Abstract
The term vascular anomaly represents a broad spectrum of vascular pathology, including proliferating vascular tumours and vascular malformations. While the treatment of most vascular anomalies is multifactorial, interventional radiology procedures, including embolic therapy, sclerotherapy and laser coagulation among others, are playing an increasingly important role in vascular anomaly management. This review discusses the diagnosis and treatment of common vascular malformations, with emphasis on the technique, efficacy and complications of different interventional radiology procedures.
Traditionally, vascular anomalies have been referred to by vernacular names1 and diagnosed on descriptive observations, including clinical appearance, location and fluid contents. This has caused misdiagnosis and improper management of vascular anomalies, leading to unsatisfactory treatment outcomes.2 However, vascular anomalies can cause functional complications, pain and disfigurement, necessitating accurate diagnosis and effective treatment.
In recent decades, there has been an effort to categorize vascular anomalies on their underlying histopathology3 and blood flow patterns. In 1996, the International Society for the Study of Vascular Anomalies (ISSVA) developed a classification system based on histopathological and blood flow characteristics, in addition to the clinical appearance and disease course.4 According to this classification system, vascular anomalies are divided into two main groups: proliferating vascular tumours (haemangiomas) and vascular malformations. Vascular malformations are further subdivided into low and high blood flow groups and complex combined groups (Table 1). These classification systems have helped clarify the distinctions between different vascular anomalies, leading to improved management and better treatment options.5
Table 1.
International Society for the Study of Vascular Anomalies classification of vascular anomalies
| Vascular tumours | Vascular malformations |
|---|---|
| Infantile haemangioma | Low-flow vascular malformations |
| Capillary malformation | |
| Congenital haemangioma (rapidly involuting congenital haemangiomas and non-involuting congenital haemangiomas) | Venous malformation |
| Lymphatic malformation | |
| Tufted angioma | High-flow vascular malformations |
| Arterial malformation | |
| Kaposiform haemangioendothelioma | Arteriovenous malformation |
| Arteriovenous fistula | |
| Spindle cell haemangioendothelioma | Complex combined vascular malformations |
| Capillary–venous malformation | |
| Pyogenic granuloma | Capillary–lymphatic malformation |
| Lymphatic–venous malformation | |
| Haemangiopericytoma | Capillary–arteriovenous malformation |
| Capillary–lymphatic–arteriovenous malformation |
Advancements in imaging technology have brought radiology to the forefront of vascular anomaly management, as imaging is vital to accurate diagnosis and treatment planning. In addition, interventional radiology continues to play an increasingly important role in the treatment of vascular anomalies. Sclerotherapy and other percutaneous treatments are now first-line treatments for many vascular anomalies, and their prominence continues to grow as new therapeutic agents and techniques emerge.
Despite these advances, the management of vascular anomalies is complex and reliant on multiple specialties, including surgery, radiology, interventional radiology and the primary care physician. In this article, we discuss the diagnosis and treatment of common vascular anomalies with emphasis on the techniques and advancements of percutaneous and image-guided therapy.
PROLIFERATING VASCULAR TUMOURS
Haemangiomas are the most common tumour in infancy with an incidence of 2–3%,6 and represent the majority of proliferating vascular tumours. According to the ISSVA classification system, haemangiomas are vascular tumours divided into infantile and congenital types, with further subdivision of congenital haemangiomas into non-involuting congenital haemangiomas (NICHs) and rapidly involuting congenital haemangiomas (RICHs).
Infantile haemangiomas (IHs) present post-natally and represent 70% of all haemangiomas. They are found on the head and neck in 60% of cases, while 25% are found on the trunk and 15% on the extremities.7 They almost always appear within the first 6 weeks of life and follow a triphasic pattern of evolution: proliferation, plateau and involution. Proliferation occurs in the first few months of life, with most IHs reaching a maximum size by 3–5 months of age.8 Most IHs begin involution by 1 year old, with complete involution in 50% of cases by the age of 5 years, 70% by the age of 7 years and nearly all by the age of 8–12 years.9 Complete involution, however, does not imply complete resolution. Up to 40% of IHs will have residual skin changes and scarring characterized by fibrofatty residuum.10
Congenital haemangiomas represent the remaining 30% of benign vascular tumours, and, unlike IH, are present at birth. The primary distinction between RICHs and NICHs is that RICHs reach maximum size by birth and involute within 12–18 months, whereas NICHs continue to grow in size in proportion with the patient and do not involute. RICH lesions can typically be followed using conservative management and only require treatment when adjacent to vital structures such as the orbits. Both RICHs and NICHs typically present as solitary lesions, most commonly on the head and neck or the extremities.11,12
Most haemangiomas are diagnosed clinically based on the lesion appearance. IHs often begin as skin blanching followed by telangiectasia and an erythematous macule. As proliferation continues, it often becomes a raised bright red/strawberry-coloured lesion with a plaque-like morphology, sometimes with central ulceration. NICHs and RICHs have similar clinical lesion morphology that is distinct from IHs. NICHs are usually pink-to-purple raised lesions with prominent telangiectasia and blue pallor either peripherally or centrally.11 RICH lesions usually present as raised grey-blue lesions with prominent telangiectasia and central depression, ulceration or scar.13
When a diagnosis of IH is confirmed, the primary treatment is observation, as >90% will involute regardless of the initial size. In general, work-up and treatment of haemangioma is required if ulceration or other factors are likely to cause scarring, when it is invading important structures and when other diagnoses such as malignancy cannot be ruled out. Another situation that requires treatment is the development of Kasabach–Merritt phenomenon. In these cases, children present with an enlarging infantile haemangioma-like lesion, profound thrombocytopenia, consumptive coagulopathy and microangiopathic haemolytic anaemia. This presentation points to tufted angiomas and more aggressive kaposiform haemangioendothelioma.
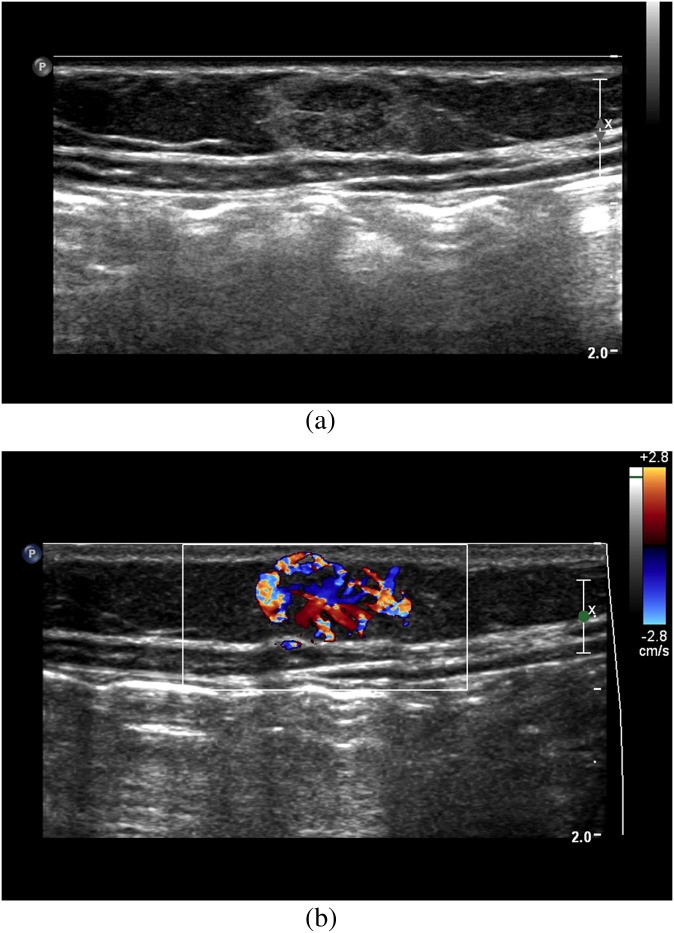
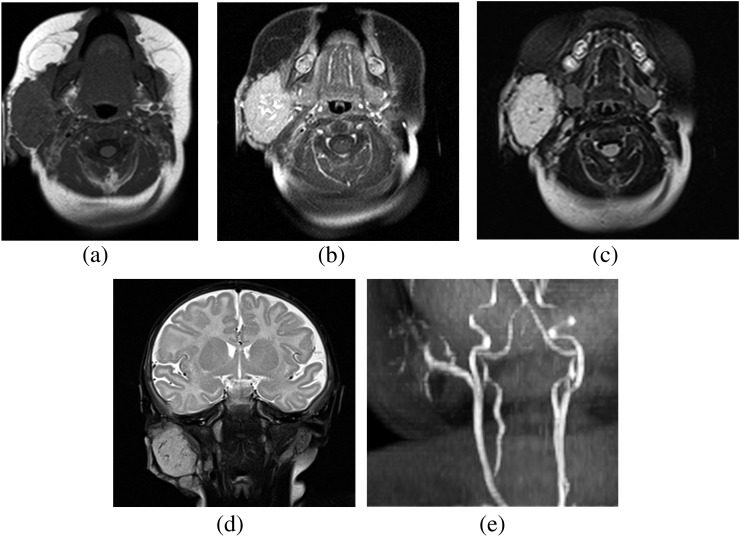
When IH cannot be confirmed clinically, imaging with MRI and ultrasound can differentiate IHs from malignant pathologies. Ultrasound is useful during the proliferative phase in showing a well-circumscribed mass with variable echogenicity (Figure 1a). Doppler ultrasound typically demonstrates high-flow vessels in and adjacent to the mass, with the arterial feeder recognized by increased colour flow, high Doppler shift and low resistance (Figure 1b). MRI can also help confirm IHs and is particularly valuable for pre-operative planning in head and neck cases. IHs are best appreciated on fat-saturated T1 weighted images with contrast, showing intense contrast enhancement within the mass and adjacent serpiginous flow voids (Figure 2a,b). T2 weighted imaging of the mass typically appears mildly hyperintense relative to surrounding muscles (Figure 2c,d). MR angiography (MRA) also serves as an important diagnostic modality in posterior fossa malformations–haemangiomas–arterial anomalies–cardiac defects–eye abnormalities–sternal cleft and supraumbilical raphe syndrome in which haemangiomas present in concert with cerebral vascular anomalies and other abnormalities. Specifically, MRA is indicated to visualize arterial stenosis, agenesis and aneurysms (Figure 2e).
Figure 1.
(a) Ultrasound of an anterior abdominal wall infantile haemangioma shows a mixed echogenic well-defined mass. (b) Colour Doppler ultrasound of the same mass demonstrates increased flow within the lesion.
Figure 2.
MRI of a right parotid gland infantile haemangioma. (a) Pre-contrast T1 weighted image shows a lobulated well-defined hypointense mass, which demonstrates intense enhancement on post-contrast image (b). (c, d) MRI of a right parotid gland infantile haemangioma. T2 weighted short tau inversion–recovery images show a lobulated well-defined isointense to mildly hyperintense mass within the right parotid gland. (e) MR angiography of the neck demonstrates ectatic arterial branches of the right facial artery supplying to the right parotid haemangioma.
