Abstract
Objective:
The aim of our study was to assess the performance of acoustic radiation force impulse (ARFI) imaging to differentiate benign from malignant thyroid nodules.
Methods:
182 patients who needed thyroid surgery were examined. All patients and 50 healthy volunteers underwent ARFI sonoelastography, which quantitatively analysed the elasticity and hardness of the nodule's centre and periphery.
Results:
ARFI values showed a statistical significance between malignant nodules and benign nodules and common thyroid parenchyma, in both the centre and periphery of nodules (p < 0.01). There was no significant difference between benign nodules and common thyroid parenchyma in either the nodule's centre or periphery (p > 0.05). There was no significant difference between the nodule's centre and periphery of the elastic parameters in both the benign and malignant nodules. There was a statistically significant difference among the two areas (the central group and the peripheral group) under the receiver operating characteristic curve, and the optimal model was the peripheral group. For differentiation of malignant from benign nodules, the sensitivity and specificity were 96.3% and 96.2%, respectively, when 2.545 m s−1 was chosen as a cut-off value in the peripheral group.
Conclusion:
ARFI imaging may be helpful to differentiate benign nodules from malignant thyroid nodules. The selecting measurement position is important in ARFI imaging, and it has good diagnostic value in clinical applications.
Advances in knowledge:
This study shows the diagnostic contribution of ARFI imaging in thyroid lesions.
Thyroid cancer is the most common endocrine malignancy, and its incidence has increased in recent years.1 It comprises different clinical and histological features in respect to different treatments.2 The diagnostic method for thyroid cancer has very quickly progressed in recent years, but the pre-operative misdiagnosis rate is 40–70%.
A newer ultrasound elastography technique called acoustic radiation force impulse (ARFI), which is performed under direct visual guidance, has recently been verified to measure the stiffness of many tissues in vivo, for example in the liver.3,4 In our study, we investigated the mechanical properties of focal thyroid disease with ARFI. The purposes of this study were to assess the effectiveness of ARFI quantification in the diagnosis of focal thyroid nodules and differentiation of benign from malignant thyroid lesions by quantification of their stiffness.
METHODS AND MATERIALS
Patients
Our study was approved by the ethics review board of our institution. Patients provided signed informed consent. Between August 2009 and February 2011, 182 patients (88 male patients and 94 female patients) with thyroid nodules referred to the Qilu Hospital of Shandong University, Jinan, China, were enrolled in our study. A detailed medical history was obtained from all patients. Mean age of the patients was 53.67 years (range, 27–83 years). Mean diameter of the nodules was 3.97 cm (range, 3.0–5.5 cm). There were 69 malignant nodules, including papillary thyroid carcinoma (n = 40), follicular thyroid carcinoma (n = 13), poorly differentiated thyroid carcinoma (n = 8) and medullary thyroid carcinoma (n = 8). The 122 benign nodules included 58 nodular goitres and 55 thyroid adenomas.
All patients were operated on after the ARFI examination within 3 days or 1 week, and the pathological findings were obtained in 1 week.
Control group
50 healthy adult volunteers (25 males and 25 females) were examined with ARFI imaging and served as a control group to obtain median ARFI velocity measurements. The mean age of the volunteers (45 years; age range, 35–68 years) was not significantly different from that of the patient group.
Ultrasound techniques
ARFI elastography was performed in all patients using a Siemens Acuson S2000™ ultrasound system (Siemens Healthcare, Erlangen, Germany) at the time of their regular ultrasound performed by an experienced radiologist, who had 10 years' experience in China. The operator could select the depth at which thyroid elasticity was evaluated by placing a “measuring box” (10 mm long and 5 mm wide) in the desired place. The patients were examined in dorsal decubitus. The front of the patient's cervical part (to avoid cardiac motion) was scanned, with minimal scanning pressure applied by the operator, while the patients were asked to stop breathing for a moment, to achieve accurate and reproducible measurements. We performed five measurements in each patient and healthy adult; the former were measured simultaneously in the centre and periphery of the nodules. A median value was calculated, the result being measured in metres per second. If the nodules were heterogeneous, we made an attempt to avoid the areas where the ARFI values might differ, such as regions of calcification and liquefaction.
