Abstract
We recently reported that HLA-G1-transfected antigen-presenting cells (HLA-G1+ APCs) were capable of inhibiting alloproliferative responses. The aim of the present work was to further study the function and the mechanisms of action of HLA-G1+ APCs. We show here that HLA-G1+ APCs are immunoinhibitory cells that (i) inhibit the proliferation of CD4+ T cells, (ii) shed HLA-G1 molecules that might provide extra, non-antigen-specific, inhibitory or proapoptotic signals, (iii) induce CD4+ T cell anergy, or at least long-term unresponsiveness, and (iv) cause the differentiation of CD4+ T cells into suppressive cells. Thus, HLA-G+ APCs might (i) be involved in the direct suppression of immune responses and (ii) contribute to long-term efficient immune escape or tolerance.
HLA-G is a nonclassical HLA class I antigen that is characterized by a tissue-restricted expression, a low polymorphism, and seven proteic isoforms (HLA-G1 to -G7) (1). HLA-G1 has a structure similar to that of classical HLA class I molecules: a heavy chain noncovalently associated with β-2 microglobulin and a nonameric peptide. Functional assays have demonstrated that HLA-G was able to inhibit allogeneic proliferation of T cells (2–5), and natural killer cell cytotoxicity (6–10), as well as antigen-specific T cell cytotoxicity (5, 11). HLA-G exerts its direct immunoinhibitory function through three inhibitory receptors, ILT2/CD85j, ILT4/CD85d, and KIR2DL4/CD158d (12–14).
Under pathological conditions, HLA-G is expressed (i) by allografts (15–17) and by infiltrating monocuclear cells within the transplanted tissues (17), (ii) during inflammatory diseases and by lesion-infiltrating HLA-G+ antigen-presenting cells (APCs) (5, 18, 19), (iii) by tumor tissues (20–24) and by tumor-infiltrating APC (24, 25), and (iv) on monocytes and T cells from HIV patients (26).
The function of HLA-G+ APCs is still unclear, but our hypothesis is that APCs expressing such a potent inhibitory molecule might be immunoregulatory. The aim of the present study was (i) to investigate the inhibitory potential and the mechanisms of action of HLA-G-expressing APCs and (ii) to determine whether HLA-G-expressing APCs were immunotolerogenic and capable of inducing the maturation/differentiation of suppressor cells. For this purpose, we generated HLA-G1-transfected APC lines (HLA-G1+ APC) and studied their functions as suppressor cells in allogeneic reactions. In this work, we first demonstrate that HLA-G1+ APC suppress by 80% the allogeneic responses of peripheral blood mononuclear cells (PBMCs) and purified CD4+ T cells. Second, we show that HLA-G1+ APC induce CD4+ T cells' long-term unresponsiveness and promote the differentiation/maturation of CD4+ T cells into suppressor cells. Our data indicate that HLA-G1+ APCs might represent a subset of immunocompetent cells with immunosuppressive function involved in the regulation of immune responses.
Materials and Methods
Cell Preparation and Culture. PBMCs obtained from heparinized whole blood of healthy volunteer adult donors were separated by density-gradient centrifugation over Ficoll/Histopaque (Amersham Biosciences, Piscataway, NJ). PBMCs were washed twice and suspended in RPMI medium 1640 (GIBCO) supplemented with fungizone (Sigma-Aldrich), 1 μg/ml gentamicin, l-glutamine (GIBCO), and 10% heat-inactivated human serum AB.
For purification of CD4+ T cells, anti-CD4-coated Dynabeads (Dynal, Great Neck, NY) were used. Magnetic beads were detached from the CD4+ T cells surface by incubation of the isolated cells overnight in a 37°C, 5% CO2, humidified incubator in culture medium.
For large scale allostimulation experiments, 2 × 107 responder cells were cocultured with γ-irradiated allogeneic unseparated PBMCs or cell lines at a responder:stimulator ratio of 1:1 and 1:0.5, respectively, and at a final concentration of responder cells of 106 cells per ml.
Cell Lines and Transfectants. KG1a cells (American Type Culture Collection), LCL 721.221 cells (LCL, American Type Culture Collection), and U937 cells (American Type Culture Collection) were used in this study. All cell lines were maintained in RPMI medium 1640 supplemented with 10% heat-inactivated FCS, 2 mM l-glutamin, 1 μg/ml gentamicin, and fungizone (Sigma-Aldrich). All transfected cell lines were selected in media containing 1 mg/ml geneticin (G418, Sigma-Aldrich).
For generation of transfectant cell lines, the pRc/RSV vector (Invitrogen) and the pRc/RSV vector containing the full length of the HLA-G1 gene (7) (pRc/RSV-G1) were transfected by electroporation (Bio-Rad Gene Pulsar at 240V, 960 μF) into KG1a cells (KG1a-RSV and KG1a-G1 cells), LCL cells (LCL-RSV and LCL-G1 cells), and U937 cells (U937-RSV and U937-G1 cells). HLA-G1-transfectant lines were monitored for the expression of HLA-G1 by flow cytometry, and were later sorted on the basis of HLA-G1 expression on a FACSVantage SE cell sorter (Becton Dickinson) by using anti HLA-G MEM-G/09 mAb. KG1a-G1 clonal lines were generated by using a FACSVantage SE cell sorter.