When treatment is required for IHs, propranolol is the first-line therapy. Response is usually seen within several days of starting treatment, as propranolol reduces the expression of vascular endothelial growth factor and other proangiogenic factors,14 while also inducing apoptosis of vascular endothelial cells.15 Before the advent of propranolol for IH, corticosteroids were the mainstay of treatment. In cases where propranolol may be contraindicated as with bradycardia or hypotension, oral prednisolone treatment provides complete involution in approximately 30% of cases and halts progression in 40% of cases.16
Surgical intervention is generally reserved for IHs at increased risk for large scarring or located near the orbit. Scarring is a major concern when haemangiomas demonstrate ulceration or bleeding. In these cases, surgical resection should be performed during the proliferative phase, as the scar from excision is likely to be smaller than if left to grow untreated. In addition, IHs near the eye, particularly the upper eyelid, should be removed as soon as possible to prevent invasion into the orbit and possible loss of vision. In these cases, a trial of medical therapy can be attempted first, but surgery should not be delayed if the haemangioma proves unresponsive.
CAPILLARY MALFORMATIONS
Capillary malformations (CMs), also known as port wine stains, have an incidence of 3 per 1000 newborns.17 They are typically isolated findings, although they can be linked with more serious disease (Table 2).18 Sturge–Weber syndrome is the most well-known associated disease and presents with ipsilateral angioma formation, glaucoma, seizures, mental retardation and arteriovenous malformation (AVM). Thus, any new diagnosis of CM should include a work-up for more serious underlying disease.
Table 2.
Clinical syndromes associated with vascular malformations
| Clinical syndrome | Vascular malformation | Location |
|---|---|---|
| Sturge–Weber | Capillary malformation | Trigeminal distribution, leptomeninges, choroid, oral mucosa |
| Klippel–Trenaunay | Capillary malformation, venous malformation, lymphatic malformation, arteriovenous malformation | Extremities, pelvis, trunk |
| Parkes Weber | Arteriovenous malformation, capillary malformation | Extremities, trunk |
| Hereditary haemorrhagic telangiectasia | Telangiectasia, visceral arteriovenous malformation, angioma | Skin, mucous membranes, brain, spinal cord, visceral organs |
| Von Hippel–Lindau | Haemangioma | Retina, cerebellum |
| Blue rubber bleb naevus | Cavernous venous malformation | Skin, GI tract, spleen, liver, CNS |
| Kasabach–Merritt | Cavernous venous malformation | Trunk, extremities |
| Maffucci's | Arteriovenous malformation, cavernous lymphangioma | Fingers, toes, extremities, viscera |
CNS, central nervous system; GI, gastrointestinal.
Data taken from Gloviczki et al.18
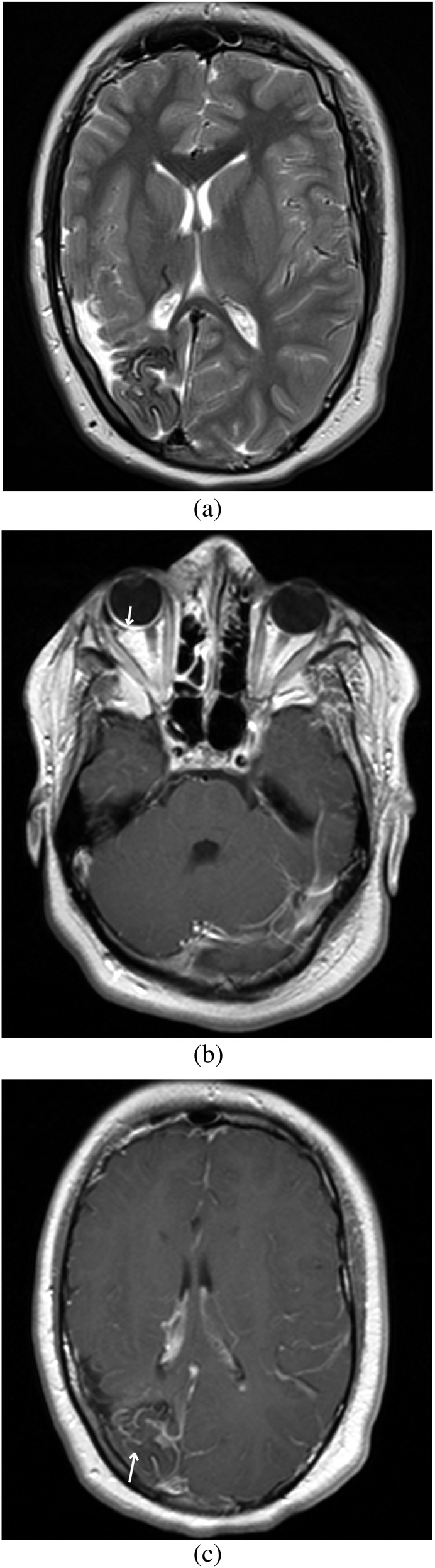
CMs are diagnosed clinically, with the identification of a flat, pink, blanching lesion. However, further work-up with MRI is required when CM occurs on the face, as this increases the risk of having Sturge–Weber syndrome (Figure 3a,b). In a recent study of 289 patients with facial CM, 15 (5%) were diagnosed with Sturge–Weber sydrome.19 Furthermore, patients with CMs appearing within the V1 (first branch of the fifth cranial nerve) distribution of the trigeminal nerve (forehead and area adjacent to the orbit) have increased risk of Sturge–Weber syndrome ranging from 7% to 28%.20,21 In these cases, imaging is required to assess for neurological involvement. Contrast-enhanced T1 weighted MRI classically shows leptomeningeal vascular malformation (Figure 3c), while dilation and enhancement of the ipsilateral choroid plexus is often seen in older children and adults.22
Figure 3.
MRI of brain. (a) T2 weighted axial image demonstrates atrophy of the right parietal and occipital lobes with gyriform hypointensity indicative of calcification in a known case of Sturge–Weber syndrome. (b) T1 weighted post-contrast axial image demonstrates thick enhancement of the right retinal region, suggestive of retinal angiomatosis (arrow) in a known case of Sturge–Weber syndrome. (c) T1 weighted post-contrast axial image demonstrates atrophy of the right parietal and occipital lobes with leptomeningeal enhancement suggestive of pial capillary malformation (arrow), with dilation and enhancement of the ipsilateral choroid plexus.
CMs are treated in early childhood to prevent inevitable thickening and hypertrophy leading to disfigurement. The gold standard treatment is pulsed dye laser therapy, whereby haemoglobin absorbs laser light and converts it to heat causing blood vessel coagulation. Through a process called selective photothermolysis, which determines proper laser wavelength, duration, fluency and spot size, photodynamic therapy selectively treats the lesion while sparing the surrounding tissue.23
While pulsed dye laser is still the preferred initial treatment for CM, the results are often suboptimal. Multiple laser therapy sessions are required and only 10% of patients experience complete resolution,24 while 20–30% of CMs are completely resistant.25 Treatment failures result from increased epidermal melanin concentration, increased vascular density within the lesion and increased vessel depth. These variables reduce optical penetration depth and fluency, thereby reducing energy transfer to levels below those needed for coagulation.26 To mitigate these factors, new techniques, including vacuum-assisted laser therapy27 and epidermal cooling,28,29 have been developed. Although these techniques utilize different mechanisms, all serve to improve light delivery to the CM, thus increasing the temperature within the vessels to augment coagulation. Several alternative modalities, including intense pulse light and photodynamic therapy, have proven beneficial for treatment-resistant CMs, although a significant response or complete resolution is only seen in approximately 50% of patients for both modalities.30–33
VENOUS MALFORMATIONS
Venous malformations (VMs) are the most common vascular malformation, accounting for 44–64% of all vascular malformations.34 They are classified according to the Hamburg classification35 as truncular or extratruncular, with 40% of lesions localized to the extremities, 20% on the trunk and 40% on the head and neck.34 VMs consist of small or large dysplastic venous channels with minimal connection to adjacent veins. These slow-flow lesions usually occur sporadically and are focal in 99% of cases.36 Clinically, they present as bluish lesions that expand with valsalva manoeuvre and following compression. In addition, they often cause bleeding and adjacent skin colour changes, and they lead to pain when there is congestion or clot within the VM.
VMs are classified into focal and diffuse lesions to aid treatment planning and determine prognosis. Focal VMs reside within one layer of tissue: muscle, skin or mucosa. Typically, these lesions are sequestered and drain into normal adjacent conducting veins through small channels. By contrast, diffuse malformations involve multiple tissue layers and usually include muscle, subcutaneous fat and skin. Unlike focal VMs, diffuse VMs communicate with main conducting veins, increasing the risk of systemic toxicity following treatment. While focal VMs are effectively treated with sclerotherapy, diffuse VMs require multiple treatment sessions and are more likely to recur.
VMs are typically benign and are managed conservatively with compression devices to prevent thrombosis and pain. Only when VMs lead to pain or swelling, involve the joints or cause disfigurement do they require further diagnostic work-up for definitive treatment. Pre-treatment analysis of VMs determines the size of the lesion, its involvement with adjacent tissue and the vessel diameter and flow rate, as each has prognostic implications for treatment efficacy and complication risks. Specifically, diffuse VMs with high-flow communication to conducting veins or adjacent arteries correlate with increased risk of systemic toxicity and lesion recurrence.
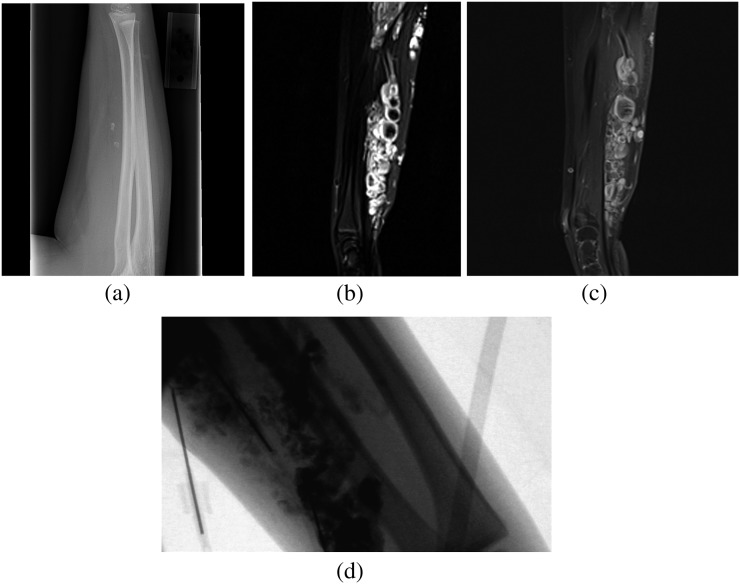
MRI is the primary imaging modality for assessing VMs. T2 weighted short tau inversion–recovery (STIR) imaging provides the best sequence for assessing the extent of the VM and the vascular flow rate and will demonstrate a soft tissue with phleboliths (Figure 4a). Additionally, lesions with large vascular channels appear cyst-like, hyperintense and septated, whereas lesions with smaller vascular channels are more solid with intermediate signal intensity (Figure 4b). On T1 weighted imaging, the lobulated area has variable signal intensity with regional fat hypertrophy. Following contrast injection, T1 weighted imaging demonstrates homogeneous or heterogeneous enhancement within the lesion (Figure 4c).37 MRI is also useful in determining adjacent tissue involvement, as vascular malformations can be circumscribed or trans-spatial with infiltrates into adjacent soft tissue or the lymphatic system.
