Abstract
Tumour hypoxia is increasingly recognized as a major deleterious factor in cancer therapies, as it compromises treatment and drives malignant progression. This review seeks to clarify the oxygen levels that are pertinent to this issue. It is argued that normoxia (20% oxygen) is an extremely poor comparator for “physoxia”, i.e. the much lower levels of oxygen universally found in normal tissues, which averages about 5% oxygen, and ranges from about 3% to 7.4%. Importantly, it should be recognized that the median oxygenation in untreated tumours is significantly much lower, falling between approximately 0.3% and 4.2% oxygen, with most tumours exhibiting median oxygen levels <2%. This is partially dependent on the tissue of origin, and it is notable that many prostate and pancreatic tumours are profoundly hypoxic. In addition, therapy can induce even further, often unrecognized, changes in tumour oxygenation that may vary longitudinally, increasing or decreasing during treatment in ways that are not always predictable. Studies that fail to take cognizance of the actual physiological levels of oxygen in tissues (approximately 5%) and tumours (approximately 1%) may fail to identify the real circumstances driving tumour response to treatment and/or malignant progression. This can be of particular importance in genetic studies in vitro when comparison to human tumours is required.
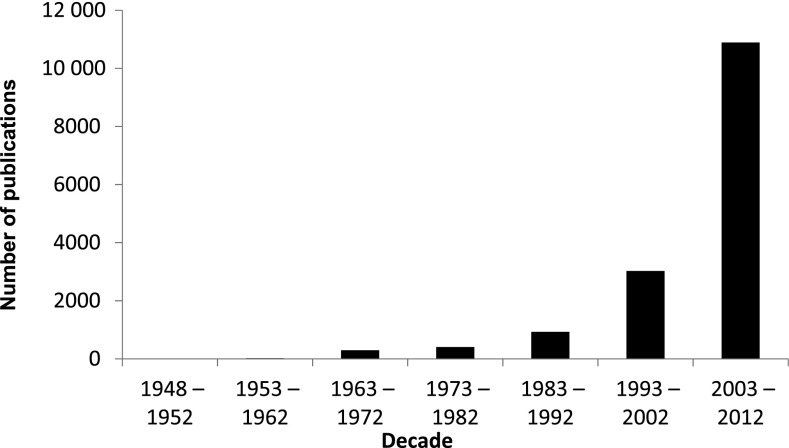
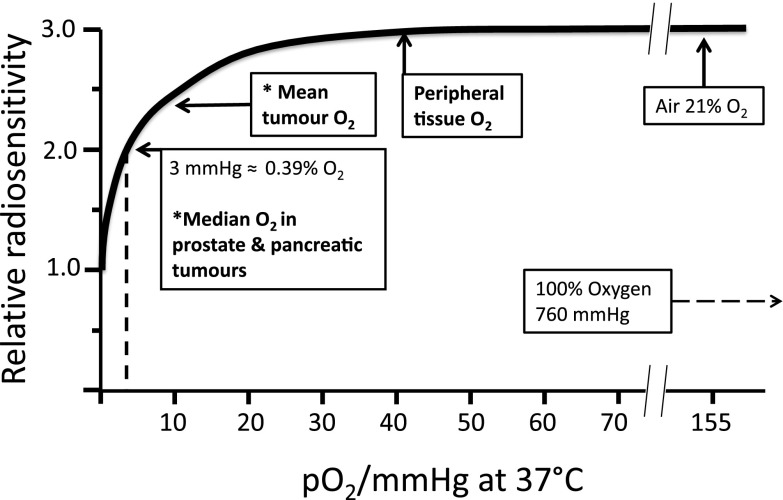
In the 1950s, tumour hypoxia was barely acknowledged, as evidenced by the paucity of publications on this subject; this is in contrast to the 10 000 articles that have been published over the last decade (Figure 1). However, since the seminal publications of Gray et al,1,2 the radiobiology and radiotherapy (RT) communities have acknowledged the importance that oxygen plays to RT responses. There is abundant and convincing evidence that RT is more effective against better-oxygenated cells, whereas hypoxic cells are significantly more radioresistant, with a marked fall in sensitivity below 2.6% oxygen (20 mmHg), which is particularly precipitate below 0.5% oxygen (3 mmHg) (Figure 2).
Figure 1.
The number of publications on tumour hypoxia as listed in PubMed, over the previous six decades. [The numbers for 1948–52 (3) and 1953–62 (20) are too small to be visible.]
Figure 2.
The relation between tissue oxygenation and radiosensitivity. Schematic representation of the radiosensitivity of tissue summarising the data from many sources. (Adapted from Hall and Giaccia3.) *Estimated approximate mean/median from published data (summarized in Table 2).
Prior to 1990, information on hypoxia was primarily obtained from excised rodent tumours treated with markers of hypoxia and/or vascular perfusion.4 When the Eppendorf electrode became available, it allowed direct oxygen measurement in situ and led to many studies which showed conclusively that oxygen levels were severely reduced in human tumours.5 The burgeoning interest in hypoxia was further fuelled by the critical finding that hypoxia selects for stress-resistant, more malignant tumour cells.6–8 This implied that tumour cells that survive hypoxic stress were likely to be a significant source of viable clonogens that can repopulate tumours with more malignant/metastatic cells. Unfortunately, most treatment protocols are less effective against hypoxic cells, which are resistant not only to RT but also to standard cytotoxic chemotherapy (CCT);9,10 although it has been reported recently that, in a few situations, hypoxia may enhance the effects of CCT.11
DEFINING TUMOUR HYPOXIA
Normoxia and physoxia
Despite the many studies on tumour hypoxia, there is considerable confusion in the use of the terms “normoxia” and “hypoxia”. Oxygenation measurements in normal tissues show that they exhibit distinct normal ranges, which vary between tissues (Table 1). However, “normoxia” is almost universally used to describe the “normal” oxygen levels in tissue culture flasks, i.e. about 20–21% oxygen (160 mmHg). Although this is not exact, as it is dependent on altitude and added CO2, for most situations, 20% is a good approximation. Despite the widespread usage of “normoxia”, this is far from an accurate comparator for peripheral tissue oxygenation. Even in lung alveoli, the oxygen level is reduced to about 14.5% oxygen (110 mmHg) by the presence of water vapour and expired CO2.13 It drops further in arterial blood and, by the time it reaches peripheral tissues, the median oxygen levels range from 3.4% to 6.8% with an average of about 6.1% (Table 2).13
Table 1.
Approximate levels of oxygen in normal tissues and tumours
| mmHg | % Oxygena | Comment |
|---|---|---|
| 760 | 100.0 | Standard atmospheric pressure |
| 160 | 21.0 | Oxygen in air at normal atmospheric pressure |
| 100 | 13.5 | Inspired oxygen pO2 in alveoli; oxygen level affected by in/outflow of gases and water vapour |
| 70 | 9.5 | Arterial blood oxygen concentration |
| 50 | 6.5 | Approximate pO2 at venous end of circulation12 |
| Suggested definitions | ||
| 38 | 5.0 | Physoxia: physiological oxygen level in peripheral tissues with an average of approximately 6% (ranging from approximately 7.5% to 4% depending on the tissue). For experimental studies, 5% is the proposed compromise since this is often used |
| 15 | 2.0 | Physiological hypoxiab—i.e. the lower level at which normal hypoxic responses are elicited (range: lower limit approximately 1%; upper limit possibly ≤5%) |
| 8 | 1.0 | Pathological hypoxiac: shows persistence of poor oxygenation suggesting disruption to normal homeostasis. Below this level pathological hypoxia applies |
| 3 | 0.4 | Radiobiological hypoxia: the oxygen level at which the cytotoxic effect of radiation is half maximal—illustrated in Figure 2 |
It is impossible to put exact figures on tissue levels. The values provided are a guide derived from several sources (see also Table 2).