Antibodies and Flow Cytometry. The following primary murine mAbs were used in flow cytometry studies: anti-CD4-ECD (phycoerythrin-Texas red-conjugated mouse IgG1, Immunotech, Marseille, France); anti-CD8-PC5 (phycoerythrin-cyanin 5-conjugated mouse IgG1, Immunotech); anti-ILT2 FITC (FITC-conjugated mouse IgG2b, BD Biosciences); anti-HLA-DR FITC (FITC-conjugated mouse IgG2b, Immunotech); anti-HLA-G MEM-G/09 FITC (FITC-conjugated mouse IgG1, Exbio, Prague); anti-pan HLA class I W6/32 (mouse IgG2a, Sigma); anti-HLA-A, -B, -C, and -E TP2599 (mouse IgG1, S. Ferrone, Roswell Park Cancer Institute, Buffalo, NY); anti-HLA-A, -B, and -C SV9985 (mouse IgG, S. Ferrone); and anti-HLA-E MEM-E/06 (mouse IgG1, V. Horejsi, Institute of Molecular Genetics, Prague). Rabbit polyclonal anti-human β-2-microglobulin antibody conjugated to horseradish peroxidase (DAKO) was used in an ELISA. The anti-pan HLA-G mAb 4H84 (mouse IgG1, M. Mc Master, University of California, San Francisco) was used in Western blot analyses. For flow cytometry analyses, cells were first incubated 30 min in PBS containing 20% human serum, washed in PBS, incubated with the primary antibodies in PBS 2% BSA for 30 min at 4°C, and washed twice in PBS 2% BSA. When necessary, phycoerythrin-conjugated F(ab′)2 Goat anti-mouse IgG (Immunotech) was used as a secondary mAb. Isotype-matched control antibodies were systematically used to evaluate nonspecific binding. Flow cytometry analyses were performed on an Epics XL cytometer (Beckman Coulter) using expo32 software (Beckman Coulter).
Immunoprecipitation and Western Blot Analysis. Immunoprecipitation of HLA-G was performed by using the mouse anti-human pan HLA class I mAb W6/32 on 10× concentrated (Ultrafree-15 centrifugal filter device, Millipore) supernatants of 24-h cultures of KG1a-RSV, KG1a-G1, LCL-RSV, LCL-G1, U937-RSV, and U937-G1 cell lines, and on KG1a-G1 cell lysates, as described (4).
Alloproliferation Assays/Mixed Lymphocyte Reaction (MLR). For alloproliferation studies, either unseparated PBMCs or purified CD4+ T cells were used as responders, and stimulator cells were either γ-irradiated unseparated PBMCs, or γ-irradiated cultured cell lines described above. Alloproliferation assays were set up as follows: 105 responder cells were cultured in 96 U-bottomed plates with 105 (PBMCs) or 5 × 104 (cell lines) irradiated stimulator cells and plated in a final volume of 150 μl per well. All samples were run in triplicate, and for each allogeneic combination, responder cells alone, irradiated stimulated cells alone, and autologous controls were included. After 6 days, cultures were pulsed with thymidine [1μCi per well (1 Ci = 37 GBq), Amersham Biosciences]. Cells were harvested 18 h later on filter mats, and thymidine incorporation into DNA was quantified on a β-counter (Wallac 1450, Amersham Biosciences).
Sensitization Procedures. When indicated, cells were sensitized for 12 h or 9 days with KG1a-RSV or KG1a-G1 stimulator cells. Sensitization was set up as MLR (see above). At the end of the sensitization period, stimulator cells were removed by using anti-CD34 mAb (KG1a-RSV-sensitized cultures) or anti-HLA-G MEM-G/09 mAb (KG1a-G1-sensitized cultures) and anti-mouse IgG-coated magnetic beads (Dynal Biotech). Before the purified cells were used, the extent of the depletion was checked by flow cytometry. Sensitized cells were used when depletion was higher than 99%. Stimulator-depleted samples were then used as responder cells or were γ-irradiated and used as third-party cells, as indicated.
ELISA. Shed HLA-G1 (sHLA-G1) concentrations in cell-free supernatants of mock- and HLA-G1-transfected cell lines were measured with an HLA-G1-specific sandwich ELISA by using anti-HLA-G1/G5 MEM-G/09 mAb for capture, and anti-human β2-microglobulin was conjugated to horseradish peroxidase for detection as described (27).
Results
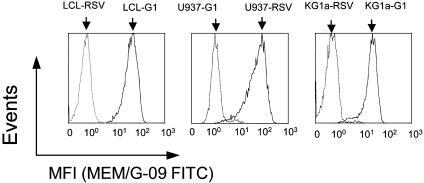
Characterization of HLA-G1-Transfected APCs. Parent and transfected cell lines were analyzed by flow cytometry for the expression of HLA-A, -B, -C, -E, -G, and -DR molecules by using anti-HLA-class I W6/32 (HLA-A, -B, -C, -E, and -G), anti-HLA-A, -B, -C, and -E TP2599, anti-HLA-A, -B, and -C SV9985, anti-HLA-E MEM-E/06, anti-HLA-G MEM-G/09, and anti-HLA-DR mAbs. Mock-transfected and HLA-G1-transfected cell lines presented the same phenotype with regard to the expression of all HLA molecules but HLA-G1 (Fig. 1).
Fig. 1.
Expression of cell-surface HLA-G by KG1a-G1, LCL-G1, and U937-G1 cell lines. pRC/RSV-transfected LCL (LCL-RSV), U937 (U937-RSV), and KG1a (KG1a-RSV) cells (dashed lines), and pRC/RSV-HLA-G1-transfected KG1a (KG1a-G1_CL3), LCL (LCL-G1), and U937 (U937-G1) cells (solid lines) were labeled by using the FITC-conjugated anti-HLA-G1 mAb MEM-G/09. Isotype-matched controls gave profiles identical to those of the HLA-G1- wild-type cells stained with MEM-G/09 and are not figured. MFI, mean fluorescence intensity.
