Abstract
Densely ionizing radiation has always been a main topic in radiobiology. In fact, α-particles and neutrons are sources of radiation exposure for the general population and workers in nuclear power plants. More recently, high-energy protons and heavy ions attracted a large interest for two applications: hadrontherapy in oncology and space radiation protection in manned space missions. For many years, studies concentrated on measurements of the relative biological effectiveness (RBE) of the energetic particles for different end points, especially cell killing (for radiotherapy) and carcinogenesis (for late effects). Although more recently, it has been shown that densely ionizing radiation elicits signalling pathways quite distinct from those involved in the cell and tissue response to photons. The response of the microenvironment to charged particles is therefore under scrutiny, and both the damage in the target and non-target tissues are relevant. The role of individual susceptibility in therapy and risk is obviously a major topic in radiation research in general, and for ion radiobiology as well. Particle radiobiology is therefore now entering into a new phase, where beyond RBE, the tissue response is considered. These results may open new applications for both cancer therapy and protection in deep space.
Biological effects of densely ionizing radiation have been studied since the beginning of radiobiology. As a matter of fact, α-particles deliver the main contribution to the background radiation dose on Earth, owing to inhalation of the indoor radon.1 Neutrons have also been extensively studied because of protection of workers in nuclear power plants; use of fast neutrons in radiotherapy; and of the exposure of the survivors of the atomic bomb in Hiroshima and Nagasaki, where a neutron component was added to γ-rays. Bacq and Alexander2 already elegantly described the radiobiology of α-particles and neutrons in their 1955 seminal book.
More recently, radiobiology research has focused on high-energy protons and heavy ions, mostly for two reasons: charged particle therapy (CPT) in oncology and radiation protection in manned space missions. In both cases, charged particles at energies >100 MeV n−1 are involved. The characteristic depth–dose distribution with the sharp Bragg peak at the end of the range can be exploited for killing tumours; on the other hand, densely ionizing radiation can effectively delay tissue morbidity. Notwithstanding the many differences in exposure conditions, CPT and space radiation protection share several research topics, including individual sensitivity, non-targeted effects, late stochastic effects and so forth.3 Research in these fields requires large high-energy accelerators and is often performed by the same research groups with a common interest in particle radiobiology. Research is rapidly moving forward owing to the diffusion of CPT centres in the USA, Europe, and Asia4 and to the growing interest in manned space exploration, now a priority for all space agencies,5 but with cosmic rays acknowledged as a potential showstopper.6 We are also facing a shift in the mainstream research topics, from comparisons of cellular end points to studies of the tissues and of the microenvironment.3,7–10
RELATIVE BIOLOGICAL EFFECTIVENESS
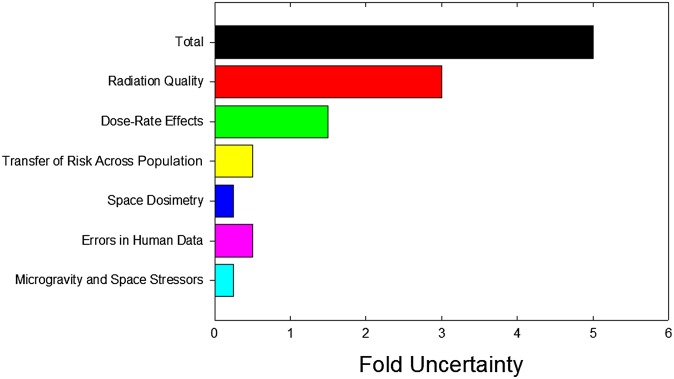
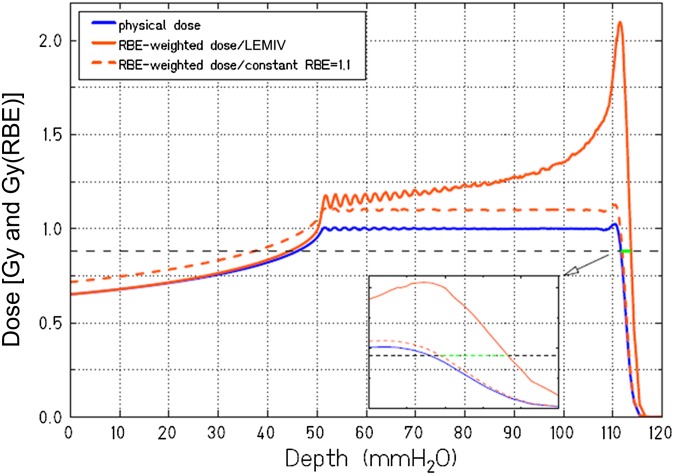
For many years, particle radiobiology was focused on measurements of relative biological effectiveness (RBE). The RBE is used to scale the data from reference radiation (X- or γ-rays) to test radiation (here energetic charged particles). The radiation weighting factors wR are among the few quantities proposed by the International Commission on Radiological Protection11 not derived from epidemiology, but from laboratory studies in radiobiology. In space travel, radiation weighting is instead based on the radiation quality factor Q, which is a continuous function of the radiation linear energy transfer (LET), rather than on radiation quality-specific wR values.12 Quality factors are derived mostly from accelerator-based experiments on relevant end points, such as carcinogenesis in animals, neoplastic transformation in vitro, mutations, chromosomal aberrations and so on. In CPT, the RBE-weighted absorbed dose is expressed in Gy(RBE), which replaces the former grey-equivalent (GyE) or cobalt-grey-equivalent (CGE).13 Despite many years of studies, starting at the Lawrence Berkeley Laboratory (LBL) in the 1970s14 and then at GSI, Darmstadt, Germany; Heavy-Ion Medical Accelerator, Chiba, Japan; Brookhaven National Laboratory, Upton, NY, and many other accelerators, uncertainty about RBE is still very high. RBE is indeed the main source of uncertainty about the estimates of cancer risk for interplanetary missions (Figure 1)15 and in dose estimation in CPT.4 In proton therapy, a constant value of 1.1 is used all along the spread-out-Bragg-peak (SOBP), but it is generally acknowledged that corrections should be used, at least in the distal part of the SOBP, where radiobiology shows that slow protons have an increased effectiveness.16 The increase of RBE with depth causes a shift of the fall-off of the proton beam, i.e. a change of the beam range (biological effective range).17,18 Depending on the SOBP specific characteristics, increases in depth of several millimetres are expected due to the increased RBE in the distal penumbra (Figure 2). The RBE has obviously a much greater impact when heavy ions are used, because in that case, the LET varies sharply along the SOBP. For carbon ions, currently used for therapy in Germany, Italy, Japan and China, the dose in the SOBP is modulated using appropriate models for the RBE.19,20 Different models are used in Europe and Japan, and the calculated doses in Gy(RBE) can differ significantly.21 Uncertainty on the RBE is often quoted as a major hindrance to a widespread use of heavy ions in radiotherapy and is a source of concern for the potential late effects. For instance, C-ions have been used very little in paediatric patients,22 mostly for the concern about high risk of secondary cancers.
Figure 1.
Estimates of uncertainties in projecting cancer risks for interplanetary space missions based on current knowledge on radiation protection on Earth. Several factors such as radiation quality of high-energy ions, space dosimetry and microgravity do not contribute on Earth and lead to large increases in risk projections. The relative biological effectiveness (RBE) is by far the main factor contributing to uncertainty. Predicting risks to individuals is difficult, as there are very few quantitative measures of individual sensitivity. Reproduced from Durante and Cucinotta15 with permission from Nature Publishing Group.
Figure 2.
Biological range extension owing to a variable relative biological effectiveness (RBE) with depth in proton therapy. The orange dashed line indicates the corrected physical spread-out-Bragg-peak using the conventional RBE = 1.1. The orange line represents the correction used with the Local Effect Model version IV (LEMIV) model.20,21 The black dashed line indicates 80% of the prescribed RBE-weighted dose. The inset shows a zoom of the distal penumbra, and the green line shows the increased range predicted by the biological model. Image and calculation courtesy of Rebecca Grün, GSI, Darmstadt, Germany. Modified with permission from the American Association of Physicists in Medicine.18
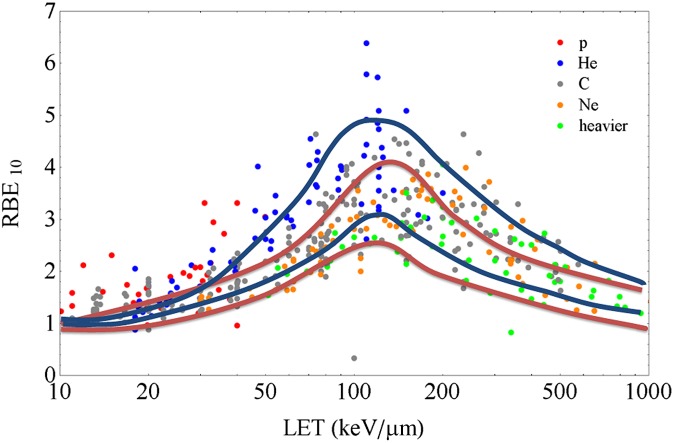
Many years of in vitro studies on the RBE for cell killing consistently show a bell-shaped dependence on LET (Figure 3) and a significant dependence also on the particle charge.23 Recent data mining of survival curves for cell cultures exposed to charged particles24,25 confirms the spread in the RBE-LET relationship originally measured at LBL.14 RBE is in fact a function of several factors, often non-independent: biological end point, tissue type, proliferation status, dose, dose rate, oxygen concentration, culture conditions (in vitro) or metabolism (in vivo) and so forth. Replicating in vitro measurements on different cell lines, as done many times since the Berkeley experiments,14 cannot reduce this intrinsic variability. Modelling will always be necessary for including RBE in the treatment planning, and here, there is room for improvements switching from phenomenological to mechanistic models.26 It is worth noting that although the uncertainty in RBE is certainly a problem in heavy ion therapy, it should not prevent the use of this treatment modality, owing to the lack of significant side effects observed so far in Japan and Europe. Centres like the National Institute for Radiological Sciences (NIRS), Heidelberg Ion Therapy Center (HIT) or Centro Nazionale Adroterapia Oncologica (CNAO), where patients are treated with C-ions, all use Phase I/II dose escalation trials to find optimal protocols.4 At the Institute of Modern Physics, Chinese Academy Sciences, Lanzhou, China, patients are treated with C-ions without any biological modulation, i.e. with an SOBP flat in dose, apparently with no major toxicity.27
Figure 3.