Normal physiological responses to hypoxia occur above about 15 mmHg (2% oxygen). Normal tissues should not get below this since homeostasis tends to return oxygen levels to physoxia. The exact oxygen level for the upregulation of hypoxia response genes is not known; it may vary between different tissue/cell types since normal tissues have different median oxygen levels.
The presence of pathological hypoxia indicates that the tissue has not been able to revert to physoxia. In normal tissues, persistence of low oxygen will cause tissue necrosis, which can have significant functional consequences. In tumours, this can also happen. Since the tumour is an abnormal growth, loss of tissue through necrosis has no known functional significance. However, hypoxia-resistant tumour cells will initially become quiescent; eventually, there will be selection for hypoxia-tolerant more malignant tumour cells.
Table 2.
Summary of reported values of the partial pressure of oxygen (pO2) in human tumours and related normal tissues
| Tumour type | n | Median tumour pO2 | Median % oxygen | n | Median normal tissue pO2 | Median % oxygen | Fold pO2 decreasea | Reference |
|---|---|---|---|---|---|---|---|---|
| Brain (6) | 104 | 13.0 | 1.7 | 104 | 26.0 | 3.4 | 2.0 | 5 |
| Head and neck cancer (13) | 592 | 10.0 | 1.3 | ND | 5.9 | 4.5 | 5 | |
| 30 | 12.2 | 1.6 | 14 | 40.0 | 5.3 | 3.3 | 14 | |
| 23 | 14.7 | 1.9 | 30 | 43.8 | 5.8 | 3.0 | 15 | |
| 65 | 14.6 | 1.9 | 65 | 51.2 | 6.7 | 3.5 | 16 | |
| Lung cancer | 6 | 14.3 | 1.9 | ND | 5.6 | 3.0 | 17 | |
| 20 | 16.6 | 2.2 | 42.8 | 5.6 | 2.6 | 18 | ||
| Breast cancer (10) | 212 | 10.0 | 1.3 | 212 | 52.0 | 6.8 | 5.2 | 5,19 |
| Cervical cancer (12) | 730 | 9.0 | 1.2 | 5 | ||||
| 48 | 42.0 | 5.5 | 4.7 | 20 | ||||
| Liver | 4 | 6.0 | 0.8 | 4 | 30.0 | 3.9 | 5.0 | 21,22 |
| Pancreatic cancer | 7 | 2.7 | 0.4 | 7 | 51.6 | 6.8 | 19.1 | 23 |
| 1 | 2.0 | 0.3 | 22.7 | 24 | ||||
| Prostate cancer | 59 | 2.4 | 0.3 | 59 | 30.0 | 3.9 | 12.5b | 25 |
| 55 | 4.5 | 0.6 | ND | 6.7b | 26 | |||
| 10 | 9.4 | 1.2 | 2 | 26.2 | 3.4 | 2.8c | 27 | |
| Vulval cancer | 29 | 11.0 | 1.4 | ND | 28 | |||
| 15 | 13.0 | 1.7 | ND | 29 | ||||
| 19 | 11.0 | 1.4 | ND | 29 | ||||
| 20 | 10.0 | 1.3 | ND | 30 | ||||
| Melanoma | 18 | 11.6 | 1.5 | 20 | 40.5 | 5.3 | 3.5 | 31 |
| Renal cell carcinoma | 3 | 10.0 | 1.3 | 3 | 37.6 | 4.9 | 3.8 | 32 |
| Rectal carcinoma | 14 | 32.0 | 4.2 | 52.0 | 6.8 | 1.6 | 21,22 | |
| 15 | 19.0 | 2.5 | 52.0 | 6.8 | 2.7 | 33 | ||
| Sarcoma (14) | 283 | 14.0 | 1.8 | 283 | 51.0 | 6.7 | 3.6 | 5 |
| Averages or total | 2257 | 10.27 | 1.4 | 685 | 45.8 | 6.0 | 4.6 | |
| Range of medians | 2.0–32.0 | 0.3–4.2 | 26.0–51.6 | 3.4–6.8 |
n, number of patients; ND, not determined.
The data included in the table are primarily a summary from a meta-analysis carried out by Vaupel et al.5 The number of studies included for each tumour type is indicated by the number in the “tumour type” column. Other data are from single studies, as referenced. The final “average” values for tumour and normal tissue oxygenation are indicative only; they are provided to illustrate the disparity between the two values. The range is considerable and reflects the different tissue origin of the tumours; despite this, there is very limited overlap with the normal tissue data. (The averages were calculated adjusting for the number of values in each cohort.)
Fold reduction of tumour vs normal tissue is based on all the data presented in the table (except prostate; see further notes).
Fold reduction calculated on contemporaneous measurements in the psoas muscle.
Data from a pilot study that included values from the “normal” prostate of two bladder cancer patients.
This clearly highlights the anomaly of the term “normoxia”. Since normal peripheral tissues are exposed to oxygen levels about 75% lower than inspired air, it is proposed that 5% oxygen (38 mmHg) is a more accurate approximation of tissue oxygenation and that this should be recognized as “physoxia” against which other experimental conditions should be compared. Researchers do not accept such an inaccurate value for other parameters such as pH, glucose etc., yet, surprisingly, they ignore the importance of controlling for oxygen, which has been known for many years to be toxic.34 It should be noted that equilibration of culture media to the oxygen level in a specific gassing mixture can take up to 3 h;35 this can be avoided if the gas mixture is in direct contact with the cell monolayer, which can be achieved if cells are grown in oxygen-permeable culture flasks (www.coylab.com). Since % oxygen is more physiologically meaningful than mmHg, it is proposed that % oxygen is a better unit for reporting oxygen levels as it more adequately illustrates the relatively low, but clearly normal, oxygen levels in many tissues; it also better highlights the particularly low levels of oxygen found in tumours. (Note: the SI unit for gas pressure is kpascal; fortuitously 100% oxygen is equivalent to 101.3 kpascals, so these units are numerically almost equivalent.)
The lower limit of physoxia is about 3% oxygen (23 mmHg) (Table 1). Homeostasis maintains physiological parameters within tight limits with individual tissues having preferred median oxygen levels (Table 2; see also Carreau et al13). This variation suggests that cells of different origins have different oxygen sensitivities, and normal tissues are also known to have a range of tolerances to reducing oxygen. Brain tissue is particularly sensitive and can only survive about 3 min without adequate oxygenation, whereas other tissues are more tolerant, e.g. kidney and liver (15–20 min), skeletal muscle (60–90 min), vascular smooth muscle (24–72 h) and hair and nails (several days).36
Physiological hypoxia
“Physiological hypoxia” can then be defined as the oxygen level at which tissues respond to maintain their preferred oxygen level. This can be by physiological means, e.g. vasodilation, increasing blood flow, and/or upregulation of hypoxia response genes.12 Since physoxia varies for individual tissues, they are likely to have different hypoxic trigger points below which this occurs. In normal tissues, this will presumably be transitory but sufficient to return the tissue to its preferred oxygen level. However, since normal tissues are ordinarily maintained at 3–7% oxygen, physiological hypoxia is likely to be in the range 2–6% oxygen. This suggests that hypoxia response elements may well upregulate at different oxygen levels in different tissues. Currently, it is difficult to envisage how “physiological hypoxia” can be measured since homeostasis should work to reverse it almost immediately, so any manifestation would be transitory. This will be maintained by a number of changes, including increases in perfusion and temporary stabilization of hypoxia inducible factor (HIF), while readjustments are made.37 When HIF1α and HIF1β expression was measured in cultured HeLa cells from 0% to 20% oxygen, a maximal response was found at 0.5% oxygen with a half maximal expression at 1.5–2% oxygen; expression was significantly low above 4% oxygen,38 confirming that HIF1 is active in the required range to control physiological responses to oxygen deprivation (discussed further below).
