Abstract
T cell receptor/CD3 ligation induces apoptosis in semimature CD4+8-HSA+ thymocytes, and this helps establish immunological tolerance and constitutes one of the safeguards against autoimmune disease. We analyzed several knockout and transgenic mouse lines and found that T cell receptor/CD3-ligation-induced killing of semimature thymocytes occurred independently of Fas and “death receptor” signaling in general but required the proapoptotic BH3-only protein Bim and could be inhibited by Bcl-2. Loss of Apaf-1 or caspase-9, which act downstream of the Bcl-2 family protein family, provided only minor protection, indicating that the “apoptosome” functions as an amplifier rather than as an essential initiator of this death program. These results reveal the mechanisms of apoptosis in negative selection of semimature thymocytes and have implications for immunological tolerance and autoimmunity.
Keywords: Bcl-2 family, Fas (APO-1/CD95), FADD/Mort1, Apaf-1, caspase-9
The stochastic processes for rearranging genes encoding antigen receptors inevitably give rise to autoreactive B and T lymphocytes that are potentially harmful. Immunological tolerance to self is safeguarded by mechanisms that cause developmental arrest, functional inactivation, or apoptotic death of self-antigen-specific lymphocytes (1).
Genetic and biochemical studies in various model organisms have led to the identification of the central components of the cell death machinery (2). The effector phase of apoptosis requires aspartate-specific cysteine proteases, termed caspases (3). Mammals have two distinct apoptosis signaling pathways (4). One is initiated when ligation of “death receptors” (e.g., Fas/APO-1/CD95), members of the tumor necrosis factor receptor (TNF-R) family with an intracellular “death domain,” causes formation of a death-inducing signaling complex (DISC) in which FADD/Mort1 adaptor proteins promote oligomerization and activation of caspase-8 (3). The other pathway is triggered by certain developmental signals, growth factor deprivation, or various stress stimuli and is regulated by the interplay of pro- and antiapoptotic members of the Bcl-2 protein family (2, 5). Bcl-2-like prosurvival molecules that share three or four regions of homology (BH regions) are essential for cell survival (2, 5). Conversely, induction of apoptosis requires members of two proapoptotic subgroups of the Bcl-2 family: Bax/Bak-like proteins, which are structurally similar to their prosurvival relatives, and BH3-only proteins, which share with the family only the short BH3 region (6).
After sequential rearrangement of their T cell receptor (TCR) β and TCRα genes, T cells developing in the thymus become subject to selection on the basis of their antigen receptor specificity. Immature CD4+8+ thymocytes with TCRα/β receptors that bind with low affinity to MHC molecules loaded with self-antigen-derived peptides mature into CD4+8- or CD4-8+ T cells and later emigrate into the peripheral lymphoid system (7). Thymocytes that lack TCRα/β or those expressing receptors that do not interact with MHC molecules undergo “death by neglect” (7), a death program that can be inhibited by Bcl-2 overexpression (8–10). Thymocytes expressing TCRα/β receptors that bind with high affinity to MHC molecules presenting self-antigen-derived peptides undergo apoptosis (1). This negative selection can occur at the CD4+8+ stage in the thymic cortex but is thought to be most prominent at the semimature CD4+8-HSA+ stage when thymocytes transit through the corticomedullary junction because this region is replete with antigen-presenting cells (11). Experiments with transgenic and knockout mouse lines have demonstrated that TCR/CD3-ligation-induced apoptosis of DP thymocytes is independent of death receptor signaling (12, 13) and instead requires the BH3-only protein Bim (14) as well as the proapoptotic multi-BH domain proteins Bax/Bak (15). Other mechanisms must also contribute to the establishment of immunological tolerance because when Bim loss (14) or Bcl-2 overexpression (9) inhibit negative selection of autoreactive thymocytes (e.g., in the anti-HY-TCR transgenic mouse model), self-antigen-specific T cells accumulating in peripheral lymphoid organs are functionally inert (anergic).
The mechanisms regulating negative selection of semimature CD4+8-HSA+ thymocytes are not well understood; they were reported to involve Fas-dependent as well as Fas-independent apoptosis signaling, depending on the strength of TCR/CD3 ligation (16). By analyzing a panel of transgenic and knockout mouse lines, we found that deletion of CD4+8-HSA+ thymocytes requires the BH3-only protein Bim but relies only partially on the Apaf-1/caspase-9 amplification system and occurs independently of death receptor signaling.
Materials and Methods
Mice. The generation and genotyping of the Bim-deficient mice (266/266del) (17), Apaf-1-deficient mice (18), caspase-9-deficient mice (19), FADD-DN transgenic mice (strains 60 and 64), expressing a truncated mutant of human FADD under control of the lck promoter (13), vav-bcl-2–69 transgenic mice (20), expressing a human bcl-2 c-DNA under control of the vav promoter at high levels in all hemopoietic cell types, and OT-II TCR transgenic mice, expressing a class II MHC-restricted TCR specific for the ovalbumin peptide 323–339 (ISQAVHAAHAEINEAGR) (21) have been described. Fas-deficient lpr mutant mice were provided by the Walter and Eliza Hall Institute breeding facility. All mouse strains were generated on an inbred C57BL/6 genetic background or had been backcrossed for more than 10 generations onto this background. For the analysis of Apaf-1-/- and caspase-9-/- thymocytes, C57BL/6-Ly5.1 mice were lethally irradiated (2 × 5.5 Gy at an interval of 3 h) and reconstituted with embryonic day 14.5 fetal liver cells from embryos produced by intercrosses of Apaf-1+/- or caspase-9+/- mice, as described (7).
