Abstract
Objective:
To evaluate the clinical efficacy and safety of simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT) for patients with locally advanced non-small-cell lung cancer (LANSCLC).
Methods:
48 patients with LANSCLC treated with SIB-IMRT from January 2010 to April 2012 were retrospectively analysed. A radiation dose of 45–63 Gy (median dose, 51.58 Gy) was delivered to the planning target volume (1.8–2.0 Gy daily fractions) simultaneously with 55.0–74.2 Gy (median dose, 63 Gy) to the planning gross tumour volume (2.00–2.25 Gy daily fractions). 45 patients received concurrent/sequential chemotherapy. The overall survival (OS), locoregional recurrence-free survival (LRFS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method. Treatment-related pneumonitis and oesophagitis were graded according to the Common Terminology Criteria for Adverse Events v. 4.0.
Results:
By 1 July 2013, 29 of the 48 patients were dead. The median follow-up time for the survivors was 28 months (19–44 months). The median OS and PFS were 21 and 14 months, respectively. The median LRFS time was not reached. The 2-year LRFS, OS and PFS were 62.5%, 45.1% and 28.0%, respectively. Two patients experienced Grade 3 treatment-related pneumonitis, two patients experienced Grade 5 treatment-related pneumonitis and two patients had ≥Grade 3 oesophagitis.
Conclusion:
SIB-IMRT appears to be an effective therapeutic option in patients with LANSCLC and warrants further evaluation with increased number of patients in prospective clinical trials.
Advances in knowledge:
This study explores the feasibility of delivering tumoricidal doses of radiation to primary lesions in non-small-cell lung cancer.
The true value of radiotherapy confined to the thorax is indisputable in the treatment of locally advanced non-small-cell lung cancer (LANSCLC). However, even with standard chemoradiation, it is difficult to achieve durable local control, and this contributes to the high morbidity and mortality of patients with non-small-cell lung cancer (NSCLC).1 Despite a demonstrated positive association between the radiation dose and tumour control,2 results of the Radiation Therapy Oncology Group (RTOG) 0617 clinical (Phase III) trial showed that the overall survival (OS) of Stage III NSCLC patients given high-dose (74 Gy) conformal radiation therapy with concurrent chemotherapy was no better than that of patients given the standard dose (60 Gy).3
The unpublished data showed that the higher dose had no further effects on improving the OS during the RTOG 0617 trial, and the reason still remained unclear, the radiation-induced decline in quality of life may be responsible for this.4 Thus, researchers turned their attention to focus on better ways of delivering radiation to tumours, while sparing surrounding normal structures. Initially, the radiation dose applied to the gross tumour was identical to that directed at targeted nodal areas, but logic suggests that the dose required to control subclinical lesions should be lower than that of the primary disease.
Simultaneous integrated boost (SIB) is a recent modality applied in conjunction with intensity-modulated radiotherapy (IMRT) in the treatment of malignancies.5,6 Simultaneous integrated boost–intensity-modulated radiotherapy (SIB-IMRT) simultaneously delivers a higher dose to the primary disease and a relatively lower dose to the subclinical disease or selected other regions. However, outcomes for SIB-IMRT in LANSCLC remain to be determined.
Herein, we retrospectively analysed clinical outcomes of patients with LANSCLC treated with SIB-IMRT to evaluate the feasibility of this technology and to provide evidence in support of future clinical study.
METHODS AND MATERIALS
Study patients
The medical records of all NSCLC patients who received definitive SIB-IMRT from 1 January 2010 to 31 April 2012 at our centre were reviewed. Inclusion criteria were the presence of newly diagnosed and pathologically confirmed NSCLC, treatment with SIB-IMRT with or without chemotherapy, Karnofsky performance status score ≥70 (capable of normal activity) and life expectancy of more than 6 months. Patients who received postoperative radiotherapy, prior thoracic radiation or thoracic surgery, or had other coexisting primary tumours or distant metastasis were excluded. The Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) approved this study.
The pre-treatment work-up included complete history, physical examination, basic laboratory studies, CT scan of the chest and abdomen with contrast, single-photon emission CT scan of bone and head CT scan or MRI. TNM staging was defined at the time of pathological diagnosis using the current American Joint Committee on Cancer criteria (seventh edition).