Pathological hypoxia
Having identified the approximate range of “physiological hypoxia”, this helps to delineate the oxygen levels which are found in pathology. Indeed it begs the question, why in pathological tissues do the homeostatic mechanisms not respond effectively to reverse the falling oxygen levels? In ischaemic disease, which can be either chronic (e.g. in diabetes, reduced lung function etc.) or acute (e.g. stroke, coronary artery occlusion etc.), re-establishment of homeostasis may not be possible owing to the loss/occlusion/reduced flow of vessels feeding the tissue in question. However, in tumours there is often enhanced angiogenesis; yet the oxygen levels (even in untreated tumours) are significantly lower, ranging from 0.3% to 4.2% oxygen (2–32 mmHg), with almost all falling below 2% (Table 2). It is generally recognized that the tumour vasculature is chaotic and is composed of leaky vessels with blind ends, shunts and a tendency to collapse.39 Clearly, the vasculature fails miserably to maintain the oxygen levels, which are well below the adjacent normal tissues (Table 2), despite evidence in many situations that HIF1 is up-regulated.40
It is clear, therefore, that tumours are well adapted to grow and expand in this persistently oxygen-depleted tumour micro-environment (TME). In defining “pathological hypoxia”, there are no absolutes; however, the reality is that all tumours tend to have median tumour oxygen levels <2% and, within that estimation, individual measurements can vary from about 6% (very rarely) down to zero with a significantly marked skewing towards the lower end of this range; frequently, most values are well below 1.3% oxygen (10 mmHg), especially in the more hypoxic tumours. Many examples of this non-gaussian distribution are found in the publications cited in Table 2.
In tumours, it appears that the homeostatic processes are disrupted for two main reasons. Firstly, the vasculature is of very poor quality and cannot adequately and reliably provide oxygen to the growing tumour. Indeed, if putative tumour cells were sensitive to low oxygen they would die as the oxygen levels are insufficient. This leads to the other main reason: clearly, the tumour cells do not die, showing that a sizeable proportion of them are significantly hypoxia tolerant. In part, this may be attributed to their switch to glycolysis for the supply of most of their energy requirements, a feature of tumours that was identified many years ago by Warburg.41 In addition, exposure to prolonged pathological hypoxia will select for hypoxia-tolerant tumour cells that are stress resistant and more malignant (see below). It is difficult to be precise about the exact level of oxygen at which this occurs; however, it is almost certainly <1% oxygen (7.5 mmHg) and may well be significantly lower. It is noteworthy how well tumour cells adapt to significantly low oxygen levels. In one study, hypoxia only caused death of tumour cells when oxygen levels were <0.01% (0.075 mmHg) for more than 24 h.42 In our studies, a proportion of LNCaP tumour cells survived exposure in vitro to 48 h or longer of 0.1% oxygen.43 More recently, we have shown that the median oxygen level of bicalutamide-treated LNCaP prostate xenografts remained below 0.1% oxygen for more than 10 days.44 Overall, survival in this extreme stress will drive selection for malignant phenotypes that are governed by a Darwinian selection process.45
Variability in tumour oxygenation
Tumour oxygenation is normally reported as a median value; however, there is significant heterogeneity within individual tumours.5 In addition, microregional oxygenation is unstable, and oxygen levels fluctuate within the tumour depending on the functionality and proximity of local blood vessels.46 Indeed, it has been shown in rat tumours that some of the variation in oxygenation can be attributed to changes in red cell flux.47 The “better-oxygenated” tumour cells around functioning capillaries will receive some oxygen, although it is rarely as much as that received by normal cells (Table 2). However, it is sufficient to allow cell division and tumour growth, which is almost certainly boosted by the enhanced level of glycolysis mentioned above.41 Indeed, it may also be facilitated by an associated reduction in mitochondrial activity.48 As the cells divide and move away from the capillaries, they receive less oxygen and the more distal cells are chronically hypoxic;49 eventually, the cells die and the tissue becomes necrotic. In histological sections, the viable cells are often seen as “cords” of actively growing cells around perfused blood vessels up to about 150 µm, although this distance is another variable which is variously quoted to range from 70 to 200 µm.2,47,50,51 The variability is probably related to two main factors: (i) the oxygen requirement of a particular tumour cell type and (ii) its hypoxia tolerance. The more metabolically active the cells, the smaller the tumour cords will be. Once the cells become pathologically hypoxic, the proportion of cells in this fraction will depend on their hypoxia tolerance. The more tolerant they are, the longer they will remain quiescent, yet still viable, resulting in a proportionately more hypoxic tumour with a larger hypoxic fraction. Conversely, hypoxia-sensitive tumour cells will die more quickly, so the hypoxic fraction will be smaller.
In addition, since the blood vessels are inadequate and the lymphatic drainage is nearly non-existent, the interstitial pressure fluctuates, causing intermittent vascular collapse. The cells around a collapsed blood vessel will become “acutely hypoxic”; how long this lasts can vary but it has been shown in animal tumours to range from 20 min to several hours.52,53 Clearly, this is a dynamic situation and again may be much longer/shorter than the figures quoted since the figures relate to the times selected in the published studies (reviewed by Bayer and Vaupel54). Cells in this compartment (if they do not die) are still likely to be in cycle, especially if the acute hypoxia is short. They will be capable of repopulating the tumour more quickly than the “chronically hypoxic” quiescent cells. However, they will be protected from RT (owing to lack of oxygen) or CCT (owing to lack of delivery), if the vessels are closed during the treatment exposure period. There is some evidence to suggest that it is the acutely hypoxic cells that are more likely to contribute to malignant progression.54–56
Variations in tumour oxygenation readings are therefore to be expected; indeed, individual readings do vary across a tumour, albeit at oxygen levels that are mainly in the pathological range.46 In clinical studies, median levels in different tumour types from different studies are often, though not always, similar (Table 2). This gives confidence that the measurements are real and the medians, although based on a significant spread of individual readings, do provide a genuine indication of the median oxygenation in the tumour mass. In LNCaP xenografts, we have found that the median oxygen level in vehicle-treated tumours is significantly reproducible (discussed below).44 In humans, it has been shown that the median oxygen level in normal breast tissue was remarkably constant irrespective of the level of haemoglobin in the blood. This was in contrast to breast tumours, which showed both a markedly lower level of pO2 than the normal tissue and also a fall, in this already low level, as the haemoglobin concentration decreased.57
Tumour hypoxia and malignant progression
Not unsurprisingly, it is now clear that hypoxia causes a multitude of genetic changes predominantly, but not exclusively, mediated through HIF1 and HIF2.40 As discussed above, in normal cells, HIF1 expression is involved in maintaining tissue oxygenation within normal limits. Its response is designed to be almost instantaneous since the active transcription factor HIF1 is composed of the constitutively expressed HIF1β and the unstable protein HIF1α. The latter is constantly produced and broken down, thereby keeping its level significantly low in physoxic cells. As soon as the oxygen level falls, the removal of HIF1α is inhibited allowing the formation of the HIF1 complex; this immediately precipitates a plethora of changes, which in normal cells elicits a return to physoxic conditions.37,58 However, in tumours, HIF1 expression often persists irrespective of the oxygen level; this suggests that there is an adaptive response in tumour cells that makes them much less dependent on oxygen. In some tumour cells, this can be a constitutive change in HIF expression, and, in others, it is caused by a genetic change to one or more of the complex array of proteins that closely control HIF expression in normal cells.37,58 This establishes a very different phenotype from normal (physoxic) cells. Advantageously, the adapted tumour cells acquire a much reduced requirement for oxygen; this leads to a markedly improved ability to survive in hypoxic conditions that is associated with their ability to use glycolysis to provide for their energy needs.41,48