Cell Culture and Reagents. Purified thymocytes were cultured in the high glucose version of DMEM supplemented with 13 μM folic acid, 250 μM l-asparagine, 50 μM 2-mercaptoethanol, and 10% FCS (Trace Biosciences, Sydney). For the induction of TCR/CD3-mediated apoptosis, sorted thymocytes (0.5–1 × 105) were incubated in 96-well plates that had been coated first with rabbit anti-hamster Ig antibodies (50 μg/ml) overnight at 4°C and then with graded concentrations of hamster anti-mouse CD3ε (clone 145–2C11) or hamster anti-mouse TCRβ (clone H57-597.2.1) mAb in the presence or absence of fixed concentrations of hamster anti-mouse CD28 (clone 37.51) mAb.
Cell Sorting and Immunofluorescence Staining. Thymocyte subpopulations were isolated by incubating single-cell suspensions from mouse thymus glands with rat anti-mouse HSA mAb (clone J11d), followed by staining with FITC-conjugated mouse anti-rat Ig mAb (clone Mar 18.5). Cells were then washed twice before incubation with Cy5-conjugated rat anti-CD8 mAb (clone YTS169) and phycoerythrin-conjugated rat anti-CD4 mAb (clone H129.19) in PBS/10% FCS and 2% normal rat serum for 30 min on ice. Alternatively, HSA+ thymocytes were stained with a biotinylated rat anti-mouse HSA mAb (clone MI/69) yielding identical results. Cell sorting was performed on a MoFlo high-speed cell sorter (DakoCytomation). The vital dye propidium iodide (Sigma; 1 μg/ml) was used to exclude dead cells. Immature CD4+8+ DP, semimature CD4+CD8-HSA+, and mature CD4+CD8-HSA- thymocytes were collected and subjected to cell death analysis in culture or used for protein extraction for Western blot analysis.
To calculate absolute numbers of different thymocyte subsets after i.v. injection of mice with anti-CD3ε (145.2C11) or anti-TCRβ (H59–597) mAb, single-cell suspensions from thymus glands were counted, stained with mAbs to CD4, CD8, and HSA, and analyzed in a FACSCalibur flow cytometer (BD Biosciences). Absolute numbers of cells within a subset were calculated by multiplying the total cell number of a thymus with the percentage of this subset.
Cell Death Assays. The percentages of viable and dead cells in culture were determined by staining cell suspensions with 2 μg/ml propidium iodide plus FITC-coupled annexin V and analyzing the samples in a FACScan (BD Biosciences).
Statistical Analysis. Statistical analysis was performed by using the Fisher protected least significant difference test and statview 4.1 software (Abacus Concepts, Berkeley, CA). P < 0.05 was considered a statistically significant difference.
Results
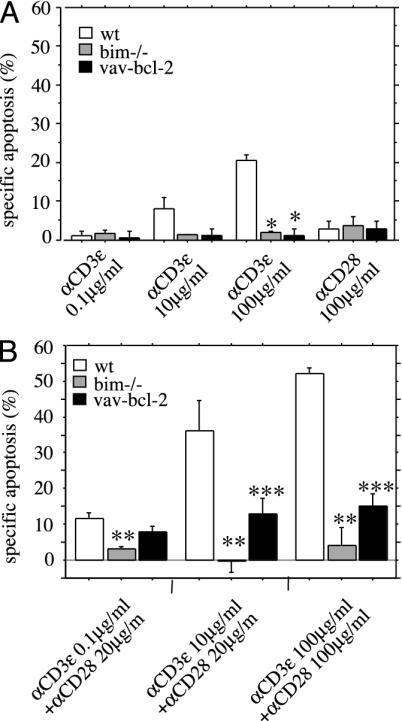
Bim Is Essential for TCR/CD3-Ligation-Induced Apoptosis of Semimature Thymocytes. It has been reported that only TCR stimulation with very high concentrations of superantigen or crosslinking anti-TCR/CD3 antibody kills semimature thymocytes through activation of Fas, whereas a lower intensity of stimulation kills through a Fas-independent mechanism (16). We investigated the role of the Bcl-2-regulated pathway in TCR/CD3-ligation-induced apoptosis of semimature thymocytes. CD4+8-HSA+ cells were isolated from WT mice, mice expressing a bcl-2 transgene in all hemopoietic cell types (20), and mice lacking the BH3-only protein Bim (17, 22). These cells were then cultured for 20 h in plates coated with graded concentrations of anti-CD3ε mAb in the absence or presence of optimal doses of anti-CD28 mAb. TCR/CD3 ligation killed WT semimature thymocytes, and this death was almost completely blocked by bcl-2 transgene expression or loss of Bim (Fig. 1). Even at very high concentrations of anti-CD3ε plus anti-CD28 antibodies, Bim-deficient and bcl-2 transgenic semimature thymocytes remained refractory to apoptosis (Fig. 1). Bim-deficiency also inhibited deletion induced by stimulation with the anti-TCRβ antibody H57.59.2.1 (plus or minus anti-CD28 mAb) (data not shown) or the superantigen Staphylococcus enterotoxin B (SEB), which triggers T lymphoid cells expressing TCRs containing vβ8 but not those containing vβ6 (Fig. 6, which is published as supporting information on the PNAS web site). Collectively, these results indicate that TCR-stimulation-induced apoptosis of semimature thymocytes is mediated by the Bcl-2-regulated pathway and requires the BH3-only protein Bim.