Figure 4.
(a) Radiograph of the right forearm, lateral view, shows several calcified foci suggestive of phleboliths. (b) MRI of the right forearm. T2 weighted coronal and sagittal short tau inversion–recovery images show multifocal well-defined multilobulated hyperintense soft-tissue lesions in the right forearm, which show robust enhancement on the fat-saturated T1 post-contrast sagittal image (c) suggestive of venous malformation. (d) Injection of foamed 3% sodium tetradecyl sulfate with contrast into a right forearm venous malformation under fluoroscopic guidance.
CT and Doppler ultrasound also provide useful adjuncts for VM evaluation. CT is the modality of choice to identify phleboliths and when there is concern of bony invasion, although it lacks the soft-tissue resolution to serve as an adequate primary imaging modality. Doppler ultrasound in conjunction with MRI enhances characterization of vascular anatomy and venous flow. Lesions with small vascular channels are more echogenic and less compressible than lesions with large vascular channels (Figure 5). In addition, phleboliths will appear as hyperechoic foci with acoustic shadowing.38 Rarely, magnetic resonance venography imaging can also be useful if there is suspicion of associated intracranial venous anomalies.
Figure 5.
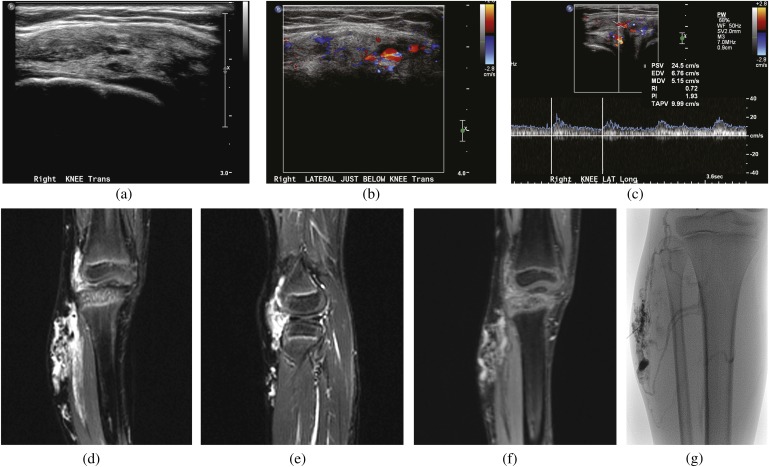
(a) Ultrasound of a right knee venous malformation: transverse image shows a mixed echogenic soft-tissue lesion which shows intrinsic colour flow (b) and low spectral waveforms within it (c). On MRI T2 short tau inversion–recovery coronal and sagittal images, this lesion is multifocal, well-defined T2 hyperintense involving subcutaneous and intramuscular soft tissue (d, e) and shows robust enhancement on post-contrast imaging (f). (g) Injection of 98% concentrated ethanol into a right knee venous malformation under fluoroscopic guidance.
Sclerotherapy is the first-line treatment for VMs and functions by destroying vascular endothelial cells within the lesion (Figures 4d and 5g). The goal of sclerotherapy is to maximize both the agent concentration and duration within the endothelial vessel lumen. The low flow rate of VMs makes sclerotherapy an effective treatment, allowing effective concentrations of sclerosing agents to remain nearly constant when delivered directly to a VM.
Concentrated ethanol is one of the most common and well-studied sclerosing agents for VMs. Composed of 98% ethanol, it is the most aggressive sclerosing agent and works by inducing protein precipitation within vascular endothelial cells leading to cell dehydration and instant thrombosis.39 This approach has proved to be an effective treatment for VMs, with one large study reporting a 95% success rate after 2 years of follow-up.40
Although ethanol is the most potent sclerosing agent and probably the most effective, it is associated with the highest complication rates.41 Skin complications, including necrosis, pain and blistering, are the most common side effects occurring in 8% of patients.40,42 Local ethanol toxicity can also cause peripheral nerve injury in 2–10% of patients.40,43 In addition, systemic side effects can occur when concentrated ethanol makes its way into the systemic circulation. Compartment syndrome,44 arrhythmia,45 pulmonary embolism,46 pulmonary hypertension with right heart failure47 and haemoglobinuria48 after ethanol sclerotherapy have all been reported in the literature.
To reduce complication rates, care should be taken to minimize ethanol introduction into the systemic circulation by reducing venous outflow from the lesion and limiting the ethanol dose. Proximal tourniquets, compression stockings and balloon occlusion are all acceptable methods of reducing outflow in lesions with significant draining veins.49 Research on ethanol dosing schemes is focused primarily on AVMs, but a generally acceptable ethanol dose is 1 ml kg−1 body weight and limits infusion rates to 0.1 ml kg−1 per 10 min.39 More recent studies of AVM patients have shown bolus ethanol injections of >0.14 ml kg−1 body weight can significantly increase pulmonary artery pressure, with the most prominent rise occurring both immediately after the first bolus and again during post-procedure recovery.50,51 Therefore, care should be taken in determining the total dose and the bolus dose of ethanol given during treatment, particularly when VMs have prominent draining veins. In addition, pulmonary artery pressure monitoring and nitroglycerin infusion are recommended during post-procedure recovery.51 Furthermore, adequate hydration before therapy helps manage haemoglobinuria, and sodium bicarbonate fluid in 5% dextrose solution should be run at twice the maintenance rate following the procedure until the urine is clear. Despite these protective measures, ethanol sclerotherapy should not be the first-line therapy for cutaneous lesions when there are major nerves adjacent to the lesion or when there is high-flow communication between the VM and the systemic circulation.
Common alternatives to concentrated ethanol are sodium tetradecyl sulfate (STS) and polidocanol. Like ethanol, these agents damage vascular endothelial cells causing fibrosis, thrombosis and collapse of the vessel.52,53 The major benefit of STS and polidocanol is that they are safer than ethanol. However, the improved safety profile of these agents comes at the cost of lower potency than ethanol. In previous studies, STS has been associated with success rates of 43–84%,52,54–56 whereas polidocanol has similarly reported ranges of 45–84%.57,58 Importantly, the definition of an adequate response as well as the lesion size, anatomical location and patient population is highly variable among previous studies, and, thus, it is difficult to ascertain the true efficacy of STS and polidocanol for VM treatment.
The main safety benefit of STS and polidocanol is that they are not considered neurolytic agents and do not place patients at risk for pulmonary hypertension.52,59 The risk of major complications is difficult to ascertain given a paucity of literature focused directly on STS and polidocanol treatment of VMs, although rare occurrences of anaphylaxis60 and cardiovascular deterioration61,62 have occurred with both. However, the majority of complications with both STS and polidocanol are minor, with pain at the injection site and local skin necrosis being the most common53,63,64 and likely related to tissue extravasation with damage of adjacent vessels at the injection site.64,65 A recent study has shown greater efficacy with foamed vs liquid injection of polidocanol,57 and new guidelines suggest both polidocanol and STS should be administered in foam form.66 The use of foam instead of liquid allows greater volume coverage and increased surface area contact with the vessel endothelium, while also slowing flow through the lesion to maximize contact with the vessel.67 Together, this permits lower doses of STS and polidocanol to be used, thus lowering the complication rate. The agents are typically foamed using the Tessari technique before administration. In this technique, a three-way stopcock adjoins two plastic syringes and the sclerosing agent plus 4–5 parts air are vigorously mixed between the two syringes to produce a microfoam.68 Although the application of foam STS and polidocanol improves therapeutic response, they remain less effective than ethanol sclerotherapy. However, they may be a better initial therapy than ethanol because of the lower risk of major complications, with ethanol reserved for larger less superficial VMs,52,69 and those resistant to STS and polidocanol.
When sclerotherapy is indicated, the initial work-up should begin with a thorough discussion with the patient regarding the goals of treatment and side effects. Regardless of the agent used, the patient should be aware that the initial goal is not total cure but a reduction in lesion size and associated symptoms. Patients should also be aware that multiple therapy sessions are likely to be required70 and may require treatment with multiple types of sclerosing agents.
Depending on the sclerosant used and the age of the patient, sclerotherapy of VMs may be performed under general anaesthesia or conscious sedation. Ethanol and STS generally require general anaesthesia because of immediate pain and swelling associated with their use; weaker agents such as bleomycin, morrhuate-sodium and hypertonic saline can usually be tolerated under conscious sedation by adult patients. Under sterile conditions, a needle or cannula such as an angiocath (usually 20–25 gauge) is inserted into the VM under direct vision or ultrasound guidance. If the malformation is close to the skin surface, the needle or cannula should be tracked through at least 1–2 cm of normal tissue before entering the lesion to reduce the likelihood of skin blistering and leakage around the puncture site. When blood return is noted, iodinated contrast is injected under fluoroscopy to confirm that the needle is in the malformation, to ensure there is no extravasation into normal tissues and to look for communication between the malformation and normal veins. If such a communication occurs, it can often be prevented by manual compression of the communicating normal vein; rarely, it may be necessary to place a platinum or fibre coil to block the venous communication. Once a proper needle location is confirmed, the sclerosing agent is injected under fluoroscopic observation. Since these agents are not radio-opaque, they need to be mixed with a substance such as ethiodized oil, room air or carbon dioxide. Opacification of the sclerosant using iodinated contrast is usually avoided because it dilutes and weakens the agent; exceptions are doxycycline and bleomycin, which are packaged as a powder. Thrombosis and vascular occlusion will occur shortly after the sclerosant is injected, preventing aspiration of blood from the needle. The ability to aspirate blood indicates that the treatment is incomplete and that more agent should be given within that vascular territory.71 An alternative method is to insert one or more additional needles or cannulae into targeted areas of the malformation after it has been opacified by the initial injection of iodinated contrast. The injection of the foamed or non-foamed sclerosant is then monitored by direct fluoroscopy or digital fluoroscopic angiographic angiography until it exits through the added needle or cannula confirming that treatment of the malformation is complete.72,73 For palpable lesions, attention should be paid to the amount of induration of the VM during sclerotherapy,74 and the cessation of treatment should occur when lesions become firm, as this suggests complete closure of the vascular channels. Finally, the physician should always look for signs of local extravasation or systemic circulation of the sclerosing agents. Common clues include adjacent skin erythema and blanching or significant changes in patient haemodynamics, including hypertension and arrhythmias.
