Abstract
Objective
The objective of this study was to compare whether different sources of cognitive complaint (i.e., subjective and informant) predict diagnostic conversion in nondemented older adults.
Methods
Participants from the National Alzheimer’s Coordinating Center had a baseline diagnosis of normal cognition (NC; n=4414, 73±8 years, 69% female) or mild cognitive impairment (MCI; n=1843, 74±8 years, 52% female). Multinomial logistic regression related baseline cognitive complaint (no-complaint, self only, informant only, or both self-and informant) to diagnostic outcome (reversion, stable or conversion).
Results
At follow-up, 14% of NC participants converted to MCI/dementia (3.5±1.8 years), and 41% of MCI participants converted to dementia (3.0±1.6 years). Among NC participants, self-complaint only (OR=2.1; 99%CI=1.5–2.9, p<0.001), informant-complaint only (OR=2.2; 99%CI=1.2–3.9, p<0.001), and both self-and informant-complaint (OR=4.2; 99%CI=2.9–6.0, p<0.001) were associated with diagnostic conversion, compared to no-complaint. Among participants with MCI—compared with no-complaint, informant-complaint only (OR, 2.2; 99% CI, 1.2–4.3, P = .002), and both self- and informant-complaint (OR, 2.9; 99% CI, 1.8–4.8; P < .001)—were associated with conversion.
Conclusions
Cognitive complaints are related to conversion among non-demented older adults. Complaint from both (i.e. mutual complaint) sources was most predictive of diagnostic outcome, followed by informant complaint, highlighting the need for obtaining informant corroboration to enhance prognosis and distinguish underlying pathological processes from normal cognitive aging. Self-complaint was inconsistently related to diagnostic outcome.
Keywords: Mild cognitive impairment, Alzheimer’s disease, cognitive complaints, prognosis, conversion
1. Introduction
Mild cognitive impairment (MCI) is widely regarded as a prodromal stage of dementia1 because many individuals diagnosed with MCI convert to Alzheimer’s disease (AD).2 Current MCI diagnostic criteria require quantitative evidence of neuropsychological impairment, relative preservation of functional abilities, and a concern regarding a change in cognition, reported by the patient or someone close to the patient (i.e., an informant) or noted by a clinician.3 Prior research suggests that self-reported cognitive complaints predict cognitive decline4 and diagnostic outcome.5 However, self-reported cognitive complaints are highly prevalent among older adults6 and lack specificity (i.e., cognitively normal elders frequently endorse cognitive problems).7 Also, elders with an underlying neurodegenerative disease sometimes lack insight8 and may self-assess their cognition incorrectly. One solution to overcome limitations associated with self-reported cognitive complaint is use of an informant or an individual who knows the patient well. Emerging evidence suggests that informant complaint may be a better predictor of cognitive progression than self complaint.9,10
There has been limited empirical consideration of how the combination of self and informant cognitive complaints relate to diagnostic outcomes. Such information would be helpful in refining the diagnostic profile of MCI. It is certainly plausible that pooling information from sources may be a more valuable clinical prognostic indicator than either information source alone. The current study aims to reconcile the prognostic properties of cognitive complaint in relation to longitudinal diagnostic outcomes of individuals with normal cognition (NC) and MCI by considering a combination of self and informant cognitive complaints. Leveraging the