HLA-G1+ APCs Inhibit Alloproliferative Responses. The allostimulatory capacity of HLA-G1-transfected APC lines were investigated by using freshly isolated PBMCs as responder cells. First, we evaluated the capability of 10 HLA-G1+ APC clonal lines (KG1a-G1) to inhibit the alloproliferative responses of PBMCs from two donors. In this experiment, the alloproliferation induced by HLA-G1+ APC lines was compared with that induced by HLA-G1- APC (KG1a-RSV cells). All HLA-G1+ APC lines inhibited the alloproliferative responses of PBMCs from both donors by >57.7% and 70.5%, respectively (Table 1).
Table 1. Allogeneic proliferative responses induced by HLA-G1+ APC and HLA-G1- APC clonal cell lines.
| PBMC I†
|
PBMC II†
|
|||
|---|---|---|---|---|
| APC lines* | cpm | % inhibition‡ | cpm | % inhibition§ |
| None | 520 | 650 | ||
| KG1a-RSV | 7,591 | 10,345 | ||
| KG1a-G1_CL1 | 2,286 | 70 | 5,844 | 44 |
| KG1a-G1_CL2 | 3,085 | 59 | 5,512 | 47 |
| KG1a-G1_CL3 | 2,154 | 72 | 2,316 | 78 |
| KG1a-G1_CL4 | 1,639 | 78 | 1,956 | 81 |
| KG1a-G1_CL5 | 3,914 | 48 | 3,153 | 70 |
| KG1a-G1_CL6 | 2,947 | 61 | 3,234 | 69 |
| KG1a-G1_CL7 | 4,205 | 45 | 1,577 | 85 |
| KG1a-G1_CL8 | 2,773 | 63 | 1,385 | 87 |
| KG1a-G1_CL9 | 6,159 | 19 | 2,746 | 73 |
| KG1a-G1_CL10 | 2,919 | 62 | 2,779 | 73 |
Raw proliferation (cpm) and percentage of proliferation inhibition (% inhibition) are presented for two donors and 10 clonal KG1a-G1 cell lines. Alloproliferation induced by the KG1a-RSV control line for each donor was used to determine the percentage of inhibition induced by the KG1a-G1 lines.
Irradiated stimulator cells
Responder cells
x̄ ± SE = 57.7 ± 5.3
x̄ ± SE = 70.5 ± 4.6
For subsequent studies, the HLA-G1+ APCs KG1a-G1_CL3 and KG1a-G1_CL10 lines, which inhibited alloproliferative responses to high and comparable levels, were chosen. These two lines were tested against a panel of eight donors. Both HLA-G1+ APC lines inhibited the alloproliferation of PBMCs from all donors tested by ≈80% (Table 2).
Table 2. Allogeneic proliferative responses of eight unseparated PBMC induced by HLA-G1- APCs and two HLA-G1+ APC clonal cell lines.
| APC†
|
|||||
|---|---|---|---|---|---|
| KG1a-RSV
|
KG1a-G1_CL3
|
KG1a-G1_CL10
|
|||
| PBMC* | cpm | cpm | % inhibition‡ | cpm | % inhibition§ |
| I | 26,552 | 2,552 | 90 | 5,756 | 78 |
| II | 33,611 | 17,439 | 48 | 7,120 | 79 |
| III | 35,038 | 3,806 | 89 | 6,704 | 81 |
| IV | 11,001 | 1,914 | 83 | 2,250 | 80 |
| V | 8,651 | 4,576 | 47 | 2,523 | 71 |
| VI | 12,576 | 1,801 | 86 | 2,862 | 77 |
| VII | 19,258 | 4,530 | 76 | 3,016 | 84 |
| VIII | 47,203 | 3,165 | 93 | 3,503 | 93 |
Raw proliferation data (cpm) and percentage of alloproliferation inhibition (% inhibition) induced by HLA-G1-transfected KG1a-G1_CL3 and KG1a-G1_CL10 are presented for eight donor responder PBMCs. Alloproliferation induced by the KG1a-RSV control line was used to determine the percentage of inhibition induced by the HLA-G1-transfected cells.
Responder cells
Stimulator cells
x̄ ± SE = 76.5 ± 6.6
x̄ ± SE = 80.4 ± 2.2
Finally, to confirm the results obtained with the KG1a cell line, the inhibitory capability of other HLA-G1+ APCs was evaluated. When LCL transfectants were used as allogeneic stimulator cells against unseparated PBMCs from two donors, an inhibition of alloproliferation of 80% was observed for LCL-G1 vs. LCL-RSV (data not shown). These results are in accordance and are comparable with those obtained for the previous HLA-G1+ APC lines.
HLA-G1+ APCs Inhibit the Alloproliferation of Purified CD4+ T Cells. We next investigated the direct effect of HLA-G1+ APCs on the CD4+ T cells. Purified CD4+ T cells from two donors were stimulated with irradiated HLA-G1- APCs (KG1a-RSV) or HLA-G1+ APCs (KG1a-G1). HLA-G1+ APCs induced an inhibition of the alloproliferative response of these two T cell populations of 50% and 80%, respectively (Table 3). This inhibition of CD4+ T cell alloproliferative responses is similar to that of unseparated PBMCs. Before use in alloproliferation assays, purified naíve CD4+ T cells were analyzed by flow cytometry for the expression of the HLA-G1 receptor ILT2/CD85j, because ILT2/CD85j is the only of the three HLA-G receptors that is expressed by naíve CD4+ T cells (28, 29), at least intracellularly (30). No expression of ILT2/CD85j was detected at the surface of the purified naíve CD4+ T cells (data not shown).
Table 3. Allogeneic proliferative responses of purified CD4+ T cell lines induced by HLA-G1- and HLA-G1+ APCs.
Raw proliferation data (cpm) and percentage of alloproliferation inhibition (% inhibition) induced by HLA-G1-transfected KG1a-G1_CL3 are presented for purified CD4+ T cells from two donors. Alloproliferation induced by the KG1a-RSV control line was used to determine the percentage of inhibition induced by the HLA-G1-transfected cells.
