Abstract
Purpose.
We hypothesize that culturing immortalized human meibomian gland epithelial cells in serum-containing medium will induce their differentiation. The purpose of this investigation was to begin to test our hypothesis, and explore the impact of serum on gene expression and lipid accumulation in human meibomian gland epithelial cells.
Methods.
Immortalized and primary human meibomian gland epithelial cells were cultured in the presence or absence of serum. Cells were evaluated for lysosome and lipid accumulation, polar and neutral lipid profiles, and gene expression.
Results.
Our results support our hypothesis that serum stimulates the differentiation of human meibomian gland epithelial cells. This serum-induced effect is associated with a significant increase in the expression of genes linked to cell differentiation, epithelium development, the endoplasmic reticulum, Golgi apparatus, vesicles, and lysosomes, and a significant decrease in gene activity related to the cell cycle, mitochondria, ribosomes, and translation. These cellular responses are accompanied by an accumulation of lipids within lysosomes, as well as alterations in the fatty acid content of polar and nonpolar lipids. Of particular importance, our results show that the molecular and biochemical changes of immortalized human meibomian gland epithelial cells during differentiation are analogous to those of primary cells.
Conclusions.
Overall, our findings indicate that immortalized human meibomian gland epithelial cells may serve as an ideal preclinical model to identify factors that control cellular differentiation in the meibomian gland.
Keywords: meibomian gland, epithelial cells, differentiation, gene expression, lipids
Our study demonstrates that serum stimulates human meibomian gland epithelial cells to differentiate. This action is associated with significant alterations in gene expression, lipid accumulation and the fatty acid content of polar and nonpolar lipids.
Introduction
The differentiation of meibomian gland epithelial cells is critically important for the health and well-being of the ocular surface. These cells produce meibum, a lipid-rich mixture that is released by the gland at the eyelid margins. Meibum spreads onto the tear film and serves to stabilize this film, prevent its evaporation, and promote visual acuity.1,2 Interference with this lipid-related production and secretion, such as occurs in meibomian gland dysfunction (MGD), leads to tear film instability and evaporation1–3 and is the major cause of dry eye disease throughout the world.1,4
Unfortunately, despite the importance of meibomian gland epithelial cells, almost nothing is known about the control of their differentiation. Insofar as we know today, meibomian gland epithelial cell differentiation in vivo, as with other sebaceous gland epithelial cells, begins with small, undifferentiated cells located in the acinar periphery. These cells contain large numbers of free ribosomes and mitochondria, and a poorly developed smooth endoplasmic reticulum (SER) and Golgi apparatus. As cells mature and start their migration toward the lateral ductules, the SER and Golgi become more prominent, lysosomes are generated, and lipids begin to accumulate. Ultimately, cells terminally differentiate, a process associated with a dramatic increase in volume, a profusion of lipid-filled vesicles, and nuclear pyknosis. Cells then undergo holocrine secretion, which involves cellular autophagy, apoptosis, disintegration, and release of lipid-laden contents into the ductules.1,5–9
A preclinical model of this cellular differentiation process in vitro would be ideal. Such a model not only would permit the discovery of agents that regulate this cellular maturation, but also allow the development of new pharmaceutical approaches for the treatment of MGD.
We hypothesize that immortalized human meibomian gland epithelial cells can serve as this preclinical model in vitro. We further hypothesize that exposure of these cells to serum will induce differentiation and provide the opportunity to identify factors that control this process. The purpose of this investigation was to begin to test our hypotheses, and explore the impact of serum on gene expression and lipid accumulation in human meibomian gland epithelial cells.
Methods
Meibomian Gland Epithelial Cell Culture Procedures
Primary and immortalized human meibomian gland epithelial cells10,11 were cultured in keratinocyte serum-free medium (SFM) containing 5 ng/mL epidermal growth factor (EGF) and 50 μg/mL bovine pituitary extract (BPE). After reaching 70% to 90% confluence, cells were placed in SFM or serum-containing medium (10% fetal bovine serum in equal volumes of Dulbecco's modified Eagle's medium and Ham's F12 with 10 ng/mL EGF) for varying intervals. Media and serum were purchased from Invitrogen-Gibco (Grand Island, NY, USA). The use of human tissues was approved by the Institutional Review Boards of the Schepens Eye Research Institute and Massachusetts Eye and Ear and adhered to the tenets of the Declaration of Helsinki.