National Alzheimer’s Coordinating Center (NACC) database, we hypothesized that individuals with both self and informant complaint (i.e. mutual complaint) have a greater risk of converting over a follow-up period (i.e., from NC to MCI or MCI to dementia) than elders with no cognitive complaint or only one type of complaint (i.e., self or informant complaint only). As therapeutic targets to slow or arrest the pathological process of AD are expected to emerge in the coming years,11 it is essential to identify individuals at greatest risk for conversion to dementia. Early intervention could prevent or delay the onset of clinical dementia, preserve functional independence, and minimize cognitive decline for at risk elders.12
2. Methods
2.1 Setting and participants
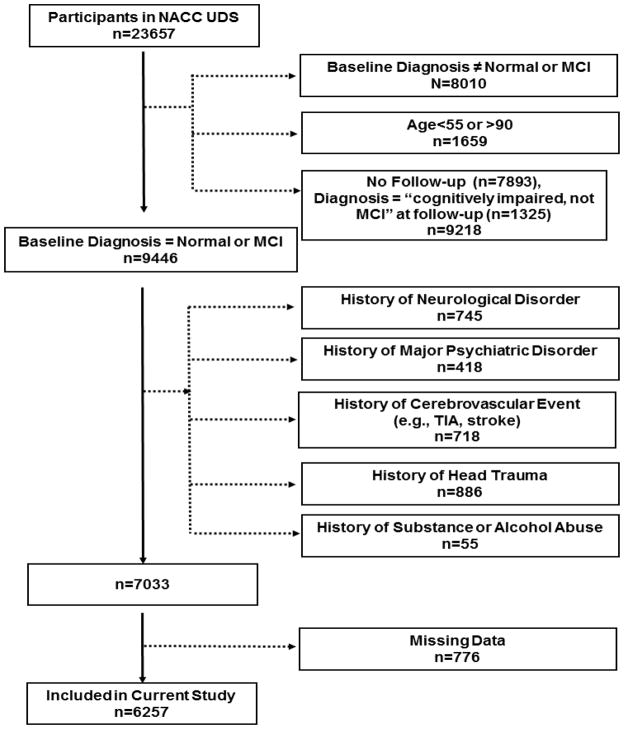
As previously reported,13 NACC maintains a database of participant information collected from ~30 national Alzheimer’s Disease Centers (ADCs) previously or currently funded through the National Institute on Aging. In 2005, NACC implemented the Uniform Data Set (UDS), a standard evaluation protocol, including clinical demographic information, medical history, neurological examination, and neuropsychological evaluation.14 Participants 55 to 90 years of age, evaluated between 9/01/2005 and 12/01/2012, and diagnosed at first visit with NC or MCI were included in the current study. Participant selection and exclusion details are provided in Figure 1 and resulted in a sample of 6261 participants for the current study. The study was approved by local Institutional Review Boards prior to data access and analysis.
Figure 1. Participant Enrollment & Exclusion Details.
Note: The exclusion numbers provided are not mutually exclusive. Missing data include complaint status at baseline. Cognitively impaired patients without MCI were excluded because of nonstandardized classification criteria. NACC UDS, National Alzheimer’s Coordinating Center Uniform Data Set; MCI, mild cognitive impairment, TIA, transient ischemic attack.
2.2 Cognitive complaint
Cognitive complaint was defined based on clinician-facilitated interviews with the participant and informant. From this interview, the clinician dichotomously reported the presence or absence of cognitive complaint for the participant (self) and the informant.
2.3 Cognitive diagnostic classification
The cognitive diagnosis for each participant is based upon clinician judgment or a multi-disciplinary consensus team using information from the comprehensive UDS work-up. Classifications relevant to the current study include:
NC is defined by (a) Clinical Dementia Rating (CDR)15=0 (no dementia), (b) no deficits in activities of daily living directly attributable to cognitive impairment, and (c) no evidence of objective cognitive impairment. Normal cognition is defined as standard scores falling 1.5 standard deviations within the age-adjusted normative mean16 on neuropsychological tests assessing language, attention, memory, and executive functioning.
MCI determinations are based upon Peterson et al. criteria17 and defined as (a) a CDR≤0.5 (reflecting mild severity of impairment), (b) relatively spared activities of daily living, (c) objective cognitive impairment in at least one cognitive domain (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean in memory, language, attention, or executive functioning) or a significant decline over time on the neuropsychological evaluation, (d) Mini Mental State Examination (MMSE) score ≥23,18,19 (e) report of a cognitive change by the patient or informant or as observed by a clinician, and (f) absence of a dementing syndrome (defined below).