Fig. 1.
TCR/CD3-ligation-induced apoptosis of semimature CD4+8-HSA+ thymocytes is inhibited by Bcl-2 overexpression or loss of the BH3-only protein Bim. Semimature CD4+8-HSA+ thymocytes from WT, bim-/-, and vav-bcl-2 transgenic mice were purified by immunofluorescent staining with surfacemarker-specific mAbs and cell sorting. Cells were cultured for 20 h in plates coated with graded concentrations of anti-CD3ε mAb in the absence (A) or presence (B) of optimal doses of anti-CD28 mAb. The total amount of apoptosis induced by a given treatment was assessed by staining cells with FITC-labeled annexin V plus propidium iodide and flow cytometric analysis. The amount of apoptosis induced specifically by TCR/CD3 ligation was calculated by the following equation: (TCR/CD3 stimulation induced apoptosis - spontaneous apoptosis) ÷ (100 - spontaneous apoptosis)%. Bars represent arithmetic means ± SE of three to four independent experiments for animals of each genotype and treatment regime. *, P < 0.0001 (WT vs. bim-/- or vav-bcl-2); **, P < 0.0059 (WT vs. bim-/-); ***, P < 0.035 (WT vs. vav-bcl-2).
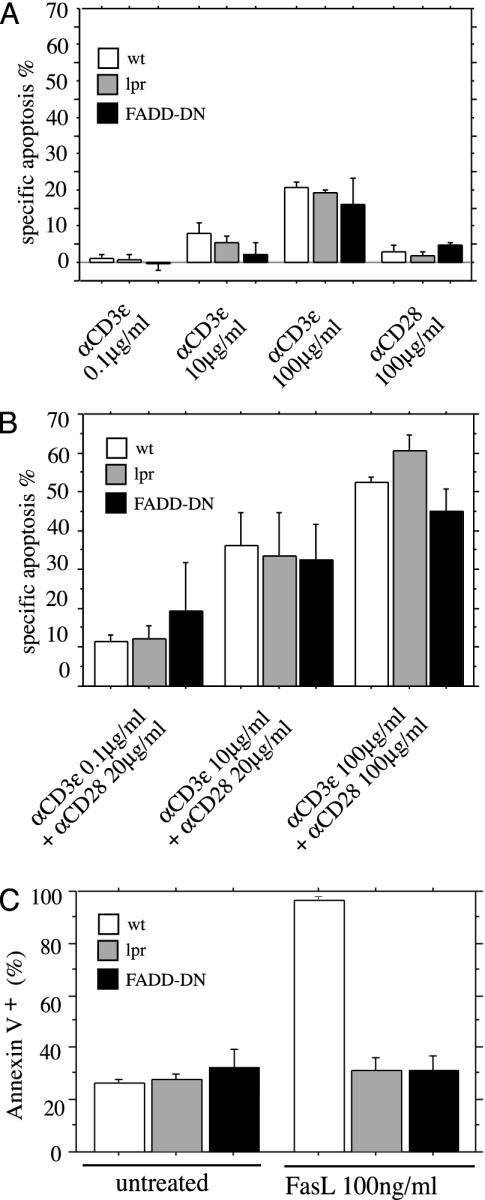
Death Receptors Are Dispensable for TCR/CD3-Ligation-Induced Apoptosis of Semimature Thymocytes. Near-complete inhibition of TCR/CD3-ligation-induced apoptosis by expression of a bcl-2 transgene or loss of Bim came as a surprise because killing of semimature CD4+8-HSA+ thymocytes was reported to be impaired in Fas-deficient lpr mice when high doses of TCR/CD3 crosslinking antibody or SEB were applied (16). We therefore investigated whether both the death-receptor-induced as well as the Bcl-2-regulated signaling pathways function in negative selection of semimature thymocytes. We isolated CD4+8-HSA+ cells from WT mice, Fas-deficient lpr mice, and mice expressing a dominant negative mutant of FADD (FADD-DN), which blocks death-receptor-induced apoptosis (13), and treated them with anti-CD3ε plus anti-CD28 antibodies. We observed that semimature CD4+8-HSA+ thymocytes from Fas-deficient lpr and FADD-DN transgenic mice were equally susceptible to TCR/CD3-mediated killing as WT cells (Fig. 2 A and B). Increasing the concentration of anti-CD3ε and anti-CD28 antibodies, even up to 100 μg/ml each, did not reveal resistance of mutant lpr or FADD-DN-expressing thymocytes to TCR/CD3-mediated apoptosis (Fig. 2B), although they were, as reported (13), refractory to Fas-induced cell death (Fig. 2C). Similarly, semimature thymocytes from Fas-deficient lpr mice were normally deleted by stimulation with the anti-TCRβ antibody H57.59.2.1 (data not shown) or SEB (Fig. 6). These results demonstrate that Fas, and FADD-transduced death receptor signaling in general, is dispensable for TCR/CD3-ligation-induced apoptosis of semimature thymocytes in vitro.