Variability of the relative biological effectiveness (RBE) (calculated at 10% survival level) vs linear energy transfer (LET) (dose-averaged in water, in keV μm−1) in vitro data. Points are extracted from the large Particle Irradiation Data Ensemble (PIDE) database (https://www.gsi.de/bio-pide) developed at GSI, Darmstadt, Germany.24 Different colours correspond to different ions as shown in the legend. The blue curves describe the approximated band of Chinese hamster V79 survival data as collected from Sørensen et al.25 The red curves include the data on different in vitro cell lines collected at Lawrence Berkeley Laboratory.14 DC, dendritic cell.
HYPOFRACTIONATION
Thanks to tremendous improvements in image-guided radiotherapy (IGRT), there is nowadays a tendency to reduce the number of fractions and increase the dose per fraction (hypofractionation).28 The advantages for the patient and for the economy are enormous. X-ray stereotactic body radiation therapy (SBRT) and CPT are both pushing hypofractionation towards the region of 1–3 fractions (oligofractionation) with a very high dose/fraction (up to 25–30 Gy). For non–small cell lung cancer (NSCLC) and oligometastases, SBRT has proven high control rates, durable local control and little normal tissue complications.29 At very high dose, the vascular injury, i.e. damage to the endothelial cells supplying the cancer tissue with oxygen and nutrients, may become a dominant pathway for tumour suppression.30 Damage to the tumour stroma at high doses was originally demonstrated in fibrosarcoma and melanoma grown in genetically modified mice, where vascular endothelial cell apoptosis was shown >10 Gy per fraction.31 The ceramide pathway orchestrated by acid sphingomyelinase is a major pathway for the apoptotic response. In later clinical work involving the use of single fraction high-dose spinal SBRT, investigators from the Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY) recorded pronecrotic response after doses in the range of 18–24 Gy, a radiographical change consistent with a devascularizing effect.32 Although the engagement of the vascular component in radiation response can be crucial at very high doses, it should be underlined that according to the conventional linear–quadratic model used in fractionated radiotherapy, hypofractionation leads to very high biologically effective doses (BED). This was not possible in the past because of the damage to the normal tissue, but it can now be spared, at least for parallel organs, with the modern image-guided techniques. In NSCLC, BED correlates with the tumour control probability (TCP) over a wide range of fractionated conformal radiotherapy and SBRT regimes.33 The question remains therefore open, whether oligofractionation can be only justified by the improved physical dose distribution in IGRT and, consequently, very high BED,34 or it requires a different radiobiological mechanism involving vascular damage and possibly reperfusion.35
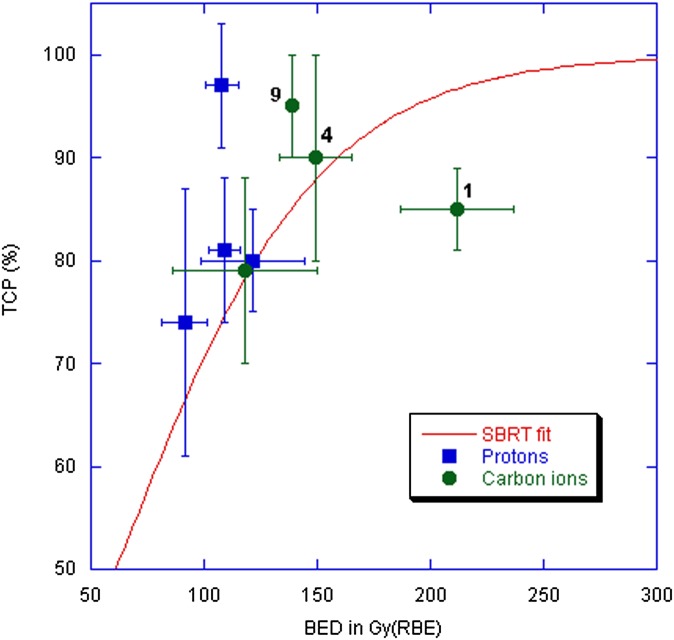
The high conformity granted by the Bragg peak makes CPT ideal for radiosurgery. Particle radiobiology research at high doses is needed to support and guide oligofractionation in hadrontherapy. Experiments in three-dimensional cultures of human endothelial cells36,37 or lung cancer cells injected with basement membrane matrix into nude mice38 showed reduced angiogenesis and vasculogenesis capability after exposure to high-energy charged particles, suggesting that the vascular damage may be particularly effective with protons or heavy ions. Hypofractionation with protons and C-ions is under way for NSCLC in several centres. Published TCP data for CPT in NSCLC39–46 are plotted in Figure 4, along with the curve derived from X-ray SBRT trials.33,34 CPT TCP are generally consistent with the X-ray SBRT TCP. One major deviation is observed in the single-fraction treatment at NIRS in Japan,46 which corresponds to BED > 200 Gy(RBE). RBE in the Japanese treatment plan is assumed to be independent from the dose per fraction.19 This can lead to overestimations at high dose per fraction up to a factor of 2.21 In fact, the single-fraction trial for NSCLC at NIRS46 gives a TCP consistent with a BED around 140 Gy X-rays (Figure 4). Moreover, the hypofractionated C-ion trial45,46 reported no side effects, whereas a small but significant fraction of complications was reported in all other trials. On the other hand, some proton data seem to point to a higher RBE than expected, especially the trial at the University of Tsukuba.42 This would be consistent with an RBE > 1.1 for protons in NSCLC. Slow protons are more effective than X-rays in the inactivation of human tumour cell lines.47 A recent experiment in a human NSCLC cell line reported an RBE = 1.9 for 3.9 MeV protons compared with X-rays.48 The Tsukuba proton beam line used for the NSCLC treatment42 has been used for in vitro experiments in different human cell lines.49 Apoptosis induction was greater than two-fold the level induced by 10 MeV X-ray.49 Other experiments have shown that the signalling cascade following exposure to protons can substantially differ from the damage response to photons.50
Figure 4.
Tumour control probability (TCP) vs biological effective dose (BED) for different stereotactic body radiation therapy (SBRT) and charged particle therapy trials for non–small cell lung cancer (NSCLC). The red curve represents the fit to the X-ray SBRT data as reported in Mehta et al33 using the common linear–quadratic formula 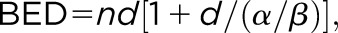 where d is the dose given in every fraction, n the number of fractions, and α/β = 8.6 for NSCLC. Data points are calculated from published trials with protons or C-ions.43–46 The number of fractions is shown next to the high BED C-ions data points. The single-fraction C-ion data point with the highest BED is described in Tsujii and Kamada,46 while the highest TCP has been achieved at Tsukuba University, Ibaraki, Japan, in a trial on 58 (T1/T2, 30/28) patients treated with 66 Gy(RBE)/10 fractions for peripherally located and 72.6 Gy(RBE)/22 fractions for centrally located tumours.42 BED for CPT was calculated using the formula above with d in Gy(RBE) as reported in the publications and the same α/β ratio as for X-rays.
where d is the dose given in every fraction, n the number of fractions, and α/β = 8.6 for NSCLC. Data points are calculated from published trials with protons or C-ions.43–46 The number of fractions is shown next to the high BED C-ions data points. The single-fraction C-ion data point with the highest BED is described in Tsujii and Kamada,46 while the highest TCP has been achieved at Tsukuba University, Ibaraki, Japan, in a trial on 58 (T1/T2, 30/28) patients treated with 66 Gy(RBE)/10 fractions for peripherally located and 72.6 Gy(RBE)/22 fractions for centrally located tumours.42 BED for CPT was calculated using the formula above with d in Gy(RBE) as reported in the publications and the same α/β ratio as for X-rays.
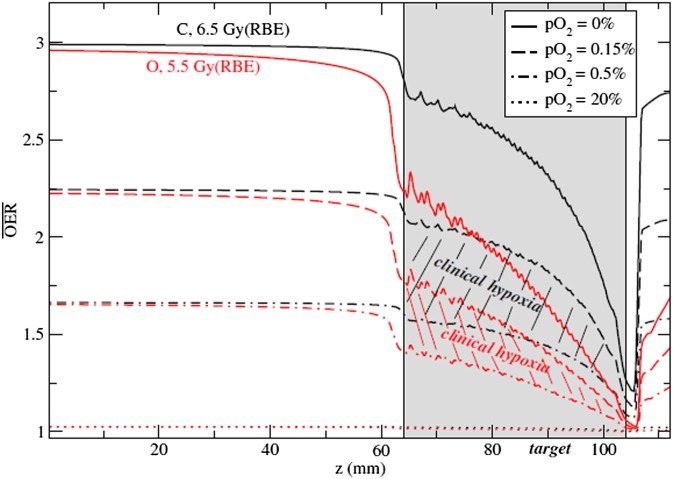
A potential advantage of CPT in hypofractionation is the reduced oxygen enhancement ratio (OER) using high-LET radiation. Hypoxia is one of the main factors reducing local controls in solid tumours,51 and fractionation in radiotherapy has one of the main reasons in the possibility of re-oxygenation of the hypoxic areas. Re-oxygenation will be reduced in hypofractionation and, finally, lost in single-fraction/high-dose radiosurgery. Combinations with hypoxic sensitizers have been proposed for SBRT.52 Owing to the reduction of OER using heavy ions,14,53 CPT may be an alternative. Targeting specifically hypoxic regions can be achieved with strategies of dose-54 or LET-painting.55 For oligofractionation or single fraction/high dose treatments, use of ions heavier than carbon (such as 16O) may be beneficial, because with these ions, the OER can be further reduced in the clinically relevant hypoxia region53 (Figure 5). Interestingly, SBRT commonly uses a non-uniform dose distribution in the tumour to achieve sharp edges on the border of the target volume.56 The excess dose in the central region of the target may indeed be already acting, in current SBRT practice, as a dose-painting for the hypoxic fraction.57
Figure 5.
Comparison of the computed oxygen enhancement ratio (OER) along a spread-out-Bragg-peak for carbon (black curves) and oxygen (red curves) at different pO2 levels. The hatched areas represent the interesting clinical regions for hypoxia (0.15% < pO2 < 0.5%). Doses indicated are prescribed relative biological effectiveness (RBE)-weighted doses in the target to achieve iso-survival. Plans for use of 16O in addition to 12C and 1H in the clinics are currently under way at Heidelberg Ion Therapy Center (Heidelberg, Germany). Reproduced from Scifoni et al53 with permission from IOP Publishing.