The most common post-procedure side effects of sclerotherapy are pain and inflammation. The degree and duration of pain vary depending on the size and location of the lesion and sclerosing agent used, although paracetamol is usually adequate for pain control.75 Inflammation is greatest within the first 24 h, and non-steroidal anti-inflammatory drugs are usually adequate anti-inflammatory medications. Dexamethasone at a rate of 0.1 mg kg−1 every 8 h is sometimes required for larger lesions or after ethanol sclerosis, as this can cause greater swelling.76 Despite these side effects, most patients are able to return home the same evening as the procedure.
The follow-up management of sclerotherapy patients should be tailored to the specific patient and varies depending on the size, characteristics and location of their VM, as well as the sclerosing agent used. Fibrosis of the VM typically continues for 1–2 months following treatment. Thus, to maximize each treatment, follow-up visits should be scheduled every 2–3 months to assess results and determine future treatment options. Repeat MRI and ultrasound should be employed to assess interval changes in lesion size and blood flow.77,78 However, the ultimate determining factor of treatment success and future therapy should be based on patient satisfaction and symptomatic improvement and not imaging findings.
LYMPHATIC MALFORMATIONS
Lymphatic malformations (LMs) account for approximately 2.8/100 000 hospital admissions79,80 and present before the age of 2 years in 90% of cases.81 They are low-flow vascular malformations consisting of malformed lymphatic channels within the lymphatic system and are cystic in nature. LMs can be divided by size into macrocystic (>1 cm) and microcystic (< 1 cm) groups. Typically, microcystic LMs are more common and present later than macrocystic LMs, which are usually present at birth.
The appearance of LMs depends on their location. Superficial LMs are typically skin-coloured masses and ballotable on palpation, whereas they appear as red or yellow blisters when involving mucous membranes.82 LMs have a predilection for the head and neck with 70–80% of cases in this location, and an additional 20% occurring in the axilla and a minority of cases occurring in the superior mediastinum, mesentery, retroperitoneum, pelvis and lower limbs.83 They typically grow slowly, although rarely LMs can rapidly expand in size after intralesion haemorrhage or superinfection.80 In these cases, LMs can become life threatening if they cause adjacent mass effect and compression of vital structures.
The evaluation of LMs is highly reliant on imaging to discern the size and characteristics of the lesion, as these directly dictate treatment strategies and outcome. As with other vascular anomalies, MRI is the best imaging modality for diagnosing and defining LMs because of its superior soft-tissue resolution. Microcystic and macrocystic LMs have distinct appearances on MRI owing to the size difference in their cystic component. On T2 weighted MRI and T2 weighted STIR imaging, LMs are typically well defined, lobulated and hyperintense (Figure 6a,b). T1 weighted imaging of macrocystic LMs typically shows a septated cystic mass that ranges from hypointense37 to isointense84 and that is often heterogeneous owing to proteinaceous fluid85 or fluid–fluid levels.86 Post-contrast T1 weighted images are also important tools for differentiating macrocystic LMs from VMs, as LMs typically do not enhance after contrast injection unlike VMs (Figures 6c,d and 7).84 By contrast, the small cystic compartments of microcystic LMs appear as diffuse hypointensity on T1 weighted imaging and diffuse hyperintensity on T2 weighted imaging.37
Figure 6.
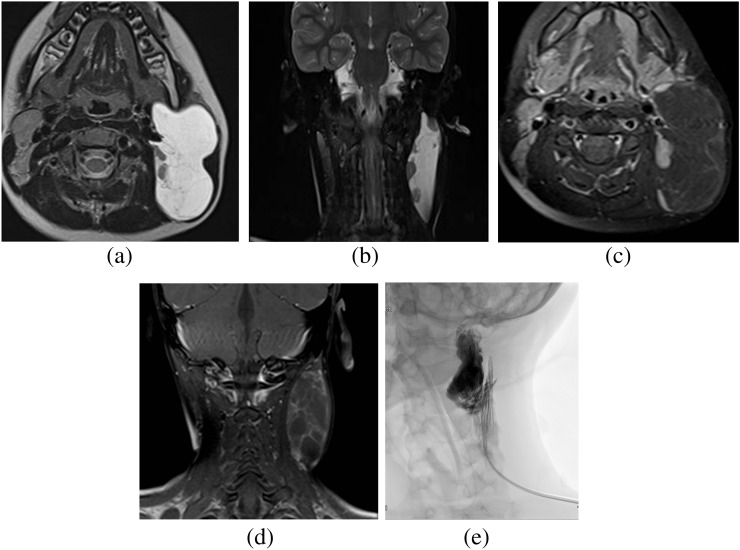
MRI of neck. (a, b) T2 weighted axial and T2 weighted short tau inversion–recovery coronal images show a well-defined lobulated and macrocystic T2 hyperintense lesion around the left angle of the mandible. (c, d) There is enhancement only in the internal septa on T1 fat-saturated post-contrast images, suggestive of a macrocystic lymphatic malformation. (e) Injection of dehydrated alcohol into a 6-French drain after cystic drainage. The dehydrated alcohol was allowed to dwell for 15 min before being removed and the procedure repeated.
Figure 7.
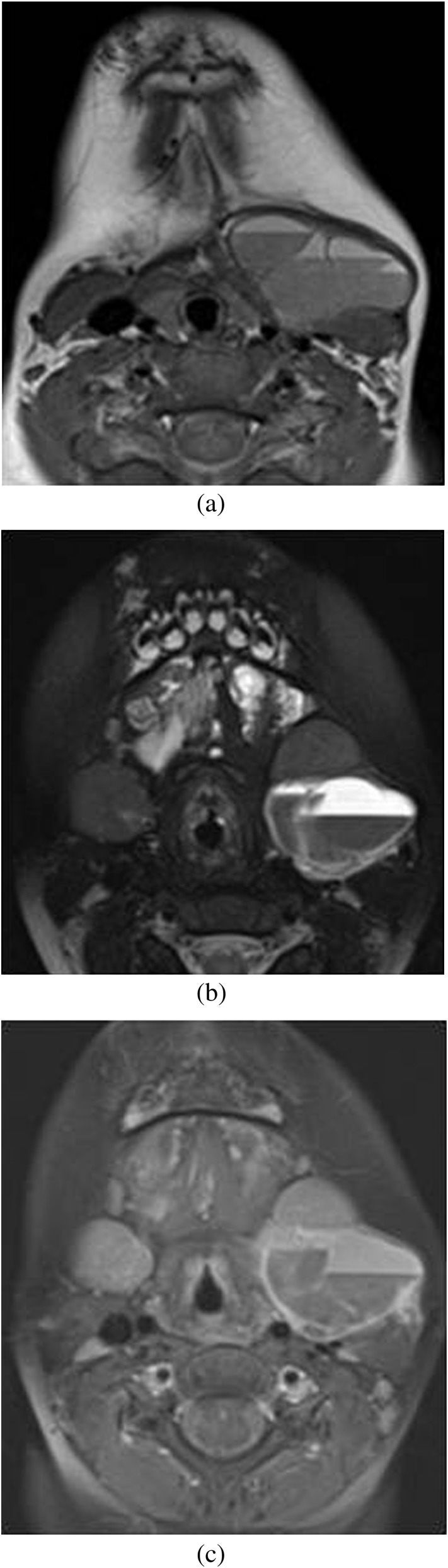
(a) MRI of neck. T1 weighted axial image shows a well-defined macrocystic lesion anterior to the proximal left internal and external carotid arteries, with internal fluid–fluid levels, multiple septa and intrinsic T1 hyperintensity. Additionally, there are well-defined T2 hyperintense multifocal lesions in the left base of the tongue and pre-mandibular soft tissue in the midline, seen on the T2 weighted axial short tau inversion–recovery image suggestive of venous malformation (b). These show peripheral enhancement of the cystic lesion and intrinsic enhancement of the adjacent abnormal T2 hyperintense soft-tissue lesion on T1 fat-saturated post-contrast image (c), suggestive of venolymphatic malformation.
Rarely, other imaging modalities may be needed to further define the lesion or differentiate an LM from other vascular anomalies. Ultrasound is a particularly useful method for discriminating LMs from VMs. Macrocystic LMs typically manifest as anechoic spaces divided by septa,87 whereas microcystic LMs appear hyperechoic owing to their small cavity size.88 Ultrasound is also useful for evaluating prior haemorrhage and demonstrating fluid–fluid levels. In addition, Doppler examination of LMs demonstrates no flow through the lesion, which helps distinguish them from VMs, where flow is seen in 85% of cases.38 Conventional radiology can also be useful in the work-up of large LMs near bone, as they can cause bony hypertrophy and bone warping.89 Although usually not necessary for the diagnosis of LMs, CT imaging demonstrates a fluid-filled low-attenuation mass with little contrast enhancement.85 In cases of intralesion haemorrhage, fluid–fluid levels may be visible on CT.
Treatment indications for LMs are similar to those of other vascular malformations, with lesions located in life-threatening areas such as retropharyngeal LMs requiring prompt treatment. Any LM that has experienced a haemorrhagic or infectious event should also be treated and requires antibiotics and steroids in addition to definitive treatment of the LM. Relative indications for treatment include patient discomfort, impaired mobility, aesthetic dissatisfaction or any other cause that significantly impairs quality of life. Importantly, lesion size also plays a significant role in dictating management. The large cyst compartments within macrocystic LMs provide accessible targets for percutaneous intervention and are effectively treated by sclerotherapy. By contrast, microcystic LMs are difficult to treat and are typically managed conservatively.
Sclerotherapy is the most common treatment modality for macrocystic LMs. Commonly used sclerosing agents are similar to those used for VMs and include ethanol, STS, bleomycin and doxycycline. Currently, there is no consensus regarding what sclerosing agent is most effective in treating macrocystic LMs, although bleomycin is perhaps the most studied sclerosing agent. The rate of successful bleomycin treatment, defined as >50% reduction in lesion size, varies between 80% and 95%.90–93 Yet, the percentage of patients who experience complete disappearance of their lesion is considerably lower, ranging between 36% and 53%,90,92,93 although one study reported complete removal of the lesion in 87% of patients.91 Bleomycin is well tolerated by most patients with local inflammation as the most common side effect.94 Furthermore, the risk of pulmonary fibrosis and interstitial pneumonia is negligible because the sclerotherapy dose of bleomycin is well below the 400-mg cumulative dose associated with these lung findings.95
Doxycycline has also been shown to be an effective sclerosant agent with complete lesion resolution seen in 70–83% of patients with macrocystic LMs.96,97 Doxycycline is associated with tooth discoloration in young children, acidosis, hypoglycaemia and prominent local inflammation and pain. However, the complication rate after sclerotherapy with doxycycline is low97,98 and is likely to be proportional to the total dose of doxycycline used.98 Ethanol is also an effective therapy, although post-procedural pain and increased risk of permanent complications should limit its use as a first-line therapy.99 Finally, OK-432 (pincinabil) is an emerging sclerosing agent that effectively treats LMs with minimal post-procedure fibrosis.100–102 Currently, OK-432 is not approved for use within the USA, although there is an ongoing multicentre trial being conducted.103
Microcystic LMs are much less amenable to therapy because of their small cyst size and are typically managed conservatively. However, microcystic LMs that become symptomatic or threaten vital structures and those that cause significant aesthetic complications should be treated. Surgery is the preferred first-line management although microcystic LMs recur in 40% of patients with incomplete excision and in 17% of patients with complete resection.104 Sclerotherapy is typically ineffective in treating microcystic LMs, although positive treatment outcomes have been reported with doxycycline,105 bleomycin91 and OK-432.106 In addition, an intermediate response in 50% of cases has also been observed.94 Thus, sclerotherapy may provide benefit to patients with symptomatic and/or large microcystic LMs.