Treatment planning
Simulation CT scanning was conducted using helical CT, a 3-mm slice thickness (CT Brilliance; Philips Medical Systems, Best, Netherlands) and intravenous contrast. All patients were immobilized in a supine position (with a thermoplastic mask on the chest) during simulation and radiotherapy. The scanned area was from the angulus mandibulae to the bottom of the L1 vertebral body. These images were transferred to a three-dimensional (3D) planning system (ADAC Pinnacle® 8.0m3; Philips Medical Systems, Bothell, WA). The primary tumour was delineated using a lung window. Mediastinal windows were used for delineation of the medial border of centrally located primaries, the involved lymph nodes and adjacent normal organs. A radiation resident conducted the contouring, which was verified by a chief radiation oncologist.
Gross tumour volume was defined as any visible primary lesions on simulation CT, and all lymph nodes with a diameter ≥1 cm in short axis were included. Clinical target volume was defined as the high-risk lymph nodal regions, including adjacent regions of involved lymph nodes (e.g. 2 left (L), 5, and 7 would be included in the clinical target volume if 4L was involved), and the ipsilateral hilar in accordance with the new lymph node map of the International Association for the Study of Lung Cancer, including the gross tumour volume with a 0.5–0.8 cm margin. Another 0.5–0.8 cm margin was added to create the planning target volume (PTV). The gross PTV was formed by including a 0.5-cm margin around the gross tumour volume (PTVG).
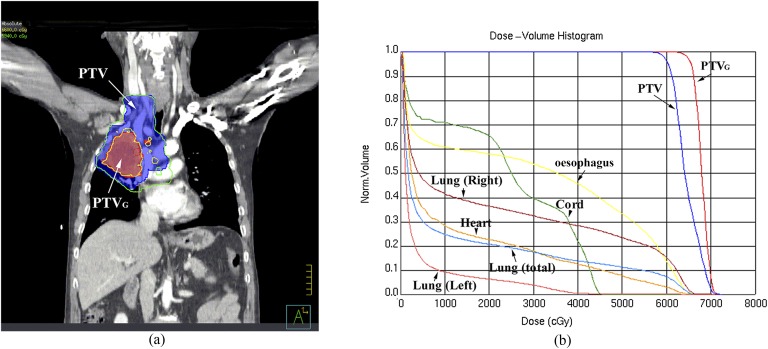
Treatments were designed using computerized radiation dosimetry and delivered by 6 MV X-rays from a Varian 120-leaf multileaf collimator linear accelerator (Varian® Medical Systems, Palo Alto, CA). Each patient received individualized doses according to normal tissue constraints (for more details, see below), concurrent chemotherapy (total dose and dose fraction would be reduced when concurrent chemotherapy was used), pulmonary function and Karnofsky performance status (higher total dose and dose fraction would be provided for patients with better pulmonary function and Karnofsky performance status). In addition under the above conditions, the PTVG of each patient was given a dose as high as possible. The final dose and dose fractions were decided by each patient's attending radiation oncologists. The prescribed radiation dose was 45–63 Gy (median dose, 51.58 Gy) to the PTV at 1.8–2 Gy per day and 55–74.2 Gy (median dose, 63 Gy) to the PTVG at 2–2.25 Gy per day and was delivered to ≥95% of the PTV or PTVG, respectively. A representative dose distribution using SIB-IMRT with 66 Gy to the PTVG is shown in Figure 1.
Figure 1.
Representative simultaneous integrated boost intensity-modulated radiotherapy with 66 Gy to the gross tumour volume (PTVG). (a) Representative dose distributions. PTVG line, field receiving 6600 cGy; planning target volume (PTV) line, field receiving 5940 cGy. (b) Dose–volume histogram. Norm, normal.
Each treatment plan consisted of five static fields with the following normal tissue constraints: (1) total lung, V5 (i.e. the percentage of lung volume receiving ≥5 Gy) was ≤60% and V20 was ≤35%; (2) the volume of heart receiving ≥40 Gy was ≤30%; (3) the volume of oesophagus receiving ≥50 Gy was ≤50%, the maximum dose to the oesophagus was ≤66 Gy; and (4) the maximum spinal cord dose was ≤45 Gy. The V20 of the total lung and the dose to the spinal cord were determinative for plan ranking and acceptance, whereas the heart and oesophageal dose–volume parameters were reported but were only decisive for plan acceptance when violated.
Follow-up and toxicity assessment
The follow-up schedule began from the time of first treatment. The last follow-up was 1 July 2013. Follow-up examinations every 3 months included basic laboratory studies, liver and renal function tests, chest CT and abdomen B-ultrasound. Examinations were performed immediately when symptoms were observed. Patterns of the first failure were recorded for patients who suffered progression of the disease. Treatment-related pneumonitis and oesophagitis were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0 grade.