Lysosome and Neutral Lipid Staining
After designated time intervals, immortalized human meibomian gland epithelial cells were cultured for 30 minutes in the presence of LysoTracker Red DND-99 (Invitrogen-Gibco) to label lysosomes.12 Cells then were fixed in paraformaldehyde, processed for staining with LipidTOX green neutral lipid stain (Invitrogen-Gibco) and 4′,6-diamidino-2-phenylindole (DAPI) blue nuclear stain (Invitrogen-Gibco), and mounted with ProLong Gold antifade, as described previously.11 Cells were imaged for LysoTracker (excitation/emission, 577/590 nm) and LipidTox (excitation/emission, 495/505 nm) staining with a Nikon Eclipse E800 (Nikon Instruments, Melville, NY, USA).
Cellular Lipid Analyses
Following the designated culture period, primary and immortalized human meibomian gland epithelial cells were treated with 0.25% trypsin and EDTA (Invitrogen-Gibco), washed with PBS twice, then centrifuged. Cell pellets were stored at −80°C until thawing for lipid analyses. Lipids were extracted by the method of Folch et al.13
For neutral lipid (i.e., cholesterol, cholesterol esters, wax esters) analysis, a lipid aliquot (100 μg) was further extracted with a Bond Elut NH2 Solid Phase Extraction 50 mg cartridge (Agilent Technologies, Columbia, MD, USA). In brief, the aliquot was dried, resuspended in 1:1 hexane:isopropanol and applied to the conditioned cartridge. The flow-through was collected, dried, resuspended in mobile phase A, and injected with internal standards into a LUNA C18(2) 100Å 150 × 1.00 mm HPLC column (Phenomenex, Torrance, CA, USA) using a Surveyor Autosampler and LC-pump (Thermo Fisher Scientific, Waltham, MA, USA), and analyzed in triplicate. Elution of the C18 column was performed with (A) 95% methanol, 5% 100 mM ammonium formate, pH 4.75 and (B) 95% isopropanol, 5% 100 mM ammonium formate, pH 4.75. The flow rate was set at 50 μL/min and the gradient was: 0 minutes 50% B, 5 minutes 50% B, 20 minutes 100% B, 95 minutes 100% B, 96 minutes 0% B with a 24-minute re-equilibration time at each run's end. Full scan spectra from a mass spectrometer (MS) were obtained by using a positive ion electrospray MS and a Orbitrap Velos Pro (Thermo Fisher Scientific) at 60,000 resolution. Neutral lipid standards used in these analyses included 1-heptadecanoyl-rac-glycerol (17:0 MG) > 99%; 1,2-dilauroyl-sn-glycerol (12:0/12:0 DG) > 99%; 1,3-ditetradecanoyl-2-(9Z-hexadecenoyl)-glycerol (14:0/16:1/14:0 TG) > 99%; cholest-5-en-3β-yl pentadecanoate (15:0 cholesterol ester) > 99%; and 1-oleoyl-N-heptadecanoyl-D-erythro-sphingosine (17:0/d18:1 Ceremide) > 99% (Avanti Lipids, Alabaster, AL, USA); cholesterol-D7 > 98% (Cambridge Isotope Lab, Andover, MA, USA); and oleyl laurate wax ester (Nu-Check-Prep, Inc., Elysian, MA, USA). The neutral lipids standards were mixed to 0.4 ng/μL triglycerides, 1 ng/μL diglyceride/cholesterol ester/wax ester, 2 ng/μL monoglyceride/ceremide, and 20 ng/μL cholesterol, and then diluted 1:1 with the sample extract. Statistical analysis of cholesterol levels was performed with Student's unpaired t-test.