Fig. 2.
TCR/CD3-ligation-induced apoptosis of semimature CD4+8-HSA+ thymocytes is independent of Fas- and FADD-transduced death receptor signaling. Semimature CD4+8-HSA+ thymocytes from WT, Fas-deficient lpr, and FADD-DN transgenic mice were purified by immunofluorescent staining with surface-marker-specific mAbs and cell sorting. Cells were cultured for 20 h in plates coated with graded concentrations of anti-CD3ε mAb in the absence (A) or presence (B) of optimal doses of anti-CD28 mAb, left untreated (C), or treated with FLAG-FasL (100 ng/ml) crosslinked with anti-FLAG mAb (1 μg/ml) (C). Apoptosis was assessed after 20 h as described in Fig. 1. Data represent arithmetic means ± SE of three to four independent experiments for animals of each genotype and for each treatment regime. No significant differences were observed in A, comparing WT vs. lpr (P > 0.52) and WT vs. FADD-DN (P > 0.163), or B, comparing WT vs. lpr (P > 0.171) and WT vs. FADD-DN (P > 0.218).
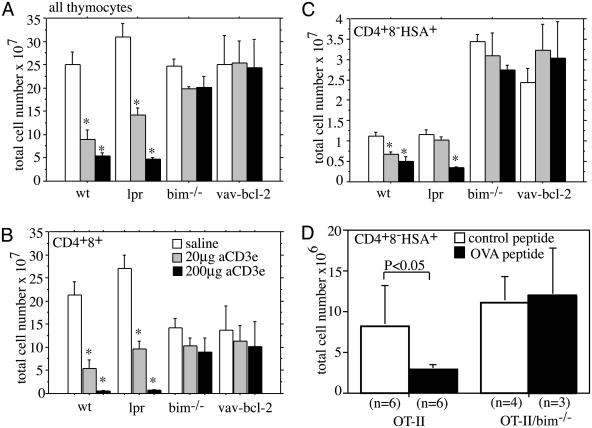
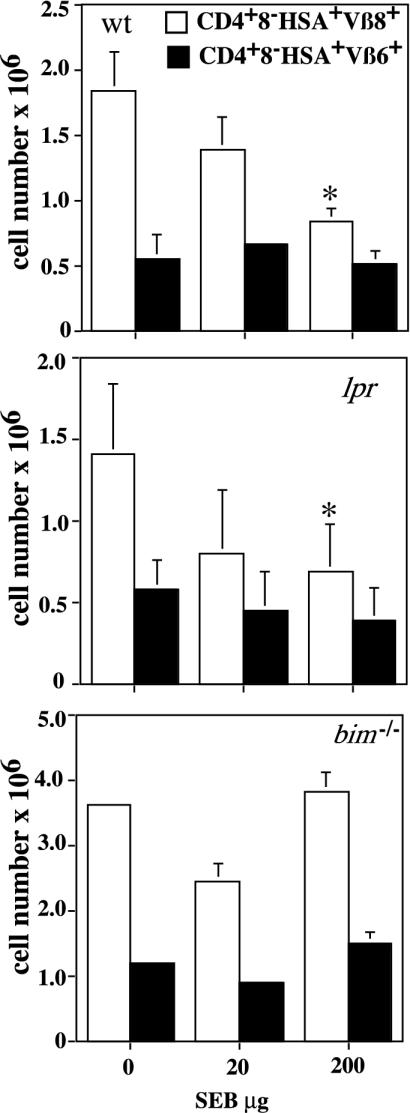
Loss of Bim or Bcl-2 Overexpression Inhibits TCR/CD3-Ligation-Induced Apoptosis of Semimature Thymocytes in Vivo. To assess the contributions of the Bcl-2-regulated cell death pathway and death receptor signaling to TCR/CD3-ligation-induced killing of semimature thymocytes in vivo, we injected WT, Bim-deficient, bcl-2 transgenic, and Fas-deficient lpr mice i.v. with two different concentrations of anti-CD3ε antibody. Deletion of immature CD4+8+ DP and semimature CD4+8-HSA+ cells was assessed after 40 h by counting total thymocyte numbers and determining the proportions of cell subsets by immunofluorescent staining with antibodies to the relevant cell-surface markers (CD4, CD8, and HSA). This analysis revealed that the total thymocyte number (Fig. 3A) and the number of CD4+8+ cells (Fig. 3B) decreased in WT and lpr mice in a manner dependent on the dose of anti-CD3ε antibody. As shown before (14), loss of Bim or Bcl-2 overexpression provided complete or near-complete protection against this death stimulus (Fig. 3 A and B). As also reported before (14), untreated bim-/- and vav-bcl-2 transgenic mice had about two times more mature CD4+8-HSA- thymocytes compared to the WT and lpr animals (Fig. 3B, P < 0.004). Moreover, we observed that the numbers of semimature CD4+8-HSA+ cells from WT and lpr mice decreased in a manner dependent on the dose of anti-CD3ε mAb injected (Fig. 3C). In contrast, no deletion of semimature thymocytes was seen in bim-/- or vav-bcl-2 transgenic mice. Similar results were obtained when animals were injected with a TCRβ-specific antibody (H57–597.2.1) (data not shown). We also found that injection of OTII TCR transgenic mice with the cognate peptide (ovalbumin 323–339) caused deletion of ≈65% of the semimature thymocytes, and this deletion was blocked completely by loss of Bim (Fig. 3D). In addition, i.p. injection of 20 or 200 μg of SEB induced deletion of ≈15–20% or ≈50%, respectively, of TCRvβ8+ semimature thymocytes from WT and mutant lpr mice but no deletion in bim-/- animals (Fig. 4). These results demonstrate that TCR/CD3-stimulation-induced killing of semimature CD4+CD8-HSA+ thymocytes in vivo requires the BH3-only protein Bim and can be inhibited by Bcl-2 but occurs independently of Fas-signaling.