Although the sclerosing agents used for LMs overlap with VMs, the sclerotherapy technique used differs in several key aspects. First, individual cysts should be identified with ultrasound guidance, and each cyst cannulated with angiocatheters or pigtail catheters for larger cysts. Fluid within the cyst should then be completely aspirated before injecting the sclerosing agent. This allows analysis of the aspirant to confirm the diagnosis. In addition, complete removal of the fluid allows an increased volume of sclerosing agent to be injected while also increasing the surface area of the cyst wall in contact with the drug. After fluid removal, the sclerosing agent is administered to the cystic compartment under fluoroscopy. Sclerosing agents are left within the cyst for a variable amount of time depending on which agent is used and, typically, multiple treatments of injection and drainage are required (Figure 6e).94,105 For large lesions with multiple cysts, the catheter is often secured in place, injecting and draining cysts sequentially for several days.
Post-procedural inflammation and oedema are the most common complications of sclerotherapy and can sometimes be significant.90 When lesions are near vital structures or may compromise the airway, bleomycin should be used because it is associated with lower amounts of inflammation.94 However, the benefits and risks of treatment should be weighed for LMs located near vital structures, as almost all sclerotherapy procedures will incur some degree of oedema, in addition to the risks of infection and bleeding.
ARTERIOVENOUS MALFORMATIONS
AVMs are vascular malformations composed of arteries and veins that directly communicate through a central nidus. Thus, AVMs bypass the high resistance of capillary beds, leading to a high-flow lesion that shunts blood from arterial to venous circulation. The lesions are present at birth in 40% of cases,107 and their clinical course can be traced according to the Schobinger clinical staging system (Table 3).108 They typically present as latent lesions during childhood that evolve into a warm pink-bluish skin lesion with a pulsatile thrill in adolescence. Over time, the lesion continues to expand leading to dystrophic skin changes, bleeding, ulceration and tissue necrosis. If left untreated, high flow into the venous system eventually leads to volume and pressure overload within the heart and subsequent heart failure.
Table 3.
Schobinger clinical staging system of arteriovenous malformations
| Stage | Description |
|---|---|
| (I) Quiescence | Pink-bluish stain, warmth and arteriovenous shunting are revealed by Doppler scanning. The arteriovenous malformation mimics a capillary malformation or involuting haemangioma |
| (II) Expansion | Same as Stage I plus enlargement, pulsation, thrill, bruit and tortuous/tense veins |
| (III) Destruction | Same as Stage II plus dystrophic skin changes, ulceration, bleeding, persistent pain or tissue necrosis. Osteolytic lesions may occur |
| (IV) Decompensation | Same as Stage III plus high-output congestive heart failure and left ventricular hypertrophy |
Like other vascular malformations, imaging is vital to characterizing AVMs and subsequent treatment planning. However, high-flow AVMs have a markedly different appearance from low-flow vascular malformations. On MRI, AVMs appear as a tangled mesh of dilated arteries and veins connected by linear or focal shunts. These vessel networks demonstrate flow void on T1 and T2 weighted spin echo imaging (Figure 8a,b)85 and appear hyperintense on T2 weighted gradient echo imaging and angiography, indicating rapid flow through the lesion.109 Unlike other vascular malformations, AVMs lack enhancement of adjacent soft tissue on T2 weighted imaging except when significant oedema is present.37,86 T1 weighted post-contrast images are also useful in defining the AVM morphology, demonstrating contrast enhancement of multiple vessels with early venous enhancement.110 MRA has also proved an important asset in the pre-treatment work-up of AVMs. MRA has high temporal resolution that demonstrates arterial feeders, shunting volume and the location and size of the nidus (Figure 8c).111,112 In addition, MRA has proven invaluable to post-procedural assessment of shunt occlusion by accurately demonstrating venous filling times.111
Figure 8.
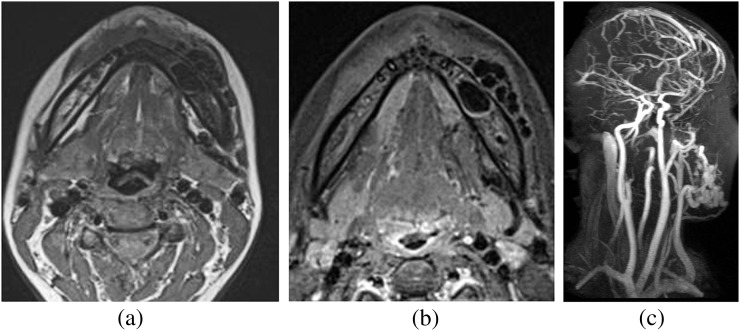
MRI of neck. (a) T1 weighted axial image shows a tangle of flow voids in and around the left body of the mandible, which is also seen on the T2 weighted short tau inversion–recovery axial image (b), suggestive of arteriovenous malformation. On contrast-enhanced MR angiography of head and neck (c), it shows a feeding artery from the facial artery and drainage into the external jugular vein.
AVMs also have distinct findings on other imaging modalities. Ultrasound and Doppler imaging show high systolic and diastolic flow, prominent arteriovenous shunting and arterial waveforms within venous structures suggesting pulsatile flow.85 Unlike other vascular malformations, CT imaging can also provide useful information in AVM assessment. Contrast-enhanced helical CT allows accurate structural assessment of arteries, veins and the nidus, in addition to providing flow analysis important for pre-procedural planning.113 Finally, conventional radiography will sometimes demonstrate erosive and intraosseous changes to adjacent bones.89,114
High-flow lesions like AVMs are the only vascular malformations that require diagnostic angiography.6 The classic appearance of AVMs on angiography demonstrates multiple dilated feeding arteries with early opacification of enlarged draining veins. Angiography also allows visualization of the nidus, which defines the point where feeding arteries first opacify outflow veins.41,115 When embolization is done concurrently with angiography, additional feeding arteries often become apparent after the original vessels are embolized.
Treatment of AVMs depends on the severity of related symptoms and lesion location. Patients with Schobinger stage I and II AVMs are typically managed conservatively with initial diagnostic work-up and subsequent annual follow-up evaluation. Definitive treatment for AVMs is generally reserved for stage III and IV lesions, which typically corresponds with the development of recurrent bleeding, pain, tissue necrosis or high-output cardiac failure. In a study by Liu et al,116 extracranial AVM progression requiring treatment was more commonly seen in adolescence than childhood. The same study also demonstrated an 82.6% risk of progression before adulthood, reiterating that most patients will require treatment early in life.
Embolization alone or in conjunction with surgical resection is the primary treatment option for AVMs. The high-flow nature of these lesions makes them difficult to treat, as sufficient dosage and lesion contact time must be maintained in the presence of collateral flow through high-output vessels. The goal of embolization is to occlude the nidus while preventing the formation of collateral flow to the AVM. As such, embolization should occur only within the nidus and very distal segment of feeding arteries, as proximal embolization may preclude access to more distal feeding branches that may recanalize from other sources. Angiography should be performed after embolization of every feeding vessel to identify other feeding vessels and ensure complete disruption of flow to the nidus. When possible, percutaneous direct puncture of the nidus should also be performed for direct embolization.110 In cases where embolization of the feeding arteries and/or direct embolization of the nidus is not feasible or is unsuccessful, embolization of the nidus by retrograde injection of the embolic agent in an occluded dominant draining vein is often successful. Once complete occlusion of the nidus has occurred, patients should undergo surgery for complete resection, as this offers the best chance for preventing recurrence.
Common embolic agents for AVM treatment include ethanol, n-butyl cyanoacrylate glue (n-BCA), Onyx® (Microtherapeutics, Inc., Irvine, CA) and coils. However no consensus exists regarding the most effective treatment, and each agent has its own pros and cons. The sclerosing agent ethanol has been reported to provide complete cure in 40–88% of patients.46,117,118 Yet, ethanol embolization is associated with high complication rates of 16–52%.46,119 Furthermore, ethanol can cause fatal pulmonary hypertension, and this risk is likely to be increased in the setting of high-flow AVMs. n-BCA and Onyx may provide a safer alternative to ethanol while still providing an effective treatment. The efficacy of Onyx is well reported for intracranial AVMs, demonstrating a 63% reduction in AVM volume and >50% reduction in volume occurring in 96% of patients.120 In addition, Onyx and n-BCA have demonstrated fewer complications than ethanol therapy, with morbidity and mortality rates of 4.8–17.0% and 0–2%, respectively.120–122 Yet, these agents are associated with a revascularization rate ranging from 11.8% to 18.0% in embolized vessels,123,124 suggesting that these are not effective single agents for curative treatment.
Coil embolization is an additional option for embolization of AVMs. The efficacy of coil embolization varies by type of coil used and the location and morphology of the AVM. However, the general mechanism of coil embolization involves direct occlusion of arteries and veins leading to thrombosis. This method has been criticized because it does not cause damage to blood vessel endothelium, thus allowing a higher rate of recanalization.125 For this reason, sclerosing agents such as ethanol are often combined with coil embolization.126 Complications associated with coil embolization include coil reflux, AVM perforation and systemic embolization of a coil, although these are likely to be rare events.126,127 Yet, regardless of which agent is used, embolization treatment alone is rarely curative without subsequent surgery.46
CONCLUSION
The role of imaging and interventional radiology has become increasingly vital to the management of vascular malformations, with significant success in improving patient morbidity and mortality. Despite the advent of improved imaging and treatment modalities, the cure rate remains suboptimal for most types of vascular malformations. This underscores the need for further research into new sclerosing and embolic agents to continue improving care.