Responder cells
Stimulator cells
CD4+ T cells purified from donor VIII
CD4+ T cells purified from donor IX
Alloproliferation Inhibition by HLA-G1+ APCs Is Not Mediated by HLA-G1 Shedding. Shedding of HLA molecules is a process common to all classical HLA class I molecules. To find out whether HLA-G1 was shed from the surface of the transfectant cell lines, we the presence of HLA-G in the 24-h culture supernatants. By using an HLA-G1-specific ELISA, we detected the presence of HLA-G1 in the culture supernatants of all HLA-G1+ APCs, but not in those of any HLA-G- APCs (Fig. 2). These results were confirmed by immunoprecipitation followed by Western blotting (Fig. 3).
Fig. 2.
Concentrations of sHLA-G1 in culture supernatants of HLA-G1- and HLA-G1+ APCs. Concentrations of sHLA-G1 were measured by ELISA in supernatants of 24-h cultures of HLA-G1- APCs (KG1a-RSV, U937-RSV, and LCL-RSV) and HLA-G1+ APCs (KG1a-G1_CL10, U937-G1, and LCL-G1). For quantification, serial dilutions of purified HLA-G in culture medium were used as a standard curve.
Fig. 3.
HLA-G-specific Western blot analysis of HLA-G1- and HLA-G1+ APC culture supernatants. The presence of sHLA-G1 was analyzed in 24-h culture supernatants of HLA-G1- APCs (KG1a-RSV, LCL-RSV, and U937-RSV) and HLA-G1+ APCs (KG1a-G1_CL3, LCL-G1, and U937-G1). Immunoprecipitation with anti-pan HLA class I W6/32 mAb and Western blot analysis by using the anti-pan HLA-G 4H84 mAb was carried on concentrated culture supernatants as described in Materials and Methods. The molecular weight of the HLA-G1 heavy chain is indicated.
To determine whether the inhibition of alloproliferation induced by HLA-G1+ APCs was due to shed HLA-G1, we compared the alloinhibitory effect of HLA-G1- and HLA-G1+ APC culture supernatants by using them as culture medium in MLR. No inhibition of alloproliferation was observed in MLR performed with HLA-G1+ APC culture supernatants (data not shown). This result indicates that, in our system, the inhibitory effect of HLA-G1+ APCs was due to a direct, cell-to-cell contact mechanism, and not to the release of HLA-G1 in the culture medium.
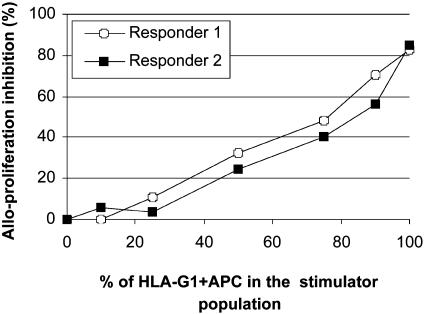
The HLA-G1+ APCs Mediate an Alloproliferation Inhibition That Correlates with the Proportion of HLA-G1+ APCs Among Stimulators. It has been shown in cytotoxicity assays in which targets were a mixture of HLA-G+ and HLA-G- cells, that as few as 10% HLA-G+ target cells in the mixture were sufficient to inhibit cytotoxic T cell-specific activity by 50%, and that a 50/50 mixture of HLA-G+ and HLA-G- target cells inhibited cytotoxic T cell-specific lysis completely (23). We investigated whether these findings applied to inhibition of alloproliferation as well.
Responder cells from two donors were stimulated by a mixture of HLA-G1- APCs (KG1a-RSV) and HLA-G1+ APCs (KG1a-G1) at various ratios (Fig. 4). There was no proliferation inhibition when HLA-G1+ APCs made up 10% of the HLA-G1- APC: HLA-G1+ APC mixture. However, there was a linear correlation between the ratio of HLA-G1- APC: HLA-G1+ APC cells in the stimulator mixture and the level of alloproliferation after this value (responder I: R2>0.98 for a percentage of HLA-G1+ APCs ranging from 10% to 100%, and Responder II: R2 >0.99 for a percentage of HLA-G1+ APCs ranging from 25% to 90%). The fact that the correlation was linear lead to the hypothesis that HLA-G1+ APCs induced a nonresponsiveness of T cells that was not temporary. Indeed, the T cells that statistically encountered HLA-G1+ APC cells first did not just ignore them to be stimulated by HLA-G1- APC cells in the culture, but seemed to have lost their capacity to proliferate altogether.
Fig. 4.
Linear correlation between the percentage of HLA-G1-expressing stimulator cells and inhibition of alloresponses. Responder PBMCs from two donors were stimulated at a responder:stimulator ratio of 1:1 by mixtures of irradiated KG1a-RSV and KG1a-G1_CL3 cells at various ratios. Alloproliferation was measured at day 6 as described in Materials and Methods.