Animal experiments comparing SBRT and CPT at high dose per fraction are necessary to clarify this issue and for a full exploitation of CPT in radiosurgery for both malignant and benign diseases. In fact, CPT can be potentially used for several non-cancer diseases58 including atrial fibrillation (Figure 6), a common heart disease now treated by catheter ablation. These applications require extensive research in particle radiobiology for different tissue, such as heart, nerves, blood vessels and so on. Very little data are available for the response of these tissues to particle, whereas clinical results are used to establish X-ray tolerance doses in SBRT.59
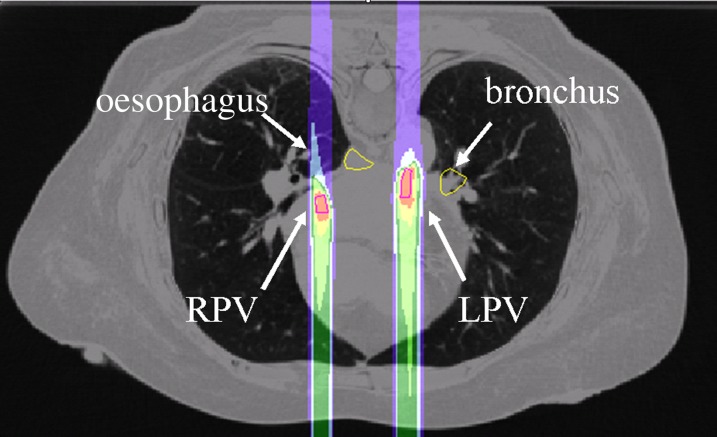
Figure 6.
An in silico study for the application of heavy ions in the treatment of atrial fibrillation based on four-dimensional CT targets, the left pulmonary vein (LPV) and right pulmonary vein (RPV) with high-energy 12C-ions. Atrial fibrillation would require a five-dimensional treatment plan for compensation of both breathing motion and heartbeat.58 The radiobiological response of the vein and cardiac tissue are very little known and, therefore, the choice of the dose level is at present uncertain. Image and calculation courtesy of Anna Constantinescu, GSI, Darmstadt, Germany.
Sensitivity of different tissues to high doses of charged particles is also important for protection of astronauts from intense solar particle events (SPEs), which can deliver high doses, even lethal, to unprotected crews.6 Animal experiments in minipigs60 suggest that the RBE for acute radiation syndrome of protons in simulated SPE is >1. Ferrets exposed to SPE-like protons had increase in fibrin clots, prothrombin time and partial thromboplastin time values post-irradiation,61 suggesting a proton radiation-induced coagulopathy, which represents a risk for SPE in space but may find important applications in CPT.
COMBINED TREATMENTS
Although local control is generally very high with CPT, in most malignancies, radiotherapy must be combined to systemic therapies to control metastasis and increase survival. Combined radio + chemotherapy protocols are already used in many cancers, such as glioblastoma multiforme (GBM) or pancreas cancer. However, very few radiobiology studies specifically address the potential synergistic interaction of the drugs and ion irradiations. In vitro experiments on GBM provided useful indications on the combination of different drugs with C-ions.62
Equally important is the combination of particle therapy with immunotherapy,63 which is now rapidly gaining practice in clinics with great expectations for cancer cure. Abscopal effects, defined as shrinkage of metastatic lesions far from the irradiation field during radiotherapy of a primary malignancy, have been reported for many years,64 but although immune-related effects were assumed to play a role, this was hitherto not immunologically proven until a recent case of a female melanoma patient at MSKCC, receiving immune adjuvant anti-CTLA4 therapy for 1 year, resulting in disease progression, requiring focal irradiation of her spinal metastasis (28.5 Gy and 6 MV X-rays in three fractions). In the CT scan, 4 months after irradiation, vanishing of multiple metastases out of the irradiation field occurred.65 Changes in cellular and molecular parameters indicate a comprehensive immune reaction against the tumour. More cases of abscopal effects with anti-CTLA4 treatment concurrent with radiotherapy were reported later, including one complete response (no cancer visible in PET scan).66 This is clear clinical evidence of immune-mediated abscopal effect, formerly observed in different animal models.67 The mechanism underlying this effect is now fairly well understood.68 Radiation triggers cell death via DNA damage. Ceramide formation and mitochondrial damage, leading to release of mitochondrial cytochrome C and consecutive caspase activation, cause cell apoptosis. In addition to apoptosis, cells can be effectively eliminated following DNA damage by necrosis, mitotic catastrophe, autophagy and premature senescence, and these pathways are prevalent in most solid cancers.69 Radiation damage response leads to the release of damage-associated molecular patterns, which induce dendritic cell (DC) maturation and tumour antigen uptake, resulting in priming and clonal expansion of cytotoxic lymphocytes (CTLs) in the lymph node, which harbour the T-cell receptor matching specifically to the antigen. From the lymph nodes, CTL can travel to the tumour. There, release of granzyme results in caspase activation and mitochondrial membrane permeabilization. At that point, immunity converges with the same cell death pathways as induced directly by irradiation (Figure 7). The immune-mediated cell killing is supported further by radiation through upregulation of HSP70, which aids in transferring granzyme into the cell, an increased antigen presentation via major histocompatibility complex-I and upregulation of NKG2D (natural killer group 2, member D) ligands, facilitating tumour cell killing by natural killer (NK) cells. The induction of NKG2D ligands may be the reason of the clinically65,66 observed sensitization of unresponsive tumours to anti-CTLA4 immunotherapy70 (Figure 8).
Figure 7.
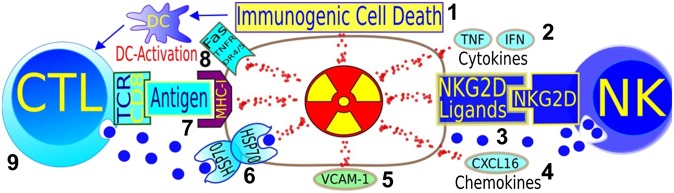
Pathways where radiation can synergize with immune adjuvant therapy for cancer. (1) Immunogenic cell death is promoted by ionizing radiation, through dendritic cell activation and, consequently, T-cell expansion. (2) Cytokines play a role in radiation therapy success. (3) NKG2D-ligands, sensitizing stressed cells to natural killer cells (innate immunity) are upregulated by radiation. (4) Chemokines can be induced by radiation, attracting effector T-cells to the tumour. (5) Radiation-induced interferon-gamma-dependent upregulation of cell adhesion molecule also influences antitumour immunity. (6) Heat shock proteins sensitize to cytotoxic granzymes. (7) Radiation can lead to enhanced expression of major histocompatibility complex (MCH)-I and to de novo expression of neoantigens. (8) Death receptors can be upregulated by irradiation. (9) CD8 T-cells are essential for the success of radiotherapy. Image courtesy of Norman Reppingen, Technical University of Darmstadt, Darmstadt, Germany. CTL, cytotoxic T cell; DC, dendritic cell; IFN, interferon; TCR, T cell receptor; TNF, tumour necrosis factor.
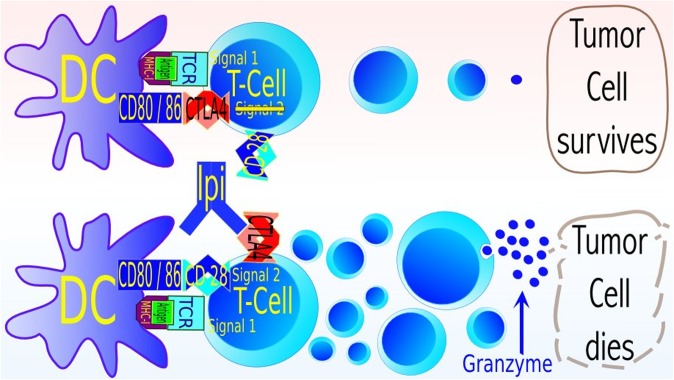
Figure 8.
Simplified model of the mechanism of anti-CTLA4 treatment with ipilimumab. The clonal expansion of tumour-specific T-cells is controlled by “immune checkpoints”. A strong signal via major histocompatibility complex-I leads to a more pronounced expression of the immune inhibitory molecule CTLA4. CTLA4 competes with higher affinity with the surface receptor CD28 for CD80 and CD86, which is a part of Signal 2, and necessary for the successful mounting of an immune response. Binding of CD80 and CD86 to CTLA4 is antagonizing the function of helper T-cells and cytotoxic T-cells and activating immune suppressive regulatory T-cells, conclusively leading to suppression of immune responses (upper row of cells). Therefore, blocking of CTLA4 leads to an increased expression of effector molecules (granzyme, cytokines) and an enhanced expansion of cytotoxic T-cells (lower row), increasing the “amplitude” of an immune response. These cells could be directed against the tumour, improving treatment success, but also against normal cells leading to severe treatment side effects. Radiation could assist to direct the immune processes toward treatment success,70 as evidently seen in some animal models and preliminarily in clinical results.65,66 Image courtesy of Norman Reppingen, Technical University of Darmstadt, Darmstadt, Germany. TCR, T cell receptor.
These studies beg the question of whether abscopal effects and combined immunotherapy and radiotherapy, can be enhanced by charged particles.68 Carbon ions significantly reduce lung metastasis count in LM8 osteosarcoma mouse models and a model of squamous cell carcinoma in immune competent C3H mice.71 A recent study showed that heavy ion irradiation could confer tumour rechallenge resistance, which was dependent on CD8+ T-cells and influenced by NK cells in this system, showing synergy with dendritic cell treatment approaches,72 an encouraging outcome for future protocols combining immunotherapy and CPT.73
RADIOGENOMICS
The ultimate goal of radiogenomics is to develop a genetic risk profile individualization of radiation dose prescriptions to optimize tumour control, while minimizing normal tissue damage.74 Genome-wide association studies have already been successful in finding novel genetic variants with high risk of developing some common diseases, for instance breast cancer.75 Radiogenomics uses a similar approach to predict the sensitivity of the normal76 and cancer77 tissue to radiation.
Normal tissue
Radiogenomics is extremely attractive for the assessment of patients' normal tissue response as long as the genetic component plays a major role in determining radiosensitivity. This is clearly the case for those syndromes, such as ataxia-telangectasia (AT) or Nijmegen breakage syndrome, where germline mutations of DNA repair genes induce extreme radiosensitivity.78 These diseases are, however, rare, confined to a limited number of families, phenotypically obvious and certainly not responsible for the observed variability in radiotherapy response. It is interesting to note that, while AT patients are homozygous for mutation in the ATM protein, a fraction about 3% of the population is heterozygous for ATM79 and a similar fraction is hypersensitive in radiotherapy trials. ATM haploinsufficiency results in increased radiosensitivity in mice,80,81 thus, suggesting that this subgroup may be the one more at risk of side effects in radiotherapy and radiation-induced cancer. ATM is one of the candidate genes to be screened as potential biomarkers of radiation response. Radiogenomics has used the candidate-gene approach to look for variations in many other genes involved in radiation response, e.g. those in the pathways of DNA repair (BRCA1/2), cytokine production (TGFβ), scavenging of free radicals (SOD2) and so forth.82 Genetic variations include single nucleotid polymorphism (SNP),83 copy number variations84 and other epigenetic modifications. Unfortunately, although several studies have initially reported associations between radiation toxicity and SNP, a prospective study aimed to validate failed to detect any of the previously reported associations,85 suggesting that SNP may be irrelevant for individual sensitivity.
