as gemcitabine31 (Figure 4b). Significantly, the authors showed that most, if not all, of the increased EPCs in the blood after paclitaxel treatment could be ascribed to increased SDF-1 in the blood, as treatment with SDF-1 neutralizing antibodies abrogated the increase in SDF-1 levels (Figure 4c). In addition, the SDF-1 neutralizing antibodies also enhanced the antitumour efficacy of paclitaxel but not gemcitabine (Figure 4d). Consistent with this, they also demonstrated that the treatment efficacy of paclitaxel but not gemcitabine was greater in Id1 ± Id3 −/− (Id) mutant mice, which cannot mobilize EPCs from the bone marrow32 but are not deficient for other bone marrow-derived proangiogenic cells, including TAMs.33 We have also observed an increase in circulating EPCs in tumour-bearing mice following tumour irradiation and the incorporation of these circulating EPCs into the vasculature of the regrowing tumour after irradiation (Russell and Brown, 2013, personal communication).
Figure 4.
Circulating levels of growth factors after paclitaxel (PTX) or gemcitabine (GEM) treatment and effect of anti-SDF-1 antibody (Ab) on endothelial progenitor cells (EPCs) and tumour growth. (a) Non-tumour-bearing C57BL/6 mice (n = 4 mice per group) were treated with PTX or GEM. 4 h later, mice were bled by cardiac puncture and plasma was collected to measure vascular endothelial growth factor (VEGF)-A, SDF-1α and granulocyte-colony stimulating factor levels by enzyme-linked immunosorbent assay. (b) Analysis of SDF-1α content stored in isolated circulating platelets from C57BL/6 mice 4 h after treatment with PTX or GEM at the maximum tolerated dose (MTD). (c) Non-tumour-bearing C57BL/6 mice (n = 5 mice per group) were treated with SDF-1α neutralizing antibodies. 24 h later, mice were treated with PTX or GEM. After 4 and 24 h, mice were bled from the retro orbital sinus for evaluation of viable endothelial progenitor cells by flow cytometry. (d) C57BL/6 mice bearing Lewis lung carcinoma (LLC) tumours (500 mm3) were treated with polyclonal SDF-1α neutralizing antibodies in combination with either PTX or GEM. Control mice received non-specific antiserum treatment. Data are expressed as mean ± standard deviation. 0.05 > *p > 0.01; **p < 0.01. From Shaked et al31 with permission. G-CSF, granulocyte colony-stimulating factor.
It is as yet a matter of conjecture as to how CD11b+ cells and EPCs interact to form blood vessels. However, the fact that MMP-9 is crucial to this process6,34 strongly suggests that degradation and/or remodelling of the extracellular matrix is involved possibly in releasing VEGF and/or facilitating blood vessel formation by the EPCs. Relevant to this is that several investigators have shown that CD11b+ cells promote the influx of EPC into damaged normal tissue and stimulate subsequent blood vessel growth.35,36
Relevance of vasculogenesis inhibition to the radiotherapy of human cancers
It is always tempting to extrapolate preclinical findings to the clinic. We must, however, be aware of significant differences between our preclinical models and human cancers. Two important differences between the above data and the clinical situation are obvious: (1) the results obtained for the GBM studies were performed in nude mice and therefore deficient in a functioning immune response. Since we are dealing with bone marrow CD11b+ myeloid cells, which are often characterized as myeloid-derived suppressor cells that suppress T-cell function, this is an important caveat to the studies. (2) The tumours were implanted (either subcutaneously or intracranially), which is not the way human tumours develop. There could thus be major differences in the vasculature of the preclinical and clinical tumours that could affect the results.