Statistical analyses
Statistical analyses were conducted using SPSS® v. 12.0 software (SPSS Inc., Chicago, IL). Because of the heterogeneity of variance, the group comparisons were performed using the Kruskal–Wallis test. The Tamhane's T2 method was used between the central and peripheral groups. The diagnostic performance of the median velocity measured using ARFI sonoelastography was assessed by analysing the receiver operating characteristic (ROC). Logistic regression analysis was applied on the central group and the peripheral group.
RESULTS
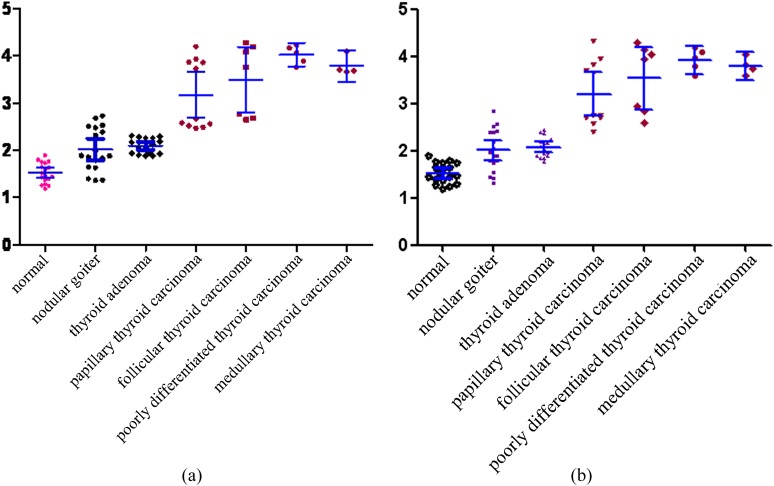
The size and ARFI values of the central and periphery groups in all nodules are summarised in Table 1 and Figure 1a,b
Table 1.
Acoustic radiation force impulse (ARFI) values of thyroid nodules
| Groups | No. lesions (no. patients) | Size of lesions, mean (range; cm) | ARFI values, median (SD; m s−1) | Range of ARFI values (m s−1) | |
|---|---|---|---|---|---|
| Nodular goitre | Centre | 19 (14) | 3.90 (3.6–4.2) | 1.93 (0.44) | 1.36–2.73 |
| Periphery | 19 (14) | 1.89 (0.46) | 1.32–2.85 | ||
| Thyroid adenoma | Centre | 17 (17) | 4.10 (3.5–4.7) | 2.07 (0.22) | 1.88–2.31 |
| Periphery | 17 (17) | 2.10 (0.16) | 1.76–2.45 | ||
| Papillary thyroid carcinoma | Centre | 12 (12) | 3.95 (3.7–4.2) | 2.73 (0.68) | 2.47–4.20 |
| Periphery | 12 (12) | 2.67 (0.73) | 2.39–4.32 | ||
| Follicular thyroid carcinoma | Centre | 8 (8) | 3.85 (3.6–4.1) | 3.94 (0.72) | 2.65–4.28 |
| Periphery | 8 (8) | 3.76 (0.76) | 2.59–4.29 | ||
| Poorly differentiated thyroid carcinoma | Centre | 6 (6) | 4.05 (3.8–4.3) | 4.07 (0.20) | 3.76–4.23 |
| Periphery | 6 (6) | 3.96 (0.24) | 3.58–4.19 | ||
| Medullary thyroid carcinoma | Centre | 4 (4) | 4.10 (3.8–4.4) | 3.69 (0.21) | 3.66–4.10 |
| Periphery | 4 (4) | 3.77 (0.18) | 3.59–4.03 | ||
SD, standard deviation.
Figure 1.
(a) The central group; (b) The periphery group. Acoustic radiation force impulse (ARFI) values of the central and periphery groups in all nodules; the wave velocity range of the malignant thyroid nodule group was significantly higher than the control group and the benign group. (a) Scatter plots of ARFI values for benign and malignant thyroid nodules in the centre. The long horizontal bar shows mean value, and the two short horizontal bars show the standard deviation (SD). (b) Scatter plots of ARFI values for benign and malignant thyroid nodules in the periphery. The long horizontal bar shows mean value, and the two short horizontal bars show the SD.
The differences of the shear wave velocity in the benign thyroid nodule group
The shear wave velocity range of the benign nodule group was relatively narrower than the malignant group, and an overlap could be seen in each other. The shear wave velocity range of nodular goitres was greater than other benign cases, which had no statistical significance (p > 0.05) between the other benign lesion nodules.
