Abstract
Objectives
This study extended and updated a meta-analysis of the association between exposure to dichlorodiphenyltrichloroethane (DDT) and the risk of breast cancer.
Methods
We reviewed the published literature on exposure to DDE and breast cancer risk to update a meta-analysis from 2004. The total of 35 studies included 16 hospital-based case–control studies, 11 population-based case–control studies, and 10 nested case–control studies identified through keyword searches in the PubMed and EMBASE databases.
Results
The summary odds ratio (OR) for the identified studies was 1.03 (95% confidence interval 0.95–1.12) and the overall heterogeneity in the OR was observed (I2 = 40.9; p = 0.006). Subgroup meta-analyses indicated no significant association between exposure to DDE and breast cancer risk by the type of design, study years, biological specimen, and geographical region of the study, except from population-based case–control studies with estimated DDE levels in serum published in 1990s.
Conclusion
Existing studies do not support the view that DDE increases the risk of breast cancer in humans. However, further studies incorporating more detailed information on DDT exposure and other potential risk factors for breast cancer are needed.
Keywords: breast cancer, dichlorodiphenyldichloroethylene, meta-analysis, pesticide exposure, systematic review
1. Introduction
Dichlorodiphenyltrichloroethane (DDT) is a synthetic chemical that includes p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT), p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), and p,p′-dichlorodiphenyldichloroethane (p,p′-DDD or p,p′-TDE). DDE (dichlorodiphenyldichloroethylene) is the main metabolite of DDT, which is rapidly converted into DDE in biological systems [1]. After identifying its insecticidal function, DDT was widely used to prevent malaria and some agricultural pests worldwide. Although the use of DDT was banned in most developed countries in the early 1970s, DDT was still used in some developing countries, such as India, Indonesia, and Mexico, until the 1990s to control the mosquitoes that cause malaria [1,2].
DDT is bioaccumulated in the lipid component of biological systems through the food chain because it is highly lipophilic and is resistant to degradation. Therefore, despite its prohibition in many countries, DDT is still present in the environment and the food chain. DDE in particular has a very long half-life and is of toxicological importance. The half-lives of DDT and DDE in humans have been estimated to be between 6 years and 10 years [3]. The DDT and DDE accumulated in the lipid components, such as adipose tissue, are slowly released into the bloodstream [4]. DDT and its metabolites have been associated with adverse effects including obesity, type 2 diabetes mellitus, and carcinogenicity [5–7]. These chemicals can affect various tissues through mechanisms involving the steroidogenic pathway such as antiandrogenic or estrogenic activity, and receptor-mediated changes in protein synthesis [8–10].
Since DDT and DDE were first reported to be related to breast cancer in 1993 [11], there has been increased attention on the association between exposure to DDT and the risk of breast cancer. Although many epidemiological studies have been conducted to investigate the relationship between DDT exposure and breast cancer risk, there is a large heterogeneity between studies and the findings are not conclusive. Because a meta-analysis study showed no evidence of an association between DDT exposure and breast cancer risk [12], several new epidemiological studies have been published about the relationship between the body burden of DDT and breast cancer risk [13–18].
In the work reported here, we aimed to provide an update of a systematic review and meta-analysis to estimate the association between DDE exposure and the risk of breast cancer based on study characteristics.
2. Materials and methods
2.1. Study selection
We searched and reviewed the PubMed and EMBASE databases to identify eligible epidemiological studies published in English up to August 2012 using selected common keywords related to DDT exposure and the risk of breast cancer. The reference lists of the identified papers and previous literature reviews were carefully examined for additional studies. The combination of keywords such as DDT, chlorphenotane, dichlorodiphenyldichloroethylene, DDE, p,p′-DDE, 1,1-dichloro-2,2-bis(4 chlorophenyl)ethylene, hydrocarbons, chlorinated, organochlorines, organochlorine pesticides, breast cancer, and breast neoplasm were entered as both medical subject heading (MeSH) terms and text words. The subject of the papers was limited to humans for all databases. We included epidemiological studies that met the following criteria: (1) studies that presented original data from case–control or cohort studies; (2) the outcome of interest was clearly defined as breast cancer; (3) the exposure of interest was DDT or DDT metabolites; and (4) studies that provided measurements with relative risk estimates and 95% confidence intervals (CIs), odds ratios (ORs) and 95% CIs, or values in cells of a 2 × 2 table (e.g., number of cases and controls in exposure categories from which the OR could be calculated). If the data were duplicated or shared in more than one study, only the most recent or more comprehensive study was included in the analysis.