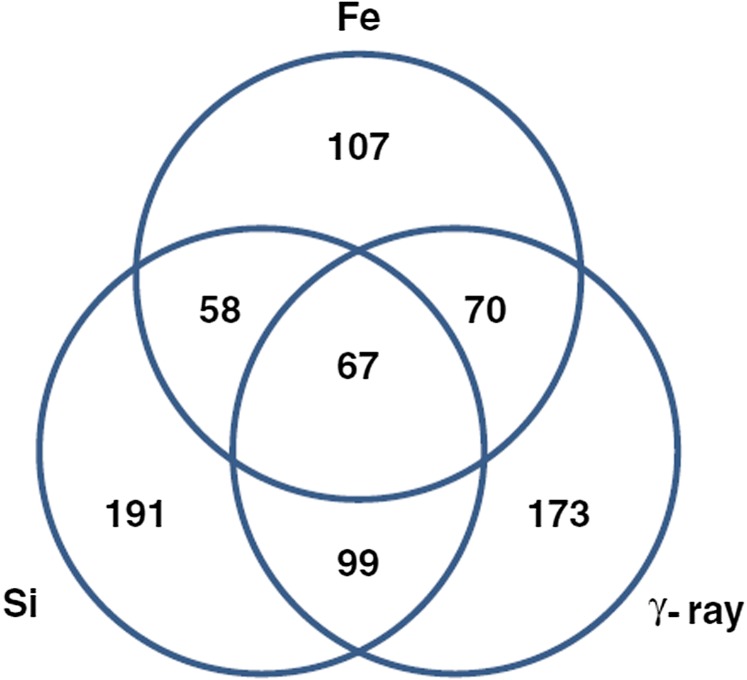
Few studies deal with radiation quality in radiogenomics. Haploinsufficiency for ATM results in accelerated cataractogenesis in mice exposed to both X-rays and heavy ions, and the RBE is higher for heterozygotes compared with wild-type.86 In a recent microarray hybridization analysis of irradiated normal human bronchial epithelial cells,87 unsupervised clustering analysis of gene expression segregated samples according to the radiation quality (Fe-ions, Si-ions or γ-rays) followed by the time after irradiation (0–24 h), whereas dose (0.5–3 Gy) was not a significant parameter for segregation. The great majority of genes with modified expression where uniquely associated to one radiation quality (Figure 9). Even if preliminary, these data suggest that gene profiling may identify pathways uniquely associated to CPT compared with X-ray therapy.
Figure 9.
Differentially expressed genes in human bronchial epithelial cells exposed to γ-rays or heavy ions. Venn diagram shows the numbers of genes, either specific to or overlapping with different radiation types. Courtesy of Michael D. Story (UT Southwestern, Dallas, TX). Reproduced from Ding et al87 with permission from BioMed Central.
Tumour tissue
The integration of molecular data with local control data can allow the generation of biomarker signatures that predict tumour response to therapy.77 The method has been applied to in vitro survival curves of different human tumour cell lines. Microarray profiling identified 22 genes differentially expressed in radiosensitive cancer cells and 18 genes associated with resistant cell lines.88 In a molecular regulatory network model based on mRNA expression profiles, it has been possible to identify 10 hubs of interactions associated to radiosensitivity.89 It is not clear whether these patterns can be used for all cancers, or they are tissue-specific. Individual NSCLC patients' responses to chemotherapy are highly variable,90 and similar spread is measured irradiating NSCLC cell lines.91 Molecular markers, including epidermal growth factor receptor, K-ras, vascular endothelial growth factor, mammalian target of rapamycin and anaplastic lymphoma kinase have been proposed as potential biomarkers of response of NSCLC to ionizing radiation.92 For head-and neck-squamous cell carcinoma, a recent study shows that Ku80 overexpression was an independent predictor for both local recurrence and mortality following radiotherapy.93 The results are easy to interpret, Ku80 being a key molecule for DNA double-strand break repair.94
The variance in radiosensitivity associated to the genetic background is reduced when cells are exposed to densely ionizing radiation.95 The interindividual variability in radiosensitivity can be assessed with organotypic tissue slices from the human tumours.96 A recent study showed interindividual variability for GBM slices treated with temozolomide and a smaller variability after C-ions.97 The RBE decreases for cells with high α/β ratios, i.e. by decreasing intrinsic radioresistance and, eventually, reaches unity for very radiosensitive cell lines.98 For this reason, high-LET heavy ions are preferentially used for radioresistant tumours. This is also a very attractive feature for targeting specific resistant sub-volumes in the tumour, such as the cancer stem cell niche.99 Expression of molecular biomarkers of radioresistance should therefore represent an indication for CPT.
Radiogenomics in tumour tissues can be used for synthetic lethal genetic screen. Two genes are synthetic lethal if mutation of either one is compatible with viability but mutation of both leads to death.100 So, targeting a gene that is synthetic lethal to a cancer-relevant mutation should kill only cancer cells and spare normal cells.101 Synthetic lethality, therefore, provides a conceptual framework for the development of cancer-specific cytotoxic agents102 and can be extended in combined therapies, targeting combination of genes conferring radioresistance to cancers.103
Carcinogenesis
If gene expression has a key role in determining sensitivity of the normal and tumour tissue, it can also be argued that it affects the risk of radiation-induced cancer. The question is whether radiation carcinogenesis is a purely stochastic process or if there exists a sub-population of sensitive individuals.104 Should this be the case, radioprotection would be useless for many normal individuals and insufficient for the (few) sensitive subjects. In the analysis of cancer risk in children from 160 families irradiated in Israel after World War II for an epidemic of tinea capitis, it was found that the increased meningioma incidence in irradiated children was determined by a subgroup of only 17 families (11%), where 4 of 5 children developed meningioma.105 In addition, the large European cohort study GENE-RAD-RISK106 has recently concluded that in carriers of BRCA1/2 mutations, any exposure to diagnostic radiation before the age of 30 years is associated with an increased risk of breast cancer. The relative risk in the mutant carrier was 1.9 and increased with dose.106
Genetic predisposition may also drive the risk from exposure to charged particles. This is a critical issue for paediatric patients, a group that is often referred to proton therapy because much normal tissue can be spared compared with X-rays and, therefore, toxicity is reduced.107 For these patients, the risk of second cancer is, however, substantial and the genetic predisposition to cancer very likely because the tumour was expressed at a young age.108 This problem will be discussed in the next section.
LATE EFFECTS
Risk of late morbidity is a major issue for charged particle radiobiology. For space travel, cancer and non-cancer late effects represent the major showstopper for long-term interplanetary missions beyond low-Earth-orbit (LEO).15 In CPT, as noted above, the problem is mostly related to second cancers in paediatric patients.108
Carcinogenesis
Epidemiology in subjects exposed to energetic charged particles is very limited. No increased cancer incidence is observed in the astronauts' cohort, consistent with the low-dose exposure in LEO experienced so far by crews on the International Space Station and other space missions.15 For hadrontherapy, comparison of chromosomal aberrations in peripheral blood lymphocytes of patients treated either with C-ions or X-rays for similar tumours generally shows a higher damage after conventional radiotherapy compared with heavy ion treatment, both for passive modulation109 and active raster scanning.110 These results, apparently in contradiction with the increased effectiveness of heavy ions in the induction of chromosomal aberrations,111 are caused by the different treatment planning. With heavy ions, the integral dose is reduced because, thanks to the Bragg peak, only a few fields (1–3) at different angles are used compared with conformal and intensity-modulated therapy with X-rays (up to 9–10 fields). Therefore, the volume of normal tissue exposed is strongly reduced, and therefore less chromosomal aberrations observed, and a reduced late morbidity expected. Preliminary results from a cohort study of 558 patients treated with proton radiation from 1973 to 2001 at the Harvard Cyclotron Laboratory, Cambridge, MA, showed a trend towards a reduced risk of second malignancies, consistent with the reduced integral dose to the normal tissue.112 Voxel-phantom models able to predict the second cancer risk in different organs (Figure 10) with different treatment modalities are now available113,114 and could guide the choice of the oncologists in defining the best treatment options for paediatric patients. The epidemiological data used in these models are, however, insufficient and the uncertainty is consequently very high.108 In vitro and in vivo experiments remain mandatory for understanding the mechanisms and reducing uncertainties.
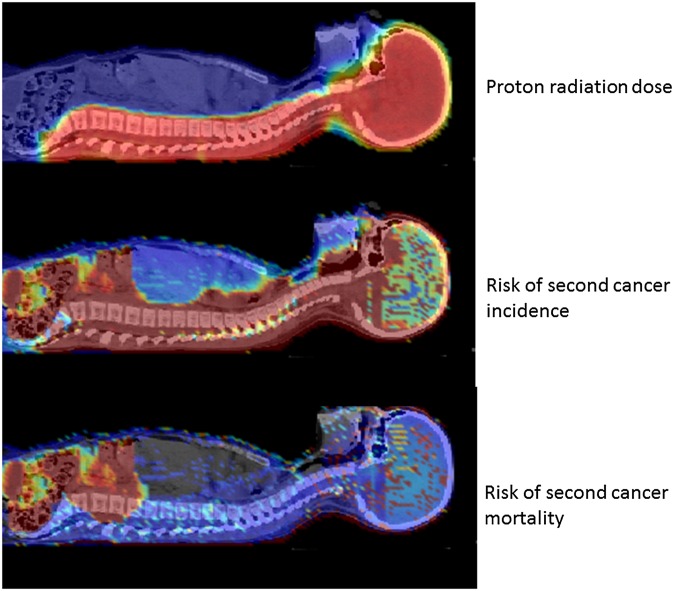
Figure 10.