The switch to a HIF1-regulated phenotype promotes selection for hundreds of genes, many of which are associated with a more malignant phenotype. For example, there is a switch to a more angiogenic phenotype with upregulation of genes, such as vascular endothelial growth factor (VEGF) and interleukin 8 (IL8), whereas angiogenesis inhibitors are downregulated, e.g. angiostatin and endostatin. Other genes/pathways implicated in this hypoxic response include nuclear factor κ B, activator protein-1, mammalian target of rapamycin kinase and the unfolded protein response.59–61 Although these genes/pathways are activated independently, indicating redundancy in oxygen-sensitive pathways, there is also evidence that they can respond to hypoxia in an integrated manner.62
Early studies have shown that hypoxia selected for cells with defects in apoptosis.6 Further reports have confirmed that hypoxia can impose a selection pressure that allows clonal variant expansion in vitro43,63,64 and in vivo.55 Studies in our laboratory showed that mice bearing LNCaP xenografts exposed to bicalutamide-induced hypoxia had increased metastasis to the lungs; this correlated with an increase in Bcl2 and reduction in Bax.44 Gene amplification has also been reported in rodent tumour cells exposed ex vivo or in vivo to hypoxia; this was associated with an increase in metastases.65,66 In both tissue culture and animal models, acute hypoxia/reoxygenation have been linked to the induction of DNA strand breaks and clearly, if these breaks are not repaired, they will result in further mutations. Indeed, there is considerable evidence that DNA repair processes in tumours are also modified by hypoxia and that this is related to increases in genetic instability.67
Other studies have shown that hypoxia can increase malignant progression/metastasis by upregulating metastasis-related genes, such as osteopontin, lysyl oxidase, CXCR4, IL8 and VEGF and many others, primarily through the stabilization of HIF1.68–70 This may also be associated with an increase in MDM2, which is an inhibitor of p53, and in vivo this leads to apoptosis resistance and increased metastasis formation.71,72 Recently, it has been shown that radiation treatment can also select for tumour cells that overexpress HIF1. Following irradiation, in vivo HIF1-overexpressing cells relocate towards the tumour blood vessels; inhibition of HIF1 blocks this effect and also reduces regrowth of the tumour.73
It is impossible to discuss all of the genetic changes reported in response to hypoxia (for more detailed reviews see 59,74–76). However, there is one issue that needs comment. Genetic changes caused by hypoxia are often measured in vitro and compared with “normoxia”. This is most frequently described, or presumed if undefined, as air containing 5% CO2 (i.e. approximately 20% O2); surprisingly, it is rare to find any comment about the validity of this assumption. However, as discussed above, it would be more relevant to normal tissue if control cells were maintained in physoxia, i.e. 5% O2, and compared with physiological hypoxia (1–3%) and pathological hypoxia (0.5–0.1%). (These figures are given as ranges, as the oxygen level pertinent to a particular investigation will depend on the origin of the tumour and normal tissue of interest—see Table 2 for relevant values.) However, oxygen levels are infrequently taken into account in genetic studies in vitro, although it is likely to be critical to clearly defining and comparing what occurs in whole tissues or tumours.
The lack of correlation between in vitro cells and solid tumours is confirmed in a recent study that identified hundreds of androgen receptor binding sites (ARBSs) in human prostate tumour biopsies. The majority of these ARBSs were not identified in LNCaP prostate tumour cells grown in vitro; however, many were found in the same cells grown as xenografts in androgen-deprived (castrated) mice. A 16-gene signature set was identified from the human biopsies that outperformed a larger signature derived from cultured cells; it was also identified in the xenografted LNCaP tumours but not in LNCaP cells grown in vitro, indicating that the TME has a major influence in the control of androgen receptor signalling in prostate tumours.77 This again emphasizes that caution should be used when comparing data from in vitro studies, especially when cells are grown in “normoxia”.
Oxygen homeostasis in the tumour microenvironment and response to treatment
Why do homeostatic mechanisms not respond to restore oxygen homeostasis in tumours? It is clear that angiogenesis is stimulated, but the vasculature formed is insufficient to maintain oxygen at physoxic levels despite extensive capillary formation in many tumours. If, in response to a prolonged hypoxic stress, the tumour cells adapt/mutate to produce even more amplified levels of pro-angiogenic factors, then they will provide the tumour with a survival advantage. Once the pro-angiogenic factors get to critical levels, then the vasculature will improve (possibly normalize)78 and the tumour will regrow. Unfortunately, when this happens, it is likely to be repopulated with pro-angiogenic, hypoxia-tolerant, more malignant tumour cells.
This is exactly what happened when mice bearing LNCaP tumours were treated daily with bicalutamide, a drug widely used in locally advanced prostate cancer. Untreated LNCaP xenografts were poorly oxygenated (0.8% oxygen; 6 mmHg); this was similar to oxygen levels found in clinical studies (0.9% oxygen, 7 mmHg; Table 2). When mice were treated daily with bicalutamide for 28 days, the oxygen level dropped precipitately over 1–3 days to ≤0.1% oxygen; this profound hypoxia was maintained for more than 10 days. However, during the next 10 days, oxygen levels increased, returning to almost pre-treatment levels. When tumours were grown in vivo in window chambers, a marked loss of blood vessels was seen in the first 14 days of bicalutamide treatment followed by an angiogenic burst. This distinctive biphasic response was attributed to a small fall, and then a larger increase, in pro-angiogenic factors, including VEGF and most markedly IL8. Clearly, the tumour cells can survive exposure to the profound hypoxic insult and, despite the androgen blockade, growth inhibition was eventually reversed by the production of sufficient pro-angiogenic factors to stimulate neovascularization. The tumour cells were clearly able to switch use of their limited energy supply to synthesize these critical factors. After 28 days of treatment, excised tumour cells were more invasive and more resistant to docetaxel than tumour cells excised from a vehicle-treated mouse. In addition, mice treated with bicalutamide had a marked increase in metastatic spread to the lungs, although this could be blocked successfully by treatment on Day 7 with a single dose of banoxantrone (AQ4N), a prodrug that specifically targets hypoxic cells.44
The initial antivascular effect of the anti-androgen bicalutamide is not widely recognized, although previous studies, mostly using castration models, have provided much evidence that it is likely to occur (discussed in 44). Our studies showed that prostate tumour cells are very hypoxia tolerant. In other tumour types, this may vary somewhat; however, it should be noted that pancreatic tumour cells may be particularly hypoxia tolerant because they survive oxygen levels ≥19-fold lower than those found in normal pancreatic tissue (Table 2).23 When patient-derived xenografts were established orthotopically in nude mice, the extent of hypoxia, measured using the hypoxic marker EF5, predicted for aggressive growth and spontaneous metastasis.79 Human pancreatic tumours are particularly treatment resistant, a characteristic that has been attributed to their extensive stroma.80 It is tempting to speculate that treatment resistance may also result from the selection for cells that have the ability to survive oxygen levels much lower than those found in the normal pancreas and which, consequently, have a particularly malignant phenotype.