Dementia was defined as meeting criteria for AD,20 or other dementias21,22,23,24–26 defined as (a) objective cognitive impairment (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean) in at least two cognitive systems (i.e., memory, language, attention or executive functioning), and (b) cognitive impairment contributes directly to impaired activities of daily living.27
2.4 Cognitive diagnostic outcome classification
Diagnostic outcome was defined by comparing the participant’s baseline (i.e., first UDS visit) diagnosis with their most recent UDS follow-up visit diagnosis. Among the NC participants, diagnostic outcome was defined as (1) stable (NC to NC) or (2) conversion (NC to MCI or NC to dementia). Among the MCI participants, diagnostic outcome was defined as (1) reversion (MCI to NC), (2) stable (MCI to MCI), or (3) conversion (MCI to dementia).
2.5 Statistical analysis
For all participants, baseline cognitive complaint was defined using four mutually exclusive levels: (1) no cognitive complaint, (2) self cognitive complaint only, (3) informant cognitive complaint only, or (4) both self and informant cognitive complaint, i.e. mutual complaint. Baseline clinical characteristics, including age, sex, race, education, follow-up period (time from first to most recent UDS visit), and global cognitive functioning (as assessed by the MMSE18), were compared across the four complaint categories separately for each diagnostic group (i.e., NC, MCI) using chi-square and analysis of variance tests.
For hypothesis testing, multinomial logistic regression was used to relate complaint status (using no complaint as the referent) to diagnostic outcome adjusting for age, sex, race, education, follow-up period, and MMSE. Restricted cubic splines with 5 knots were used to model continuous variables (age, education, MMSE, and years of follow-up) to relax the linearity assumption of covariate effects. Nonlinear terms of continuous variables were removed if no significant trends were detected. Two-way interactions were considered (i.e., race*sex, race*complaint, sex*complaint) and only interactions with significant effects remained in the model. For the NC participants, the multinomial logistic regression was reduced to logistic regression for binary variables as there were only two diagnosis outcome levels. The model then analyzed the effect of complaint status on the odds of conversion versus remaining stable. For the MCI participants, the multinomial logistic regression compared the effect of complaint status across three categories of diagnosis outcome with stable as the referent. Significance was set a-priori at p<0.01, and results are presented as odds ratios (OR) with 99% confidence intervals (CI). Analyses were conducted using R 2.12.1 (www.r-project.org).
3. Results
3.1 Participant characteristics
At baseline, participants included n=4414 NC participants and n=1843 individuals with MCI. Among NC participants, the four cognitive complaint subgroups did not differ with respect to age, race, or education, but differed on sex (χ2=36, p<0.001), MMSE (F(3,4410)=15.0, p<0.001, and length of follow-up (F(3,4410)=7.8, p<0.001. Among MCI participants, the complaint subgroups did not differ with respect to age, education, or length of follow-up period but differed with respect to sex (χ2=18, p<0.001), race (χ2=77, p<0.001), and MMSE (F(3,1839)=12.0, p<0.001). See Table 1 for details.
Table 1.