Dose and risk distribution for second cancer. Images of a 9-year old girl who received craniospinal irradiation for medulloblastoma using passively scattered proton beams at MD Anderson Cancer Center. The colour scale illustrates the difference for absorbed dose, incidence and mortality cancer risk in different organs. Radiation absorbed dose depends strongly on patient anatomy and treatment factors. Risk of second cancer incidence and mortality varies strongly with radiation dose, but, importantly, it also varies strongly between organs, with the patient's age at exposure and attained age, sex, genetic profile, and other factors. Reproduced from Newhauser and Durante108 with permission from Nature Publishing Group.
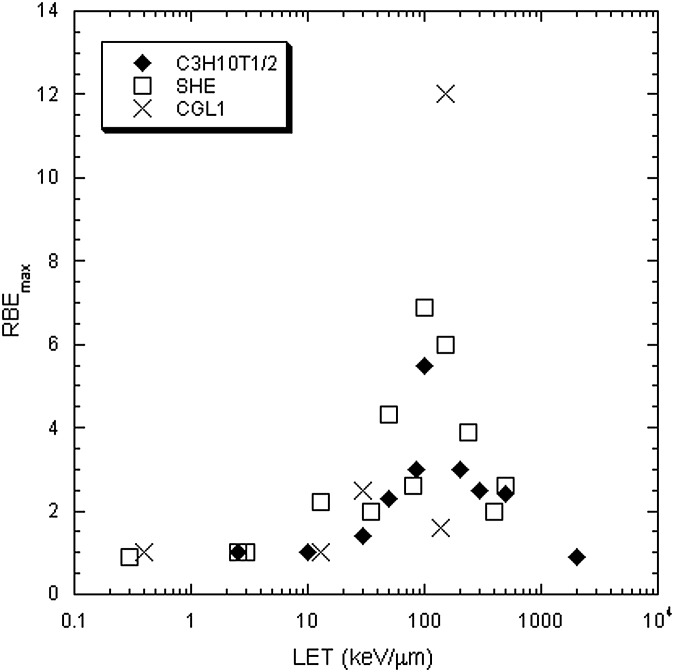
Neoplastic transformation in vitro by heavy ions has been studied in immortalized mouse C3H 10T1/2 fibroblasts,115 primary Syrian hamster embryo fibroblasts,116 and in the HeLa X human skin fibroblast hybrid cell line CGL1.117 All these systems are very artificial and great caution should be exercised in extrapolating data to the in vivo situation, especially since each cancer may display a different behaviour in vivo. Nevertheless, the general trend confirms the RBE-LET relationship observed for cell killing, mutations and chromosomal aberrations, i.e. a bell-shaped curve with a peak around 100–200 keV μm−1 (Figure 11).
Figure 11.
Relative biological effectiveness (RBE) for in vitro neoplastic transformation plotted vs the linear energy transfer (LET) of different charged particles. Data on C3H 10T1/2 mouse fibroblasts are from Yang et al;115 Syrian hamster embryo (SHE) fibroblasts were used in Han et al;116 and HeLaxskin fibroblasts hybrid cells (CGL1) were studied in Elmore et al.117
Animal studies report the induction of different tumours in rodents and have been recently reviewed.118 In many cases, the trend for solid tumour induction is not in contradiction with the in vitro data in Figure 11, although generally RBE values are lower than those observed with fission neutrons. However, in vivo data point to quantitative and qualitative differences in the RBE for different types of cancers. For instance, recent work at the Brookhaven National Laboratory accelerator has shown that the RBE of 1 GeV n−1 Fe-ions for cancer induction in mice is about 1 for leukaemia and about 20 for hepatocellular carcinoma.119 How can the RBE be so different? The reason is most likely because of the different nature of liquid and solid cancers. Although leukaemia is strongly related to specific chromosomal aberrations (in mouse, deletion of PU.1 gene in chromosome 2), solid cancers are associated to genomic instability, i.e. to late tissue effects. Radiation can act both as an initiator of the carcinogenic process (e.g. by inducing mutations or chromosome aberrations) or in promotion (related to tissue inflammation). For liquid cancers, radiation could act as an initiator, whereas for solid cancer, as a promoter. Although heavy ions are more effective than X-rays in the induction of chromosomal rearrangements, most of the aberrations are lethal, and the RBE drops to about one in the surviving population.120 On the other hand, heavy ions are very effective in the induction of inflammation,121 and this process is a driver for promotion of carcinogenesis.122,123
Non-cancer effects
Recent epidemiological studies suggest that non-cancer late effects, particularly, cardiovascular diseases, may contribute to health risk after moderate to low doses of ionizing radiation.124 The radiobiology of charged particles for these end points and tissues is scarcely known, as already noted for potential applications of CPT in radiosurgery. The major degenerative late effects that could result from exposure to high-energy charged particles are:
– acute and late damage to the central nervous system (CNS)
– cataract formation
– cardiovascular diseases
– other diseases related to accelerated senescence, including digestive and respiratory diseases, endocrine and immune system dysfunction.
CNS complications, including necrosis and leukoencephalotopy, have been reported in patients treated for brain tumours both by chemotherapy, X-rays125 or protons.126 However, exposure to heavy ions seems to produce a distinct damage in the CNS also at low doses, perhaps by single particle traversals. First evidence of these effects came from reports of visual phosphenes, described as sudden “light flashes”, first reported by Buzz Aldrin after the first Moon landing and then by almost all the astronauts in the Apollo programme.127 Light flashes are believed to be produced by a direct interaction of an energetic charged particle with the retina or the eye, as confirmed by studies in space128 and in dedicated experiments in cancer patients treated with C-ions using spot-scanning.129 The observation of light flashes brought attention to the possible effects of charged particles on the brain function. Calculations suggest that for a 3-year mission to Mars at solar minimum, 2–13% of the “critical sites” of cells in the CNS would be directly hit at least once by iron ions, and roughly 20 million of 43 million hippocampal cells and 230 000 of 1.8 million thalamus cell nuclei would be directly hit by one or more particles with Z > 15 on such a mission,130 without considering the additional contribution caused by δ-rays. Evidence of behavioural effects induced by doses as low as 0.2 Gy of Fe-ions was produced in a series of experiments in Sprague-Dawley rats.131 A review of behavioural end points in rats exposed to different ions conclude that low doses of heavy ions can indeed produce behavioural alterations in rats, including disruption in motor behaviour, taste aversion learning, spatial learning and conditioned place preference.132 The large uncertainty on these behavioural end points and the interindividual variability in the animal experiments make, however, very difficult any reliable risk estimates. There could be a dose threshold, and it seems to be charge- and energy-dependent, and defining an RBE is even more difficult, considering that most of these end points are not observed at all with X-rays.131 These observations have therefore triggered a number of cellular and molecular studies on neural cells and tissue, to gain understanding of the mechanisms of heavy ion-induced damage in the brain. These studies are ongoing, and despite some evidence of the involvement of reactive oxygen species (ROS) and neuroinflammation,133 they are still preliminary and do not presently allow drawing a firm conclusion.
On the contrary, we have now clear evidence that space radiation induces ocular cataract in astronauts,134 and indeed lens opacification is the only proven space-radiation effects actually observed in crews of space missions. Recent epidemiological evidence does not support the existence of dose threshold for radiation-induced lens opacification, although the shape of the dose–response curve is still unclear.135 However, animal studies show that the RBE of heavy ions for cataract induction is as high as 50 at doses <100 mGy,136 thus explaining the high effectiveness for accelerated caratactogenesis in space.
Apart from cataractogenesis, the uncertainty on other, more harmful, non-cancer late effects, especially cardiovascular diseases, is much higher. There is now clear epidemiological evidence that high radiation doses induce late cardiovascular diseases. A clear correlation between radiation dose to the heart in breast cancer patients and late ischaemic heart diseases has been recently shown in a cohort of Swedish and Danish females treated by radiotherapy for breast cancer.137 A-bomb survivors' data also support these results.138 It is not clear, however, whether this cardiovascular risk has a threshold at low doses. This is very important for both radiotherapy and space exploration. Proton therapy can efficiently spare the heart during the treatment of left-side breast cancer,139 whereas with X-rays, a large fraction of the heart is generally exposed. Breast cancer is therefore becoming a major application of protontherapy.140–142 Breast cancer is indeed the most frequent cancer in females, and if proven, superior CPT could have a much larger use than in the past. The advantage here is not in the TCP, but in the reduced normal tissue toxicity, and this advantage, already considered the main justification for paediatric use of protons,107 is likely to be decisive in the future of CPT. In space, a mission to Mars would bring the total dose around 1 Sv,143 a region where epidemiological data suggest an increase of cardiovascular diseases.138 An examination of possible biological mechanisms indicates that the most likely target of radiation-induced cardiovascular damages are endothelial cells and subsequent induction of an inflammatory response.144 Again, inflammation and, in general, non-targeted effects mediated by the microenvironment are the major pathways in radiation response of the organisms,122 driving both cancer and non-cancer late effects, as well as for radiation therapy.145
Generally speaking, the epidemiological evidence of non-cancer late effects in exposed individuals is consistent with radiation-induced acceleration of the ageing process. As a matter of fact, it has been shown that radiation accelerates age-related diseases such as endocrine dysfunctions,146 digestive and respiratory diseases,138 immune system function impairment.147 What can be the mechanism relating radiation exposure to ageing? Premature cellular senescence can be induced by radiation and other genotoxic agents, and this process is driven by telomere shortening.148 Even single nuclear traversals of energetic charged particles can induce accelerated senescence of cultured human fibroblasts.149 The link between radiation exposure and accelerated ageing may be mediated by a persistent oxidative stress.150 Indirect evidence of ROS involvement is provided by experiments on protection from late non-cancer effects using antioxidants.151 For example, accelerated radiation-induced formation of aortic lesions occurred in mice that were on a high-fat diet, whereas smaller lesions were observed in their irradiated transgenic littermates that over-expressed CuZn-superoxide dismutase, which is expected to decrease chronic oxidative stress.152
CONCLUSIONS
Radiobiology research, in general, is underfunded both in the USA and Europe. However, it is clear that charged-particle radiobiology studies are needed both for cancer therapy and radiation protection in space. In CPT, medical physics can improve the precision and quality of the treatments, but IGRT is rapidly progressing in X-ray therapy as well. Only radiobiology can identify areas of potential breakthrough in radiotherapy, i.e. specific tumours, fractionation schedules and combined treatments where the benefit of CPT will be decisive for the patient. In space travel, cosmic radiation is recognized as a potential showstopper. Passive shielding is not a solution to the problem, and active (magnetic) shielding is still not technologically feasible. Again, radiobiology studies are mandatory for reducing uncertainties in late radiogenic risk and developing biomedical countermeasures. These ambitious goals should be pursued with innovative research going beyond the traditional RBE measurements. In this review, we have identified several new key topics in particle radiobiology, such as effects of high doses (hypofractionation), radiogenomics, combined treatments (with chemo- and immunotherapies), and studies on late effects focused on the response of the microenvironment. This research needs funding and accelerator facilities willing to invest in research. There are already several accelerator facilities involved in biomedical research for clinical153 and space154 radiobiology, and others have been proposed.155 The exploitation of these facilities is, however, dependent on the funding available for users interested in high-energy particle radiobiology. In the USA, both the US National Cancer Institute (NCI) and the US Department of Energy (DOE) are considering further investments in CPT, including radiobiology.156 In Europe, Horizon 2020 should be the platform to continue the funding gathered in past years through the European Network for LIGht ion Hadron Therapy (ENLIGHT) platform157 and related European Union-funded projects.158 The potential gain of this research is likely to be very high.