Statistical analyses
The clinical end points of this study were survival rates [OS, locoregional recurrence-free survival (LRFS) and progression-free survival (PFS)] and toxicities due to treatment (pneumonitis and oesophagitis). Locoregional failures included recurrences of primary tumour or regional lymph node, which were diagnosed based on CT or positron emission tomography images. Regional lymph nodes included those from the supraclavicular to the mediastinal lymph node stations. Survival was defined as starting from the date of pathological diagnosis and ending at death of the patient or censored at the date of last follow-up (if the patient was still alive). Patients who were censored at the last follow-up were considered censored for the OS rate. Patients who censored at the last follow-up, or died without evidence of locoregional recurrence or progression, were considered censored for the LRFS or PFS rates. Survival probabilities were determined using the Kaplan–Meier method.
The curative effect was evaluated in accordance with guidelines for evaluating response to treatment in solid tumours published collectively by several international health agencies in the year 2000.7
RESULTS
Patient population and treatment
48 patients with inoperable NSCLC and treated with SIB-IMRT were enrolled in the present study (Table 1). All patients were in locally advanced stages of the disease (20 with Stage IIIa and 28 with Stage IIIb).
Table 1.
Demographics and clinicopathological characteristics of 48 patients
| Characteristics | na | % |
|---|---|---|
| Age | ||
| Median, range (years) | 58, 43–82 | |
| Gender | ||
| Male | 37 | 77.1 |
| female | 11 | 22.9 |
| Smoking history (pack years)b | ||
| Never | 15 | 31.3 |
| 1–20 | 6 | 12.5 |
| 21–40 | 10 | 20.8 |
| 41–60 | 9 | 18.8 |
| >60 | 8 | 16.7 |
| Karnofsky performance status | ||
| 60 | 2 | 4.2 |
| 70 | 7 | 14.6 |
| 80 | 23 | 47.9 |
| 90 | 16 | 33.9 |
| Disease stage | ||
| IIIa | 20 | 41.7 |
| IIIb | 28 | 58.3 |
| Tumour histology | ||
| Squamous cell carcinoma | 31 | 64.6 |
| Adenocarcinoma | 10 | 20.8 |
| Not specified | 7 | 14.6 |
| Tumour location | ||
| Left upper lobe | 12 | 25.0 |
| Left lower lobe | 7 | 14.6 |
| Right upper lobe | 14 | 29.2 |
| Right mid-lobe | 5 | 10.4 |
| Right lower lobe | 10 | 20.8 |
| Tumour location | ||
| Peripheral | 16 | 33.3 |
| Central | 32 | 66.7 |
| Treatment choice | ||
| RT alone | 3 | 6.3 |
| RT + adjuvant chemotherapy | 1 | 2.1 |
| Concurrent chemoradiotherapy alone | 0 | 0.0 |
| Concurrent + adjuvant chemotherapy | 5 | 10.4 |
| Induction chemotherapy + RT | 9 | 18.8 |
| Induction + RT + adjuvant chemotherapy | 11 | 22.9 |
| Induction + concurrent chemotherapy | 12 | 25.0 |
| Induction + concurrent + adjuvant chemotherapy | 7 | 14.6 |
| Target volume median, range (cm3) | ||
| PTV | 438.3, 204.9–1116.2 | |
| PTVG | 131.0, 10.9–509.2 | |
| Treatment dose median, range (Gy) | ||
| PTV | 51.58, 45.0–63.0 | |
| PTVG | 63.0, 55.0–74.2 | |
| PTVG dose (Gy) | ||
| 55–59 | 8 | 16.7 |
| 60–64 | 20 | 41.7 |
| 65–69 | 16 | 33.3 |
| ≥70 | 4 | 8.3 |
PTV, planning target volume; PTVG, gross tumour volume; RT, radiation therapy.
n denotes the number of patients, unless otherwise indicated.
1 pack year = smoking 20 cigarettes per day for 1 year.
Most of the patients (93.8%) received 2–8 cycles of platinum-based chemotherapy and 24 (50.0%) received concurrent chemoradiotherapy. The most commonly used regimens were paclitaxel plus platinum and docetaxel plus platinum. The actual dose to PTVG was ≥60 Gy in 40 patients (83.3%) and 55–60 Gy in 8 patients (16.7%).