If exposure to drugs which cause hypoxia can select for hypoxia-tolerant/more malignant tumour cells, it is possible that any treatment that causes increased and prolonged hypoxia can do the same thing. This may be the foremost reason why vascular targeting drugs, used as single agents, are not as successful as originally expected. Indeed, considerable redundancy in angiogenic pathways has been observed and revascularization has also been found after initial early antivascular responses (for review see 81). Most anticancer treatments either (i) target blood vessels directly or (ii) target the tumour cells that support the functioning, however inadequately, of the tumour vasculature. Therefore, it is possible that many treatments cause early (often unrecognized) antivascular effects and an associated increase in hypoxia. Since the oxygen levels in most tumours are already in the pathological range, this may well result in a critical hypoxic insult.
We have shown this in PC3 prostate tumours with the cytotoxic drug docetaxel, which caused an early antiangiogenic effect that was further potentiated with dexamethasone. This may explain why there is a short-term (although not long-term) efficacy of this combination in patients with metastatic prostate cancer.82 As discussed above, the antiandrogen bicalutamide has a similar initial and profound effect on tumour vasculature, an effect that we have also found with other mechanistically different antitumour drugs (our unpublished data). However, tumours can adapt to this hypoxic insult and they recover with a more pro-angiogenic, and potentially malignant, phenotype.44,83,84 This problem may be overcome in part, at least, by combination with other CCT. Hypoxia-activated prodrugs (HAPs) (discussed below) offer an additional approach, since they specifically target hypoxic cells. Clearly, a greater understanding of longitudinal changes in tumour oxygenation induced by current therapies is required, to more effectively schedule drug combinations, including HAPs.
THE IMPORTANCE OF TUMOUR HYPOXIA ON SURVIVAL OUTCOMES FOLLOWING CANCER THERAPY
In the last 20 years, evidence has shown that median oxygen levels vary between tumour types (Table 2), and variability is found even within the same histological group.5 There is now considerable clinical evidence that tumours with a higher proportion of hypoxic cells have a poor prognosis (reviewed by Vaupel et al5). This was found even in patients treated by surgical excision, where all of the hypoxic cells would, at least in theory, be removed.85,86 In assessing the data summarized in Table 2, it should be noted that general anaesthesia can improve tumour oxygenation and potentially cause an underestimation of tumour hypoxia; therefore, if at all possible, it is preferable to use some form of local anaesthesia when measuring tumour oxygenation.24,27,87 Estimation of oxygenation in the prostate provides an additional problem, since the tumour often infiltrates throughout the gland, which may affect measurement of “normal” oxygen levels. It has been reported that the “normal” tissue is not significantly different in oxygen level from tumours;26,88 however, it is possible that the normal tissue is influenced by the presence of tumour. In one study, the normal prostate was assessed in two patients prior to surgery for bladder cancer and they showed a mean of 3.4% oxygen (26.2 mmHg).27 This indicates that the level of oxygenation in the normal prostate is low, compared with other normal tissues; however, it is significantly higher than the low levels found in untreated prostate tumours of approximately 0.5% oxygen (3.90 mmHg). In other studies, this group used psoas muscle as their reference normal tissue, which had a similar median pO2 of 4% oxygen (30 mmHg). They found the level of tumour to muscle pO2 was a strong predictor of prognosis.25,27,87,89,90
All of the studies listed in Table 2 have shown that a patient had a markedly poorer prognosis when their tumour was more hypoxic. It is likely that these patients will have a larger number of hypoxia-tolerant, more malignant cells free in the circulation and/or already in secondary sites, factors which will impact on recurrence and survival. Tumours are also known to contain small subpopulations of cancer stem cells (CSCs), which are thought to repopulate tumours following treatment.91,92 It is perhaps not surprising that hypoxic niches are a preferred location for CSCs93,94 and that HIF1 has an important role in control of these cells.95,96 Indeed, it has been stressed that physiologically relevant levels of oxygen are critical for the culturing of stem cells including CSCs.97 Whether it is predominantly the CSCs or a subpopulation of more malignant tumour cells that contribute to treatment resistance, clearly hypoxia has a role to play in protecting both these populations and selecting for the most hypoxia-tolerant stress-resistant cells.
The hypoxic fraction and treatment response
RT is a potential treatment for >50% of cancer patients.98 For several reasons, RT is normally administered as a fractionated regimen; this is because there is improved targeting of previously hypoxic cells, as they reoxygenate between fractions.3 In the 1980s, radiation sensitizers were developed to enhance the effects of RT through improving the radiosensitivity of hypoxic tumour cells. For a number of reasons, most of these drugs are no longer in use. However, nimorazole was found to provide a significant enhancement of the effects of radiation, and it is still used in some European countries, predominantly Denmark.99 A meta-analysis has been performed of clinical trials designed to improve hypoxic cell eradication, through either improved oxygenation strategies or direct cytotoxicity. Significant improvement has been shown using several outcome measures, yet, regrettably, these approaches have not been widely adopted by the clinical community.100
Overcoming the radioresistance of hypoxic tumours has also been attempted using a variety of methods that improve tumour oxygenation.101 One of the earliest methods was to use hyperbaric oxygen. However, this was found to have logistical limitations and carried the risk of additional adverse events. A recent meta-analysis has shown that there is some evidence for its utility, however there is currently little interest in this approach.102 Tumour oxygenation has also been improved through increasing red cell numbers by treatment with erythropoietin (EPO). However, results have been equivocal, and, in some cases, the outcome was worse.103–105 This was unexpected but may be linked to the presence of EPO in tumour cells, which has been shown to independently predict for a worse prognosis.105,106 Additionally, since the effect of EPO develops over several days, the hypoxic fraction may adjust as the oxygenation improves, although the evidence for this is equivocal.107,108
A more successful approach for improving tumour oxygen is the use of accelerated radiotherapy in combination with carbogen (95% oxygen, 5% CO2) breathing and oral nicotinamide (ARCON).109 A study of 345 patients with laryngeal cancer compared accelerated RT alone against ARCON. There was no improvement in local tumour control at 5 years, however regional control was significantly better with ARCON (93%) than with accelerated RT (86%, p = 0.04); this was specific to patients with hypoxic tumours (p = 0.01). Toxicity was the same in both arms of the trial.110 The utility of ARCON has also been shown in a Phase II trial for bladder cancer.111
Antiangiogenic/antivascular agents offer a more specific way to starve a tumour of oxygen and nutrients.112 However, this approach will almost certainly increase hypoxia, even if only for a limited period. In some situations, this approach has led to a normalization of blood vessels, which conversely tends to improve tumour oxygenation and hence improve sensitivity to RT and CCT.78 Although, initially, vascular targeting provides for better tumour control the longer term outcome is not so clear.112,113 It is possible that responses may depend on whether tumours go through an initial hypoxic phase while readjusting to the exposure to the drugs. If there is a significant period of enhanced hypoxia this may leave the tumour repopulated with more hypoxia-tolerant, more malignant cells. It is also possible that some tumours are intrinsically resistant to angiogenesis inhibitors and/or can adapt to use other pathways to promote angiogenesis.114
Direct targeting of hypoxic tumour cells with hypoxia-activated prodrugs
Hypoxic tumour cells can be targeted directly using HAPs, also called bioreductive drugs.11,112,113,115 These chemically disparate agents are grouped together based on the principle that they are only metabolized in hypoxic cells (usually below about 1% oxygen), providing for tumour specificity, since hypoxia is rare in normal tissues.116,117 Combining HAPs with standard RT and CCT has much to recommend it, since they kill the most malignant cells while only causing a limited (if any) increase in systemic toxicity. Several HAPs have entered clinical trials although, as yet, none has been successfully licenced. Tirapazamine (TPZ) is the most extensively studied HAP, and it was shown to provide good antitumour enhancement in Phase II clinical trials.118 This was not confirmed in Phase III trials, which failed to report a significant increase in survival in comparison with conventional therapy.119,120 However, these patients were not categorized based on their level of tumour hypoxia. When patients were stratified on the basis of hypoxia-induced hepatocyte growth factor and IL8, elevated plasma levels were found to correlate with a beneficial response to TPZ combined with chemoradiotherapy.121 This highlights the need for patient stratification on the grounds of hypoxia, preferably using a non-invasive technique that can follow oxygenation changes longitudinally. Many new radiological techniques are currently in development; it is hoped that non-invasive approaches that can accurately and reliably image tumour hypoxia will become available in the next few years. This has recently been reviewed by Horsman et al.122
AQ4N is a unique HAP, since it has the additional advantage of forming a stable cytotoxin as a result of an irreversible reduction in hypoxic cells.118 AQ4N was showing promise in clinical trials but was withdrawn for commercial reasons. It is now superseded by OCT1002, which has similar, if not better, characteristics (our unpublished data); this compound is currently in pre-clinical development. An analogue of TPZ, SN30000 (also called CEN 209), is currently awaiting commencement of a clinical trial.117,123 Other HAPs already in clinical trial include TH-302124 and Proacta.125,126 If successful, these new HAPs could provide for a direct method to eradicate hypoxic cells, improve tumour control and reduce metastasis. Clearly, scheduling of treatment combinations should be informed by the knowledge of tumour oxygenation before, and also during, treatment to ensure the drugs are used when they will have their maximal effect.