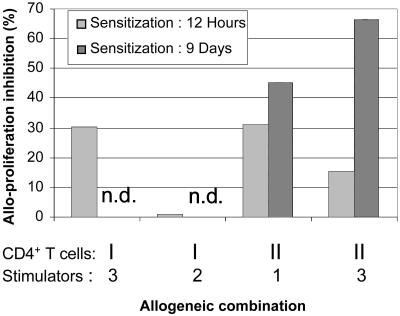
Stimulation by HLA-G1+ APCs Induces Long-Term CD4+ T Cell Unresponsiveness. We investigated whether the HLA-G1+ APCs mediated an inhibition of the alloproliferation of T cells that was temporary and depended on the presence of HLA-G1 on stimulator cells, or whether the HLA-G1+ APCs induced a more prolonged T cell nonresponsiveness. Purified CD4+ T cells from two donors were stimulated 12 h with irradiated HLA-G1- APCs (KG1a-RSV) or HLA-G1+ APCs (KG1a-G1). After this sensitization, the stimulator cells were removed by magnetic sorting, and the alloproliferative capability of the sensitized CD4+ T cells was evaluated in response to irradiated PBMCs from two allogeneic donors. CD4+ T cells sensitized 12 h with HLA-G1- APCs responded to the new allostimulators to a level comparable with that of nonsensitized CD4+ T cells from the same donors. On the contrary, CD4+ T cells sensitized with HLA-G1+ APCs showed a significant decrease in alloproliferation in three of four MLR. As can be seen in Fig. 5, the alloproliferation of CD4+ T cells from donor I sensitized with HLA-G1+ APCs was 70% of that of HLA-G1- APCs sensitized CD4+ T cells in one of the allogeneic combinations, whereas no difference was observed for the other combination. For donor II, the alloproliferation of CD4+ T cells sensitized with HLA-G1+ APCs was 68% of that of HLA-G1- APCs sensitized CD4+ T cells for one combination and 83% for the other. These data indicate that HLA-G1+ APCs can induce a prolonged inhibition of CD4+ T cell responsiveness, even whether the level of this inhibition seems to be affected by the quality of the second stimulation.
Fig. 5.
Stimulation by HLA-G1+ APC long-term CD4+ T cell unresponsiveness. Purified CD4+ T cells from two donors were sensitized with KG1a-RSV or KG1a-G1_CL3 cells for 12 h or 9 days. After this period, the alloproliferative responses of sensitized CD4+ T cells were tested in MLR against two allogeneic donor PBMCs. The graph presents the inhibition of alloproliferation of cells sensitized with KG1a-G1_CL3. Inhibition was calculated by using alloproliferation levels obtained for KG1a-RSV-sensitized CD4+ T cells (“no inhibition” levels). The origin of the CD4+ T cells as well as the allogeneic donors they were tested against are indicated. light shaded bar, CD4+ T cells sensitized 12 h; darker shaded bar, CD4+ T cells sensitized 9 days; n.d., not done.
To find out whether a maturation process was involved in the inhibition of responsiveness mediated by HLA-G1+ APCs, the same experiment as above was performed for one of the two donors (donor II) with a sensitization of 9 days instead of 12 h. The alloproliferative responses of CD4+ T cells sensitized 9 days with HLA-G1+ APCs against two donor PBMCs were 54% and 33% of the alloproliferative responses of CD4+ T cells sensitized with HLA-G1- APCs against the same stimulators (Fig. 5). These results indicate that a maturation/differentiation process is most likely involved in the inhibition of responsiveness mediated by HLA-G1+ APC.
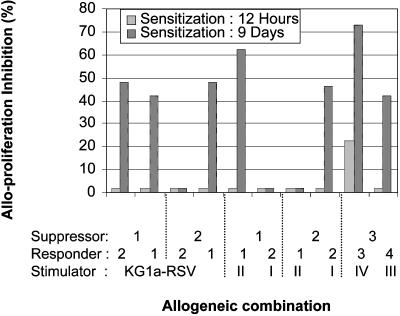
Stimulation by HLA-G1+ APCs Induces Suppressive CD4+ T Cells. Immunosuppressive T cells such as CD4+CD25+ regulatory T cells or CD8+CD28- suppressor T cells can inhibit alloproliferative responses by means of various mechanisms (31–34). To find out whether HLA-G1+ APCs induced the differentiation of immunosuppressive T cells, PBMCs or purified CD4+ T cells were sensitized 12 h or 9 days by irradiated HLA-G1- or HLA-G1+ APCs, as described above. After sensitization, the stimulatory cells were removed and the sensitized cells were irradiated, added to MLR, and their immunosuppressive functions were compared. Irradiated PBMCs or CD4+ T cells sensitized with HLA-G1- APCs never inhibited the alloproliferation of the responder cells. As can be seen in Fig. 6, unseparated PBMCs or purified CD4+ T cells sensitized 12 h with HLA-G1+ APCs had little or no suppressive effect and decreased the alloproliferative response by 21% in only one allogeneic combination. On the contrary, unseparated PBMCs or purified CD4+ T cells sensitized 9 days with HLA-G1+ APCs suppressed the alloproliferative response in 7 of 10 allogeneic combinations, regardless of the stimulators used, by an average of 52% (a range of 43–73%). These data show that HLA-G1+ APCs induced the differentiation of CD4+ T cells into suppressive CD4+ T cells.
Fig. 6.
Sensitization by HLA-G1+ APCs induces suppressive CD4+ T cells. Purified CD4+ T cells were prestimulated with KG1a-RSV or KG1a-G1_CL3 cells for 12 h or 9 days. After this period, the sensitized cells were purified, irradiated, and added to MLRs as third-party cells (suppressors), at a final stimulator:responder:irradiated sensitized cells ratio of 1:1:1. The graph presents the inhibition of alloproliferation induced by irradiated, KG1a-G1_CL3-sensitized CD4+ T cells. Inhibition was calculated by using alloproliferation levels obtained when irradiated, KG1a-RSV-sensitized cells were added to the MLR combinations (“no inhibition” level). The origin of the sensitized cells as well as the allogeneic donors they were tested against are indicated. Light shaded bar, 12-h sensitization; dark shaded bar, 9-day sensitization.