For polar lipid analysis, a lipid aliquot (10 μg) and an internal standard mixture were injected into a LUNA 3 μ Silica(2) 100Å 150 × 1.00 mm column (Phenomenex) using a Surveyor Autosampler and LC-pump (Thermo Fisher Scientific), and analyzed by light chromatography/mass spectrometry (LC/MS) in triplicate and LC/MS2/MS3 in singlicate. Elution of the Silica column was carried out with (A) 58:40:02, isopropanol:hexane:100 mM ammonium formate, pH 4.75 and (B) 50:40:10, isopropanol:hexane:100 mM ammonium formate, pH 4.75. The flow rate was 50 μL/min and the gradient was: 0 minutes 0% B, 15 minutes 0% B, 40 minutes 100% B, 50 minutes 100% B, 51 minutes 0% B, with a 40-minute re-equilibration time at the end of each run. Full scan MS spectra were obtained using negative ion electrospray MS and a LTQ Orbitrap Velos (Thermo Fisher Scientific) at 60,000 resolution. The data-dependent MS2 and MS3 scans were acquired on the most intense fragment ion of the top 5 intense ions in the full MS spectra. This process was used to facilitate the identification of the fatty acid composition of individual polar lipid molecular species. The chromatographic elution time for each lipid group was determined by observing the elution of the internal standard and the highest molecular weight species. The extracted ion current of each lipid molecular species was processed with SIEVE software (Thermo Fisher Scientific), and the lipid identity was determined by the retention time and exact mass (5 parts per million [ppm]). Identities were verified by using the fragment ions and the extracted ion chromatogram profiles. The phospholipid standards purchased from Avanti Lipids included: dimyristoyl phosphocholine (14:0/14:0 PC) > 99%; dimyristoyl phosphoethanolamine (14:0/14:0 PE) > 99%; dimyristoyl phosphoserine (14:0/14:0 PS) > 99%; dimyristoyl phosphoglycerol (14:0/14:0 PG) > 99%; dimyristoyl phosphate (14:0/14:0 PA) > 99%; dilauroyl sphingosylphosphocholine (d18:1/12:0 SM) > 99%;1-myristoyl phosphate (14:0 LPA) > 99%; 1-heptadecenoyl phosphoserine (14:0 LPS) > 99%; 1-myristoyl phosphoethanolamine (14:0 LPE) > 99%; and 1-myristoyl phosphoglycerol (14:0 LPG) > 99%. The polar lipids standards were mixed to 0.1 ng/μL of PG, 1 ng/μL of PE, 2 ng/μL of LPG/LPE/PS/LPS/LPA/SM, and 20 ng/μL of PA/PC.
For all lipid groups except phosphatidylcholine, lysophosphatidylcholine, and sphingomyelin, the ion detected was the molecular species minus one proton. For the excepted species, the ion detected was the molecular ion minus methane plus acetate. The average intensities of all ions were obtained from four replicate measurements.
Microarray Procedures
Total RNA was isolated from cells, analyzed for integrity and processed for the measurement of mRNA levels at Asuragen (Austin, TX, USA), as previously published.14 Our experiments used Illumina HumanHT-12 v3 and v4 Expression BeadChips (Illumina, San Diego, CA, USA). Data were acquired with Illumina BeadStudio software, and used background subtraction and cubic spline normalization. Standardized hybridization intensity values were adjusted by adding a constant, so that the lowest intensity value for any sample was equal to 16.15
Normalized data were evaluated without log transformation and statistical analyses were performed with Student's t-test (2-tailed, unpaired). These analyses were conducted with GeneSifter software (Geospiza, Seattle, WA, USA), which also yielded gene ontology, KEGG pathway, and z-score (zsc) reports organized according to the guidelines of the Gene Ontology Consortium (available in the public domain at http://www.geneontology.org/GO).16 Gene comparisons between groups were facilitated by using the GeneSifter intersector program (Geospiza; available in the public domain at www.public.genesifter.net). All data from the Illumina BeadChips may be obtained and downloaded from the National Center for Biotechnology Information's Gene Expression Omnibus (available in the public domain at http://www.ncbi.nlm.nih.gov/geo) via series accession numbers GSE18099 and GSE 37089.
Real-Time PCR Procedures
The differential expression of selected genes was verified by using quantitative real-time PCR (qPCR) methods. The cDNAs were generated by using SuperScript III Reverse Transcriptase (Invitrogen-Gibco) and random hexamer primers (Invitrogen-Gibco). The qPCR reactions were carried out in triplicate with TaqMan Gene Assays (Applied Biosystems, Inc., Foster City, CA, USA), TaqMan-specific primers and probes for lipocalin 2 (Hs.204238), claudin 1 (Hs.439060), acetyl-coenzyme A acetyltransferase 2 (Hs.571037), ornithine decarboxylase 1 (Hs.467701), and GAPDH (4326317E). Differential gene expression was calculated by following the Comparative Ct method, as described in Applied Biosystems User Bulletin 2 (updated 2001).
Results
Effect of Serum on Gene Expression in Human Meibomian Gland Epithelial Cells
To determine whether serum upregulates genes associated with cellular differentiation, we cultured primary and immortalized human meibomian gland epithelial cells in SFM for 5 days (“undifferentiated” cells), another 2 days to 70% to 80% confluence, followed by an additional 14 days in either SFM or serum-containing medium. Cells then were processed for analyses with Illumina BeadChips and Geospiza software.