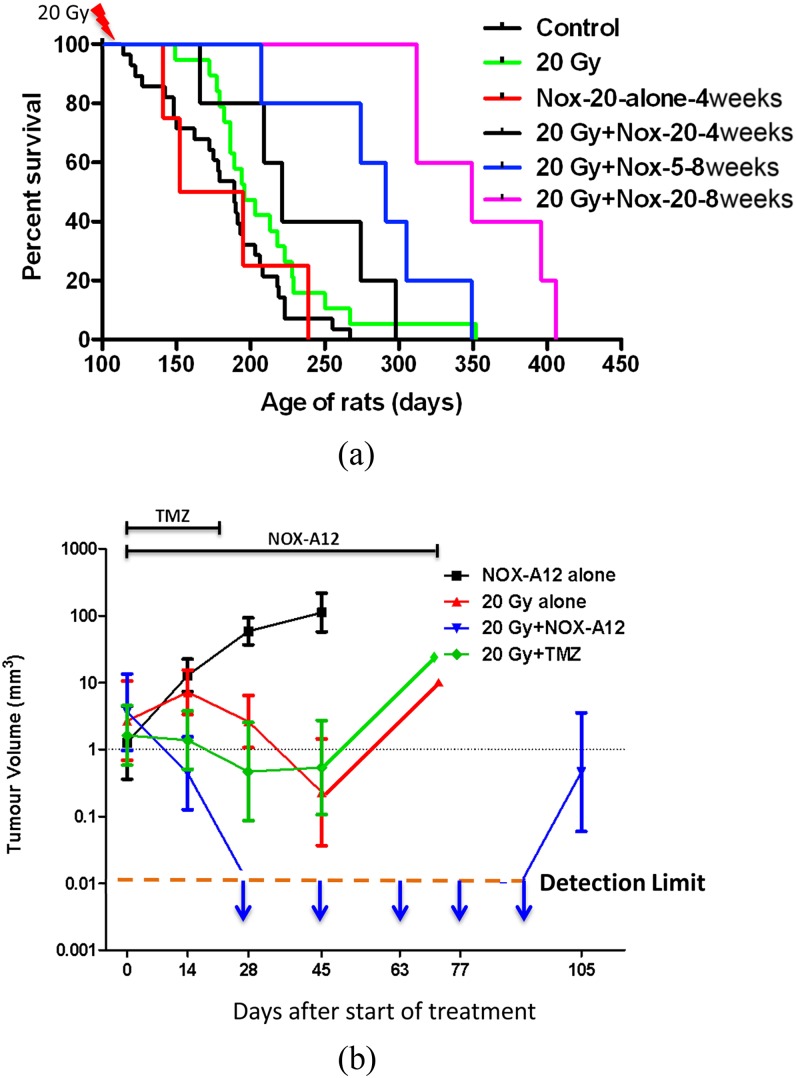
To bridge the gap between the preclinical results and the clinical situation, we set about to repeat the results with a more clinically relevant model of brain cancer in which the tumours develop naturally in the brains of immune competent rats. For this, we used ethylnitrosourea (ENU)-induced brain tumours in the Sprague-Dawley rat, a model that has proved to be extremely resistant to anticancer therapy in prior studies by a variety of investigators.37,38 Furthermore, macroscopic tumours that develop in this model frequently contain high levels of VEGF, haemorrhage and focal necrosis—all general characteristics of the most malignant glioblastomas. After in utero exposure to ENU on Day 17–18 of gestation, the pups appear healthy for >100 days during which time they begin to demonstrate neurological distress and die progressively from brain tumours from Day 120 after birth. The key advantages of this model are that the tumours arise autochthonously in immune competent hosts and have a genetic diversity and aggressiveness comparable with human brain tumours.39 To perform these studies, we used NOX-A12, a specific inhibitor of SDF-1.40 We sorted pups from ENU-treated mothers at Day 115 after birth, which is just before the first rats start to die from their brain tumours. Our data (Figure 5a) demonstrate that NOX-A12-mediated SDF-1 blockade is effective in inhibiting or delaying death of the rats following the single dose of 20 Gy whole-brain irradiation. It can also be seen from this figure that SDF-1 inhibition did not change the survival time of the unirradiated rats and that the efficacy of the treatment depended on the drug dose and particularly on the time period over which the drug was delivered (with 8 weeks being superior to 4 weeks). However, both the doses and time periods were similar to exposures that have been described to be safe and well tolerated in humans.