Discussion
This work was aimed at elucidating the function of HLA-G+ APCs that are present during the course of various pathologies. In particular, given the strong inhibitory effect of HLA-G on the function of immune cells and the central role played by APCs in initiating and supporting immune responses, we wanted to investigate whether HLA-G1+ APCs were immunoinhibitory cells with suppressive function. To address these issues, we generated HLA-G1-transfected APC lines and used them as stimulator cells in allogeneic reactions against PBMCs and purified CD4+ T cells. This in vitro model was used to investigate the direct inhibitory function of HLA-G1+ APCs, and then to gain insight into the long-term effects of these cells on CD4+ T cells.
Our data show that HLA-G1+ APCs used as stimulator cells strongly inhibit the alloproliferative responses of PBMCs as well as those of purified CD4+ T cells. In an immune response, the interaction between APCs and CD4+ T cells is a crucial event that leads to the activation and proliferation of antigen-specific CD4+ T cells, and their differentiation into CD4+ T helper cells. These cells actively support the activation and multiplication of antigen-specific CD8+ T cells and their maturation into cytotoxic T cells. Therefore, the inhibition of the activation, proliferation, and differentiation of antigen-specific CD4+ T cells by HLA-G1+ APCs would lead to an almost complete abortion of the immune response, and to an impaired generation of cytotoxic T cells.
It is interesting to note that no ILT2/CD85j was detected at the surface of purified CD4+ T cells, whereas alloproliferation was nevertheless inhibited by HLA-G1+ APCs. ILT2/CD85j is the only of the HLA-G receptors described so far that is expressed by CD4+ T cells. These results might indicate that another, yet unknown, HLA-G receptor is expressed by CD4+ T cells, or that HLA-G1 has an inhibitory effect on the APCs themselves through the receptors they bear. Indeed, it was shown in a murine model that, acting through the murine homologue of ILT4, HLA-G can impair APC maturation, which leads to decreased cellular immune responses (35, 36). In our system, HLA-G might act in such a fashion, decrease the stimulatory capabilities of APCs, and so reduce CD4+ T cell alloproliferation.
As shown in this work, HLA-G1+ APCs released HLA-G1 molecules in their environment (sHLA-G1). Even though in our system, membrane-bound HLA-G1 had a vastly predominant inhibitory effect over that of sHLA-G1, it was shown that sHLA-G1 induced the apoptosis of activated CD8+ natural killer and T cells (37, 38). It is therefore possible that HLA-G1 shedding by HLA-G1+ APCs constitutes a back-up inhibitory mechanism: sHLA-G1 would target CD8+ T cells that would have bypassed the inhibition mediated by HLA-G1+ APCs, in an antigen-nonspecific fashion.
The effects of HLA-G1+ APC-mediated inhibition on the capability of CD4+ T cells to be restimulated were investigated. Our data show that CD4+ T cell populations that had been sensitized 12 h with HLA-G1+ APCs had lost between 19% and 35% of their capability to respond to a new allogeneic stimulation, as compared with that of CD4+ T cells sensitized with HLA-G1- APCs. After 9 days of sensitization, this effect was even more pronounced, with the level of alloproliferation of CD4+ T cells sensitized with HLA-G1+ APCs being 35–55% of that of CD4+ T cells sensitized with HLA-G1- APCs. These results show that HLA-G1+ APCs do not only act as inhibitors of CD4+ T cell activation. Indeed, by rendering the CD4+ T cells unable to respond to further stimulation, HLA-G1+ APCs induce a long-term antigen-specific unresponsiveness apparently similar to T cell anergy. Induction of antigen-specific CD4+ T cell long-term unresponsiveness is one way to inactivate some T cell specificities from the functional antigenic repertoire, thus promoting a lasting immune ignorance/tolerance to some antigens. The fact that the inhibition of functional reactivity was more pronounced after a sensitization of 9 days than 12 h may be due to two nonexclusive reasons. (i) Only part of the CD4+ responder T cells would recognize the alloantigens presented by HLA-G1+ APCs and be sensitive to HLA-G1+ APC inhibition. This finding means that after a 12-h culture, CD4+ T cells would remain that had not been rendered unresponsive by HLA-G1+ APCs. These cells would have retained the alloreactivity of naíve CD4+ T cells. Thus, the difference in alloreactivity between HLA-G1+ APC-sensitized and HLA-G1- APC-sensitized CD4+ T cell cultures would represent the proportion of HLA-G1+ APC-specific CD4+ T cells within the responder population. After 9 days, however, CD4+ T cells that did not recognize the alloantigens presented by the APCs would have died by lack of stimulation. In this case, the differences in alloreactivity between CD4+ T cells sensitized with HLA-G1+ and HLA-G1- APCs would truly represent the effect of HLA-G1+ APCs on antigen-specific CD4+ T cells. (ii) The differences observed between 12 h- and 9 day-sensitized CD4+ T cells might indicate that a maturation process, which would take more that 12 h, is involved in the acquisition of nonresponsiveness by CD4+ T cells.
The maturation/differentiation of CD4+ T cells sensitized with HLA-G1+ APCs into regulatory/suppressor cells was investigated. Our data show that CD4+ T cells sensitized 12 h with HLA-G1+ APCs had little or no suppressive function, but that CD4+ T cells that had been sensitized 9 days by HLA-G1+ APCs strongly inhibited the alloproliferative response of autologous or allogeneic CD4+ T cells in MLR. These results indicate that stimulation of CD4+ T cells by HLA-G1+ APCs induces their differentiation into immunosuppressive cells. The precise characterization and the mechanism of action of such suppressive CD4+ T cells warrant future investigation.