2.2. Data extraction
All studies for which an abstract was present were reviewed and extracted independently by two evaluators (E.S.C. and Y.K.) according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [19]. Disagreements between evaluators about selected studies were resolved by discussion. The following data were extracted from the eligible studies and included in the final analysis: first author's name, publication year, study years, country, study design, number of participants (cases and controls), type of biological specimen, and OR with 95% CIs for association between the exposure of DDT and breast cancer.
2.3. Statistical analysis
Meta-analytic techniques that weight the logarithm of the OR of each study by a function of its variance were used to calculate a summary estimate. Meta-analyses were performed on the total data set and separately for the type of design (hospital-based case–control, population-based case–control, and nested case–control), study years (2000s, 1990s, 1980s, 1970s, and 1960s), biological specimen (serum, plasma, and adipose tissue), and geographical region of the study (North America, Europe, Asia, and South America). A random effect model was used to estimate pooled ORs regarding the potential heterogeneity of the study populations. Statistical heterogeneity between studies was assessed with the Q-statistics and quantified by I2, which measured the percentage of total variation in included studies [20]. Significant heterogeneity was defined as the Q-statistics test p < 0.1 or I2 greater than 50%. We assessed potential publication bias by examining funnel plots and using Egger's test. All the statistical analyses were performed using the Stata 12.0 software (StataCorp, College Station, TX, USA).
3. Results
The PubMed and EMBASE search yielded 530 papers and 44 papers remained after screening based on the inclusion criteria. On reviewing of the full text of the remaining 44 papers, we identified 35 papers on the exposure to DDE and the risk of breast cancer. Two papers each consisted of two subpopulations and we treated the data of each subgroup as a separate study (Figure 1).
Figure 1.
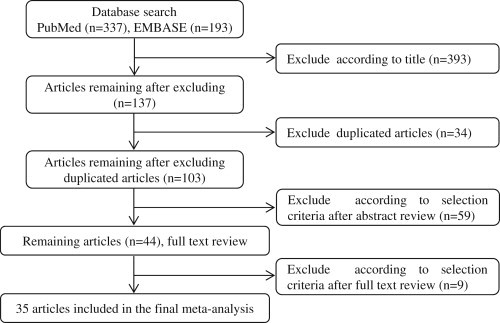
Process used for literature search.
Table 1 gives the details of the 35 studies that were included in the meta-analysis. All were case–control studies and of these 10 were prospective (nested case–control) and 16 were hospital based case–control studies, and 11 were population based case–control studies, which consist of 8160 cases and 9280 controls. Five studies indicated a significant positive association with the risk of breast cancer, whereas no significant association was observed in 32 studies. Twenty-two studies conducted in the USA and Canada, eight in Europe, three in Asia, and four in South America. In most studies, the level of DDE was measured in serum samples.
Table 1.