APPENDICES
| Vascular anomalies overview |
|---|
| Infantile haemangiomas |
| Presentation: appear within 6 weeks of life. Begin as telangiectasia that progress to a raised strawberry-coloured plaque |
| Imaging: Doppler ultrasound: well-circumscribed mass with variable heterogeneity with associated high-flow vessels and an arterial feeder. MRI/MR angiography: contrast enhancement with adjacent flow voids. Useful in cases requiring surgical management |
| Treatment: typically resolve spontaneously with minimal residual skin discoloration or scarring. Propranolol is first-line treatment for small lesions not adjacent to vital structures. Surgery reserved for lesions at risk for significant scarring and those located near the orbit |
| Prognosis: lesions requiring surgery have good prognosis if treated in proliferative phase before scarring or damage to adjacent tissue occurs |
| Congenital haemangiomas (rapidly involuting congenital haemangiomas and non-involuting congenital haemangiomas) |
| Presentation: both present at birth and most commonly found on head, neck and extremities |
| Rapidly involuting congenital haemangiomas (RICHs)—raised grey-blue lesion with prominent telangiectasia and central depression, ulceration or scar |
| Non-involuting congenital haemangiomas (NICHs)—pink/purple macule with prominent telangiectasia and central blue pallor |
| Imaging: similar to infantile haemangioma. Ultrasound may show vascular aneurysms and intravascular thrombi, which helps differentiate from infantile haemangioma |
| Treatment: RICH—observation. NICH—surgical response (limited evidence suggests poor response to embolization) |
Proliferating vascular tumors
| Capillary malformation |
|---|
| Presentation: “port wine stain”. Flat, pink, blanching lesion |
| Imaging: only required for facial lesions or other finding suspicious for Sturge–Weber suspected. Head MRI: leptomeningeal vascular malformation, calcification, retinal angiomatosis |
| Treatment: First line: pulsed dye laser; typically requires multiple sessions. Alternatives: intense pulse light; photodynamic therapy |
| Prognosis: highly variable: 10% complete response; 20–30% are completely resistant |
| Venous malformation |
| Presentation: blue-tinted lesions that expand with Valsalva and compression. Associated with frequent bleeding, adjacent skin discoloration and pain |
| Imaging: T2weighted short tau inversion–recovery: preferred MR sequence to evaluate lesion size and vascular flow rate. Typically demonstrates hyperintense, septated, cystic lesions, often with phleboliths. T1 weighted MR with contrast demonstrates heterogeneous enhancement |
| Treatment: First line: compression devices for benign lesions. Sclerotherapy is first line for painful lesions and those causing disfigurement. Common agents include: concentrated ethanol, foamed sodium tetradecyl sulfate (STS), foamed polidocanol |
| Prognosis: Sclerotherapy is often curative for focal venous malformations (VMs), whereas diffuse VMs at increased risk of recurrence and require multiple treatments |
| Microcystic lymphatic malformations (<1 cm) |
| Presentation: present at birth, most commonly on the head and neck. Skin-coloured masses ballotable to palpation, with red or yellow blisters when involving the mucous membrane |
| Imaging: T1 weighted MRI: diffuse hypointensity. T2 weighted MRI: diffuse hyperintensity |
| Treatment: surgical resection. Reserved for lesions at risk of significant complication |
| Prognosis: Curative treatment rare and recurrence common |
| Macrocystic lymphatic malformations (>1 cm) |
| Presentation: less common, appear later in life and are more prone to haemorrhage and infection |
| Imaging: T1weighted MRI: septated cystic mass with hypo- to isointense heterogeneity that do not enhance with contrast. T2weighted MRI: well-defined, lobulated, hyperintense mass |
| Treatment: Sclerotherapy: bleomycin, ethanol, STS and doxycycline. Reserved for lesions at risk of significant complication |
| Prognosis: varies with lesion characteristics and sclerosing agent used. Typically good response, but rarely achieve complete resolution |
| Arteriovenous malformation |
| Presentation: warm pink-bluish lesions with a pulsatile thrill, can progress to dystrophic skin changes, bleeding, ulceration and necrosis. Associated heart failure in severe cases |
| Imaging: T1and T2weighted MRI: mesh of flow voids often with visible shunt. CT angiography and MR angiography: both enable arteriovenous malformation (AVM) flow analysis by determining the size and location of the AVM nidus, arterial feeders and shunt volume. Angiography: dilated arteries with early filling of enlarged draining veins beginning at the nidus |
| Treatment: embolization with or without surgical resection. Embolic agents: ethanol, n-butyl cyanoacrylate glue, onyx, coils. No definitive consensus exists regarding the most effective embolic agent |
| Prognosis: dependent on size, location and flow rate of lesion. Embolization followed by surgical resection provides best chance for curative treatment |
REFERENCES
- 1.Fevurly RD, Fishman SJ. Vascular anomalies in pediatrics. Surg Clin North Am 2012; 92: 769–800. doi: 10.1016/j.suc.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Marler JJ, Mulliken JB. Vascular anomalies: classification, diagnosis, and natural history. Facial Plast Surg Clin North Am 2001; 9: 495–504. [PubMed] [Google Scholar]
- 3.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982; 69: 412–22. [DOI] [PubMed] [Google Scholar]
- 4.Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 1997; 24: 701–10. [DOI] [PubMed] [Google Scholar]
- 5.Lee BB, Bergan JJ. Advanced management of congenital vascular malformations: a multidisciplinary approach. Cardiovasc Surg 2002; 10: 523–33. [DOI] [PubMed] [Google Scholar]
- 6.Ernemann U, Kramer U, Miller S, Bisdas S, Rebmann H, Breuninger H, et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol 2010; 75: 2–11. doi: 10.1016/j.ejrad.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 7.Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg 1983; 18: 894–900. [DOI] [PubMed] [Google Scholar]
- 8.Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 2008; 122: 360–7. doi: 10.1542/peds.2007-2767 [DOI] [PubMed] [Google Scholar]
- 9.Leaute-Labreze C, Sans-Martin V. [Infantile hemangioma]. Presse Med 2010; 39: 499–510. doi: 10.1016/j.lpm.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 10.Greene AK. Management of hemangiomas and other vascular tumors. Clin Plast Surg 2011; 38: 45–63. doi: 10.1016/j.cps.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Enjolras O, Mulliken JB, Boon LM, Wassef M, Kozakewich HP, Burrows PE. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg 2001; 107: 1647–54. [DOI] [PubMed] [Google Scholar]
- 12.Berenguer B, Mulliken JB, Enjolras O, Boon LM, Wassef M, Josset P, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol 2003; 6: 495–510. [DOI] [PubMed] [Google Scholar]
- 13.Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr 1996; 128: 329–35. [DOI] [PubMed] [Google Scholar]
- 14.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 2010; 163: 269–74. doi: 10.1111/j.1365-2133.2010.09848.x [DOI] [PubMed] [Google Scholar]
- 15.Sommers Smith SK, Smith DM. Beta blockade induces apoptosis in cultured capillary endothelial cells. In Vitro Cell Dev Biol Anim 2002; 38: 298–304. doi: [DOI] [PubMed] [Google Scholar]
- 16.Bennett ML, Fleischer AB Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol 2001; 137: 1208–13. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics 1976; 58: 218–22. [PubMed] [Google Scholar]
- 18.Gloviczki P, Noel AA, Hollier LH. Arteriovenous fistulas and vascular malformations. In: Ascher E, Hollier LH, Strandness DE, Towne JB, Calligaro K, Kent KC, et al., eds. Haimovici's vascular surgery. 5th edn. Oxford, UK: Blackwell Science; 2004. pp. 991–1014. [Google Scholar]
- 19.Piram M, Lorette G, Sirinelli D, Herbreteau D, Giraudeau B, Maruani A. Sturge-Weber syndrome in patients with facial port-wine stain. Pediatr Dermatol 2012; 29: 32–7. doi: 10.1111/j.1525-1470.2011.01485.x [DOI] [PubMed] [Google Scholar]
- 20.Ch'ng S, Tan ST. Facial port-wine stains: clinical stratification and risks of neuro-ocular involvement. J Plast Reconstr Aesthet Surg 2008; 61: 889–93. doi: 10.1016/j.bjps.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 21.Tallman B, Tan OT, Morelli JG, Piepenbrink J, Stafford TJ, Trainor S, et al. Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications. Pediatrics 1991; 87: 323–7. [PubMed] [Google Scholar]
- 22.Comi AM. Presentation, diagnosis, pathophysiology, and treatment of the neurological features of Sturge-Weber syndrome. Neurologist 2011; 17: 179–84. doi: 10.1097/NRL.0b013e318220c5b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983; 220: 524–7. [DOI] [PubMed] [Google Scholar]
- 24.Lanigan SW. Port-wine stains unresponsive to pulsed dye laser: explanations and solutions. Br J Dermatol 1998; 139: 173–7. [DOI] [PubMed] [Google Scholar]
- 25.Renfro L, Geronemus RG. Anatomical differences of port-wine stains in response to treatment with the pulsed dye laser. Arch Dermatol 1993; 129: 182–8. [PubMed] [Google Scholar]
- 26.Aguilar G, Choi B, Broekgaarden M, Yang O, Yang B, Ghasri P, et al. An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains. Ann Biomed Eng 2012; 40: 486–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco W, Childers M, Nelson JS, Aguilar G. Laser surgery of port wine stains using local vacuum [corrected] pressure: changes in calculated energy deposition (Part II). Lasers Surg Med 2007; 39: 118–27. doi: 10.1002/lsm.20464 [DOI] [PubMed] [Google Scholar]
- 28.Hammes S, Roos S, Raulin C, Ockenfels HM, Greve B. Does dye laser treatment with higher fluences in combination with cold air cooling improve the results of port-wine stains? J Eur Acad Dermatol Venereol 2007; 21: 1229–33. doi: 10.1111/j.1468-3083.2007.02246.x [DOI] [PubMed] [Google Scholar]