Our results showed that serum clearly stimulates the activity of genes related to cell differentiation. As demonstrated in Table 1, serum exposure significantly (P < 0.05) altered the expression of over 2750 genes in primary and immortalized human meibomian gland epithelial cells. This effect was found irrespective of whether serum-associated responses were compared to those of undifferentiated or SFM-treated cells. The magnitude of serum's influence was quite remarkable. For example, serum exposure induced 19- to 456-fold increases in the activity of genes encoding retinoic acid receptor responder 1, chemokine (C-C motif) ligand 28 (CCL28), insulin-like growth factor binding protein 3, matrix metallopeptidase 12, phospholipase A2, and prominin (Table 2). Many of these genes are associated with cell differentiation. Serum also enhanced by up to 5.9-fold the gene expression of transforming growth factor β2, increased the mRNA levels of netrin 4 and collagen, type V, α2 by 2.5- to 21.1-fold, and induced the appearance of polymeric immunoglobulin receptor mRNA (pIgR; data not shown). Especially noteworthy was our finding that these molecular responses were very similar between primary and immortalized human meibomian gland epithelial cells (Tables 2–7). Indeed, serum elicited the same responses (i.e., significant up- or downregulation compared to “undifferentiated”) in over 2500 genes in both primary and immortalized human meibomian gland epithelial cells.
Table 1.
Influence of Serum on Gene Expression in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Group |
Genes ↑ |
Genes ↓ |
Total Genes |
| Undiff vs. serum | |||
| Primary | 2125 | 2358 | 4483 |
| Immortalized | 2025 | 2787 | 4812 |
| SFM vs. serum | |||
| Primary | 1368 | 1814 | 3182 |
| Immortalized | 1602 | 1187 | 2789 |
Data were evaluated without log transformation. The expression of listed genes was significantly (P < 0.05) up- (↑) or (↓) downregulated by serum exposure, compared to the undifferentiated (“Undiff”) or SFM control groups.
Table 2.
Effect of Serum on Gene Expression in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Accession # |
Gene |
Ratio |
Ontology |
| Upregulation | |||
| NM_000478 | Alkaline phosphatase | 24.7, 11.9, 12.3, 11.9 | Anatomical structure morphogenesis |
| NM_019846 | chemokine (C-C motif) ligand 28 | 15.7, 19.1, 18.9, 17.6 | Chemotaxis |
| NM_000598 | Insulin-like growth factor binding protein 3 | 54.2, 33.8, 41.3, 20.8 | Regulation of cell growth |
| NM_002426 | Matrix metallopeptidase 12 | 50.1, 45.6, 43.0, 54.8 | Proteolysis |
| NM_007069 | Phospholipase A2, group XVI | 55.4, 17.9, 24.3, 9.0 | Lipid catabolic process |
| NM_006017 | Prominin 1 | 32.4, 31.9, 32.4, 31.9 | Cell differentiation |
| NM_206963 | Retinoic acid receptor responder (tazarotene induced) 1 | 456.0, 207.0, 30.6, 99.5 | Negative regulation of cell proliferation |
| NM_019554 | S100 calcium binding protein A4 | 24.1, 20.8, 12.3, 9.8 | Epithelial to mesenchymal transition |
| NM_003357 | Secretoglobin, family 1A, member 1 | 31.2, 18.3, 29.3, 17.7 | Negative regulation of transcription from RNA polymerase II promoter |
| NM_030754 | Serum amyloid A2 | 140.9, 32.2, 7.2, 43.6 | Acute phase response |
| NM_003186 | Transgelin | 62.0, 107.5, 8.1, 11.9 | Anatomical structure development |
| Downregulation | |||
| NM_001218 | Carbonic anhydrase XII | 11.9, 9.8, 8.8, 14.4 | 1-Carbon metabolic process |
| NM_002775 | HtrA serine peptidase 1 | 5.7, 6.5, 4.9, 7.5 | Negative regulation of TGF β receptor signaling pathway |
Relative ratios were calculated by comparing the degree of gene expression in primary and immortalized meibomian gland epithelial cells treated with serum, compared to the undifferentiated or SFM control groups. The ratios, left to right, are from the following comparisons: primary cells, serum versus undifferentiated; immortalized cells, serum versus undifferentiated; primary cells, serum versus SFM; immortalized cells, serum versus SFM.
Table 3.