Clinical Characteristics by Cognitive Complaint Category
| Cognitively Normal
Participants | ||||||
|---|---|---|---|---|---|---|
| No Complaint n=3300 | Self Complaint n=656 | Informant Complaint n=139 | Self + Informant Complaint n=319 | p value | Total n=4414 | |
| Age, mean (SD), y | 72.6 (8.2) | 72.7 (8.4) | 73.9 (8.4) | 72.8 (7.4) | 0.35 | 72.7 (8.1) |
| Sex (% Female) | 70 | 70 | 57 | 56 | <0.001§,#**,†† | 69 |
| Race (% White) | 80 | 77 | 87 | 81 | 0.015 | 80 |
| Education, mean (SD), y | 15.8 (5.7) | 16.0 (7.2) | 15.1 (3.4) | 15.9 (8.8) | 0.09 | 15.8 (6.1) |
| Baseline MMSE score†, mean (SD) | 29.0 (1.4) | 28.9 (1.4) | 28.6 (1.7) | 28.5 (1.6) | <0.001§,¶,#,** | 28.9 (1.4) |
| Follow-up Period‡, mean (SD), y | 3.6 (1.8) | 3.3 (1.7) | 3.4 (1.7) | 3.2 (1.7) | <0.001§ | 3.5 (1.8) |
| Conversion Status Frequency | ||||||
| Stable/Persistent (%) | 91 | 83 | 80 | 71 | --- | 88 |
| Progressive/Converters (%) | 9 | 17 | 20 | 29 | --- | 12 |
| Mild Cognitive Impairment Participants | ||||||
|
| ||||||
| No Complaint n=263 | Self Complaint n=197 | Informant Complaint n=151 | Self + Informant Complaint n=1232 | p value | Total n=1843 | |
|
| ||||||
| Age, mean (SD), y | 74.3 (7.2) | 74.4 (7.9) | 76.3 (7.1) | 74.2 (7.5) | 0.02 | 74.4 (7.5) |
| Sex (% Female) | 57 | 64 | 45 | 50 | <0.001**,†† | 52 |
| Race (% White) | 68 | 67 | 80 | 85 | <0.001‡‡,§§ | 81 |
| Education, mean (SD), y | 15.8 (9.6) | 15.0 (3.4) | 15.3 (3.2) | 15.8 (5.7) | 0.04 | 15.6 (6.1) |
| Baseline MMSE score†, mean (SD) | 28.0 (1.8) | 27.5 (1.8) | 27.6 (1.9) | 27.3 (1.9) | <0.001§,¶ | 27.4 (1.9) |
| Follow-up Period‡, mean (SD), y | 3.1 (1.7) | 3.0 (1.8) | 3.2 (1.6) | 2.9 (1.6) | 0.21 | 3.0 (1.6) |
| Conversion Status Frequency | ||||||
| Reverters (%) | 32 | 25 | 8 | 6 | --- | 12 |
| Stable/Persistent (%) | 51 | 55 | 50 | 45 | --- | 47 |
| Progressive/Converters (%) | 17 | 20 | 42 | 49 | --- | 41 |
Note: Complaint categories are mutually exclusive and defined based on clinician-facilitated interviews with the participant and informant. MMSE=Mini Mental State Examination,
MMSE score range from 0–30 with lower score=worse performance;
Follow-up period is time from the first visit until time to the last visit; Post-hoc group comparisons with Bonferroni correction:
no complaint > both complaints;
no complaint > self complaint;
no complaint > informant complaint;
self complaint > informant complaint;
self complaint > both complaints;
no complaint < both complaints;
self complaint < both complaints
3.2 Cognitive complaint & NC diagnostic outcome
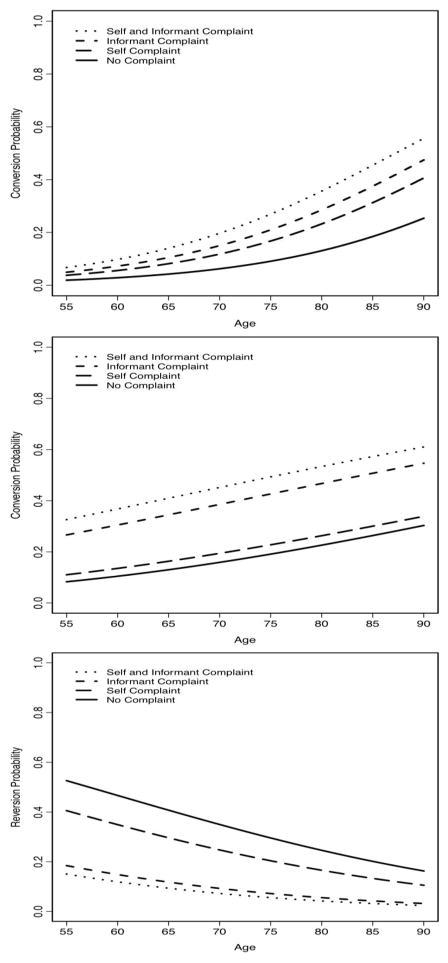
All the nonlinear effects of age, education, MMSE, and follow-up periods were not statistically significant (all p-values>0.17). Similarly, all interactions were not statistically significant (all p-values>0.27). Therefore, the final model included only main effects of the baseline characteristics with linear effects for continuous variables. Compared to NC participants with no cognitive complaint, participants with self complaint only (OR=2.1; 99%CI=1.5–2.9, p<0.001), informant complaint only (OR=2.2; 99%CI=1.2–3.9, p<0.001), and both self and informant complaint (OR=4.2; 99%CI=2.9–6.0, p<0.001) were at increased risk of converting to MCI or dementia. Compared to NC participants with self complaint, both self and informant complaint were at increased risk of converting to MCI or dementia (OR=1.8; 99%CI=1.2–2.9, p<0.001). See Table 2. The probability of conversion against age by years of follow-up was plotted for each complaint category, and participants with both complaints have the highest probability of conversion, followed by participants with informant complaint only, self complaint only and no complaint. See Figure 2a.