In the light of these findings, the presence of HLA-G+ APCs might have different significance, depending on physiopathological status, and their effect might either be beneficial or deleterious. For instance, HLA-G expression by transplanted tissues was detected after heart transplantation and kidney-liver combined transplantation. In both cases, expression of HLA-G correlated with a reduced number of acute rejection episodes and no chronic rejection (15–17). In both of these studies, HLA-G was expressed by some accepted transplants in the absence of inflammation or infiltration, and seric-soluble HLA-G5/G6 of unknown source was detected as well. In the case of kidney-liver combined transplantation, infiltrating HLA-G+ APCs were found within some HLA-G- transplants, and their presence was associated with inflammation. Given the data presented above, it is possible that HLA-G+ APCs were not detected within HLA-G- nonrejected transplants because tolerization for the transplants was already achieved. Tolerogenic HLA-G+ APCs might have existed earlier, or might have existed elsewhere and be the source of seric HLA-G5/G6. On the other hand, the presence of HLA-G+ APCs within HLA-G- transplanted tissue might reveal (i) an attempt by the immune system to stop an ongoing rejection reaction and/or (ii)an ongoing “tolerization” reaction.
HLA-G+ APCs have been detected in pathologies such as cancer. In these cases, HLA-G+ APCs, acting as suppressive immune effectors might have a deleterious effect and inhibit local antitumoral responses. Further, by their tolerogenic action, they might actively induce long term antitumoral unresponsiveness and be in part responsible for tumoral immune escape.
Taken together, our data show that HLA-G1+ APCs are immunoinhibitory cells that (i) inhibit the proliferation of CD4+ T cells; (ii) shed HLA-G1 molecules that might provide extra, non-antigen-specific, inhibitory, or proapoptotic signals; (iii) induce CD4+ T cells anergy, or at least long-term unresponsiveness; and (iv) cause the differentiation of CD4+ T cells into suppressive cells and spread antigen-specific inhibition. Thus, by their local action, HLA-G+ APCs might be involved in the suppression of immune responses and by their in long-term effects, in efficient immune escape or tolerance. Depending on physiopathological status, HLA-G+ APCs might therefore constitute a help to boost or a threat to eliminate.
Acknowledgments
This work was supported by the Commissariat `l a'Energie Atomique and the Etablissement Français des Greffes.
Abbreviations: APC, antigen-presenting cell; PBMC, peripheral blood mononuclear cell; MLR, mixed lymphocyte reaction; sHLA-G1, shed HLA-G1.
References
- 1.Carosella, E. D., Moreau, P., Le Maoult, J., Le Discorde, M., Dausset, J. & Rouas-Freiss, N. (2003) Adv. Immunol. 81, 199-252. [DOI] [PubMed] [Google Scholar]
- 2.Riteau, B., Menier, C., Khalil-Daher, I., Sedlik, C., Dausset, J., Rouas-Freiss, N. & Carosella, E. D. (1999) J. Reprod. Immunol. 43, 203-211. [DOI] [PubMed] [Google Scholar]
- 3.Bainbridge, D. R., Ellis, S. A. & Sargent, I. L. (2000) J. Reprod. Immunol. 48, 17-26. [DOI] [PubMed] [Google Scholar]
- 4.Lila, N., Rouas-Freiss, N., Dausset, J., Carpentier, A. & Carosella, E. D. (2001) Proc. Natl. Acad. Sci. USA 98, 12150-12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiendl, H., Mitsdoerffer, M., Hofmeister, V., Wischhusen, J., Weiss, E. H., Dichgans, J., Lochmuller, H., Hohlfeld, R., Melms, A. & Weller, M. (2003) Brain 126, 176-185. [DOI] [PubMed] [Google Scholar]
- 6.Rouas-Freiss, N., Marchal, R. E., Kirszenbaum, M., Dausset, J. & Carosella, E. D. (1997) Proc. Natl. Acad. Sci. USA 94, 5249-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouas-Freiss, N., Goncalves, R. M., Menier, C., Dausset, J. & Carosella, E. D. (1997) Proc. Natl. Acad. Sci. USA 94, 11520-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil-Daher, I., Riteau, B., Menier, C., Sedlik, C., Paul, P., Dausset, J., Carosella, E. D. & Rouas-Freiss, N. (1999) J. Reprod. Immunol. 43, 175-182. [DOI] [PubMed] [Google Scholar]
- 9.Menier, C., Riteau, B., Dausset, J., Carosella, E. D. & Rouas-Freiss, N. (2000) Hum. Immunol. 61, 1118-1125. [DOI] [PubMed] [Google Scholar]
- 10.Riteau, B., Rouas-Freiss, N., Menier, C., Paul, P., Dausset, J. & Carosella, E. D. (2001) J. Immunol. 166, 5018-5026. [DOI] [PubMed] [Google Scholar]
- 11.Le Gal, F. A., Riteau, B., Sedlik, C., Khalil-Daher, I., Menier, C., Dausset, J., Guillet, J. G., Carosella, E. D. & Rouas-Freiss, N. (1999) Int. Immunol. 11, 1351-1356. [DOI] [PubMed] [Google Scholar]