Summary of papers included in the meta-analysis for DDT exposure and breast cancer risk
| Author (year) | Study years | Country | Design | n (cases/controls) | Biological specimen | OR (95% CI) |
|---|---|---|---|---|---|---|
| Aronson (2000) [26] | 1995–1997 | Canada | Hospital CC | 217/213 | Adipose tissue | 1.10 (0.78–1.55) |
| Charlier (2004) [14] | 2001–2002 | Belgium | Population CC | 231/290 | Serum | 2.21 (1.41–3.48) |
| Cohn (2007) [27] | 1959–1967 | USA | Hospital CC | 129/129 | Serum | 1.29 (0.85–1.96) |
| Dello Lacovo (1999) [28] | 1997–1998 | Italy | Population CC | 170/195 | Serum | 1.02 (0.68–1.54) |
| Demers (2000) [29] | 1994–1997 | Canada | Population CC | 315/307 | Plasma | 0.91 (0.70–1.17) |
| Demers (2000) [29] | 1994–1997 | Canada | Hospital CC | 315/219 | Plasma | 1.01 (0.74–1.39) |
| Dorgan (1999) [30] | 1977–1987 | USA | Nested CC | 105/207 | Serum | 0.70 (0.47–0.99) |
| Gammon (2002) [15] | 1996–1997 | USA | Population CC | 643/427 | Serum | 1.20 (0.76–1.90) |
| Gatto (2007) [31] | 1995–1998 | USA | Population CC | 355/327 | Serum | 1.05 (0.82–1.35) |
| Helzlsouer (1999) [32] | 1974 | USA | Nested CC | 235/235 | Serum | 0.94 (0.71–1.25) |
| Helzlsouer (1999) [32] | 1989 | USA | Nested CC | 105/105 | Serum | 0.88 (0.56–1.38) |
| Hoyer (1998) [33] | 1976 | Denmark | Nested CC | 237/469 | Serum | 0.88 (0.56–1.37) |
| Hoyer (2000) [34] | 1976–1978/1981–1983 | Denmark | Nested CC | 240/477 | Serum | 1.04 (0.70–1.55) |
| Ibarluzea (2004) [16] | 1996–1998 | Spain | Hospital CC | 198/260 | Adipose tissue | 1.16 (0.83–1.62) |
| Itoh (2009) [35] | 2001–2005 | Japan | Population CC | 349/349 | Serum | 0.74 (0.48–1.13) |
| Iwasaki (2008) [17] | 1990–1995 | Japan | Nested CC | 139/278 | Plasma | 1.23 (0.80–1.90) |
| Krieger (1994) [36] | 1964–1969 | USA | Nested CC | 150/150 | Serum | 1.31 (0.82–2.09) |
| Laden (2001) [37] | 1989–1990 | USA | Nested CC | 372/372 | Plasma | 0.79 (0.61–1.01) |
| Liljegren (1998) [38] | 1993–1995 | Sweden | Hospital CC | 43/35 | Adipose tissue | 0.40 (0.10–1.20) |
| Lopez-Carrillo (1997) [39] | 1994–1996 | Mexico | Hospital CC | 141/141 | Serum | 0.68 (0.43–1.07) |
| McCready (2004) [18] | 1995–1997 | Canada | Hospital CC | 68/52 | Adipose tissue | 2.48 (1.08–5.71) |
| Mendonca (1999) [40] | 1995–1996 | Brazil | Hospital CC | 162/331 | Serum | 1.05 (0.75–1.46) |
| Millikan (2000) [41] | 1993–1996 | USA | Population CC | 748/659 | Plasma | 1.07 (0.86–1.32) |
| Moysich (1998) [42] | 1986–1991 | USA | Population CC | 154/192 | Serum | 1.15 (0.74–1.79) |
| Olaya-Contreras (1998) [21] | 1995–1996 | Colombia | Hospital CC | 153/153 | Serum | 1.56 (1.02–2.39) |
| Pavuk (2003) [43] | 1997–1999 | USA | Hospital CC | 24/85 | Serum | 1.49 (0.45–4.87) |
| Raaschou-Nielsen (2005) [44] | 1993–1997 | Denmark | Nested CC | 363/363 | Adipose tissue | 0.87 (0.69–1.10) |
| Romieu (2000) [22] | 1990–1995 | Mexico | Population CC | 120/126 | Serum | 2.02 (1.14–3.57) |
| Rubin (2005) [45] | 1981–1987 | USA | Population CC | 63/63 | Serum | 0.97 (0.41–2.32) |
| Schecter (1997) [46] | 1994 | Vietnam | Hospital CC | 21/21 | Serum | 0.69 (0.23–2.07) |
| Stellman (2000) [47] | 1994–1996 | USA | Hospital CC | 232/323 | Adipose tissue | 0.94 (0.66–1.33) |
| van't Veer (1997) [48] | 1991–1992 | Five European countries | Hospital CC | 265/341 | Adipose tissue | 0.75 (0.52–1.08) |
| Wolff (1993) [11] | 1985–1991 | USA | Population CC | 58/171 | Serum | 2.30 (1.31–4.04) |
| Wolff (2000) [49] | 1994–1996 | USA | Hospital CC | 151/317 | Serum | 0.86 (0.61–1.22) |
| Wolff (2000) [50] | 1987–1992 | USA | Nested CC | 110/213 | Serum | 0.83 (0.50–1.37) |
| Zheng (1999) [51] | 1994–1997 | USA | Hospital CC | 304/304 | Adipose tissue | 1.02 (0.73–1.41) |
| Zheng (2000) [52] | 1995–1997 | USA | Hospital CC | 475/502 | Serum | 1.01 (0.79–1.28) |
CC = case-control study; CI = confidence interval; OR = odds ratio.