Fig. 3.
Bim deficiency and Bcl-2 overexpression but not loss of Fas prevent TCR/CD3-stimulation-induced killing of semimature CD4+8-HSA+ thymocytes in vivo. WT, mutant lpr, bim-/-, and vav-bcl-2 transgenic mice were injected with 20 μgor200 μg anti-CD3ε antibody, or as a control with saline, and killed after 40 h. Total numbers of all thymocytes (A), the CD4+8+ DP population (B), and the CD4+8-HSA+ (C) subset were calculated by multiplying the total thymic cellularity with the relative percentages obtained by immunofluorescent staining with surface-marker-specific mAbs and flow cytometric analysis. Data represent arithmetic means ± SE of three to five independent experiments with animals of each genotype and treatment regime. A statistically significant reduction in thymocyte number induced by anti-CD3ε injection within each group (genotype) is indicated by an asterisk (P < 0.005). (D) OT-II TCR transgenic and OT-II/bim-/- mice were injected twice (at 0 and 24 h) with 0.5 mg of cognate (ovalbumin 323–339, ISQAVHAAHAEINEAGR) or control (human proinsulin 52–65, SLQPLALEGSLQKR) peptide. Mice were killed 40 h after the first injection. Total numbers of the CD4+CD8-HSA+ subset were calculated by multiplying the total thymic cellularity with the percentages of this subset obtained by flow cytometric analysis. Data represent arithmetic means ± SE from three to six mice for each genotype and treatment.
Fig. 4.
Bim deficiency but not loss of Fas prevents SEB-induced killing of semimature TCRvβ8+ CD4+8-HSA+ thymocytes in vivo. WT, mutant lpr, and bim-/- mice were injected with 20 or 200 μg of SEB, or as a control with saline, and killed after 40 h. Total numbers of TCRvβ8+ CD4+8-HSA+ and TCRvβ6+ CD4+8-HSA+ thymocytes (which serve as a control because they are not activated by SEB) were calculated by multiplying the total thymic cellularity with the relative percentages obtained by immunofluorescent staining with surface-marker-specific mAbs and flow cytometric analysis. Data represent arithmetic means ± SD of three to five independent experiments with animals of each genotype and treatment regime with WT and lpr mice and two independent experiments with bim-/- mice. A statistically significant reduction in thymocyte number induced by SEB injection within each group (genotype) is indicated by an asterisk (P < 0.04).