The differences of the shear wave velocity in the malignant thyroid nodule group
The wave velocity range of the malignant group was wider than that of the benign group and the control group, which overlapped each other, but there was no statistically significant difference in each group (p > 0.05).
The differences of the shear wave velocity in the benign thyroid nodule group and in the common control group
There was a smaller overlap in the shear wave velocity range of the benign group and the control group, and no statistically significant difference between the two groups (p > 0.05).
The differences of the shear wave velocity in the malignant thyroid nodule group and in the common control group
From Table 1 and Figure 1, we can see that the wave velocity range of the malignant nodule group was significantly higher than that of the control group, and there was a significant difference between the two groups (p < 0.01).
The differences of the shear wave velocity in the benign thyroid nodule group and in the malignant thyroid nodule group
There was no significant overlap between the benign group and the malignant group, only nodular goitres had a small overlap with the malignant group. There was a statistically significant difference between the two groups (p < 0.01), being in the centre and periphery of the nodules.
The specificity and sensitivity of acoustic radiation force impulse for identifying benign and malignant thyroid nodules
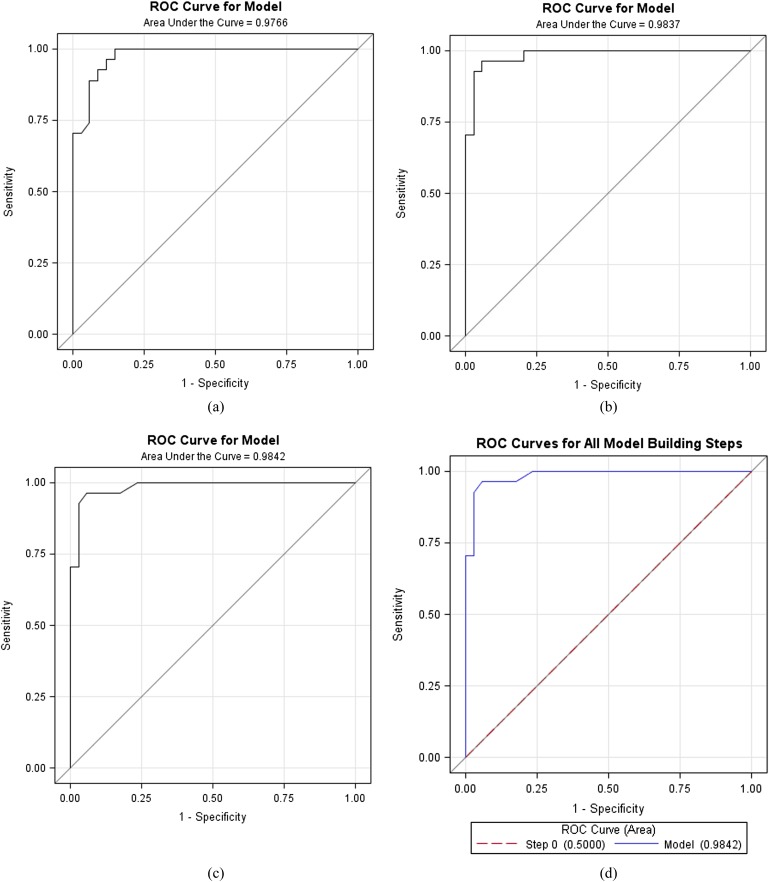
To evaluate the diagnostic performance of the ARFI values in the differentiation of benign from malignant nodules, ROC analysis was performed. For the two groups of the ROC curve, the logistic regression analysis was applied because of the high correlation of the central group (X1) and the peripheral group (X2). There was no statistically significant difference between the two areas under the ROC curve (p < 0.05). Since the X2 model was larger than the X1 model, the optimal model was only the X2 model in stepwise regression. The sensitivity and specificity values were 96.3% and 96.2%, respectively, when 2.545 m s−1 was chosen as a cut-off value in the peripheral group (Figure 2).
Figure 2.
(a) Only X1 (central group); (b) X1, X2 (central, peripheral); (c) only X2 (peripheral); (d) stepwise. (a) Only the central group was the model, the area under the receiver operating characteristic (ROC) curve for differentiation of the benign and malignant nodules is 0.9766. (b) The central group and the peripheral group were the model, the area under the ROC curve for differentiation of the benign and malignant nodules is 0.983. (c) Only the peripheral group was the model, the area under the ROC curve for differentiation of the benign and malignant nodules is 0.9842. (d) For stepwise regression, the optimal model is the X2 model.