Overall, there was no significant association between the exposure to DDE and the risk of breast cancer in the meta-analysis of all case–control studies (OR 1.03, 95% CI 0.95–1.12; Figure 2A) and there was some evidence for heterogeneity (p = 0.006, I2 = 40.9). However, no significant publication bias was observed in the selected studies (Begg's funnel plot was symmetric; Egger's test, p for bias = 0.145; Figure 2B).
Figure 2.
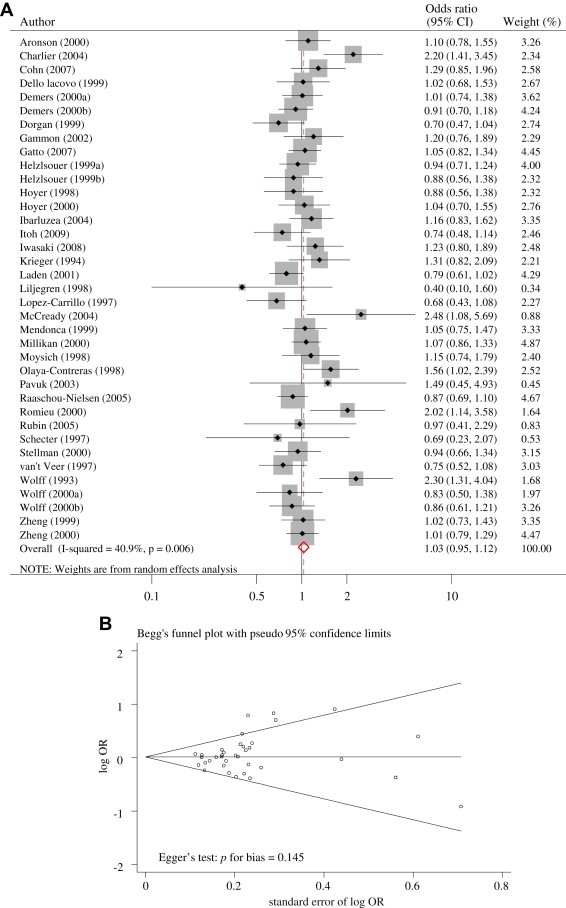
(A) Odds ratios (ORs) for DDT exposure and breast cancer. (B) Funnel plot of all included studies.
To resolve the heterogeneity, we performed subgroup meta-analyses by the type of study design, study years, type of biological specimen, and country (Table 2). We found a borderline statistically significant summary OR for population-based case–control studies with 1.19 (95% CI 0.99–1.44), although there was a considerable heterogeneity based on the 11 studies (I2 = 61.3). However, there was no significant association in other subgroup meta-analysis.
Table 2.