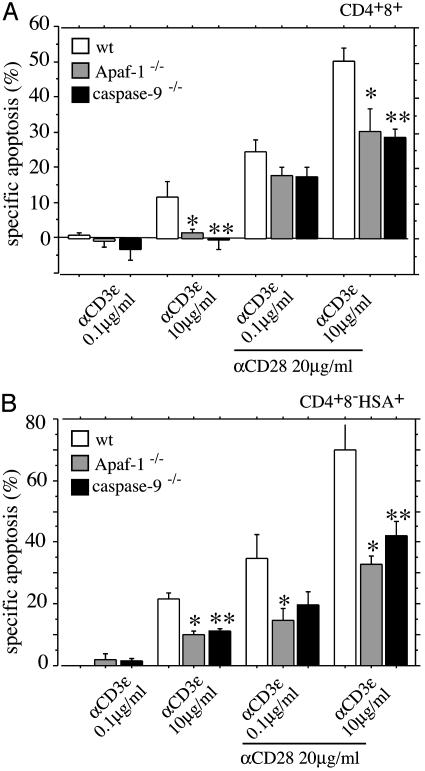
Apaf-1 and Caspase-9 Are only Partially Required for TCR/CD3-Ligation-Induced Killing of Semimature CD4+8-HSA+ Thymocytes. Apaf-1-mediated activation of caspase-9 occurs in cells exposed to death stimuli that trigger the Bcl-2-regulated (mitochondrial) apoptosis signaling pathway (5). In some cell types, such as the developing neurons, Apaf-1 and caspase-9 are essential for cell killing (5), but these apoptosis regulators are dispensable for developmentally programmed death during lymphopoiesis (7). We wanted to examine the role of Apaf-1 and caspase-9 in TCR/CD3-ligation-induced apoptosis of semimature thymocytes. Because Apaf-1 and caspase-9 deficiencies cause embryonic lethality (5), we reconstituted lethally irradiated WT (C57BL/6-Ly5.1) mice with fetal liver stem cells from embryonic day 14.5 Apaf-1-/-, caspase-9-/-, or, as a control, WT embryos (all donors were C57BL/6-Ly5.2). After >8 weeks of reconstitution, semimature, donor-derived thymocytes (CD4+8-HSA+Ly5.1-) were isolated by cell sorting and cultured in the presence or absence of plate-bound anti-CD3ε plus anti-CD28 antibodies. Cell survival analysis by annexin V plus propidium iodide staining after 20 h of culture showed that WT thymocytes from reconstituted mice died to a similar extent as did thymocytes from unmanipulated WT animals (compare data from Fig. 5 with those from Fig. 1). Consistent with a previous report (23), Apaf-1-/- CD4+8+ DP thymocytes had some, albeit relatively small, resistance to TCR/CD3-stimulation-induced apoptosis (Fig. 5). For example, stimulation with 10 μg/ml anti-CD3ε plus 20 μg/ml anti-CD28 mAbs killed, on average, 50% of WT versus 29% of Apaf-1-/--deficient or 27% of caspase-9-deficient CD4+8+ DP thymocytes (Fig. 5A). This treatment killed 69% of WT, 31% of Apaf-1-/--deficient, and 41% caspase-9-deficient CD4+8-HSA+ semimature thymocytes (Fig. 5B). Thus, loss of Apaf-1 or caspase-9 provided only minor protection against TCR/CD3-stimulation-induced apoptosis in immature CD4+8+ or semimature CD4+8-HSA+ thymocytes in culture. This finding indicates that the apoptosome may not be essential for this pathway to cell death but functions as an amplification mechanism in the caspase cascade.
Fig. 5.
Loss of Apaf-1 or caspase-9 has a minor effect on TCR/CD3-stimulation-induced apoptosis of semimature CD4+8-HSA+ thymocytes in vitro. Immature CD4+8+ DP (A) and semimature CD4+8-HSA+ thymocytes (B) from lethally irradiated C57BL/6-Ly5.1 mice reconstituted with fetal liver stem cells from embryonic day 14.5 WT, Apaf-1-/-, or caspase-9-/- embryos were purified by immunofluorescent staining with surface-marker-specific antibodies and cell sorting. Cells were cultured for 20 h in plates coated with graded concentrations of anti-CD3ε mAb in the presence or absence of optimal doses of anti-CD28 mAb. Apoptosis was assessed as described in Fig. 1. Bars represent arithmetic means ± SE of three to five independent experiments for animals of each genotype and for each treatment regimen. Significant differences in A: *, P < 0.0394 (WT vs. Apaf-1-/-); **, P < 0.0435 (WT vs. caspase-9-/-). Significant differences in B: *, P < 0.026 (WT vs. Apaf-1-/-); **, P < 0.0309 (WT vs. caspase-9-/-).
Discussion
The deletion of potentially autoreactive T cells developing in the thymus is one of several mechanisms that safeguard immunological tolerance to self-antigens. This deletion is triggered by stimulation of the TCR/CD3 complex and can occur at two distinct stages of T cell development, the immature CD4+8+ stage and the semimature CD4+8-HSA+ stage. Experiments with transgenic and knockout mice have indicated that deletion of immature CD4+8+ thymocytes is mediated by the Bcl-2-regulated cell-death pathway (9, 24) and requires the BH3-only protein Bim (14) but is independent of Fas- and FADD-transduced death receptor signaling in general (12, 13).