Clinical outcomes
The complete plus partial response and stable plus progressive disease rates of primary tumours at 3 months post-radiotherapy were 64.6% and 31.3%, respectively, with no records in two patients (4.2%).
The median duration of follow-up was 19.5 months (range, 6–44 months) for all patients and 28 months (range, 19–44 months) for the survivors. At last follow-up, 22.9% of all patients were alive with no evidence of recurrence, 16.7% were alive with recurrence and 52.1% died from recurrence or associated causes. The rest (8.3%) died due to other causes.
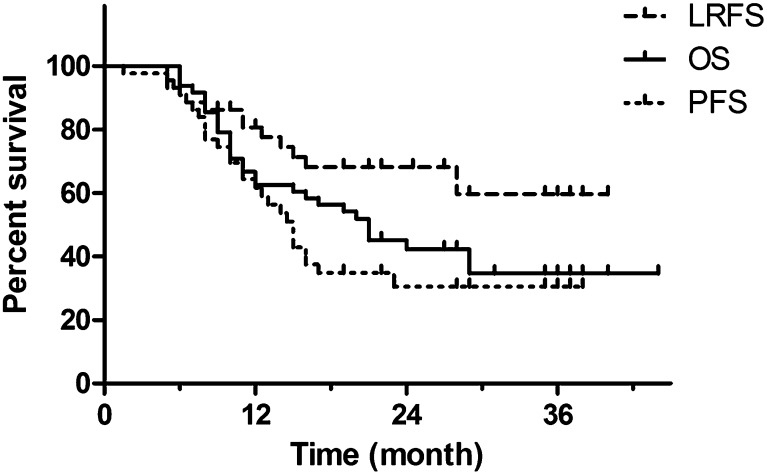
The median LRFS was not reached, whereas the median OS and PFS were 21 and 14 months, respectively. The 2-year LRFS, OS and PFS were 62.5%, 45.1% and 28.0%, respectively (Figure 2).
Figure 2.
Overall survival (OS), locoregional recurrence-free survival (LRFS) and progression-free survival (PFS) curves for 48 locally advanced non-small-cell lung cancer patients who received simultaneous integrated boost intensity-modulated radiotherapy.
31 patients (64.6%) experienced recurrence. In 13 patients (27.1%), locoregional recurrence was found, and 17 patients (35.4%) showed distant metastasis. Of the 13 patients who had locoregional recurrence, 1 suffered regional lymph node failure, which was located outside the PTV, 1 had elective nodal failure inside the PTV and 11 had recurrence of the primary tumour. Disease recurred in both a locoregional and a distant site in the one remaining patient.
Toxicity
Four (8.3%) and two (4.2%) patients experienced severe (grade ≥3) treatment-related pneumonitis and oesophagitis, respectively (Table 2).
Table 2.
Pneumonitis and oesophagitis incidence rates
| Toxicity | CTCAE v. 4.0 grade |
||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4 | 5 | |||
| Pneumonitis, n, % | 38, 79.2% | 6, 12.5% | 2, 4.2% | 0, 0% | 2, 4.2% | ||
| Oesophagitis, n, % | 42, 87.5% | 4, 8.3% | 2, 4.2% | 0, 0% | 0, 0.0% | ||
CTCAE v. 4.0, Common Terminology Criteria for Adverse Events v. 4.0.
DISCUSSION
Despite various efforts during the past few decades, local control and OS after radiation therapy remains relatively poor and unchanged for patients with LANSCLC. Our results suggest that it may be feasible to escalate the radiation dose to the gross tumour, while delivering a relatively lower dose to the subclinical lesions. To the best of our knowledge, the present study is among the few clinical reports of treatment outcome for the use of SIB-IMRT in LANSCLC. Some researchers8–10 simultaneously delivered a higher dose per fraction to the primary disease and a relatively lower dose to the subclinical disease or selected other regions by using 3D conformal radiotherapy (3DCRT), and the clinical results showed this regimen was feasible. Compared with published data,1,11,12 our results showed a relatively longer median survival time (21 months) for locally advanced Stage III NSCLC, although only a portion of the patients received concurrent chemoradiotherapy. The reasons were unclear; we believe that the relatively good LRFS that benefited from the high doses of radiation may be responsible for this, which need to be determined in the future studies.