DISCUSSION
Thyroid cancer, a familiar cancer in the head and neck region, has the highest incidence of endocrine neoplasms, an approximate 7–21% palpable mass in the thyroid, of which about 5% is thyroid cancer.5 Clinically, the benign and malignant thyroid nodules have many similar characteristics, but their treatment and prognosis are completely different, so early identification of the clinical thyroid nodule properties, especially the distinction of the benign from the malignant nodules, is very important. Many methods have been used to test the thyroid neoplasms pre-operatively; ultrasound with its non-invasive real-time monitoring, economy and convenient operation has become the most common method in a clinic. However, the traditional ultrasound examination, including colour Doppler ultrasonography and energy Doppler ultrasonography, can not accurately distinguish the nature of a thyroid nodule, even if combined with CT and MRI examination.
Elasticity and hardness are significant physical parameters of tissues. All tissues are inherently viscoelastic, and the hardness is to a large extent related to their structural properties and the organizational micro and macro forms. Elastic changes are often related to abnormal pathological conditions, the different organizational structures and the same structure of different pathological status make a distinct difference in elasticity and hardness.6–8 The feasibility of using acoustic radiation force methods to detect the mechanical properties of tissues has been investigated by several groups.9–11 The thyroid nodules have benign and malignant lesions, which have different elasticity and hardness with different pathological patterns. ARFI can be used to measure the different viscoelasticity and hardness of the thyroid lesions by testing the velocity of tissues, which reflects the different pathological types.
ARFI imaging involves targeting an anatomical region to be examined for its elastic properties by using short-duration acoustic pulses (push pulses), which produce shear waves that spread away from the region of interest, and to generate localized micron-scale displacements in tissue.12,13 Simultaneously, tracking beams are applied adjacent to the push pulse path, which are sensitive to >1/100 of the wavelength of sound. These beams are continuously transmitted until the passing shear wave front is detected. The time between the generation of the shear wave and the detection of the peak is used to compute the shear wave velocity, which can reflect the elasticity and hardness of tissues.4,14,15
The shear wave velocity increases with stiffness, and the stiffer a region in the tissue, the greater the shear wave velocity as it travels through this region.14,15 Thus, the measured shear wave speed is an intrinsic and a reproducible property of tissues.16
Our investigation indicates that there was a significant difference (p < 0.01) in the ARFI values (shear wave velocity) between malignant and benign lesion nodules and normal controls, whether in the centre or periphery of the nodules. For differentiation of malignant from benign nodules, the sensitivity and specificity were 96.3% and 96.2%, respectively, when 2.545 m s−1 was chosen as a cut-off value. This provides a powerful imaging guarantee to clinically judge the nature of the thyroid nodule before the operation. Thus, in the future, patients with median velocities of >2.545 m s−1 should be closely followed up because it is likely that they are malignant nodules and that thyroid needle biopsy should be undertaken. On the other hand, there is a possibility that patients with low median velocity might have benign nodules. Therefore, in the future, patients with a low median velocity measured using ARFI might be spared from undergoing thyroid biopsy.
It can be concluded that the pathological changes of the organizational form can affect the shearing velocity of a wave according to the forming principles of the shear wave. In our study, malignant nodules showed an extremely wider range than the benign nodules and the common control group in shearing velocity of the wave owing to many pathological types, for example calcification, diffluence and vascular perfusion. The nodular goitre as a special benign thyroid nodule also has a wider range than the other benign thyroid nodules because of its multiple pathological morphologies.17,18
There were not many details regarding the measurement position in the previous literature, whereas our study measured the shear wave velocity in nodular lesions in the centre and periphery of nodules. And there was a statistically significant difference through the two ROC curve areas (the centre and periphery groups) for diagnosing the benign and malignant nodules. So in the future, it is necessary to pay much attention to the measurement position of the lesions, especially in the periphery of the nodules.
In our preliminary studies, ARFI could non-invasively quantify thyroid elasticity and hardness and reflect the pathological character of thyroid lesions, and might be helpful to differentiate benign from malignant lesions. At the same time, we considered that additional studies correlating with other such elastographic techniques and with signs of thyroid lesions, for example the shape of the lesions with calcification or not and tumour vascularity, will be required to assist the clinical value of ARFI technology in the differential diagnosis of thyroid lesions in further studies.