Table 2.
Odds Ratios for Diagnostic Outcome
| Normal Cognition | ||||||
|---|---|---|---|---|---|---|
| Self Complaintŧ | p value | Informant Complaintŧ | p value | Self + Informant Complaintŧ | p value | |
| Convert vs. Stableŧŧ | 2.1 [1.5–2.9]* | <0.001 | 2.2 [1.2–3.9]* | <0.001 | 4.2 [2.9–6.0]* | <0.001 |
| Mild Cognitive Impairment | ||||||
| Self Complaintŧ | p value | Informant Complaintŧ | p value | Self + Informant Complaintŧ | p value | |
| Revert vs. Stableŧŧ | 0.8 [0.4–1.5] | 0.37 | 0.3 [0.1–0.6]* | <0.001 | 0.2 [0.1–0.4]* | <0.001 |
| Convert vs. Stableŧŧ | 1.0 [0.5–1.9] | 0.85 | 2.2 [1.2–4.3]* | 0.002 | 2.9 [1.8–4.8]* | <0.001 |
Note: Data presented as Odds Ratio [99% Confidence Interval];
p<.01;
predictor referent=no complaint;
outcome referent=stable, Complaint categories are mutually exclusive and defined based on clinician-facilitated interviews with the participant and informant;
Figure 2.
depicts the predicted probability of diagnostic outcome for each cognitive complaint group by age. Figure 2a reflects the likelihood of conversion in individuals with normal cognition. Figure 2b reflects the likelihood of conversion in individuals with MCI as compared to remaining diagnostically stable. Figure 2c reflects the likelihood of reversion in individuals with MCI as compared to remaining diagnostically stable.
Among the NC participants, we secondarily examined how type of complaint related to each of the two diagnostic conversion outcomes (i.e., MCI and dementia). Compared to NC complaints with no complaint, participants with self complaint only (OR=2.3; 99%CI=1.6–3.2, p<0.001) and both self and informant complaint (OR=3.7; 99%CI=2.5–5.7, p<0.001) were at increased risk of converting to MCI. Compared to NC participants with no complaint, participants with informant complaint only (OR=4.7; 99%CI=2.1–10.5, p<0.001) and both self and informant complaint (OR=5.7; 99%CI=3.1–10.6, p<0.001) were at increased risk of converting to dementia.
3.3 Cognitive complaint & MCI diagnostic outcome: reversion versus stable
Similar to the final model for NC participants, the model for MCI participants included main effects of baseline characteristics only with linear effects for age, education, MMSE, and years of follow-up. MCI participants with informant complaint (OR=0.3; 99%CI=0.1–0.6, p<0.001) and both self and informant complaint (OR=0.2; 99%CI=0.1–0.4, p<0.001) were less likely to revert to normal than remain stable as compared to MCI participants with no complaint. Furthermore, compared to participants with self complaint only, MCI participants with both self and informant complaint were less likely to revert to normal than remain stable (OR=0.3; 99%CI=0.2–0.6, p<0.001). See Table 2. The probability of reversion against age was plotted using the same participant profile as for NC participants. See Figure 2b.