It is widely believed that deletion of autoreactive T cells occurs prominently at the semimature CD4+8-HSA+ stage of development because these cells are in close contact with antigen-presenting cells at the corticomedullary junction (11). It is therefore important to define the mechanisms that mediate their apoptosis. Previous studies reported that signaling through the death receptor Fas is required for killing of semimature CD4+8-HSA+ thymocytes when the TCR/CD3 complex is stimulated with high doses of superantigen or crosslinking antibody but that a different process kills cells at low intensity TCR/CD3 stimulation (16). We found that TCR/CD3-ligation-induced apoptosis of semimature CD4+8-HSA+ thymocytes was blocked by loss of the BH3-only protein Bim or Bcl-2 overexpression, regardless of the intensity of TCR/CD3 stimulation (Fig. 1). Neither Bim deficiency (17) nor Bcl-2 overexpression (4) inhibits Fas-induced apoptosis in lymphocytes. Because Fas receptor signaling (16) and, recently, also TRAIL receptor signaling (25) were reported to be required for TCR/CD3-stimulation-induced thymocyte killing, under at least certain circumstances, we expected that neither the absence of Bim nor Bcl-2 overexpression would prevent this death. A possible explanation for this conundrum could be that death receptor signaling and Bim act in parallel to mediate the deletion of semimature CD4+8-HSA+ thymocytes. We therefore reexamined the contribution of Fas- and FADD-transduced death receptor signaling to TCR/CD3-ligation-induced thymocyte killing. Consistent with our previous findings (12, 13), immature CD4+8+ thymocytes from Fas-deficient lpr mice and mice expressing a dominant negative mutant of FADD were deleted normally in response to TCR/CD3 ligation. Semimature CD4+8-HSA+ thymocytes from these animals were, as expected, resistant to Fas-induced apoptosis but were killed as efficiently as WT cells in response to TCR/CD3 ligation in vivo or in vitro, regardless of whether high- or low-intensity stimulation was applied (Figs. 2–4). We excluded the possibility that differences in isolation of thymocytes (panning versus FACS sorting) or differences in antibodies used for TCR/CD3 activation (anti-TCRβ versus anti-CD3ε mAbs) might be responsible for the discrepancies between our results and those reported earlier (16). Differences in genetic background of lpr mice could potentially account for the differences in results obtained by us (Figs. 1–4) versus those previously reported. It is, however, noteworthy that although one publication reported on lpr mice on a C3H background (16), subsequent work from that lab used lpr mice on a C57BL/6 background (26), like we did. Therefore, our data, together with studies that were unable to reproduce the findings that TRAIL is essential for thymocyte negative selection (27), demonstrate that death receptor signaling is dispensable for deletion of autoreactive thymocytes.
Deletion of semimature CD4+8-HSA+ thymocytes in vivo after injection of antibodies to CD3ε or TCRβ or SEB was clearly impaired in mice lacking Bim or expressing a bcl-2 transgene but occurred to a similar extent in WT and Fas-deficient lpr mice (Figs. 3 and 4). Interestingly, the total number of semimature CD4+8-HSA+ thymocytes did not differ between untreated WT and lpr mice but was elevated >2-fold in Bim-deficient and bcl-2 transgenic mice (Figs. 3 and 4). It appears more likely that this is a consequence of failed negative rather than enhanced positive selection because Bim loss does not enhance the efficiency of positive selection in female anti-HY-TCR transgenic mice (14). Our findings are consistent with previous studies in vivo which demonstrated that deletion of autoreactive DP thymocytes requires the BH3-only protein Bim (14) as well as the multi BH domain proapoptotic Bcl-2 family members Bax and Bak (15) but is independent of death receptor signaling (12, 13).
Because Bcl-2-regulated apoptosis signaling appeared to be essential for TCR/CD3-ligation-induced killing of semimature CD4+8-HSA+ thymocytes, we investigated the role of the known downstream effectors Apaf-1 and caspase-9 (5) in this process. Our studies showed that loss of Apaf-1 or caspase-9 protected immature CD4+8+ or semimature CD4+8-HSA+ thymocytes only weakly (compared to loss of Bim) from TCR/CD3-stimulation-induced apoptosis (Fig. 5). These results are consistent with previous observations that Apaf-1 and caspase-9 are dispensable for developmentally programmed death during lymphopoiesis (7) and that autoreactive Apaf-1-/- DP thymocytes expressing the HY TCR transgene are normally deleted in vivo, although they had a small degree of resistance to HY-peptide-induced killing in vitro (23).
In conclusion, our data indicate that the deletion of potentially deleterious autoreactive immature CD4+8+ thymocytes as well as semimature CD4+8-HSA+ thymocytes requires the BH3-only protein Bim. The deletion process is largely independent of the Apaf-1/caspase-9 apoptosome and does not require death receptor signaling. Because loss of even 50% of Bim function in bim+/- mice has significant impact on apoptosis and development of lymphocytes (17, 28), it will be interesting to investigate whether mutations in the bim gene or abnormalities in Bim protein regulation contribute to the development of autoimmune diseases in humans.
Supplementary Material
Acknowledgments
We thank Drs. J. Adams, S. Cory, A. Harris, F. Cecconi, P. Gruss, and K. Kuida for gifts of vav-bcl-2 and lck-FADD-DN transgenic mice and Bim-, Apaf-1-, and caspase-9-deficient mice; M. Robati, F. Müllauer, and K. Rossi for technical assistance; Dr. F. Battye, C. Tarlinton, V. Lapatis, and G. Böck for help with flow cytometry; A. Naughton, J. Morrow, and M. Brennsteiner for animal care; and J. Adams, S. Cory, D. Vaux, A. Harris, G. Wick, J. Wiegers, and J. Miller for helpful discussions. This work was supported by the Leukemia Research Foundation, the Austrian Science Fund (Foerderung der Wissenschafflichen Forschung to A.V.), the National Health and Medical Research Council (Canberra, Reg. Key 973002), the Dr. Josef Steiner Cancer Research Foundation (Bern), National Cancer Institute Grants CA43540 and CA80188, the Juvenile Diabetes Foundation, and Leukemia and Lymphoma Society Grant 7015-02.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SEB, Staphylococcus enterotoxin B; TCR, T cell receptor.