Meta-analysis of the effect of the exposure to DDT on the risk of breast cancer according to subgroup
| Studies included | No. of Studies | OR | 95% CI | Heterogeneity |
Egger’s test (p for bias) | |
|---|---|---|---|---|---|---|
| p-value | I2 (%) | |||||
| Type of study design | ||||||
| Hospital CC | 16 | 1.02 | 0.91 to 1.15 | 0.183 | 24.0 | 0.780 |
| Population CC | 11 | 1.19 | 0.99 to 1.44 | 0.004 | 61.3 | 0.212 |
| Nested CC | 10 | 0.90 | 0.81 to 1.01 | 0.554 | 0.0 | 0.274 |
| Study years | ||||||
| 2000s | 2 | 1.28 | 0.44 to 3.71 | 0.001 | 91.5 | – |
| 1990s | 27 | 1.03 | 0.94 to 1.24 | 0.034 | 35.9 | 0.169 |
| 1980s | 4 | 0.87 | 0.69 to 1.09 | 0.575 | 0.0 | 0.908 |
| 1970s | 2 | 0.92 | 0.73 to 1.17 | 0.808 | 0.0 | – |
| 1960s | 2 | 1.30 | 0.95 to 1.77 | 0.962 | 0.0 | – |
| Type of biologic specimen | ||||||
| Serum | 24 | 1.07 | 0.93 to 1.11 | 0.006 | 47.0 | 0.365 |
| Plasma | 5 | 0.97 | 0.85 to 1.11 | 0.325 | 14.1 | 0.910 |
| Adipose tissue | 8 | 0.98 | 0.83 to 1.16 | 0.141 | 36.0 | 0.228 |
| Country | ||||||
| North America | 22 | 1.01 | 0.92 to 1.10 | 0.185 | 21.0 | 0.524 |
| Europe | 8 | 1.02 | 0.81 to 1.29 | 0.010 | 62.0 | 0.246 |
| Asia | 3 | 0.92 | 0.63 to 1.36 | 0.226 | 32.8 | 0.900 |
| South America | 4 | 1.20 | 0.78 to 1.83 | 0.012 | 72.8 | 0.707 |
CC = case–control study; CI = confidence interval; OR = odds ratio.
Figure 3 shows the subgroup meta-analysis for population-based case-control studies with estimated DDE levels in serum published in 1990s. The OR for this subgroup indicated 1.28 (95% CI 1.00–1.65; Figure 3A), although there was a high heterogeneity (Figure 3B). In other stratified meta-analyses, there was no significant association between exposure to DDE and the risk of breast cancer (data not shown).
Figure 3.
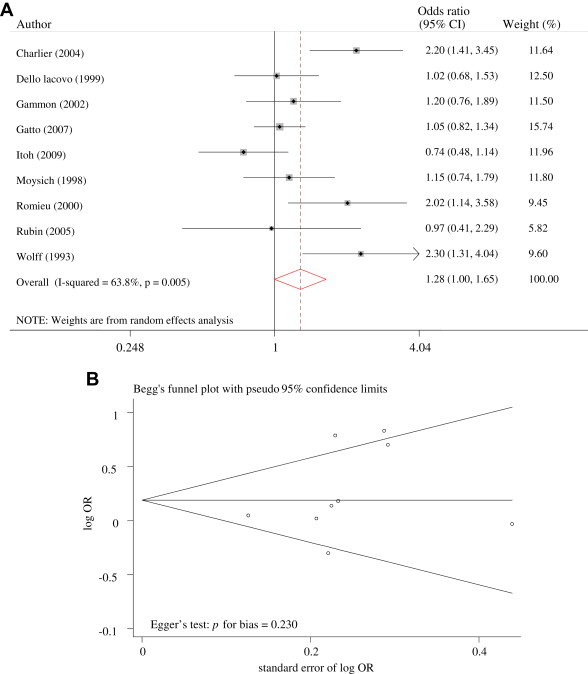
(A) Odds ratios for DDT exposure and breast cancer in population-based case–control studies. (B) Funnel plot of population-based case–control studies.
4. Discussion
We found that there was no significant evidence of an association between the risk of breast cancer and exposure to DDE with recent published literature. Subgroup meta-analyses by the type of design, study years, biological specimen, and geographical region of study also do not support a relationship between exposure to DDE and the risk of breast cancer. However, population-based case–control studies with estimated DDE levels in serum and published in the 1990s showed marginally significant findings, which need further investigation.
Many studies did not report an increased risk, despite the first publication reporting an excess of breast cancer associated with exposure to DDE [11]. Five studies [11,14,18,21,22] among the 35 pooled studies included in our meta-analysis found a positive association between exposure to DDE and the risk of breast cancer. There was moderate heterogeneity among the pooled studies. The inconsistency and heterogeneity of the studies could be explained by potential confounders or modifiers that might affect the relationship between DDE and the risk of breast cancer. One potential explanation for the huge differences in the risk of breast cancer and the moderate heterogeneity among pooled studies is that there is a delayed time between exposure and diagnosis. As DDT can remain in the body for a long period, there is a limitation to identifying accurately the exposure period and levels of exposure.