Figure 5.
Stromal cell-derived factor 1 (SDF-1) inhibition after irradiation prolongs the survival of the brain tumour-bearing rats and produces tumour remission. Rats born to mothers treated with a single injection of the carcinogen ENU on Day 18 of gestation were sham irradiated or given a dose of 20 Gy to the whole brain with shielding of the buccal cavity. (a) Rats receiving NOX-A12 were injected subcutaneously every 2 days with either 5 or 20 mg kg−1 starting soon after irradiation and continued for either 4 or 8 weeks. (b) Addition of the SDF-1 inhibitor NOX-A12 following irradiation of the ENU-induced brain tumours produces complete responses by MRI. In utero ENU-treated rats were imaged by MR starting on Day 130 of age and then repeated every 2 weeks until death. Adapted from Liu et al41 with permission. TMZ, temozolomide.
However, one aspect of the study shown in Figure 5a was not comparable to the clinical situation: namely that the brains of the rats were irradiated and started treatment with the SDF-1 inhibitor with the expectation based on population statistics that they had tumours and that all the groups had similar average tumour sizes. To make for a more clinically realistic situation, we repeated the study but, instead of assigning the rats to the various groups at 115 days of age, we monitored tumour growth by repeated MRI measurements and only assigned rats to the various treatment groups when they had visible (by MRI) tumours. This also allowed us to equalize the average tumour size at the beginning of treatment for all the groups. This assignment to the various groups occurred on Days 132–165 of age and, thus, considerably later than the first study and therefore presumably more difficult to control. In this study, we also included a group that received irradiation (20 Gy) combined with temozolomide (TMZ) (10 mg kg−1 intraperitoneally) 5 days per week for 3 weeks. The following conclusion can be drawn from the data shown in Figure 5b (colours refer to online images only):
The tumours in the rats treated with NOX-A12 alone continued to grow as expected (black line).
The tumours in the rats treated with 20 Gy + NOX-A12 (blue line) disappeared by 28 days after the start of treatment and continued to be undetectable until the appearance of 2 recurrences 105 days after the initiation of treatment.
The tumours in the rats that were given 20 Gy alone or 20 Gy + TMZ (red and green lines) behaved similarly with an initial decrease in volume to Day 45 followed by a regrowth. This shows that inhibition of SDF-1 is much more effective than the addition of TMZ with irradiation.
CLINICAL IMPLICATIONS
We also tested SDF-1 inhibition with the U251 human GBM implanted into nude mice and observed a similar extension of lifespan.41 Based on these results, we believe that a clinical trial of inhibition SDF-1 or its receptor CXCR4 in combination with standard therapy in first-line glioblastoma patients would be justified. Both the drugs tested in our studies are in clinical use. The CXCR4 antagonist AMD3100 (Plerixafor, MOZOBIL®) is indicated for combination with granulocyte-colony stimulating factor to mobilize haematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma (MM). The SDF-1 inhibitor NOX-A12 is currently in Phase II studies for the treatment of chronic lymphocytic leukaemia and MM, again based on its ability to mobilize cells (in this case cancer cells) from the bone marrow, to render them more sensitive to systemic chemotherapy. Based on our findings and on the hypothesis that the vasculogenesis pathway only becomes important to the tumour when it is starting to recur after irradiation, we believe that the important period to apply inhibitors of this pathway is following, rather than during, radiotherapy. For how long remains to be determined, but it is clear from our studies with the rat tumours (Figure 5) that 8 weeks is superior to 4 weeks of inhibition of the SDF-1 pathway. As many GBM show recurrence after 6 months, we would anticipate that optimally the inhibitors should be maintained for at least this period.
FUNDING
This work was supported by grants from the National Institutes of Health (grant numbers R01 CA128873 and 1R01 CA149318) and by a grant from Noxxon Pharma AG, Berlin.