References
- 1.Marsden, V. & Strasser, A. (2003) Annu. Rev. Immunol. 21, 71-105. [DOI] [PubMed] [Google Scholar]
- 2.Strasser, A., O'Connor, L. & Dixit, V. M. (2000) Annu. Rev. Biochem. 69, 217-245. [DOI] [PubMed] [Google Scholar]
- 3.Shi, Y. (2002) Mol. Cell 9, 459-470. [DOI] [PubMed] [Google Scholar]
- 4.Strasser, A., Harris, A. W., Huang, D. C. S., Krammer, P. H. & Cory, S. (1995) EMBO J. 14, 6136-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang, X. (2001) Genes Dev. 15, 2922-2933. [PubMed] [Google Scholar]
- 6.Huang, D. C. S. & Strasser, A. (2000) Cell 103, 839-842. [DOI] [PubMed] [Google Scholar]
- 7.Marsden, V., O'Connor, L., O'Reilly, L. A., Silke, J., Metcalf, D., Ekert, P., Huang, D. C. S., Cecconi, F., Kuida, K., Tomaselli, K. J., et al. (2002) Nature 419, 634-637. [DOI] [PubMed] [Google Scholar]
- 8.Strasser, A., Harris, A. W., Corcoran, L. M. & Cory, S. (1994) Nature 368, 457-460. [DOI] [PubMed] [Google Scholar]
- 9.Strasser, A., Harris, A. W., Von Boehmer, H. & Cory, S. (1994) Proc. Natl. Acad. Sci. USA 91, 1376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linette, G. P., Grusby, M. J., Hedrick, S. M., Hansen, T. H., Glimcher, L. H. & Korsmeyer, S. J. (1994) Immunity 1, 197-205. [DOI] [PubMed] [Google Scholar]
- 11.Surh, C. D. & Sprent, J. (1994) Nature 372, 100-103. [DOI] [PubMed] [Google Scholar]
- 12.Smith, K. G. C., Strasser, A. & Vaux, D. L. (1996) EMBO J. 15, 5167-5176. [PMC free article] [PubMed] [Google Scholar]
- 13.Newton, K., Harris, A. W., Bath, M. L., Smith, K. G. C. & Strasser, A. (1998) EMBO J. 17, 706-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouillet, P., Purton, J. F., Godfrey, D. I., Zhang, L.-C., Coultas, L., Puthalakath, H., Pellegrini, M., Cory, S., Adams, J. M. & Strasser, A. (2002) Nature 415, 922-926. [DOI] [PubMed] [Google Scholar]
- 15.Rathmell, J. C., Lindsten, T., Zong, W.-X., Cinalli, R. M. & Thompson, C. B. (2002) Nat. Immunol. 3, 932-939. [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto, H., Surh, C. D. & Sprent, J. (1998) J. Exp. Med. 187, 1427-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouillet, P., Metcalf, D., Huang, D. C. S., Tarlinton, D. M., Kay, T. W. H., Köntgen, F., Adams, J. M. & Strasser, A. (1999) Science 286, 1735-1738. [DOI] [PubMed] [Google Scholar]
- 18.Cecconi, F., Alvarez-Bolado, G., Meyer, B. I., Roth, K. A. & Gruss, P. (1998) Cell 94, 727-737. [DOI] [PubMed] [Google Scholar]
- 19.Kuida, K., Haydar, T. F., Kuan, C.-Y., Gu, Y., Taya, C., Karasuyama, H., Su, M. S.-S., Rakic, P. & Flavell, R. A. (1998) Cell 94, 325-337. [DOI] [PubMed] [Google Scholar]
- 20.Ogilvy, S., Metcalf, D., Print, C. G., Bath, M. L., Harris, A. W. & Adams, J. M. (1999) Proc. Natl. Acad. Sci. USA 96, 14943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. (1998) Immunol. Cell Biol. 76, 34-40. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor, L., Strasser, A., O'Reilly, L. A., Hausmann, G., Adams, J. M., Cory, S. & Huang, D. C. S. (1998) EMBO J. 17, 384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara, H., Takeda, A., Takeuchi, M., Wakeham, A. C., Itie, A., Sasaki, M., Mak, T. W., Yoshimura, A., Nomoto, K. & Yoshida, H. (2002) J. Immunol. 168, 2288-2295. [DOI] [PubMed] [Google Scholar]
- 24.Strasser, A., Harris, A. W. & Cory, S. (1991) Cell 67, 889-899. [DOI] [PubMed] [Google Scholar]
- 25.Lamhamedi-Cherradi, S. E., Zheng, S. J., Maguschak, K. A., Peschon, J. & Chen, Y. H. (2003) Nat. Immunol. 4, 255-260. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto, H. & Sprent, J. (2001) Nat. Immunol. 2, 1025-1031. [DOI] [PubMed] [Google Scholar]
- 27.Cretney, E., Uldrich, A. P., Berzins, S. P., Strasser, A., Godfrey, D. I. & Smyth, M. J. (2003) J. Exp. Med. 198, 491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouillet, P., Cory, S., Zhang, L.-C., Strasser, A. & Adams, J. M. (2001) Dev. Cell 1, 645-653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.