3.4 Cognitive complaint & MCI diagnostic outcome: conversion versus stable
MCI participants with informant complaint only (OR=2.2; 99%CI=1.2–4.3, p<0.001) and both self and informant complaint (OR=2.9; 99%CI=1.8–4.8, p<0.001) were more likely to progress to dementia compared to MCI participants with no complaint. MCI individuals with both self and informant complaint were more likely to convert to dementia compared to participants with self complaint only (OR=2.7; 99%CI=1.6–4.6, p<0.001). See Table 2. The probability of conversion against age was plotted using the same participant profile as for NC participants. See Figure 2c.
4. Discussion
Leveraging a large, multicenter cohort, we found that among cognitively normal older adults, a combination of both self and informant cognitive complaint (i.e. mutual complaint) was associated with a four-fold risk of progression to MCI or dementia over the follow-up period. An informant complaint alone or a self complaint alone conferred a two-fold increased risk of progression over the follow-up period (as compared to no complaint). A self complaint was less predictive of conversion than the presence of both self and informant complaint.
A similar pattern was observed in MCI participants with a combination of both self and informant complaint yielding a nearly three-fold greater risk of converting than remaining stable. The presence of just an informant complaint was related to diagnostic conversion, conferring over two times the risk for converting from MCI to dementia than remaining stable. MCI participants with both a self and informant complaint were nearly 80% less likely to revert than remain diagnostically stable over the follow-up period, and MCI individuals with only an informant complaint were 75% less likely to revert. The presence of only a participant (i.e., self) complaint was not statistically predictive of diagnostic conversion or reversion as compared to remaining stable among individuals with MCI.
Our findings are not only aligned with prior work relating self complaint in cognitively normal elders28 and informant complaint9 in MCI to diagnostic outcome but extend this work in several ways. First, our methodology integrated self and informant sources of complaint alone and in combination to assess conversion risk. In doing so, we augment existing literature by suggesting the presence of both patient and informant cognitive complaint may have important prognostic value over either one alone. Many cognitively impaired older adults go undiagnosed in the primary care setting,29 with prevalence estimates suggesting that 16% of individuals over age 70 years have MCI,30 and one in eight individuals over age 65 has dementia.31 Although there is no current cure for AD, early identification is important for a couple of reasons. First, literature supports early identification, as patients benefit from dementia pharmacological treatments (i.e., cholinesterase inhibitors).32
Second, early identification may promote lifestyle and behavioral interventions, such as increased physical activity, which have been shown to enhance cognition33,34 and decrease diagnostic conversion35,36 risk. Taken together with growing evidence supporting the benefit of early identification of dementia37 and health care policy reform requiring annual cognitive screening for all Medicare beneficiaries, our findings emphasize that integrating a family member or reliable informant into the primary care evaluation of older patients can provide important information for cognitive prognosis. In the event that both a patient and their loved one complain about memory changes, more thorough cognitive screening or a memory diagnostic referral might be warranted.
Third, we examined the prognostic value of cognitive complaints not only for diagnostic conversion10,38 but also diagnostic reversion and stability in MCI. Fluctuations in MCI are common, as many people diagnosed with MCI fail to meet diagnostic criteria one year later.39 Making accurate prognostic determinations for individuals with MCI poses a challenge for clinicians.40 However, the current results offer some resolution, as a combination of self and informant complaint in MCI yields nearly three times the risk of converting to dementia as compared to reverting to normal cognition over nearly a three year period. This finding implies a combination of complaints reflects the presence of an underlying neurodegenerative process that will progress.