CONCLUSIONS
Tumour hypoxia is a critically important parameter, which compromises treatment and promotes malignancy. It is widespread in human tumours, and it has a marked effect on responses across most treatments irrespective of their mode of action. In experimental studies, it is crucial that investigators control for oxygenation to mimic, as far as is possible, the significant low oxygen levels found in most human tumour cells; that is at or <1%, and often <0.1% oxygen (7.5 to <0.75 mmHg).
Many antitumour treatments affect blood vessels either directly or indirectly, causing even more profound (often unrecognized) treatment-induced hypoxia. Many tumour cells are well adapted to survive this hypoxic insult and unfortunately tumours often recur following treatment, repopulated by more malignant cells. One approach, to target hypoxic cells directly, is to use HAPs. Although this approach has had variable results in clinical trials, newer agents are showing promise. In conclusion, unless an effective method of eradicating hypoxic tumour cells is found, complete control of many solid tumours is unlikely to be achieved.
Conflicts of interest
SR McKeown is a director of OncoTherics Limited, the spin-out company responsible for development of OCT1002.
REFERENCES
- 1.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26: 638–48. [DOI] [PubMed] [Google Scholar]
- 2.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1995; 9: 539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall EJ, Giaccia A. Radiobiology for the radiologist. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 4.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006; 82: 699–757. doi: 10.1080/09553000601002324 [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 2007; 9: 1221–35. doi: 10.1089/ars.2007.1628 [DOI] [PubMed] [Google Scholar]
- 6.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91. doi: 10.1038/379088a0 [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol 2010; 32: 125–7. [PubMed] [Google Scholar]
- 8.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther 2011; 11: 714–23. [DOI] [PubMed] [Google Scholar]
- 9.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev 1994; 13: 139–68. [DOI] [PubMed] [Google Scholar]
- 10.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007; 99: 1441–54. doi: 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- 11.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393–410. doi: 10.1038/nrc3064 [DOI] [PubMed] [Google Scholar]
- 12.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001; 93: 266–76. [DOI] [PubMed] [Google Scholar]
- 13.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011; 15: 1239–53. doi: 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordsmark M, Bentzen SM, Overgaard J. Measurement of human tumour oxygenation status by a polarographic needle electrode. Acta Oncol 1994; 33: 383–9. [DOI] [PubMed] [Google Scholar]
- 15.Becker A, Hänsgen G, Bloching M, Weigel C, Lautenschläger C, Dunst J. Oxygenation of squamous cell carcinoma of the head and neck: comparison of primary tumors, neck node metastases, and normal tissue. Int J Radiat Oncol Biol Phys 1998; 42: 35–41. [DOI] [PubMed] [Google Scholar]
- 16.Le QT, Kovacs MS, Dorie MJ, Koong A, Terris DJ, Pinto HA, et al. Comparison of the comet assay and the oxygen microelectrode for measuring tumor oxygenation in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2003; 56: 375–83. [DOI] [PubMed] [Google Scholar]
- 17.Falk SJ, Ward R, Bleehen NM. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised pO2 histography. Br J Cancer 1992; 66: 919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 2006; 12: 1507–14. doi: 10.1158/1078-0432.CCR-05-2049 [DOI] [PubMed] [Google Scholar]
- 19.Vaupel P, Mayer A, Briest S, Höckel M. Oxygenation gain factor: a novel parameter characterizing the association between hemoglobin level and the oxygenation status of breast cancers. Cancer Res 2003; 63: 7634–7. [PubMed] [Google Scholar]
- 20.Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res 1991; 51: 6098–102. [PubMed] [Google Scholar]
- 21.Kallinowski F, Buhr HJ. Can the oxygenation status of rectal carcinomas be improved by hypoxia? In: Vaupel P, Kelleher DK, Günderoth M, eds. Tumor oxygenation. Stuttgart, Germany: Gustav Fischer; 1995. pp. 291–6. [Google Scholar]
- 22.Kallinowski F, Buhr HJ. Tissue oxygenation of primary, metastatic and xenografted rectal cancers. In: Vaupel P, Kelleher DK, Günderoth M, eds. Tumor oxygenation. Stuttgart, Germany: Gustav Fischer; 1995. pp. 205–9. [Google Scholar]
- 23.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000; 48: 919–22. [DOI] [PubMed] [Google Scholar]
- 24.Graffman S, Björk P, Ederoth P, Ihse I. Polarographic pO2 measurements of intra-abdominal adenocarcinoma in connection with intraoperative radiotherapy before and after change of oxygen concentration of anaesthetic gases. Acta Oncol 2001; 40: 105–7. [DOI] [PubMed] [Google Scholar]
- 25.Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Pinover WH, Greenberg RE, et al. Hypoxia in human prostate carcinoma: an Eppendorf pO2 study. Am J Clin Oncol 2001; 24: 458–61. [DOI] [PubMed] [Google Scholar]
- 26.Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, et al. Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58: 750–7. doi: 10.1016/S0360-3016(03)01621-3 [DOI] [PubMed] [Google Scholar]
- 27.Movsas B, Chapman JD, Horwitz EM, Pinover WH, Greenberg RE, Hanlon AL, et al. Hypoxic regions exist in human prostate carcinoma. Urology 1999; 53: 11–18. [DOI] [PubMed] [Google Scholar]
- 28.Vaupel P, Thews O, Mayer A, Höckel S, Höckel M. Oxygenation status of gynecologic tumors: what is the optimal hemoglobin level? Strahlenther Onkol 2002; 178: 727–31. doi: 10.1007/s00066-002-1081-x [DOI] [PubMed] [Google Scholar]
- 29.Vaupel P, Mayer A, Höckel M. Oxygenation status of primary and recurrent squamous cell carcinomas of the vulva. Eur J Gynaecol Oncol 2006; 27: 142–6. [PubMed] [Google Scholar]
- 30.Stone JE, Parker R, Gilks CB, Stanbridge EJ, Liao SY, Aquino-Parsons C. Intratumoral oxygenation of invasive squamous cell carcinoma of the vulva is not correlated with regional lymph node metastasis. Eur J Gynaecol Oncol 2005; 26: 31–5. [PubMed] [Google Scholar]
- 31.Lartigau E, Randrianarivelo H, Avril MF, Margulis A, Spatz A, Eschwège F, et al. Intratumoral oxygen tension in metastatic melanoma. Melanoma Res 1997; 7: 400–6. [DOI] [PubMed] [Google Scholar]
- 32.Lawrentschuk N, Poon AM, Foo SS, Putra LG, Murone C, Davis ID, et al. Assessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomography. BJU Int 2005; 96: 540–6. doi: 10.1111/j.1464-410X.2005.05681.x [DOI] [PubMed] [Google Scholar]
- 33.Mattern J, Kallinowski F, Herfarth C, Volm M. Association of resistance-related protein expression with poor vascularization and low levels of oxygen in human rectal cancer. Int J Cancer 1996; 67: 20–3. doi: [DOI] [PubMed] [Google Scholar]
- 34.Winslow RM. Oxygen: the poison is in the dose. Transfusion 2013; 53: 424–37. doi: 10.1111/j.1537-2995.2012.03774.x [DOI] [PubMed] [Google Scholar]
- 35.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol Lung Cell Mol Physiol 2001; 281: L1021–7. [DOI] [PubMed] [Google Scholar]
- 36.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ 1998; 317: 1370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassavaugh J, Lounsbury KM. Hypoxia-mediated biological control. J Cell Biochem 2011; 112: 735–44. doi: 10.1002/jcb.22956 [DOI] [PubMed] [Google Scholar]