As DDT crosses the placenta to the fetus and is secreted in breast milk [23], human exposure begins during the early prenatal period and continues during the breastfeeding neonatal period. Evidence for DDE release from fat storage tissue in humans has been provided by breastfeeding studies, which have been found to decrease the risk of breast cancer [24,25]. Exposure during the prenatal and neonatal periods may reduce the distinction between the exposed and unexposed groups and make it harder for such studies to show a true causal association. The age at exposure to chemicals such as DDE is also an important modifier in explaining the relationship between exposure and the risk of disease. Cohn et al [27] reported that DDT was associated with breast cancer only for women potentially exposed at a young age (prior to 14 years of age). Thus the relationship between age at exposure to DDT and breast cancer represents an important area in need of further research.
The other limitation is combined exposure with other chemicals in the natural environment. Many persistent organic pollutants, including DDT, are known or suspected to be endocrine disruptors. However, these chemicals do not all have the same effect; some chemicals have an agonistic role in estrogenic effects, but others have an antagonistic role. Thus current estimations may rule out the possibility that there is a particular hazard from these mixtures or one chemical, whereas exposure to several different chemicals may have a pronounced effect due to their combination.
In summary, our meta-analysis found no evidence that there is an association between exposure to DDE and the risk of breast cancer. Although our results indicate no relationship, there are still several limitations to this study, such as the delay time between exposure and diagnosis, age of exposure, the effect of susceptible populations, and combined exposure with other potential carcinogens. It is particularly important to recommend studying the relationship between DDT and breast cancer based on age of exposure and combined exposure to a number of potential carcinogens.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported by a grant (12161MFDS767) from Ministry of Food and Drug Safety, Osong, Korea in 2012.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) 2002 Sep. Toxicological profile for DDT, DDE, and DDD.http://www.atsdr.cdc.gov/toxprofiles/tp35-p.pdf [accessed 13.02.14] [PubMed] [Google Scholar]
- 2.Turusov V., Rakitsky V., Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002 Feb;110(2):125–128. doi: 10.1289/ehp.02110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff M.S. Half-lives of organochlorines (OCs) in humans [Letter] Arch Environ Contam Toxicol. 1999 May;36(4):504. doi: 10.1007/pl00006624. [DOI] [PubMed] [Google Scholar]
- 4.Lim J.S., Son H.K., Park S.K. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes (Lond) 2011 May;35(5):744–747. doi: 10.1038/ijo.2010.188. [DOI] [PubMed] [Google Scholar]
- 5.Elobeid M.A., Padilla M.A., Brock D.W. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999–2002 data. Int J Environ Res Public Health. 2010 Jul;7(7):2988–3005. doi: 10.3390/ijerph7072988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everett C.J., Frithsen I.L., Diaz V.A. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2007 Mar;103(3):413–418. doi: 10.1016/j.envres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Beard J. DDT and human health. Sci Total Environ. 2006 Feb;355(1–3):78–89. doi: 10.1016/j.scitotenv.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Kelce W.R., Stone C.R., Laws S.C. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995 Jun;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz R., Young P.C., Caperell-Grant A. Novel estrogenic action of the pesticide residue beta-hexachlorocyclohexane in human breast cancer cells. Cancer Res. 1996 Dec;56(23):5403–5409. [PubMed] [Google Scholar]