Despite being a criterion for MCI diagnosis, quantification of cognitive complaints is poorly defined. Methods assessing cognitive complaint range from a single dichotomous (yes/no) question9 to a more extensive multi-item questionnaire,38 and the individual reporting the complaint may be the patient, an informant, or the clinician. Questions vary in wording, such as “Do you have complaints about your memory?” or “Have you noticed a significant decline in your thinking?” Therefore, standardized methods assessing cognitive complaint are needed to refine MCI diagnostic profiling, further enhance prognostic implications, and decrease diagnostic fluctuations.
Cognitive complaints are quite common among older adults.7 Among our two participant groups, the presence of self complaint only was inconsistent in predicting diagnostic conversion (i.e., predictive in NC but not MCI). The observed discrepancy, consistent with prior studies,10,28 warrants some discussion. We found that among cognitively normal elders, self complaint only was related to an increased risk of converting to MCI or dementia rather than remaining stable over the follow-up period. This finding suggests an underlying biological basis for subjective cognitive complaints among cognitively normal elders, a concept supported by prior evidence linking self complaint with an in-vivo structural neuroimaging changes reflecting the earliest pathological signs of dementia (i.e., atrophy within the medial temporal lobe)41,42 and an increased presence of AD neuropathology post-mortem in non-demented individuals.43 Coupled with prior findings, our observations suggest that older adults who are known to be cognitively normal but who are complaining of cognitive changes should not be dismissed as “worried well.”
In contrast, we observed that self complaint is not related to increased risk of diagnostic conversion among MCI individuals. The discrepancy between self and informant complaint in our MCI cohort is consistent with previous literature10 and may be attributable to compromised awareness of cognitive impairment (anosagnosia) or memory loss (meta-memory) observed in both MCI8 and early AD44 populations. Areas associated with self-perception and awareness of deficits (i.e., right prefrontal cortex45 and right hippocampus46) are often affected by early AD pathology.47,48. Therefore, our observation that self complaint in MCI was not associated with increased risk of conversion may be due to inconsistencies in awareness across MCI participants.
A number of strengths are associated with this study. First, methodology incorporated a combination of complaint sources, as compared to previous research that has considered self or informant complaint alone. The current study considered multiple MCI diagnostic outcomes (i.e., reversion, stability, and conversion), which extends previous studies that have not differentiated MCI stability from reversion. The use of the NACC UDS represents a number of strengths and enhancements to the previous literature, including a large sample size, a comprehensive and standardized neuropsychological protocol, and standardized diagnostic criteria.
Despite numerous strengths, several key limitations must be considered. NACC participants, although representative of ~30 ADCs throughout the United States, are primarily White and well-educated and participants in ADCs are often from a memory clinic or self-referred for participation. These factors may lead to different baseline characteristics as compared to a population-based study and limit the generalizability of these results. Furthermore, given that estimates of conversion to MCI and dementia vary between clinic49–51 and community samples,49,51 the findings from this study may reflect sample characteristics specific to the NACC cohort. Population-based analyses aimed at understanding the prognostic value of cognitive complaint are necessary. Secondly, methods for determining cognitive complaints vary across sites. Furthermore, cognitive complaint is rated in a dichotomous nature, which does not allow for assessing complaint gradations or severity in relation to diagnostic outcome; however, the simplicity of the dichotomous rating may have more implications for active primary care settings, where an extensive cognitive complaint interview would not be feasible. Finally, the follow-up time was limited to three years on average, but this limitation is tempered by the large sample size providing ample power to detect diagnostic conversion in the limited time frame.
The current findings are an important step in understanding how cognitive complaint enhances prognostic implications for both cognitively normal and MCI individuals in primary care settings. Further research is needed to better understand best practices for assessing and quantifying cognitive complaint and the temporal nature of subjective and informant cognitive complaint onset in relation to cognitive decline or diagnostic conversion. Such research advances are necessary to refine the definition of cognitive complaint as a diagnostic entity in MCI, inform treatment decisions for those individuals at greatest risk for progression as more effective therapeutic targets become available, and provide practical information and assessment tools for primary care providers of older adults.