- 38.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996; 271: C1172–80. [DOI] [PubMed] [Google Scholar]
- 39.Nussenbaum F, Herman IM. Tumor angiogenesis: insights and innovations. J Oncol Apr 2010. Epub ahead of print. doi: 10.1155/2010/132641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 2008; 15: 678–85. doi: 10.1038/cdd.2008.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. [DOI] [PubMed] [Google Scholar]
- 42.Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res 2005; 65: 3171–8. doi: 10.1158/0008-5472.CAN-04-3395 [DOI] [PubMed] [Google Scholar]
- 43.Butterworth KT, McCarthy HO, Devlin A, Ming L, Robson T, McKeown SR, et al. Hypoxia selects for invasive, androgen independent and apoptosis-resistant LNCaP cells in vitro. Int J Cancer 2008; 123: 760–8. doi: 10.1002/ijc.23418 [DOI] [PubMed] [Google Scholar]
- 44.Ming L, Byrne NM, Camac SN, Mitchell CA, Ward C, Waugh DJ, et al. Androgen deprivation results in time-dependent hypoxia in LNCaP prostate tumours: informed scheduling of the bioreductive drug AQ4N improves treatment response. Int J Cancer 2013; 132: 1323–32. [DOI] [PubMed] [Google Scholar]
- 45.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 2012; 12: 487–93. doi: 10.1038/nrc3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol 2004; 381: 335–54. doi: 10.1016/S0076-6879(04)81023-1 [DOI] [PubMed] [Google Scholar]
- 47.Lanzen J, Braun RD, Klitzman B, Brizel D, Secomb TW, Dewhirst MW. Direct demonstration of instabilities in oxygen concentrations within the extravascular compartment of an experimental tumor. Cancer Res 2006; 66: 2219–23. [DOI] [PubMed] [Google Scholar]
- 48.Denko N. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–13. doi: 10.1038/nrc2468 [DOI] [PubMed] [Google Scholar]
- 49.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 50.Olive PL, Vikse C, Trotter MJ. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys 1992; 22: 397–402. [DOI] [PubMed] [Google Scholar]
- 51.Torres-Filho IP, Leunig M, Yuan F, Intaglietta M, Jain RK. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc Natl Acad Sci U S A 1994; 91: 2081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaplin DJ, Peters CE, Horsman MR, Trotter MJ. Drug induced perturbations in tumour blood flow: therapeutic potential and possible limitations. Radiother Oncol 1991; 20(Suppl. 1): 93–101. [DOI] [PubMed] [Google Scholar]
- 53.Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat Res 2009; 172: 653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer C, Vaupel P. Acute versus chronic hypoxia in tumors: controversial data concerning time frames and biological consequences. Strahlenther Onkol 2012; 188: 616–27. doi: 10.1007/s00066-012-0085-4 [DOI] [PubMed] [Google Scholar]
- 55.Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res 2004; 64: 2054–61. [DOI] [PubMed] [Google Scholar]
- 56.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res 2001; 61: 8903–8. [PubMed] [Google Scholar]
- 57.Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist 2008; 13: 21–6. doi: 10.1634/theoncologist.13-S3-21 [DOI] [PubMed] [Google Scholar]
- 58.Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life 2008; 60: 591–7. doi: 10.1002/iub.93 [DOI] [PubMed] [Google Scholar]
- 59.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med 2012; 18: 534–43. doi: 10.1016/j.molmed.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenza GL. HIF1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 2013; 123: 3664–71. doi: 10.1172/JCI67230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci 2012; 19: 102. doi: 10.1186/1423-0127-19-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wouters BG, Koritzinsky M. Hypoxia signaling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 2008; 8: 851–64. doi: 10.1038/nrc2501 [DOI] [PubMed] [Google Scholar]
- 63.Koshikawa N, Maejima C, Miyazaki K, Nakagawara A, Takenaga K. Hypoxia selects for high-metastatic Lewis lung carcinoma cells overexpressing Mcl-1 and exhibiting reduced apoptotic potential in solid tumors. Oncogene 2006; 25: 917–28. doi: 10.1038/sj.onc.1209128 [DOI] [PubMed] [Google Scholar]
- 64.Weinmann M, Jendrossek V, Güner D, Goecke B, Belka C. Cyclic exposure to hypoxia and reoxygenation selects for tumor cells with defects in mitochondrial apoptotic pathways. FASEB J 2004; 18: 1906–18. doi: 10.1096/fj.04-1918fje [DOI] [PubMed] [Google Scholar]
- 65.Young SD, Hill RP. Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst 1990; 82: 371–80. [DOI] [PubMed] [Google Scholar]
- 66.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A.1998; 85: 9533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. doi: 10.1038/nrc2344 [DOI] [PubMed] [Google Scholar]
- 68.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol 2000; 76: 589–605. [DOI] [PubMed] [Google Scholar]
- 69.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia induced metastasis. Nature 2006; 440: 1222–6. doi: 10.1038/nature04695 [DOI] [PubMed] [Google Scholar]
- 70.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev 2007; 26: 333–9. doi: 10.1007/s10555-007-9063-1 [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Hill RP. Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res 2004; 64: 4180–9. doi: 10.1158/0008-5472.CAN-03-3038 [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Hill RP. Hypoxia enhances metastatic efficiency in HT1080 fibrosarcoma cells by increasing cell survival in lungs, not cell adhesion and invasion. Cancer Res 2007; 67: 7789–97. doi: 10.1158/0008-5472.CAN-06-4221 [DOI] [PubMed] [Google Scholar]
- 73.Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun 2012; 3: 783. doi: 10.1038/ncomms1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis 2003; 20: 237–50. [DOI] [PubMed] [Google Scholar]
- 75.Box C, Rogers SJ, Mendiola M, Eccles SA. Tumour-microenvironmental interactions: paths to progression and targets for treatment. Semin Cancer Biol 2010; 20: 128–38. doi: 10.1016/j.semcancer.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 76.Koi M, Boland CR. Tumor hypoxia and genetic alterations in sporadic cancers. J Obstet Gynaecol Res 2011; 37: 85–98. doi: 10.1111/j.1447-0756.2010.01377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 2013; 23: 35–47. doi: 10.1016/j.ccr.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 78.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2012; 2: a006486. doi: 10.1101/cshperspect.a006486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res 2011; 71: 3110–20. doi: 10.1158/0008-5472.CAN-10-4049 [DOI] [PubMed] [Google Scholar]
- 80.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012; 18: 4266–76. doi: 10.1158/1078-0432.CCR-11-3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ribatti D. Novel angiogenesis inhibitors: addressing the issue of redundancy in the angiogenic signaling pathway. Cancer Treat Rev 2011; 37: 344–52. doi: 10.1016/j.ctrv.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 82.Wilson C, Scullin P, Worthington J, Seaton A, Maxwell P, O'Rourke D, et al. Dexamethasone potentiates the antiangiogenic activity of docetaxel in castration-resistant prostate cancer. Br J Cancer 2008; 99: 2054–64. doi: 10.1038/sj.bjc.6604804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15: 232–9. doi: 10.1016/j.ccr.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15: 220–31. doi: 10.1016/j.ccr.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Höckel M, Schlenger K, Höckel S, Aral B, Schäffer U, Vaupel P. Tumor hypoxia in pelvic recurrences of cervical cancer. Int J Cancer 1998; 79: 365–9. [DOI] [PubMed] [Google Scholar]