ACKNOWLEDGMENTS
The manuscript reports the Bacq and Alexander lecture given by the author during the 40th Annual Meeting of the European Radiation Research Society (ERRS) held in Dublin on 1–5 September 2013. The author is grateful to the ERRS for this prestigious award, which is a great honour and a sign of the relevance of charged particles in modern radiation research. I am also grateful to many authors for their contribution to several figures: Frank Cucinotta (University of Las Vegas, Las Vegas, NV) for Figure 1; Rebecca Grün (GSI, Darmstadt, Germany) for Figure 2; Thomas Friedrich (GSI) for Figure 3; Emanuele Scifoni (GSI) for Figure 5; Anna Constantinescu (GSI) for Figure 6; Norman Reppingen (TU Darmstadt, Darmstadt, Germany) for Figures 7 and 8; Michael D. Story (UT Southwestern, Dallas, TX) for Figure 9; and Wayne Newhauser (Louisiana State University, Baton Rouge, LA) for Figure 10. Research in radiobiology at GSI is supported by the Helmholtz Association (POFIII and Portfolio Technologie und Medizin) and European Space Agency (IBER contract).
REFERENCES
- 1.National Research Council. Health effects of exposure to radon: BEIR VI. Washington, DC: The National Academies Press; 1999. [PubMed] [Google Scholar]
- 2.Bacq ZM, Alexander P. Fundamentals of radiobiology. London, UK: Butterworths Scientific Publications; 1955. [Google Scholar]
- 3.Durante M. Eighth Warren K. Sinclair keynote address: heavy ions in therapy and space: benefits and risks. Health Phys 2012; 103: 532–9. doi: 10.1097/HP.0b013e318264b4b6 [DOI] [PubMed] [Google Scholar]
- 4.Loeffler JS, Durante M. Charged particle therapyoptimization, challenges and future directions. Nat Rev Clin Oncol 2013; 10: 411–24. doi: 10.1038/nrclinonc.2013.79 [DOI] [PubMed] [Google Scholar]
- 5.International Space Exploration Coordination Group (ISECG). The Global Exploration Roadmap. NASA NP-2013-06-945-HQ, 2013. Available from: http://www.globalspaceexploration.org/
- 6.Durante M, Cucinotta FA. Physical basis of radiation protection in space travel. Rev Mod Phys 2011; 83: 1245–81. [Google Scholar]
- 7.Barcellos-Hoff MH. What is the use of systems biology approaches in radiation biology? Health Phys 2011; 100: 272–3. [DOI] [PubMed] [Google Scholar]
- 8.Mothersill C, Seymour C. Changing paradigms in radiobiology. Mutat Res 2012; 750: 85–95. doi: 10.1016/j.mrrev.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Rodemann HP, Wouters BG. Molecular and translational radiation biology/oncology: what's up? Radiother Oncol 2011; 99: 257–61. doi: 10.1016/j.radonc.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Cordes N. Radiation therapy and the microenvironment. Int J Radiat Biol 2007; 83: 723–5. doi: 10.1080/09553000701799928 [DOI] [PubMed] [Google Scholar]
- 11.ICRP. The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 2007; 37: 1–332. [DOI] [PubMed] [Google Scholar]
- 12.ICRP. Assessment of radiation exposure of astronauts in space. ICRP publication 123. Ann ICRP 2013; 42: 1–339. doi: 10.1016/j.icrp.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 13.ICRU. Prescribing, recording, and reporting proton-beam therapy. ICRU report 78. J ICRU 2007; 7. [Google Scholar]
- 14.Blakely EA, Ngo FQH, Curtis SB, Tobias CA. Heavy-ion radiobiology: cellular studies. Adv Radiat Biol 1984; 11: 295–389. [Google Scholar]
- 15.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer 2008; 8: 465–72. doi: 10.1038/nrc2391 [DOI] [PubMed] [Google Scholar]
- 16.Carabe A, España S, Grassberger C, Paganetti H. Clinical consequences of relative biological effectiveness variations in proton radiotherapy of the prostate, brain and liver. Phys Med Biol 2013; 58: 2103–17. doi: 10.1088/0031-9155/58/7/2103 [DOI] [PubMed] [Google Scholar]
- 17.Carabe A, Moteabbed M, Depauw N, Schuemann J, Paganetti H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol 2012; 57: 1159–72. doi: 10.1088/0031-9155/57/5/1159 [DOI] [PubMed] [Google Scholar]
- 18.Grün R, Friedrich T, Krämer M, Zink K, Durante M, Engenhart-Cabillic R, et al. Physical and biological factors determining the effective proton range. Med Phys 2013; 40 (11): 111716. doi: 10.1118/1.4824321. [DOI] [PubMed] [Google Scholar]
- 19.Kanai T, Matsufuji N, Miyamoto T, Mizoe J, Kamada T, Tsuji H, et al. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys 2006; 64: 650–6. doi: 10.1016/j.ijrobp.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 20.Friedrich T, Grün R, Scholz U, Elsässer T, Durante M, Scholz M. Sensitivity analysis of the relative biological effectiveness predicted by the local effect model. Phys Med Biol 2013; 58: 6827–49. doi: 10.1088/0031-9155/58/19/6827 [DOI] [PubMed] [Google Scholar]
- 21.Steinsträter O, Grün R, Scholz U, Friedrich T, Durante M, Scholz M. Mapping of RBE-weighted doses between HIMAC- and LEM-based treatment planning systems for carbon ion therapy. Int J Radiat Oncol Biol Phys 2012; 84: 854–60. doi: 10.1016/j.ijrobp.2012.01.038 [DOI] [PubMed] [Google Scholar]
- 22.Combs SE, Kessel KA, Herfarth K, Jensen A, Oertel S, Blattmann C, et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol 2012; 7: 170. doi: 10.1186/1748-717X-7-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedrich T, Durante M, Scholz M. Particle species dependence of cell survival RBE: evident and not negligible. Acta Oncol 2013; 52: 589–603. doi: 10.3109/0284186X.2013.767984 [DOI] [PubMed] [Google Scholar]
- 24.Friedrich T, Scholz U, Elsässer T, Durante M, Scholz M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J Radiat Res 2013; 54: 494–514. doi: 10.1093/jrr/rrs114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørensen BS, Overgaard J, Bassler N. In vitro RBE-LET dependence for multiple particle types. Acta Oncol 2011; 50: 757–62. doi: 10.3109/0284186X.2011.582518 [DOI] [PubMed] [Google Scholar]
- 26.Friedrich T, Durante M, Scholz M. Modeling cell survival after photon irradiation based on double-strand break clustering in megabase pair chromatin loops. Radiat Res 2012; 178: 385–94. doi: 10.1667/RR2964.1 [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Sihver L. Therapeutic techniques applied in the heavy-ion therapy at IMP. Nucl Instrum Methods Phys Res B 2011; 269: 664–70. [Google Scholar]
- 28.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010; 7: 44–54. doi: 10.1038/nrclinonc.2009.188 [DOI] [PubMed] [Google Scholar]
- 29.Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013; 14: e28–37. doi: 10.1016/S1470-2045(12)70510-7 [DOI] [PubMed] [Google Scholar]
- 30.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 2005; 8: 89–91. doi: 10.1016/j.ccr.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. doi: 10.1126/science.1082504 [DOI] [PubMed] [Google Scholar]
- 32.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008; 71: 484–90. doi: 10.1016/j.ijrobp.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 33.Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: a pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol 2012; 2: 288–95. [DOI] [PubMed] [Google Scholar]
- 34.Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 85: 1159–60. doi: 10.1016/j.ijrobp.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012; 177: 311–27. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi Y, Teshima T, Kawaguchi N, Hamada Y, Mori S, Madachi A, et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res 2003; 63: 4253–7. [PubMed] [Google Scholar]
- 37.Kamlah F, Hänze J, Arenz A, Seay U, Hasan D, Juricko J, et al. Comparison of the effects of carbon ion and photon irradiation on the angiogenic response in human lung adenocarcinoma cells. Int J Radiat Oncol Biol Phys 2011; 80: 1541–9. doi: 10.1016/j.ijrobp.2011.03.033 [DOI] [PubMed] [Google Scholar]
- 38.Grabham P, Sharma P, Bigelow A, Geard C. Two distinct types of the inhibition of vasculogenesis by different species of charged particles. Vasc Cell 2013; 5: 16. doi: 10.1186/2045-824X-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush DA, Cheek G, Zaheer S, Wallen J, Mirshahidi H, Katerelos A, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013; 86: 964–8. doi: 10.1016/j.ijrobp.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Iwata H, Murakami M, Demizu Y, Miyawaki D, Terashima K, Niwa Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010; 116: 2476–85. doi: 10.1002/cncr.24998 [DOI] [PubMed] [Google Scholar]
- 41.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006; 65: 107–11. doi: 10.1016/j.ijrobp.2005.10.031 [DOI] [PubMed] [Google Scholar]
- 42.Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys 2010; 78: 467–71. doi: 10.1016/j.ijrobp.2009.07.1707 [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto T, Yamamoto N, Nishimura H, Koto M, Tsujii H, Mizoe JE, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2003; 66: 127–40. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys 2007; 67: 750–8. doi: 10.1016/j.ijrobp.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol 2007; 2: 916–26. doi: 10.1097/JTO.0b013e3181560a68 [DOI] [PubMed] [Google Scholar]
- 46.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol 2012; 42: 670–85. doi: 10.1093/jjco/hys104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belli M, Bettega D, Calzolari P, Cera F, Cherubini R, Dalla Vecchia M, et al. Inactivation of human normal and tumour cells irradiated with low energy protons. Int J Radiat Biol 2000; 76: 831–9. [DOI] [PubMed] [Google Scholar]
- 48.Wéra AC, Heuskin AC, Riquier H, Michiels C, Lucas S. Low-LET proton irradiation of A549 non-small cell lung adenocarcinoma cells: dose response and RBE determination. Radiat Res 2013; 179: 273–81. doi: 10.1667/RR3008.1 [DOI] [PubMed] [Google Scholar]
- 49.Gerelchuluun A, Hong Z, Sun L, Suzuki K, Terunuma T, Yasuoka K, et al. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV X-rays in human tumour cell lines. Int J Radiat Biol 2011; 87: 57–70. doi: 10.3109/09553002.2010.518201 [DOI] [PubMed] [Google Scholar]