- 29.Milanic M, Jia W, Nelson JS, Majaron B. Numerical optimization of sequential cryogen spray cooling and laser irradiation for improved therapy of port wine stain. Lasers Surg Med 2011; 43: 164–75. doi: 10.1002/lsm.21040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjerring P, Christiansen K, Troilius A. Intense pulsed light source for the treatment of dye laser resistant port-wine stains. J Cosmet Laser Ther 2003; 5: 7–13. [PubMed] [Google Scholar]
- 31.Raulin C, Schroeter CA, Weiss RA, Keiner M, Werner S. Treatment of port-wine stains with a noncoherent pulsed light source: a retrospective study. Arch Dermatol 1999; 135: 679–83. [DOI] [PubMed] [Google Scholar]
- 32.Qiu H, Gu Y, Wang Y, Huang N. Twenty years of clinical experience with a new modality of vascular-targeted photodynamic therapy for port wine stains. Dermatol Surg 2011; 37: 1603–10. doi: 10.1111/j.1524-4725.2011.02129.x [DOI] [PubMed] [Google Scholar]
- 33.Lu YG, Wu JJ, Yang YD, Yang HZ, He Y. Photodynamic therapy of port-wine stains. J Dermatolog Treat 2010; 21: 240–4. doi: 10.1080/09546630903200604 [DOI] [PubMed] [Google Scholar]
- 34.Loose DA. Surgical management of venous malformations. Phlebology 2007; 22: 276–82. [DOI] [PubMed] [Google Scholar]
- 35.Belov S. Classification of congenital vascular defects. Int Angiol 1990; 9: 141–6. [PubMed] [Google Scholar]
- 36.Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology 2010; 25: 224–35. doi: 10.1258/phleb.2009.009041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konez O, Burrows PE. Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am 2002; 10: 363–88. [DOI] [PubMed] [Google Scholar]
- 38.Trop I, Dubois J, Guibaud L, Grignon A, Patriquin H, McCuaig C, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 1999; 212: 841–5. doi: 10.1148/radiology.212.3.r99au11841 [DOI] [PubMed] [Google Scholar]
- 39.Yakes WF, Rossi P, Odink H. How I do it. Arteriovenous malformation management. Cardiovasc Intervent Radiol 1996; 19: 65–71. [DOI] [PubMed] [Google Scholar]
- 40.Lee BB, Do YS, Byun HS, Choo IW, Kim DI, Huh SH. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg 2003; 37: 533–8. doi: 10.1067/mva.2003.91 [DOI] [PubMed] [Google Scholar]
- 41.Legiehn GM, Heran MK. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am 2006; 37: 435–74. doi: 10.1016/j.ocl.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 42.Lee KB, Kim DI, Oh SK, Do YS, Kim KH, Kim YW. Incidence of soft tissue injury and neuropathy after embolo/sclerotherapy for congenital vascular malformation. J Vasc Surg 2008; 48: 1286–91. doi: 10.1016/j.jvs.2008.06.058 [DOI] [PubMed] [Google Scholar]
- 43.Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol 2004; 15: 431–45. [DOI] [PubMed] [Google Scholar]
- 44.Andreisek G, Nanz D, Weishaupt D, Pfammatter T. MR imaging-guided percutaneous sclerotherapy of peripheral venous malformations with a clinical 1.5-T unit: a pilot study. J Vasc Interv Radiol 2009; 20: 879–87. doi: 10.1016/j.jvir.2009.03.034 [DOI] [PubMed] [Google Scholar]
- 45.Mason KP, Michna E, Zurakowski D, Koka BV, Burrows PE. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology 2000; 217: 127–32. doi: 10.1148/radiology.217.1.r00se30127 [DOI] [PubMed] [Google Scholar]
- 46.Do YS, Yakes WF, Shin SW, Lee BB, Kim DI, Liu WC, et al. Ethanol embolization of arteriovenous malformations: interim results. Radiology 2005; 235: 674–82. doi: 10.1148/radiol.2352040449 [DOI] [PubMed] [Google Scholar]
- 47.Yakes WF, Krauth L, Ecklund J, Swengle R, Dreisbach JN, Seibert CE, et al. Ethanol endovascular management of brain arteriovenous malformations: initial results. Neurosurgery 1997; 40: 1145–52; discussion 52–4. [DOI] [PubMed] [Google Scholar]
- 48.Hammer FD, Boon LM, Mathurin P, Vanwijck RR. Ethanol sclerotherapy of venous malformations: evaluation of systemic ethanol contamination. J Vasc Interv Radiol 2001; 12: 595–600. [DOI] [PubMed] [Google Scholar]
- 49.Hyodoh H, Hori M, Akiba H, Tamakawa M, Hyodoh K, Hareyama M. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics 2005; 25: S159–71. doi: 10.1148/rg.25si055509 [DOI] [PubMed] [Google Scholar]
- 50.Shin BS, Do YS, Cho HS, Kim DI, Hahm TS, Kim CS, et al. Effects of repeat bolus ethanol injections on cardiopulmonary hemodynamic changes during embolotherapy of arteriovenous malformations of the extremities. J Vasc Interv Radiol 2010; 21: 81–9. [DOI] [PubMed] [Google Scholar]
- 51.Ko JS, Kim CS, Shin BS, Kim MJ, Lee JH, Kim KH, et al. Changes in pulmonary artery pressures during ethanol sclerotherapy for arteriovenous malformations: identifying the most vulnerable period. Clin Radiol 2011; 66: 639–44. doi: 10.1016/j.crad.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 52.Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edstrom S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol). Scand J Plast Reconstr Surg Hand Surg 1997; 31: 145–50. [DOI] [PubMed] [Google Scholar]
- 53.Duffy DM. Sclerosants: a comparative review. Dermatol Surg 2010; 36: S1010–25. doi: 10.1111/j.1524-4725.2009.01469.x [DOI] [PubMed] [Google Scholar]
- 54.Tan KT, Kirby J, Rajan DK, Hayeems E, Beecroft JR, Simons ME. Percutaneous sodium tetradecyl sulfate sclerotherapy for peripheral venous vascular malformations: a single-center experience. J Vasc Interv Radiol 2007; 18: 343–51. doi: 10.1016/j.jvir.2006.12.735 [DOI] [PubMed] [Google Scholar]
- 55.Khandpur S, Sharma VK. Utility of intralesional sclerotherapy with 3% sodium tetradecyl sulphate in cutaneous vascular malformations. Dermatol Surg 2010; 36: 340–6. doi: 10.1111/j.1524-4725.2009.01440.x [DOI] [PubMed] [Google Scholar]
- 56.Stimpson P, Hewitt R, Barnacle A, Roebuck DJ, Hartley B. Sodium tetradecyl sulphate sclerotherapy for treating venous malformations of the oral and pharyngeal regions in children. Int J Pediatr Otorhinolaryngol 2012; 76: 569–73. doi: 10.1016/j.ijporl.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 57.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg 2008; 47: 578–84. doi: 10.1016/j.jvs.2007.11.026 [DOI] [PubMed] [Google Scholar]
- 58.Gulsen F, Cantasdemir M, Solak S, Gulsen G, Ozluk E, Numan F. Percutaneous sclerotherapy of peripheral venous malformations in pediatric patients. Pediatr Surg Int 2011; 27: 1283–7. doi: 10.1007/s00383-011-2962-9 [DOI] [PubMed] [Google Scholar]
- 59.O'Donovan JC, Donaldson JS, Morello FP, Pensler JM, Vogelzang RL, Bauer B. Symptomatic hemangiomas and venous malformations in infants, children, and young adults: treatment with percutaneous injection of sodium tetradecyl sulfate. AJR Am J Roentgenol 1997; 169: 723–9. [DOI] [PubMed] [Google Scholar]
- 60.Goldman MP, Sadick NS. Complications and adverse sequelae of leg vein sclerotherapy. In: Nouri K, ed. Complications in dermatologic surgery. St Louis, MO: Mosby/Elsevier; 2008. pp. 219–42. [Google Scholar]
- 61.Marrocco-Trischitta MM, Guerrini P, Abeni D, Stillo F. Reversible cardiac arrest after polidocanol sclerotherapy of peripheral venous malformation. Dermatol Surg 2002; 28: 153–5. [DOI] [PubMed] [Google Scholar]
- 62.Potter B, Gobeil F, Oiknine A, Laramee P. The first case of takotsubo cardiomyopathy associated with sodium tetradecyl sulphate sclerotherapy. Can J Cardiol 2010; 26: 146–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cabrera J, Cabrera J Jr, Garcia-Olmedo MA, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol 2003; 139: 1409–16. doi: 10.1001/archderm.139.11.1409 [DOI] [PubMed] [Google Scholar]
- 64.Dietzek CL. Sclerotherapy: introduction to solutions and techniques. Perspect Vasc Surg Endovasc Ther 2007; 19: 317–24. [DOI] [PubMed] [Google Scholar]
- 65.Albanese G, Kondo KL. Pharmacology of sclerotherapy. Semin Intervent Radiol 2010; 27: 391–9. doi: 10.1055/s-0030-1267848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabe E, Breu F, Cavezzi A, Smith PC, Frullini A, Gillet J, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology Apr 2013. Epub ahead of print. doi: 10.1177/0268355513483280 [DOI] [PubMed]
- 67.Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg 2001; 27: 58–60. [PubMed] [Google Scholar]
- 68.Tessari L. Nouvelle technique d'obtention de la sclero-mousse. Phlebologie 2000; 53: 129. [Google Scholar]
- 69.Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg 1999; 104: 1–11. [PubMed] [Google Scholar]
- 70.Smithers CJ, Vogel AM, Kozakewich HP, Freedman DA, Burrows PE, Fauza DO, et al. An injectable tissue-engineered embolus prevents luminal recanalization after vascular sclerotherapy. J Pediatr Surg 2005; 40: 920–5. doi: 10.1016/j.jpedsurg.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 71.de Lorimier AA. Sclerotherapy for venous malformations. J Pediatr Surg 1995; 30: 188–93. [DOI] [PubMed] [Google Scholar]
- 72.Puig S, Aref H, Brunelle F. Double-needle sclerotherapy of lymphangiomas and venous angiomas in children: a simple technique to prevent complications. AJR Am J Roentgenol 2003; 180: 1399–401. doi: 10.2214/ajr.180.5.1801399 [DOI] [PubMed] [Google Scholar]
- 73.Li L, Zeng XQ, Li YH. Digital subtraction angiography-guided foam sclerotherapy of peripheral venous malformations. AJR Am J Roentgenol 2010; 194: W439–44. doi: 10.2214/AJR.09.3416 [DOI] [PubMed] [Google Scholar]
- 74.Holt PD, Burrows PE. Interventional radiology in the treatment of vascular lesions. Facial Plast Surg Clin North Am 2001; 9: 585–99. [PubMed] [Google Scholar]
- 75.Boll DT, Merkle EM, Lewin JS. Low-flow vascular malformations: MR-guided percutaneous sclerotherapy in qualitative and quantitative assessment of therapy and outcome. Radiology 2004; 233: 376–84. doi: 10.1148/radiol.2332031213 [DOI] [PubMed] [Google Scholar]
- 76.Lee CH, Chen SG. Direct percutaneous ethanol instillation for treatment of venous malformation in the face and neck. Br J Plast Surg 2005; 58: 1073–8. doi: 10.1016/j.bjps.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 77.Mimura H, Kanazawa S, Yasui K, Fujiwara H, Hyodo T, Mukai T, et al. Percutaneous sclerotherapy for venous malformations using polidocanol under fluoroscopy. Acta Med Okayama 2003; 57: 227–34. [DOI] [PubMed] [Google Scholar]
- 78.Jain R, Bandhu S, Sawhney S, Mittal R. Sonographically guided percutaneous sclerosis using 1% polidocanol in the treatment of vascular malformations. J Clin Ultrasound 2002; 30: 416–23. doi: 10.1002/jcu.10091 [DOI] [PubMed] [Google Scholar]