- 86.Rajaganeshan R, Prasad R, Guillou PJ, Poston G, Scott N, Jayne DG. The role of hypoxia in recurrence following resection of Dukes' B colorectal cancer. Int J Colorectal Dis 2008; 23: 1049–55. doi: 10.1007/s00384-008-0497-x [DOI] [PubMed] [Google Scholar]
- 87.Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, et al. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age: an Eppendorf pO(2) study. Cancer 2000; 89: 2018–24. [DOI] [PubMed] [Google Scholar]
- 88.Milosevic M, Warde P, Ménard C, Chung P, Toi A, Ishkanian A, et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res 2012; 18: 2108–14. doi: 10.1158/1078-0432.CCR-11-2711 [DOI] [PubMed] [Google Scholar]
- 89.Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C, et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology 2002; 60: 634–9. [DOI] [PubMed] [Google Scholar]
- 90.Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys 2012; 82: e433–9. doi: 10.1016/j.ijrobp.2011.05.037 [DOI] [PubMed] [Google Scholar]
- 91.Singh RP, Franke K, Wielockx B. Hypoxia-mediated regulation of stem cell fate. High Alt Med Biol 2012; 13: 162–8. doi: 10.1089/ham.2012.1043 [DOI] [PubMed] [Google Scholar]
- 92.Mao Q, Zhang Y, Fu X, Xue J, Guo W, Meng M, et al. A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. J Cancer Res Clin Oncol 2013; 139: 211–22. doi: 10.1007/s00432-012-1310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol 2009; 219: 271–5. doi: 10.1002/jcp.21690 [DOI] [PubMed] [Google Scholar]
- 94.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett 2013; 341: 63–72. doi: 10.1016/j.canlet.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 95.Zeng W, Wan R, Zheng Y, Singh SR, Wei Y. Hypoxia, stem cells and bone tumor. Cancer Lett 2011; 313: 129–36. doi: 10.1016/j.canlet.2011.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee EK, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr Opin Cell Biol 2012; 24: 232–5. doi: 10.1016/j.ceb.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 97.Wion D, Christen T, Barbier EL, Coles JA. PO(2) matters in stem cell culture. Cell Stem Cell 2009; 5: 242–3. doi: 10.1016/j.stem.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 98.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005; 104: 1129–37. doi: 10.1002/cncr.21324 [DOI] [PubMed] [Google Scholar]
- 99.Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR; Danish Head and Neck Cancer Study Group. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 2005; 6: 757–64. [DOI] [PubMed] [Google Scholar]
- 100.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 2007; 25: 4066–74. doi: 10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]
- 101.Jordan BF, Sonveaux P. Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy. Front Pharmacol 2012; 3: 94. doi: 10.3389/fphar.2012.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennett M, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy: a systematic review of randomised controlled trials. Cancer Treat Rev 2008; 34: 577–91. doi: 10.1016/j.ctrv.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 103.Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 2003; 362: 1255–60. [DOI] [PubMed] [Google Scholar]
- 104.Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99-03). Int J Radiat Oncol Biol Phys 2007; 69: 1008–17. doi: 10.1016/j.ijrobp.2007.04.063 [DOI] [PubMed] [Google Scholar]
- 105.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys 2005; 63: 25–36. doi: 10.1016/j.ijrobp.2005.04.049 [DOI] [PubMed] [Google Scholar]
- 106.Leo C, Horn LC, Rauscher C, Hentschel B, Liebmann A, Hildebrandt G, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res 2006; 12: 6894–900. doi: 10.1158/1078-0432.CCR-06-1285 [DOI] [PubMed] [Google Scholar]
- 107.Joiner B, Hirst VK, McKeown SR, McAleer JJ, Hirst DG. The effect of recombinant human erythropoietin treatment on tumour radiosensitivity and cancer-associated anaemia in the mouse. Br J Cancer 1993; 68: 720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stüben G, Thews O, Pöttgen C, Knühmann K, Vaupel P, Stuschke M. Recombinant human erythropoietin increases the radiosensitivity of xenografted human tumours in anaemic nude mice. J Cancer Res Clin Oncol 2001; 127: 346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol 2002; 3: 728–37. [DOI] [PubMed] [Google Scholar]
- 110.Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012; 30: 1777–83. doi: 10.1200/JCO.2011.35.9315 [DOI] [PubMed] [Google Scholar]
- 111.Hoskin P, Rojas A, Saunders M. Accelerated radiotherapy, carbogen, and nicotinamide (ARCON) in the treatment of advanced bladder cancer: mature results of a phase II nonrandomized study. Int J Radiat Oncol Biol Phys 2009; 73: 1425–31. [DOI] [PubMed] [Google Scholar]
- 112.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473: 298–307. doi: 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michieli P. Hypoxia, angiogenesis and cancer therapy. To breathe or not to breathe? Cell Cycle 2009; 8: 3291–6. [DOI] [PubMed] [Google Scholar]
- 114.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. doi: 10.1038/nrc2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4: 437–47. doi: 10.1038/nrc1367 [DOI] [PubMed] [Google Scholar]
- 116.McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol) 2007; 19: 427–42. doi: 10.1016/j.clon.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 117.Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer 2011; 11: 504. doi: 10.1186/1471-2407-11-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer Jul 2013. Epub ahead of print. doi: 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williamson SK, Crowley JJ, Lara PN Jr, McCoy J, Lau DH, Tucker RW, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol 2005; 23: 9097–104. doi: 10.1200/JCO.2005.01.3771 [DOI] [PubMed] [Google Scholar]
- 120.Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010; 28: 2989–95. doi: 10.1200/JCO.2009.27.4449 [DOI] [PubMed] [Google Scholar]
- 121.Le QT, Fisher R, Oliner KS, Young RJ, Cao H, Kong C, et al. Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clin Cancer Res 2012; 18: 1798–807. doi: 10.1158/1078-0432.CCR-11-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. doi: 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 123.Hicks KO, Siim BG, Jaiswal JK, Pruijn FB, Fraser AM, Patel R, et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res 2010; 16: 4946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res 2011; 17: 2997–3004. doi: 10.1158/1078-0432.CCR-10-3425 [DOI] [PubMed] [Google Scholar]
- 125.McKeage MJ, Gu Y, Wilson WR, Hill A, Amies K, Melink TJ, et al. A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC Cancer 2011; 11: 432. doi: 10.1186/1471-2407-11-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McKeage MJ, Jameson MB, Ramanathan RK, Rajendran J, Gu Y, Wilson WR, et al. PR-104 a bioreductive pre-prodrug combined with gemcitabine or docetaxel in a phase Ib study of patients with advanced solid tumours. BMC Cancer 2012; 12: 496. doi: 10.1186/1471-2407-12-496 [DOI] [PMC free article] [PubMed] [Google Scholar]