- 50.Girdhani S, Sachs R, Hlatky L. Biological effects of proton radiation: what we know and don't know. Radiat Res 2013; 179: 257–72. doi: 10.1667/RR2839.1 [DOI] [PubMed] [Google Scholar]
- 51.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. doi: 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 52.Brown JM, Diehn M, Loo BWJr. Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys 2010; 78: 323–7. doi: 10.1016/j.ijrobp.2010.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scifoni E, Tinganelli W, Weyrather WK, Durante M, Maier A, Krämer M. Including oxygen enhancement ratio in ion beam treatment planning: model implementation and experimental verification. Phys Med Biol 2013; 58: 3871–95. doi: 10.1088/0031-9155/58/11/3871 [DOI] [PubMed] [Google Scholar]
- 54.Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol 2005; 6: 112–7. doi: 10.1016/S1470-2045(05)01737-7 [DOI] [PubMed] [Google Scholar]
- 55.Bassler N, Toftegaard J, Lühr A, Sørensen BS, Scifoni E, Krämer M, et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol Sep 2013. Epub ahead of print. doi: 10.3109/0284186X.2013.832835 [DOI] [PubMed] [Google Scholar]
- 56.Salama JK, Kirkpatrick JP, Yin FF. Stereotactic body radiotherapy treatment of extracranial metastases. Nat Rev Clin Oncol 2012; 9: 654–65. doi: 10.1038/nrclinonc.2012.166 [DOI] [PubMed] [Google Scholar]
- 57.Ruggieri R, Naccarato S, Nahum AE. Severe hypofractionation: non-homogeneous tumour dose delivery can counteract tumour hypoxia. Acta Oncol 2010; 49: 1304–14. doi: 10.3109/0284186X.2010.486796 [DOI] [PubMed] [Google Scholar]
- 58.Bert C, Engenhart-Cabillic R, Durante M. Particle therapy for noncancer diseases. Med Phys 2012; 39: 1716–27. doi: 10.1118/1.3691903 [DOI] [PubMed] [Google Scholar]
- 59.Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 2011; 12: 3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanzari JK, Wan SX, Diffenderfer ES, Cengel KA, Kennedy AR. Relative biological effectiveness of simulated solar particle event proton radiation to induce acute hematological change in the porcine model. J Radiat Res 2013; in press. Epub ahead of print Sep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krigsfeld GS, Savage AR, Sanzari JK, Wroe AJ, Gridley DS, Kennedy AR. Mechanism of hypocoagulability in proton-irradiated ferrets. Int J Radiat Biol 2013; 89: 823–31. doi: 10.3109/09553002.2013.802394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrabi S, Combs SE, Brons S, Haberer T, Debus J, Weber KJ. Temozolomide in combination with carbon ion or photon irradiation in glioblastoma multiforme cell lines—does scheduling matter? Int J Radiat Biol 2013; 89: 692–7. doi: 10.3109/09553002.2013.791406 [DOI] [PubMed] [Google Scholar]
- 63.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105: 256–65. doi: 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol 1953; 26: 234–41. [DOI] [PubMed] [Google Scholar]
- 65.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–31. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 2035. doi: 10.1056/NEJMc1203984#SA1 [DOI] [PubMed] [Google Scholar]
- 67.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10: 718–26. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med 2013; 19: 565–82. doi: 10.1016/j.molmed.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 69.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 2005; 5: 231–7. doi: 10.1038/nrc1560 [DOI] [PubMed] [Google Scholar]
- 70.Demaria S, Pilones KA, Formenti SC, Dustin ML. Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Oncoimmunology 2013; 2: e23127. doi: 10.4161/onci.23127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogata T, Teshima T, Kagawa K, Hishikawa Y, Takahashi Y, Kawaguchi A, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005; 65: 113–20. [PubMed] [Google Scholar]
- 72.Matsunaga A, Ueda Y, Yamada S, Harada Y, Shimada H, Hasegawa M, et al. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer 2010; 116: 3740–8. doi: 10.1002/cncr.25134 [DOI] [PubMed] [Google Scholar]
- 73.Lumniczky K, Sáfrány G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett Aug 2013. Epub ahead of print. doi: 10.1016/j.canlet.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 74.Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009; 9: 134–42. doi: 10.1038/nrc2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet 2013; 45: 392–8, 398e1–2. doi: 10.1038/ng.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006; 6: 702–13. doi: 10.1038/nrc1950 [DOI] [PubMed] [Google Scholar]
- 77.Das AK, Bell MH, Nirodi CS, Story MD, Minna JD. Radiogenomics predicting tumor responses to radiotherapy in lung cancer. Semin Radiat Oncol 2010; 20: 149–55. doi: 10.1016/j.semradonc.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys 2009; 74: 1323–31. doi: 10.1016/j.ijrobp.2009.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med 1991; 325: 1831–6. doi: 10.1056/NEJM199112263252602 [DOI] [PubMed] [Google Scholar]
- 80.Barlow C, Eckhaus MA, Schäffer AA, Wynshaw-Boris A. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat Genet 1999; 21: 359–60. doi: 10.1038/7684 [DOI] [PubMed] [Google Scholar]
- 81.Worgul BV, Smilenov L, Brenner DJ, Junk A, Zhou W, Hall EJ. Atm heterozygous mice are more sensitive to radiation-induced cataracts than are their wild-type counterparts. Proc Natl Acad Sci U S A 2002; 99: 9836–9. doi: 10.1073/pnas.162349699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin Radiat Oncol 2008; 18: 126–35. doi: 10.1016/j.semradonc.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 83.Katsanis SH, Katsanis N. Molecular genetic testing and the future of clinical genomics. Nat Rev Genet 2013; 14: 415–26. doi: 10.1038/nrg3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wellcome Trust Case Control Consortium. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature 2010; 464: 713–20. doi: 10.1038/nature08979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnett GC, Coles CE, Elliott RM, Baynes C, Luccarini C, Conroy D, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol 2012; 13: 65–77. doi: 10.1016/S1470-2045(11)70302-3 [DOI] [PubMed] [Google Scholar]
- 86.Hall EJ, Worgul BV, Smilenov L, Elliston CD, Brenner DJ. The relative biological effectiveness of densely ionizing heavy-ion radiation for inducing ocular cataracts in wild type versus mice heterozygous for the ATM gene. Radiat Environ Biophys 2006; 45: 99–104. doi: 10.1007/s00411-006-0052-5 [DOI] [PubMed] [Google Scholar]
- 87.Ding LH, Park S, Peyton M, Girard L, Xie Y, Minna JD, et al. Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to γ-rays and different elemental particles of high Z and energy. BMC Genomics 2013; 14: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amundson SA, Do KT, Vinikoor LC, Lee RA, Koch-Paiz CA, Ahn J, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res 2008; 68: 415–24. doi: 10.1158/0008-5472.CAN-07-2120 [DOI] [PubMed] [Google Scholar]
- 89.Eschrich S, Zhang H, Zhao H. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys 2009; 75: 497–505. doi: 10.1016/j.ijrobp.2009.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92–8. [DOI] [PubMed] [Google Scholar]
- 91.Carmichael J, Degraff WG, Gamson J. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol 1989; 25: 527–34. [DOI] [PubMed] [Google Scholar]
- 92.Ausborn NL, Le QT, Bradley JD, Choy H, Dicker AP, Saha D, et al. Molecular profiling to optimize treatment in non-small cell lung cancer: a review of potential molecular targets for radiation therapy by the translational research program of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 2012; 83: e453–64. [DOI] [PubMed] [Google Scholar]
- 93.Moeller BJ, Yordy JS, Williams MD, Giri U, Raju U, Molkentine DP, et al. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin Cancer Res 2011; 17: 2035–43. doi: 10.1158/1078-0432.CCR-10-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 2001; 412: 607–14. [DOI] [PubMed] [Google Scholar]
- 95.Weyrather WK, Ritter S, Scholz M, Kraft G. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int J Radiat Biol 1999; 75: 1357–64. [DOI] [PubMed] [Google Scholar]
- 96.Merz F, Bechmann I. Irradiation of human tumor tissue cultures: optimizing ion radiation therapy. Future Oncol 2011; 7: 489–91. doi: 10.2217/fon.11.18 [DOI] [PubMed] [Google Scholar]
- 97.Merz F, Gaunitz F, Dehghani F, Renner C, Meixensberger J, Gutenberg A, et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol 2013; 15: 670–81. doi: 10.1093/neuonc/not003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawkins RB. The relationship between the sensitivity of cells to high-energy photons and the RBE of particle radiation used in radiotherapy. Radiat Res 2009; 172: 761–76. doi: 10.1667/RR1655.1 [DOI] [PubMed] [Google Scholar]
- 99.Pignalosa D, Durante M. Overcoming resistance of cancer stem cells. Lancet Oncol 2012; 13: e187–8. doi: 10.1016/S1470-2045(12)70196-1 [DOI] [PubMed] [Google Scholar]
- 100.Tucker CL, Fields S. Lethal combinations. Nat Genet 2003; 35: 204–5. doi: 10.1038/ng1103-204 [DOI] [PubMed] [Google Scholar]
- 101.Kaelin WGJr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer 2005; 5: 689–98. doi: 10.1038/nrc1691 [DOI] [PubMed] [Google Scholar]
- 102.Chan DA, Giaccia AJ. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov 2011; 10: 351–64. doi: 10.1038/nrd3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev Clin Oncol 2012; 9: 144–55. doi: 10.1038/nrclinonc.2012.3 [DOI] [PubMed] [Google Scholar]
- 104.Hall EJ. Cancer caused by x-raysa random event? Lancet Oncol 2007; 8: 369–70. doi: 10.1016/S1470-2045(07)70113-4 [DOI] [PubMed] [Google Scholar]
- 105.Flint-Richter P, Sadetzki S. Genetic predisposition for the development of radiation-associated meningioma: an epidemiological study. Lancet Oncol 2007; 8: 403–10. doi: 10.1016/S1470-2045(07)70107-9 [DOI] [PubMed] [Google Scholar]