Treatment failure in LANSCLC still occurs locally, despite administration of chemoradiation as the standard of care. Theoretically, higher doses of radiation applied to tumours should improve the tumour control rate, and studies have confirmed this. For example, a radiation dose escalation study found a positive association between tumour control and higher radiation dose, with control rates of 12%, 35% and 49% for radiation doses 63–69, 74–84 and 92–103 Gy, respectively.2 However, the result of the RTOG 0617 clinical trial showed that escalating the dose to 74 Gy from the standard 60 Gy did not improve survival in patients with Stages IIIa/IIIb NSCLC.3 Although the actual reasons for the unexpected results of the latter remains unclear, radiation-induced decline in quality of life with the high dose delivered to the PTV might account for this.4 Thus, safely applying and escalating the radiation doses to the targets, while sparing and decreasing the doses to the normal adjacent organs may be the key to improving the therapeutic outcome in NSCLC.
A dosimetric study found that, compared with the standard treatment plan, the use of SIB in patients with unresectable stage IIIa/IIIb NSCLC enabled a median dose escalation of 14.7 Gy (22%) to the target tumour, whereas there were no significant changes in doses to critical structures.13 In the present study, most patients were given ≥60 Gy to the PTVG, the median biological effective dose (α/β = 10) was 75.7 Gy (range, 67.1–89.9 Gy) and the equivalent dose in 2-Gy fractions was 63.1 Gy (55.9–74.9 Gy). With the optimal local control and the lower frequency use of concurrent chemotherapy in the study, we might have reason to believe that high doses of radiation could benefit the LRFS even without the use of concurrent chemoradiation. However, more studies are required to confirm this.
Some studies found that, when using 3DCRT without elective nodal irradiation (ENI), the elective nodal failure rate was low. This could be due to incidental radiation on the clinically uninvolved nodal regions.14–18 However, the amount of incidental radiation delivered to non-targeted elective nodes might be different in IMRT and may be a factor in the rate of elective nodal failure. Furthermore, in low-dose areas, regional recurrence still occurs.18 In our study, the elective nodal regions received radiation therapy preventatively with tolerable doses to normal adjacent organs. Therefore, elective nodal radiation of selected very-high-risk regions is standard when IMRT is used in our medical centre. We found that where ENI was used, elective nodal failure in the PTV occurred in only one patient. This may imply that the relatively lower doses of radiation delivered to elective nodal regions could be sufficient to control subclinical lesions when SIB-IMRT is used, although many factors can bias this.
Considering the prevalence and potential lethality of treatment-related pneumonitis, it may be an important treatment-related toxicity in the treatment of patients with lung cancer. In this study, we did not observe severe lung complications compared with other studies that used 3DCRT or IMRT,19–23 and we believed that SIB-IMRT is safe for the treatment of LANSCLC. However, as late responses were highly correlated with fraction size,24 the high-fraction doses delivered by SIB-IMRT could potentially cause more toxicity than with standard fractionated radiation. Therefore, late complications (such as pulmenary fibrosis) have to be carefully followed in this study.
Because of the inherent flaws in a retrospective study, confounding variables may exist that were not accounted for in this study. Moreover, because the patients were not prospectively followed up, selection bias and loss to follow-up might contribute to an underestimation of the recurrence rates, death rates and treatment-related pneumonitis and oesophagitis. We did not perform positron emission tomography-CT for lymph node staging and four-dimensional CT examinations in our study, which might have influenced clinical outcomes, although most of the primary tumours were located in the upper or middle lobes or belonged to the central type. Despite the above limitations, because there is little data available concerning the use of SIB-IMRT in NSCLC, we believe that the present study provides an intriguing justification for future study in treatments of LANSCLC.
In conclusion, our study shows that SIB-IMRT can safely increase the radiation dose to the gross tumour volume in patients with LANSCLC, while maintaining tolerable doses to adjacent organs, and it has a low elective nodal failure rate. We believe that our results should, at the very least, encourage further evaluations of the therapeutic efficacy of SIB-IMRT in NSCLC patients in future prospective clinical trials.
ACKNOWLEDGMENTS
We thank Medjaden Bioscience for assisting in the preparation of this manuscript.
FUNDING
This study was supported in part by grant #12ZCDZSY15900 (to PW) from the Tianjin Major Project in Cancer Prevention and Treatment.
REFERENCES
- 1.Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized Phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–60. doi: 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong FM, Ten HR, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63: 324–33. [DOI] [PubMed] [Google Scholar]
- 3.Bradley JD, Paulus R, Komaki R, Masters G, Forster K, Schild SE, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy +/− cetuximab for stage IIIa/IIIb non-small cell lung cancer: preliminary findings on radiation dose in RTOG 0617. Proceedings of the 53rd Annual Meeting of the American Society of Radiation Oncology; 2–6 October 2011; Miami, FL. Washington, DC: American Society for Radiation Oncology, 2011.