- 10.Lemaire G., Mnif W., Mauvais P. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006 Aug;79(12):1160–1169. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Wolff M.S., Toniolo P.G., Lee E.W. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993 Apr;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Cervantes M., Torres-Sanchez L., Tobias A. Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence. Environ Health Perspect. 2004 Feb;112(2):207–214. doi: 10.1289/ehp.112-1241830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laden F., Collman G., Iwamoto K. 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: combined analysis of five U.S. studies. J Natl Cancer Inst. 2001 May;93(10):768–776. doi: 10.1093/jnci/93.10.768. [DOI] [PubMed] [Google Scholar]
- 14.Charlier C., Foidart J.M., Pitance F. Environmental dichlorodiphenyltrichlorethane or hexachlorobenzene exposure and breast cancer: is there a risk? Clin Chem Lab Med. 2004 Feb;42(2):222–227. doi: 10.1515/CCLM.2004.040. [DOI] [PubMed] [Google Scholar]
- 15.Gammon M.D., Wolff M.S., Neugut A.I. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002 Aug;11(8):686–697. [PubMed] [Google Scholar]
- 16.Ibarluzea J.J., Fernandez M.F., Santa-Marina L. Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control. 2004 Aug;15(6):591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki M., Inoue M., Sasazuki S. Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Sci Total Environ. 2008 Sep;402(2–3):176–183. doi: 10.1016/j.scitotenv.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.McCready D., Aronson K.J., Chu W. Breast tissue organochlorine levels and metabolic genotypes in relation to breast cancer risk Canada. Cancer Causes Control. 2004 May;15(4):399–418. doi: 10.1023/B:CACO.0000027505.32564.c2. [DOI] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Olaya-Contreras P., Rodriguez-Villamil J., Posso-Valencia H.J. Organochlorine exposure and breast cancer risk in Colombian women. Cad Saude Publica. 1998;(14 Suppl 3):125–132. doi: 10.1590/s0102-311x1998000700013. [DOI] [PubMed] [Google Scholar]
- 22.Romieu I., Hernandez-Avila M., Lazcano-Ponce E. Breast cancer, lactation history, and serum organochlorines. Am J Epidemiol. 2000;152(4):363–370. doi: 10.1093/aje/152.4.363. [DOI] [PubMed] [Google Scholar]
- 23.Perera F.P., Rauh V., Whyatt R.M. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005 Aug;26(4):573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Carrillo L., Torres-Sanchez L., Moline J. Breast-feeding and serum p,p'DDT levels among Mexican women of childbearing age: a pilot study. Environ Res. 2001 Dec;87(3):131–135. doi: 10.1006/enrs.2001.4296. [DOI] [PubMed] [Google Scholar]
- 25.Bradman A.S., Schwartz J.M., Fenster L. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expos Sci Environ Epidemiol. 2007 Jul;17(4):388–399. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronson K.J., Miller A.B., Woolcott C.G. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000 Jan;9(1):55–63. [PubMed] [Google Scholar]
- 27.Cohn B.A., Wolff M.S., Cirillo P.M. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007 Oct;115(10):1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dello Iacovo R., Celentano E., Strollo A.M. Organochlorines and breast cancer. A study on Neapolitan women. Adv Exp Med Biol. 1999;472:57–66. [PubMed] [Google Scholar]
- 29.Demers A., Ayotte P., Brisson J. Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Cancer Epidemiol Biomarkers Prev. 2000 Feb;9(2):161–166. [PubMed] [Google Scholar]
- 30.Dorgan J.F., Brock J.W., Rothman N. Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA) Cancer Causes Control. 1999 Feb;10(1):1–11. doi: 10.1023/a:1008824131727. [DOI] [PubMed] [Google Scholar]
- 31.Gatto N.M., Longnecker M.P., Press M.F. Serum organochlorines and breast cancer: a case-control study among African-American women. Cancer Causes Control. 2007 Feb;18(1):29–39. doi: 10.1007/s10552-006-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helzlsouer K.J., Alberg A.J., Huang H.Y. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999 Jun;8(6):525–532. [PubMed] [Google Scholar]
- 33.Hoyer A.P., Grandjean P., Jorgensen T. Organochlorine exposure and risk of breast cancer. Lancet. 1998 Dec;352(9143):1816–1820. doi: 10.1016/S0140-6736(98)04504-8. [DOI] [PubMed] [Google Scholar]