- 106.Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Noguès C, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ 2012; 345: e5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Merchant TE. Clinical controversies: proton therapy for pediatrictumors. Semin Radiat Oncol 2013; 23: 97–108. doi: 10.1016/j.semradonc.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newhauser WD, Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer 2011; 11: 438–48. doi: 10.1038/nrc3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durante M, Yamada S, Ando K, Furusawa Y, Kawata T, Majima H, et al. X-rays vs. carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int J Radiat Oncol Biol Phys 2000; 47: 793–8. [DOI] [PubMed] [Google Scholar]
- 110.Hartel C, Nikoghosyan A, Durante M, Sommer S, Nasonova E, Fournier C, et al. Chromosomal aberrations in peripheral blood lymphocytes of prostate cancer patients treated with IMRT and carbon ions. Radiother Oncol 2010; 95: 73–8. doi: 10.1016/j.radonc.2009.08.031 [DOI] [PubMed] [Google Scholar]
- 111.Ritter S, Durante M. Heavy-ion induced chromosomal aberrations: a review. Mutat Res 2010; 701: 38–46. doi: 10.1016/j.mrgentox.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 112.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys 2013; 87: 46–52. doi: 10.1016/j.ijrobp.2013.04.030 [DOI] [PubMed] [Google Scholar]
- 113.Paganetti H, Athar BS, Moteabbed M, A Adams J, Schneider U, Yock TI. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys Med Biol 2012; 57: 6047–61. doi: 10.1088/0031-9155/57/19/6047 [DOI] [PubMed] [Google Scholar]
- 114.Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol 2013; 58: 807–23. doi: 10.1088/0031-9155/58/4/807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang TC, Craise LM, Mei MT, Tobias CA. Neoplastic cell transformation by heavy charged particles. Radiat Res Suppl 1985: 8; S177–87. [PubMed] [Google Scholar]
- 116.Han ZB, Suzuki H, Suzuki F, Suzuki M, Furusawa Y, Kato T, et al. Relative biological effectiveness of accelerated heavy ions for induction of morphological transformation in Syrian hamster embryo cells. J. Radiat Res 1998: 39; 193–201. [DOI] [PubMed] [Google Scholar]
- 117.Elmore E, Lao XY, Kapadia R, Redpath JL. Threshold-type dose response for induction of neoplastic transformation by 1 GeV/nucleon iron ions. Radiat Res.2009; 171: 764–70. doi: 10.1667/RR1673.1 [DOI] [PubMed] [Google Scholar]
- 118.Suit H, Goldberg S, Niemierko A, Ancukiewicz M, Hall E, Goitein M, et al. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat Res 2007; 167: 12–42. doi: 10.1667/RR0527.1 [DOI] [PubMed] [Google Scholar]
- 119.Weil MM, Bedford JS, Bielefeldt-Ohmann H, Ray FA, Genik PC, Ehrhart EJ, et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon (56)Fe ions. Radiat Res 2009; 172: 213–9. doi: 10.1667/RR1648.1 [DOI] [PubMed] [Google Scholar]
- 120.Durante M, George K, Cucinotta FA. Chromosomes lacking telomeres are present in the progeny of human lymphocytes exposed to heavy ions. Radiat Res 2006; 165: 51–8. [DOI] [PubMed] [Google Scholar]
- 121.Tungjai M, Whorton EB, Rithidech KN. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to ²⁸Silicon (²⁸Si) ions. Radiat Environ Biophys 2013; 52: 339–50. [DOI] [PubMed] [Google Scholar]
- 122.Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer 2013; 13: 511–8. doi: 10.1038/nrc3536 [DOI] [PubMed] [Google Scholar]
- 123.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012; 120: 1503–11. doi: 10.1289/ehp.1204982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet 2009; 374: 1639–51. doi: 10.1016/S0140-6736(09)61299-X [DOI] [PubMed] [Google Scholar]
- 126.Gridley DS, Grover RS, Loredo LN, Wroe AJ, Slater JD. Proton-beam therapy for tumors of the CNS. Expert Rev Neurother 2010; 10: 319–30. doi: 10.1586/ern.09.150 [DOI] [PubMed] [Google Scholar]
- 127.Pinsky LS, Osborne WZ, Bailey JV, Benson RE, Thompson LF. Light flashes observed by Astronauts on Apollo 11 through Apollo 17. Science 1974; 183: 957–9. doi: 10.1126/science.183.4128.957 [DOI] [PubMed] [Google Scholar]
- 128.Casolino M, Bidoli V, Morselli A, Narici L, De Pascale MP, Picozza P, et al. Space travel: dual origins of light flashes seen in space. Nature 2003; 422: 680. doi: 10.1038/422680a [DOI] [PubMed] [Google Scholar]
- 129.Schardt D, Kavatsyuk O, Krämer M, Durante M. Light flashes in cancer patients treated with heavy ions. Brain Stimul 2013; 6: 416–7. doi: 10.1016/j.brs.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 130.Curtis SB, Vazquez ME, Wilson JW, Atwell W, Kim MH. Cosmic ray hits in the central nervous system at solar maximum. Adv Space Res 2000; 25: 2035–40. [DOI] [PubMed] [Google Scholar]
- 131.Joseph JA, Shukitt-Hale B, McEwen J, Rabin BM. CNS-induced deficits of heavy particle irradiation in space: the aging connection. Adv Space Res 2000; 25: 2057–64. [DOI] [PubMed] [Google Scholar]
- 132.Rabin BM, Shukitt-Hale B, Joseph JA, Carrihill-Knoll KL, Carey AN, Cheng V. Relative effectiveness of different particles and energies in disrupting behavioral performance. Radiat Environ Biophys 2007; 46: 173–7. doi: 10.1007/s00411-006-0071-2 [DOI] [PubMed] [Google Scholar]
- 133.Rola R, Fishman K, Baure J, Rosi S, Lamborn KR, Obenaus A, et al. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat Res 2008; 169: 626–32. doi: 10.1667/RR1263.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chylack LTJr, Feiveson AH, Peterson LE, Tung WH, Wear ML, Marak LJ, et al. NASCA report 2: longitudinal study of relationship of exposure to space radiation and risk of lens opacity. Radiat Res 2012; 178: 25–32. [DOI] [PubMed] [Google Scholar]
- 135.Blakely EA, Kleiman NJ, Neriishi K, Chodick G, Chylack LT, Cucinotta FA, et al. Radiation cataractogenesis: epidemiology and biology. Radiat Res 2010; 173: 709–17. doi: 10.1667/RRXX19.1 [DOI] [PubMed] [Google Scholar]
- 136.Brenner DJ, Medvedovsky C, Huang Y, Merriam GRJr, Worgul BV. Accelerated heavy particles and the lens. VI. RBE studies at low doses. Radiat Res 1991; 128: 73–81. [PubMed] [Google Scholar]
- 137.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 138.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res 2012; 177: 229–43. [DOI] [PubMed] [Google Scholar]
- 139.Wang X, Zhang X, Li X, Amos RA, Shaitelman SF, Hoffman K, et al. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: do uncertainties outweigh potential benefits? Br J Radiol 2013; 86: 20130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bush DA, Slater JD, Garberoglio C, Do S, Lum S, Slater JM. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer 2011; 11: 241–5. doi: 10.1016/j.clbc.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 141.MacDonald SM, Patel SA, Hickey S, Specht M, Isakoff SJ, Gadd M, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013; 86: 484–90. doi: 10.1016/j.ijrobp.2013.01.038 [DOI] [PubMed] [Google Scholar]
- 142.Chang JH, Lee NK, Kim JY, Kim YJ, Moon SH, Kim TH, et al. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol 2013; 108: 209–14. doi: 10.1016/j.radonc.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 143.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013; 340: 1080–4. doi: 10.1126/science.1235989 [DOI] [PubMed] [Google Scholar]
- 144.Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol 2009; 5: e1000539. doi: 10.1371/journal.pcbi.1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009; 9: 351–60. doi: 10.1038/nrc2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boehm BO, Rosinger S, Belyi D, Dietrich JW. The parathyroid as a target for radiation damage. N Engl J Med 2011; 365: 676–8. doi: 10.1056/NEJMc1104982 [DOI] [PubMed] [Google Scholar]
- 147.Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 2008; 84: 1–14. doi: 10.1080/09553000701616106 [DOI] [PubMed] [Google Scholar]
- 148.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8: 729–40. doi: 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 149.Fournier C, Zahnreich S, Kraft D, Friedrich T, Voss KO, Durante M, et al. The fate of a normal human cell traversed by a single charged particle. Sci Rep 2012; 2: 643. doi: 10.1038/srep00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res 2007; 35: 7545–56. doi: 10.1093/nar/gkm1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003; 189: 1–20. [DOI] [PubMed] [Google Scholar]
- 152.Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol 1999; 19: 1387–92. [DOI] [PubMed] [Google Scholar]
- 153.ptcog.web.psi.ch [homepage on the internet]. Particle Therapy Co-Operative Group (PTCOG); 2009 [updated 24 Aug 2013]. Available from: http://ptcog.web.psi.ch/
- 154.Durante M, Reitz G, Angerer O. Space radiation research in Europe: flight experiments and ground-based studies. Radiat Environ Biophys 2010; 49: 295–302. doi: 10.1007/s00411-010-0300-6 [DOI] [PubMed] [Google Scholar]
- 155.Dosanjh M, Jones B, Myers S. A possible biomedical facility at the European Organization for Nuclear Research (CERN). Br J Radiol 2013; 86: 20120660. doi: 10.1259/bjr.20120660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.US Department of Energy, Department of Health & Human Services, National Institutes of Health, National Cancer Institute. DOE-NCI Workshop on Ion Beam Therapy. Summary Report. January 9–10, 2013. Available from:http://science.energy.gov/∼/media/hep/pdf/accelerator-rd-stewardship/Workshop_on_Ion_Beam_Therapy_Report_Final_R1.pdf
- 157.Dosanjh M, Jones B, Mayer R. ENLIGHT and other EU-funded projects in hadron therapy. Br J Radiol 2010; 83: 811–3. doi: 10.1259/bjr/49490647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Combs SE, Djosanjh M, Pötter R, Orrechia R, Haberer T, Durante M, et al. Towards clinical evidence in particle therapy: ENLIGHT, PARTNER, ULICE and beyond. J Radiat Res 2013; 54(Suppl. 1): i6–12. doi: 10.1093/jrr/rrt039 [DOI] [PMC free article] [PubMed] [Google Scholar]