- 4.Movsas B, Hu C, Sloan J, Bradley JD, Kavadi VS, Narayan S, et al. Quality of life (QOL) analysis of the randomized radiation (RT) dose-escalation NSCLC trial (RTOG 0617): the rest of the story. Proceedings of the 55rd Annual Meeting of the American Society of radiation Oncology; 22–25 September 2013; Atlanta, GA. Washington, DC: American Society for Radiation Oncology, 2013.
- 5.Studer G, Peponi E, Kloeck S, Dossenbach T, Huber G, Glanzmann C. Surviving hypopharynx-larynx carcinoma in the era of IMRT. Int J Radiat Oncol Biol Phys 2010; 77: 1391–6. doi: 10.1016/j.ijrobp.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 6.McCammon R, Rusthoven KE, Kavanagh B, Newell S, Newman F, Raben D. Toxicity assessment of pelvic intensity-modulated radiotherapy with hypofractionated simultaneous integrated boost to prostate for intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2009; 75: 413–20. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 8.Kepka L, Tyc-Szczepaniak D, Bujko K. Dose-per-fraction escalation of accelerated hypofractionated three-dimensional conformal radiotherapy in locally advanced non-small cell lung cancer. J Thorac Oncol 2009; 4: 853–61. doi: 10.1097/JTO.0b013e3181a97dda [DOI] [PubMed] [Google Scholar]
- 9.Uitterhoeve AL, Belderbos JS, Koolen MG, van der Vaart PJ, Rodrigus PT, Benraadt J, et al. Toxicity of high-dose radiotherapy combined with daily cisplatin in non-small cell lung cancer: results of the EORTC 08912 Phase I/II study. European Organization for Research and Treatment of Cancer. Eur J Cancer 2000; 36: 592–600. [DOI] [PubMed] [Google Scholar]
- 10.Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer 2007; 43: 114–21. doi: 10.1016/j.ejca.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 11.Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 2004; 46: 87–98. doi: 10.1016/j.lungcan.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005; 23: 5910–17. [DOI] [PubMed] [Google Scholar]
- 13.Turner LM, Howard JA, Dehghanpour P, Barrett RD, Rebueno N, Palmer M, et al. Exploring the feasibility of dose escalation positron emission tomography-positive disease with intensity-modulated radiation therapy and the effects on normal tissue structures for thoracic malignancies. Med Dosim 2011; 36: 383–8. [DOI] [PubMed] [Google Scholar]
- 14.De Ruysscher D, Wanders S, van Haren E, Hochstenbag M, Geeraedts W, Utama I, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys 2005; 62: 988–94. doi: 10.1016/j.ijrobp.2004.12.019 [DOI] [PubMed] [Google Scholar]
- 15.Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol 2007; 25: 5557–61. doi: 10.1200/JCO.2007.13.2191 [DOI] [PubMed] [Google Scholar]
- 16.Sulman EP, Komaki R, Klopp AH, Cox JD, Chang JY. Exclusion of elective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol 2009; 4: 5. doi: 10.1186/1748-717X-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Chen M, Ten HR, Chetty I, Chapet O, Hayman JA, et al. Three-dimensional conformal radiation may deliver considerable dose of incidental nodal irradiation in patients with early stage node-negative non-small cell lung cancer when the tumor is large and centrally located. Radiother Oncol 2007; 82: 153–9. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Togami T, Nishiyama Y, Ohkawa M, Takashima H. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non-small-cell lung cancer treated with involved-field radiation therapy: does incidental irradiation contribute to the low incidence of elective nodal failure? Int J Radiat Oncol Biol Phys 2010; 77: 337–43. [DOI] [PubMed] [Google Scholar]
- 19.Yorke ED, Jackson A, Rosenzweig KE, Merrick SA, Gabrys D, Venkatraman ES, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2002; 54: 329–39. [DOI] [PubMed] [Google Scholar]
- 20.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol 2003; 67: 275–83. [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee DH, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 2005; 235: 208–15. doi: 10.1148/radiol.2351040248 [DOI] [PubMed] [Google Scholar]
- 22.Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 94–102. [DOI] [PubMed] [Google Scholar]
- 23.Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol 2008; 87: 17–23. doi: 10.1016/j.radonc.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thames HJ, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys 1982; 8: 219–26. [DOI] [PubMed] [Google Scholar]