- 34.Hoyer A.P., Jorgensen T., Grandjean P. Repeated measurements of organochlorine exposure and breast cancer risk (Denmark) Cancer Causes Control. 2000 Feb;11(2):177–184. doi: 10.1023/a:1008926219539. [DOI] [PubMed] [Google Scholar]
- 35.Itoh H., Iwasaki M., Hanaoka T. Serum organochlorines and breast cancer risk in Japanese women: a case-control study. Cancer Causes Control. 2009 Jul;20(5):567–580. doi: 10.1007/s10552-008-9265-z. [DOI] [PubMed] [Google Scholar]
- 36.Krieger N., Wolff M.S., Hiatt R.A. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst. 1994 Apr;86(8):589–599. doi: 10.1093/jnci/86.8.589. [DOI] [PubMed] [Google Scholar]
- 37.Laden F., Hankinson S.E., Wolff M.S. Plasma organochlorine levels and the risk of breast cancer: an extended follow-up in the Nurses' Health Study. Int J Cancer. 2001 Feb;91(4):568–574. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1081>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Liljegren G., Hardell L., Lindström G. Case-control study on breast cancer and adipose tissue concentrations of congener specific polychlorinated biphenyls, DDE and hexachlorobenzene. Eur J Cancer Prev. 1998 Apr;7(2):135–140. [PubMed] [Google Scholar]
- 39.Lopez-Carrillo L., Blair A., Lopez-Cervantes M. Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: a case-control study from Mexico. Cancer Res. 1997 Sep;57(17):3728–3732. [PubMed] [Google Scholar]
- 40.Mendonca G.A., Eluf-Neto J., Andrada-Serpa M.J. Organochlorines and breast cancer: a case–control study in Brazil. Int J Cancer. 1999 Nov;83(5):596–600. doi: 10.1002/(sici)1097-0215(19991126)83:5<596::aid-ijc4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 41.Millikan R., DeVoto E., Duell E.J. Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Cancer Epidemiol Biomarkers Prev. 2000 Nov;9(11):1233–1240. [PubMed] [Google Scholar]
- 42.Moysich K.B., Ambrosone C.B., Vena J.E. Environmental organochlorine exposure and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998 Mar;7(3):181–188. [PubMed] [Google Scholar]
- 43.Pavuk M., Cerhan J.R., Lynch C.F. Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia. J Expo Anal Environ Epidemiol. 2003 Jun;13(4):267–275. doi: 10.1038/sj.jea.7500277. [DOI] [PubMed] [Google Scholar]
- 44.Raaschou-Nielsen O., Pavuk M., Leblanc A. Adipose organochlorine concentrations and risk of breast cancer among postmenopausal Danish women. Cancer Epidemiol Biomarkers Prev. 2005 Jan;14(1):67–74. [PubMed] [Google Scholar]
- 45.Rubin C.H., Lanier A., Kieszak S. Breast cancer among Alaska Native women potentially exposed to environmental organochlorine chemicals. Int J Circumpolar Health. 2006 Feb;65:18–27. doi: 10.3402/ijch.v65i1.17885. [DOI] [PubMed] [Google Scholar]
- 46.Schecter A., Toniolo P., Dai L.C. Blood levels of DDT and breast cancer risk among women living in the North of Vietnam. Arch Environ Contam Toxicol. 1997 Nov;33(4):453–456. doi: 10.1007/s002449900276. [DOI] [PubMed] [Google Scholar]
- 47.Stellman S.D., Djordjevic M.V., Britton J.A. Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 2000 Nov;9(11):1241–1249. [PubMed] [Google Scholar]
- 48.van't Veer P., Lobbezoo I.E., Martin-Moreno J.M. DDT (dicophane) and postmenopausal breast cancer in Europe: case-control study. BMJ. 1997 Jul;315(7100):81–85. doi: 10.1136/bmj.315.7100.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolff M.S., Berkowitz G.S., Brower S. Organochlorine exposures and breast cancer risk in New York City women. Environ Res. 2000 Oct;84(2):151–161. doi: 10.1006/enrs.2000.4075. [DOI] [PubMed] [Google Scholar]
- 50.Wolff M.S., Zeleniuch-Jacquotte A., Dubin N. Risk of breast cancer and organochlorine exposure. Cancer Epidemiol Biomarkers Prev. 2000 Mar;9(3):271–277. [PubMed] [Google Scholar]
- 51.Zheng T., Holford T.R., Mayne S.T. DDE and DDT in breast adipose tissue and risk of female breast cancer. Am J Epidemiol. 1999 Sep;150(5):453–458. doi: 10.1093/oxfordjournals.aje.a010033. [DOI] [PubMed] [Google Scholar]
- 52.Zheng T., Holford T.R., Mayne S.T. Risk of female breast cancer associated with serum polychlorinated biphenyls and 1,1-dichloro-2,2'-bis(p-chlorophenyl) ethylene. Cancer Epidemiol Biomarkers Prev. 2000 Feb;9(2):167–174. [PubMed] [Google Scholar]