In conclusion, the ARFI as an uncomplicated and non-invasive medical image diagnostic method has made a great contribution to the determination of benign and malignant nodules and also has an application prospect in clinical practice.
FUNDING
This work was supported by grants from Shandong Province Natural Science Foundation, Independent Innovation Foundation of Shandong University and Shandong Scientific Technology, and Population and Family Planning Commission of Shandong Province Science and Technology Research Projects (2010No. 14).
REFERENCES
- 1.Fayaz S, Fard-Esfahani P, Fard-Esfahani A, Mostafavi E, Meshkani R, Mirmiranpour H, et al. Assessment of genetic mutations in the XRCC2 coding region by high resolution melting curve analysis and the risk of differentiated thyroid carcinoma in Iran. Genet Mol Biol 2012; 35: 32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massimino M, Vigneri P, Fallica M, Fidilio A, Aloisi A, Frasca F, et al. IRF5 promotes the proliferation of human thyroid cancer cells. Mol Cancer 2012; 11: 21. doi: 10.1186/1476-4598-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 2008; 40: 371–8. doi: 10.1016/j.dld.2007.10.019 [DOI] [PubMed] [Google Scholar]
- 4.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256: 640–7. doi: 10.1148/radiol.10091662 [DOI] [PubMed] [Google Scholar]
- 5.Duick DS, Klopper JP, Diggans JC, Friedman L, Kennedy GC, Lanman RB, et al. The impact of benign gene expression classifier test results on the endocrinologist-patient decision to operate on patients with thyroid nodules with indeterminate fine-needle aspiration cytopathology. Thyroid 2012; 22: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2007; 5: 1214–20. doi: 10.1016/j.cgh.2007.07.020 [DOI] [PubMed] [Google Scholar]
- 7.Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One 2012; 7: e44930. doi: 10.1371/journal.pone.0044930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252: 595–604. [DOI] [PubMed] [Google Scholar]
- 9.Burnside ES, Hall TJ, Sommer AM, Hesley GK, Sisney GA, Svensson WE, et al. Differentiating benign from malignant solid breast masses with US strain imaging. Radiology 2007; 245: 401–10. doi: 10.1148/radiol.2452061805 [DOI] [PubMed] [Google Scholar]
- 10.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006; 239: 341–50. doi: 10.1148/radiol.2391041676 [DOI] [PubMed] [Google Scholar]
- 11.Hiltawsky KM, Kruger M, Starke C, Heuser L, Ermert H, Jensen A. Freehand ultrasound elastography of breast lesions: clinical results. Ultrasound Med Biol 2001; 27: 1461–9. [DOI] [PubMed] [Google Scholar]
- 12.Bota S, Sporea I, Sirli R, Popescu A, Danila M, Sendroiu M, et al. Spleen assessment by Acoustic Radiation Force Impulse Elastography (ARFI) for prediction of liver cirrhosis and portal hypertension. Med Ultrason 2010; 12: 213–17. [PubMed] [Google Scholar]
- 13.Madsen EL, Sathoff HJ, Zagzebski JA. Ultrasonic shear wave properties of soft tissues and tissuelike materials. J Acoust Soc Am 1983; 74: 1346–55. [DOI] [PubMed] [Google Scholar]
- 14.Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, et al. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis 2009; 18: 303–10. [PubMed] [Google Scholar]
- 15.Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, et al. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int 2010; 30: 538–45. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Wilson SR. Differentiation of benign from malignant liver masses with Acoustic Radiation Force Impulse technique. Ultrasound Q 2011; 27: 217–23. doi: 10.1097/RUQ.0b013e318239422e [DOI] [PubMed] [Google Scholar]
- 17.Kerdok AE, Ottensmeyer MP, Howe RD. Effects of perfusion on the viscoelastic characteristics of liver. J Biomech 2006; 39: 2221–31. doi: 10.1016/j.jbiomech.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Mazza E, Nava A, Hahnloser D, Jochum W, Bajka M. The mechanical response of human liver and its relation to histology: an in vivo study. Med Image Anal 2007; 11: 663–72. doi: 10.1016/j.media.2007.06.010 [DOI] [PubMed] [Google Scholar]