REFERENCES
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–86. doi: 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- 2.Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol 2012; 30: 648–57. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–47. [DOI] [PubMed] [Google Scholar]
- 4.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A 2009; 106: 6742–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008; 13: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer 1978; 38: 573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010; 120: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen FH, Chiang CS, Wang CC, Tsai CS, Jung SM, Lee CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res 2009; 15: 1721–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10: 858–64. doi: 10.1038/nm1075 [DOI] [PubMed] [Google Scholar]
- 11.Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E, et al. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Res 2005; 65: 4918–28. doi: 10.1158/0008-5472.CAN-04-4453 [DOI] [PubMed] [Google Scholar]
- 12.Williams KJ, Telfer BA, Xenaki D, Sheridan MR, Desbaillets I, Peters HJ, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol 2005; 75: 89–98. doi: 10.1016/j.radonc.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 13.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res 2010; 70: 5679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 1996; 184: 1101–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997; 185: 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, et al. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol 1997; 27: 1788–93. doi: 10.1002/eji.1830270729 [DOI] [PubMed] [Google Scholar]
- 17.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001; 97: 3354–60. [DOI] [PubMed] [Google Scholar]
- 18.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283: 845–48. [DOI] [PubMed] [Google Scholar]
- 19.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 2002; 3: 687–94. doi: 10.1038/ni813 [DOI] [PubMed] [Google Scholar]
- 20.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A 1997; 94: 1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996; 382: 833–35. doi: 10.1038/382833a0 [DOI] [PubMed] [Google Scholar]
- 22.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem 1994; 269: 232–37. [PubMed] [Google Scholar]
- 23.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A 1996; 93: 14726–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 2006; 203: 2201–13. doi: 10.1084/jem.20052144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell 2008; 132: 463–73. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 2006; 124: 175–89. doi: 10.1016/j.cell.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 27.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A 2010; 107: 8363–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest 2011; 121: 1969–73. doi: 10.1172/JCI44562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006; 313: 1785–87. doi: 10.1126/science.1127592 [DOI] [PubMed] [Google Scholar]
- 30.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005; 5: 423–35. doi: 10.1038/nrc1628 [DOI] [PubMed] [Google Scholar]
- 31.Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 2008; 14: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1999; 401: 670–77. doi: 10.1038/44334 [DOI] [PubMed] [Google Scholar]
- 33.Ciarrocchi A, Jankovic V, Shaked Y, Nolan DJ, Mittal V, Kerbel RS, et al. Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS One 2007; 2: e1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6: 409–21. doi: 10.1016/j.ccr.2004.08.031 [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res 2006; 99: 315–22. doi: 10.1161/01.RES.0000235986.35957.a3 [DOI] [PubMed] [Google Scholar]
- 36.Chappell JC, Song J, Klibanov AL, Price RJ. Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 2008; 28: 1117–22. [DOI] [PubMed] [Google Scholar]
- 37.Kish PE, Blaivas M, Strawderman M, Muraszko KM, Ross DA, Ross BD, et al. Magnetic resonance imaging of ethyl-nitrosourea-induced rat gliomas: a model for experimental therapeutics of low-grade gliomas. J Neurooncol 2001; 53: 243–57. [DOI] [PubMed] [Google Scholar]
- 38.Yabuno T, Konishi N, Nakamura M, Tsuzuki T, Tsunoda S, Sakaki T, et al. Drug resistance and apoptosis in ENU-induced rat brain tumors treated with anti-cancer drugs. J Neurooncol 1998; 36: 105–12. [DOI] [PubMed] [Google Scholar]
- 39.Jang T, Savarese T, Low HP, Kim S, Vogel H, Lapointe D, et al. Osteopontin expression in intratumoral astrocytes marks tumor progression in gliomas induced by prenatal exposure to N-ethyl-N-nitrosourea. Am J Pathol 2006; 168: 1676–85. doi: 10.2353/ajpath.2006.050400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayyed SG, Hagele H, Kukarni OP, Endlich K, Segerer S, Eulberg D, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 2009; 52: 2445–54. [DOI] [PubMed] [Google Scholar]
- 41.Liu S-C, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol Dec 2013. Epub ahead of print. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]