Acknowledgments
This research was supported by National Alzheimer’s Coordinating Center Junior Investigator Award #2011-JI-08 (KAG); T32-AG036697 (KAG), K23-AG030962 (Paul B. Beeson Career Development Award in Aging; ALJ), Alzheimer’s Association IIRG-08-88733 (ALJ), R01-AG034962 (ALJ), R01-HL11516 (ALJ), P30-AG013846 (Boston University Alzheimer’s Disease Core Center), U01- AG016976 (National Alzheimer’s Coordinating Center), and the Vanderbilt Memory & Alzheimer’s Center (KAG, ALJ).
References
- 1.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Ganguli M, Snitz BE, Saxton JA, Chang CC, Lee CW, Vander Bilt J, Hughes TF, Loewenstein DA, Unverzagt FW, Petersen RC. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68:761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, Dekosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, Brys M, de Leon MJ. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dementia and Geriatric Cognitive Disorders. 2007;24:177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 6.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 7.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17:181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 9.Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724–1726. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 10.Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Archives of Neurology. 1996;53:423–427. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 11.Panza F, Solfrizzi V, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, Capurso A, Imbimbo BP. Disease-modifying approach to the treatment of Alzheimer’s disease: from alpha-secretase activators to gamma-secretase inhibitors and modulators. Drugs Aging. 2009;26:537–555. doi: 10.2165/11315770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 14.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 22.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 23.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 24.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 26.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 27.American_Psychiatric_Association. Diagnostic & Statistical Manual - IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 29.Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alzheimer’s_Association. 2011 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Tinklenberg JR, Kraemer HC, Yaffe K, Ross L, Sheikh J, Ashford JW, Yesavage JA, Taylor JL. Donepezil treatment and Alzheimer disease: can the results of randomized clinical trials be applied to Alzheimer disease patients in clinical practice? Am J Geriatr Psychiatry. 2007;15:953–960. doi: 10.1097/JGP.0b013e3180986138. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 34.Menec VH. The relation between everyday activities and successful aging: a 6-year longitudinal study. J Gerontol B Psychol Sci Soc Sci. 2003;58:S74–82. doi: 10.1093/geronb/58.2.s74. [DOI] [PubMed] [Google Scholar]
- 35.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 36.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 37.Solomon PR, Murphy CA. Should we screen for Alzheimer’s disease? A review of the evidence for and against screening Alzheimer’s disease in primary care practice. Geriatrics. 2005;60:26–31. [PubMed] [Google Scholar]
- 38.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 40.van Hout H, Vernooij-Dassen M, Bakker K, Blom M, Grol R. General practitioners on dementia: tasks, practices and obstacles. Patient Educ Couns. 2000;39:219–225. doi: 10.1016/s0738-3991(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 41.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart R, Dufouil C, Godin O, Ritchie K, Maillard P, Delcroix N, Crivello F, Mazoyer B, Tzourio C. Neuroimaging correlates of subjective memory deficits in a community population. Neurology. 2008;70:1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54. [DOI] [PubMed] [Google Scholar]
- 43.Jorm AF, Masaki KH, Davis DG, Hardman J, Nelson J, Markesbery WR, Petrovitch H, Ross GW, White LR. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 44.Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43:1004–1019. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starkstein SE, Vazquez S, Migliorelli R, Teson A, Sabe L, Leiguarda R. A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Arch Neurol. 1995;52:415–420. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- 46.Marshall GA, Kaufer DI, Lopez OL, Rao GR, Hamilton RL, DeKosky ST. Right prosubiculum amyloid plaque density correlates with anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1396–1400. doi: 10.1136/jnnp.2003.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 48.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 50.Gabryelewicz T, Styczynska M, Luczywek E, Barczak A, Pfeffer A, Androsiuk W, Chodakowska-Zebrowska M, Wasiak B, Peplonska B, Barcikowska M. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22:563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 51.Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment--a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]