- 12.Colonna, M., Samaridis, J., Cella, M., Angman, L., Allen, R. L., O'Callaghan, C. A., Dunbar, R., Ogg, G. S., Cerundolo, V. & Rolink, A. (1998) J. Immunol. 160, 3096-3100. [PubMed] [Google Scholar]
- 13.Navarro, F., Llano, M., Bellon, T., Colonna, M., Geraghty, D. E. & Lopez-Botet, M. (1999) Eur. J. Immunol. 29, 277-283. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan, S. & Long, E. O. (1999) J. Exp. Med. 189, 1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lila, N., Carpentier, A., Amrein, C., Khalil-Daher, I., Dausset, J. & Carosella, E. D. (2000) Lancet 355, 2138. [DOI] [PubMed] [Google Scholar]
- 16.Lila, N., Amrein, C., Guillemain, R., Chevalier, P., Latremouille, C., Fabiani, J. N., Dausset, J., Carosella, E. D. & Carpentier, A. (2002) Circulation 105, 1949-1954. [DOI] [PubMed] [Google Scholar]
- 17.Rouas-Freiss, N., LeMaoult, J., Moreau, P., Dausset, J. & Carosella, E. D. (2003) Am. J. Transplant. 3, 11-16. [DOI] [PubMed] [Google Scholar]
- 18.Aractingi, S., Briand, N., Le Danff, C., Viguier, M., Bachelez, H., Michel, L., Dubertret, L. & Carosella, E. D. (2001) Am. J. Pathol. 159, 71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosrotehrani, K., Le Danff, C., Reynaud-Mendel, B., Dubertret, L., Carosella, E. D. & Aractingi, S. (2001) J. Invest. Dermatol. 117, 750-752. [DOI] [PubMed] [Google Scholar]
- 20.Paul, P., Rouas-Freiss, N., Khalil-Daher, I., Moreau, P., Riteau, B., Le Gal, F. A., Avril, M. F., Dausset, J., Guillet, J. G. & Carosella, E. D. (1998) Proc. Natl. Acad. Sci. USA 95, 4510-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim, E. C., Guerra, N., Lacombe, M. J., Angevin, E., Chouaib, S., Carosella, E. D., Caignard, A. & Paul, P. (2001) Cancer Res. 61, 6838-6845. [PubMed] [Google Scholar]
- 22.Urosevic, M., Kurrer, M. O., Kamarashev, J., Mueller, B., Weder, W., Burg, G., Stahel, R. A., Dummer, R. & Trojan, A. (2001) Am. J. Pathol. 159, 817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiendl, H., Mitsdoerffer, M., Hofmeister, V., Wischhusen, J., Bornemann, A., Meyermann, R., Weiss, E. H., Melms, A. & Weller, M. (2002) J. Immunol. 168, 4772-4780. [DOI] [PubMed] [Google Scholar]
- 24.Pangault, C., Le Friec, G., Caulet-Maugendre, S., Lena, H., Amiot, L., Guilloux, V., Onno, M. & Fauchet, R. (2002) Hum. Immunol. 63, 83-90. [DOI] [PubMed] [Google Scholar]
- 25.Onno, M., Le Friec, G., Pangault, C., Amiot, L., Guilloux, V., Drenou, B., Caulet-Maugendre, S., Andre, P. & Fauchet, R. (2000) Hum. Immunol. 61, 1086-1094. [DOI] [PubMed] [Google Scholar]
- 26.Lozano, J. M., Gonzalez, R., Kindelan, J. M., Rouas-Freiss, N., Caballos, R., Dausset, J., Carosella, E. D. & Pena, J. (2002) AIDS 16, 347-351. [DOI] [PubMed] [Google Scholar]
- 27.Le Rond, S., Le Maoult, J., Creput, C., Menier, C., Deschamps, M., Le Friec, G., Amiot, L., Durrbach, A., Dausset, J., Carosella, E. D. & Rouas-Freiss, N. (2004) Eur. J. Immunol. 34, 649-660. [DOI] [PubMed] [Google Scholar]
- 28.Colonna, M., Navarro, F., Bellon, T., Llano, M., Garcia, P., Samaridis, J., Angman, L., Cella, M. & Lopez-Botet, M. (1997) J. Exp. Med. 186, 1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrego, F., Kabat, J., Kim, D., Lieto, L., Maasho, K., Pena, J., Solana, R. & Coligan, J. (2002) Mol. Immunol. 38, 637-660. [DOI] [PubMed] [Google Scholar]
- 30.Saverino, D., Fabbi, M., Ghiotto, F., Merlo, A., Bruno, S., Zarcone, D., Tenca, C., Tiso, M., Santoro, G., Anastasi, G., et al. (2000) J. Immunol. 165, 3742-3755. [DOI] [PubMed] [Google Scholar]
- 31.Ng, W. F., Duggan, P. J., Ponchel, F., Matarese, G., Lombardi, G., Edwards, A. D., Isaacs, J. D. & Lechler, R. I. (2001) Blood 98, 2736-2744. [DOI] [PubMed] [Google Scholar]
- 32.Chatenoud, L., Salomon, B. & Bluestone, J. A. (2001) Immunol. Rev. 182, 149-163. [DOI] [PubMed] [Google Scholar]
- 33.Cortesini, R., LeMaoult, J., Ciubotariu, R. & Cortesini, N. S. (2001) Immunol. Rev. 182, 201-206. [DOI] [PubMed] [Google Scholar]
- 34.Chang, C. C., Ciubotariu, R., Manavalan, J. S., Yuan, J., Colovai, A. I., Piazza, F., Lederman, S., Colonna, M., Cortesini, R., Dalla-Favera, R. & Suciu-Foca, N. (2002) Nat. Immunol. 3, 237-243. [DOI] [PubMed] [Google Scholar]
- 35.Horuzsko, A., Lenfant, F., Munn, D. H. & Mellor, A. L. (2001) Int. Immunol. 13, 385-394. [DOI] [PubMed] [Google Scholar]
- 36.Liang, S., Baibakov, B. & Horuzsko, A. (2002) Eur. J. Immunol. 32, 2418-2426. [DOI] [PubMed] [Google Scholar]
- 37.Solier, C., Aguerre-Girr, M., Lenfant, F., Campan, A., Berrebi, A., Rebmann, V., Grosse-Wilde, H. & Le Bouteiller, P. (2002) Eur. J. Immunol. 32, 3576-3586. [DOI] [PubMed] [Google Scholar]
- 38.Contini, P., Ghio, M., Poggi, A., Filaci, G., Indiveri, F., Ferrone, S. & Puppo, F. (2003) Eur. J. Immunol. 33, 125-134. [DOI] [PubMed] [Google Scholar]