- 79.Smith RJ. Lymphatic malformations. Lymphat Res Biol 2004; 2: 25–31. [DOI] [PubMed] [Google Scholar]
- 80.Emery PJ, Bailey CM, Evans JN. Cystic hygroma of the head and neck. A review of 37 cases. J Laryngol Otol 1984; 98: 613–19. [DOI] [PubMed] [Google Scholar]
- 81.Bailey CM. Cystic hygroma. Lancet 1990; 335: 511–12. [PubMed] [Google Scholar]
- 82.Zhou Q, Zheng JW, Mai HM, Luo QF, Fan XD, Su LX, et al. Treatment guidelines of lymphatic malformations of the head and neck. Oral Oncol 2011; 47: 1105–9. doi: 10.1016/j.oraloncology.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 83.Puig S, Casati B, Staudenherz A, Paya K. Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol 2005; 53: 35–45. doi: 10.1016/j.ejrad.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 84.Kern S, Niemeyer C, Darge K, Merz C, Laubenberger J, Uhl M. Differentiation of vascular birthmarks by MR imaging. An investigation of hemangiomas, venous and lymphatic malformations. Acta Radiol 2000; 41: 453–7. [DOI] [PubMed] [Google Scholar]
- 85.Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 1999; 29: 879–93. [DOI] [PubMed] [Google Scholar]
- 86.Meyer JS, Hoffer FA, Barnes PD, Mulliken JB. Biological classification of soft-tissue vascular anomalies: MR correlation. AJR Am J Roentgenol 1991; 157: 559–64. doi: 10.2214/ajr.157.3.1872245 [DOI] [PubMed] [Google Scholar]
- 87.Paltiel HJ, Burrows PE, Kozakewich HP, Zurakowski D, Mulliken JB. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 2000; 214: 747–54. doi: 10.1148/radiology.214.3.r00mr21747 [DOI] [PubMed] [Google Scholar]
- 88.Sintzoff SA Jr, Gillard I, Van Gansbeke D, Gevenois PA, Salmon I, Struyven J. Ultrasound evaluation of soft tissue tumors. J Belge Radiol 1992; 75: 276–80. [PubMed] [Google Scholar]
- 89.Boyd JB, Mulliken JB, Kaban LB, Upton J III, Murray JE. Skeletal changes associated with vascular malformations. Plast Reconstr Surg 1984; 74: 789–97. [DOI] [PubMed] [Google Scholar]
- 90.Orford J, Barker A, Thonell S, King P, Murphy J. Bleomycin therapy for cystic hygroma. J Pediatr Surg 1995; 30: 1282–7. [DOI] [PubMed] [Google Scholar]
- 91.Zhong PQ, Zhi FX, Li R, Xue JL, Shu GY. Long-term results of intratumorous bleomycin-A5 injection for head and neck lymphangioma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 86: 139–44. [DOI] [PubMed] [Google Scholar]
- 92.Zulfiqar MA, Zaleha AM, Zakaria Z, Amin T. The treatment of neck lymphangioma with intralesional injection of bleomycin. Med J Malaysia 1999; 54: 478–81. [PubMed] [Google Scholar]
- 93.Sanlialp I, Karnak I, Tanyel FC, Senocak ME, Buyukpamukcu N. Sclerotherapy for lymphangioma in children. Int J Pediatr Otorhinolaryngol 2003; 67: 795–800. [DOI] [PubMed] [Google Scholar]
- 94.Perkins JA, Manning SC, Tempero RM, Cunningham MJ, Edmonds JL Jr, Hoffer FA, et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg 2010; 142: 795–803. doi: 10.1016/j.otohns.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 95.Muir T, Kirsten M, Fourie P, Dippenaar N, Ionescu GO. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int 2004; 19: 766–73. doi: 10.1007/s00383-003-1058-6 [DOI] [PubMed] [Google Scholar]
- 96.Chaudry G, Burrows PE, Padua HM, Dillon BJ, Fishman SJ, Alomari AI. Sclerotherapy of abdominal lymphatic malformations with doxycycline. J Vasc Interv Radiol 2011; 22: 1431–5. doi: 10.1016/j.jvir.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 97.Burrows PE, Mitri RK, Alomari A, Padua HM, Lord DJ, Sylvia MB, et al. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat Res Biol 2008; 6: 209–16. doi: 10.1089/lrb.2008.1004 [DOI] [PubMed] [Google Scholar]
- 98.Cahill AM, Nijs E, Ballah D, Rabinowitz D, Thompson L, Rintoul N, et al. Percutaneous sclerotherapy in neonatal and infant head and neck lymphatic malformations: a single center experience. J Pediatr Surg 2011; 46: 2083–95. doi: 10.1016/j.jpedsurg.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 99.Alomari AI, Karian VE, Lord DJ, Padua HM, Burrows PE. Percutaneous sclerotherapy for lymphatic malformations: a retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol 2006; 17: 1639–48. doi: 10.1097/01.RVI.0000239104.78390.E5 [DOI] [PubMed] [Google Scholar]
- 100.Smith RJ, Burke DK, Sato Y, Poust RI, Kimura K, Bauman NM. OK-432 therapy for lymphangiomas. Arch Otolaryngol Head Neck Surg 1996; 122: 1195–9. [DOI] [PubMed] [Google Scholar]
- 101.Ogita S, Tsuto T, Tokiwa K, Takahashi T. Intracystic injection of OK-432: a new sclerosing therapy for cystic hygroma in children. Br J Surg 1987; 74: 690–1. [DOI] [PubMed] [Google Scholar]
- 102.Rautio R, Keski-Nisula L, Laranne J, Laasonen E. Treatment of lymphangiomas with OK-432 (Picibanil). Cardiovasc Intervent Radiol 2003; 26: 31–6. doi: 10.1007/s00270-002-1980-3 [DOI] [PubMed] [Google Scholar]
- 103.Smith MC, Zimmerman MB, Burke DK, Bauman NM, Sato Y, Smith RJ, et al. Efficacy and safety of OK-432 immunotherapy of lymphatic malformations. Laryngoscope 2009; 119: 107–15. doi: 10.1002/lary.20041 [DOI] [PubMed] [Google Scholar]
- 104.Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years' experience with lymphangiomas in children. J Pediatr Surg 1999; 34: 1164–8. [DOI] [PubMed] [Google Scholar]
- 105.Shiels WE II, Kenney BD, Caniano DA, Besner GE. Definitive percutaneous treatment of lymphatic malformations of the trunk and extremities. J Pediatr Surg 2008; 43: 136–9. doi: 10.1016/j.jpedsurg.2007.09.049 [DOI] [PubMed] [Google Scholar]
- 106.Sung MW, Lee DW, Kim DY, Lee SJ, Hwang CH, Park SW, et al. Sclerotherapy with picibanil (OK-432) for congenital lymphatic malformation in the head and neck. Laryngoscope 2001; 111: 1430–3. doi: 10.1097/00005537-200108000-00020 [DOI] [PubMed] [Google Scholar]
- 107.Frieden I, Enjolras O, Esterly N. Vascular birthmarks and other abnormalities of blood vessels and lymphatics. In: Schacner LA, Hanson RC, eds. Pediatric dermatology. 3rd edn. St Louis, MO: Mosby Publishers; 2003. pp. 833–62. [Google Scholar]
- 108.Kohout MP, Hansen M, Pribaz JJ, Mulliken JB. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg 1998; 102: 643–54. [DOI] [PubMed] [Google Scholar]
- 109.Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am 2001; 39: 701–20. [DOI] [PubMed] [Google Scholar]
- 110.Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 2010; 40: 895–905. doi: 10.1007/s00247-010-1621-y [DOI] [PubMed] [Google Scholar]
- 111.Reinacher PC, Stracke P, Reinges MH, Hans FJ, Krings T. Contrast-enhanced time-resolved 3-D MRA: applications in neurosurgery and interventional neuroradiology. Neuroradiology 2007; 49(Suppl. 1): S3–13. doi: 10.1007/s00234-007-1468-6 [DOI] [PubMed] [Google Scholar]
- 112.Saleh RS, Lohan DG, Villablanca JP, Duckwiler G, Kee ST, Finn JP. Assessment of craniospinal arteriovenous malformations at 3T with highly temporally and highly spatially resolved contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2008; 29: 1024–31. doi: 10.3174/ajnr.A0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gulati MS, Paul SB, Batra A, Sarma D, Dadhwal V, Nath J. Uterine arteriovenous malformations: the role of intravenous ‘dual-phase’ CT angiography. Clin Imaging 2000; 24: 10–14. [DOI] [PubMed] [Google Scholar]
- 114.Savader SJ, Savader BL, Otero RR. Intraosseous arteriovenous malformations mimicking malignant disease. Acta Radiol 1988; 29: 109–14. [PubMed] [Google Scholar]
- 115.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg 2000; 37: 517–84. [DOI] [PubMed] [Google Scholar]
- 116.Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg 2010; 125: 1185–94. doi: 10.1097/PRS.0b013e3181d18070 [DOI] [PubMed] [Google Scholar]
- 117.Cho SK, Do YS, Kim DI, Kim YW, Shin SW, Park KB, et al. Peripheral arteriovenous malformations with a dominant outflow vein: results of ethanol embolization. Korean J Radiol 2008; 9: 258–67. doi: 10.3348/kjr.2008.9.3.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bae S, Do YS, Shin SW, Park KB, Kim DI, Kim YW, et al. Ethanol embolotherapy of pelvic arteriovenous malformations: an initial experience. Korean J Radiol 2008; 9: 148–54. doi: 10.3348/kjr.2008.9.2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin BS, Do YS, Lee BB, Kim DI, Chung IS, Cho HS, et al. Multistage ethanol sclerotherapy of soft-tissue arteriovenous malformations: effect on pulmonary arterial pressure. Radiology 2005; 235: 1072–7. doi: 10.1148/radiol.2353040903 [DOI] [PubMed] [Google Scholar]
- 120.Loh Y, Duckwiler GR; Onyx Trial Investigators. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Clinical article. J Neurosurg 2010; 113: 733–41. [DOI] [PubMed] [Google Scholar]
- 121.Pierot L, Januel AC, Herbreteau D, Barreau X, Drouineau J, Berge J, et al. Endovascular treatment of brain arteriovenous malformations using onyx: results of a prospective, multicenter study. J Neuroradiol 2009; 36: 147–52. [DOI] [PubMed] [Google Scholar]
- 122.Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Vinuela F. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001; 48: 984–95. [DOI] [PubMed] [Google Scholar]
- 123.Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 1996; 85: 19–28. doi: 10.3171/jns.1996.85.1.0019 [DOI] [PubMed] [Google Scholar]
- 124.Natarajan SK, Born D, Ghodke B, Britz GW, Sekhar LN. Histopathological changes in brain arteriovenous malformations after embolization using Onyx or N-butyl cyanoacrylate. Laboratory investigation. J Neurosurg 2009; 111: 105–13. doi: 10.3171/2008.12.JNS08441 [DOI] [PubMed] [Google Scholar]
- 125.Lee BB, Lardeo J, Neville R. Arterio-venous malformation: how much do we know? Phlebology 2009; 24: 193–200. doi: 10.1258/phleb.2009.009032 [DOI] [PubMed] [Google Scholar]
- 126.Do YS, Kim YW, Park KB, Kim DI, Park HS, Cho SK, et al. Endovascular treatment combined with emboloscleorotherapy for pelvic arteriovenous malformations. J Vasc Surg 2012; 55: 465–71. doi: 10.1016/j.jvs.2011.08.051 [DOI] [PubMed] [Google Scholar]
- 127.Prasad V, Chan RP, Faughnan ME. Embolotherapy of pulmonary arteriovenous malformations: efficacy of platinum versus stainless steel coils. J Vasc Interv Radiol 2004; 15: 153–60. [DOI] [PubMed] [Google Scholar]