Effect of Serum Exposure on Cellular Component Ontologies in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Ontology |
Undifferentiated vs. Serum |
SFM vs. Serum |
||||||
|
Pri Genes |
Pri
z-Score |
Imm Genes |
Imm
z-Score |
Pri Genes |
Pri
z-Score |
Imm Genes |
Imm
z-Score |
|
| Upregulation | ||||||||
| Cytoplasmic membrane-bounded vesicle | 97 | 3.14 | 89 | 2.99 | 68 | 3.2 | 81 | 4.07 |
| Cytoplasmic vesicle | 105 | 3.5 | 101 | 3.94 | 72 | 3.29 | 89 | 4.64 |
| Endomembrane system | 213 | 5.4 | 183 | 3.99 | 161 | 6.65 | 176 | 6.57 |
| Endoplasmic reticulum | 144 | 4.14 | 120 | 2.6 | 105 | 4.69 | 132 | 6.7 |
| Endoplasmic reticulum membrane | 90 | 3.41 | 81 | 3.03 | 71 | 4.69 | 81 | 5.17 |
| Endoplasmic reticulum part | 101 | 3.55 | 86 | 2.51 | 82 | 5.27 | 95 | 6.02 |
| Golgi apparatus | 162 | 7.25 | 143 | 6.26 | 107 | 5.9 | 128 | 7.26 |
| Golgi apparatus part | 106 | 6.71 | 88 | 5.04 | 68 | 5.1 | 84 | 6.65 |
| Golgi cisterna | 16 | 2.96 | 15 | 2.95 | 13 | 3.5 | 16 | 4.32 |
| Golgi cisterna membrane | 13 | 2.51 | 11 | 2 | 10 | 2.73 | 13 | 3.72 |
| Golgi lumen | 6 | 2.48 | 6 | 2.72 | 4 | 2.04 | 5 | 2.56 |
| Golgi membrane | 84 | 5.56 | 73 | 4.65 | 61 | 5.56 | 72 | 6.53 |
| Golgi stack | 24 | 4.29 | 20 | 3.41 | 18 | 4.31 | 21 | 4.87 |
| Lysosome | 65 | 7.66 | 54 | 6.11 | 31 | 3.24 | 30 | 2.31 |
| Lytic vacuole | 65 | 7.66 | 54 | 6.11 | 31 | 3.24 | 30 | 2.31 |
| Membrane-bounded vesicle | 100 | 3.32 | 92 | 3.2 | 70 | 3.34 | 84 | 4.33 |
| Vacuole | 69 | 8.26 | 69 | 7.73 | 38 | 3.88 | 34 | 2.25 |
| Vesicle | 111 | 3.88 | 106 | 4.23 | 76 | 3.59 | 94 | 5.02 |
| Downregulation | ||||||||
| Cytosolic large ribosomal subunit | 28 | 12.9 | 22 | 8.67 | 29 | 15.54 | 15 | 9.42 |
| Cytosolic small ribosomal subunit | 29 | 14.19 | 20 | 8.2 | 32 | 18.23 | 13 | 8.44 |
| Large ribosomal subunit | 20 | 10.75 | 17 | 7.96 | 13 | 7.55 | 5 | 3 |
| Mitochondrial envelope | 84 | 5.93 | 84 | 4.31 | 69 | 5.78 | 37 | 2.8 |
| Mitochondrial large ribosomal subunit | 11 | 7.27 | 9 | 5.06 | 7 | 4.94 | 4 | 3.29 |
| Mitochondrial nucleoid | 15 | 6.37 | 11 | 3.52 | 7 | 2.62 | 5 | 2.38 |
| Mitochondrial part | 149 | 10.63 | 151 | 8.66 | 112 | 8.65 | 51 | 2.84 |
| Mitochondrion | 265 | 12.63 | 292 | 12.05 | 202 | 10.51 | 102 | 4.34 |
| Organellar large ribosomal subunit | 11 | 7.27 | 9 | 5.06 | 7 | 4.94 | 4 | 3.29 |
| Ribonucleoprotein complex | 220 | 24.93 | 201 | 19.29 | 163 | 20.3 | 60 | 6.66 |
| Ribosome | 113 | 22.93 | 93 | 16.03 | 98 | 22.68 | 35 | 8.33 |
Designated ontologies were chosen after analyses of nontransformed data. A z-score is a statistical rating of the relative expression of gene ontologies, and indicates how much a given ontology is over (positive)- or under (negative)-represented in a specified gene list. In other words, a z-score is a normalized difference using the expected value and standard deviation of the number of genes.17 Positive z-scores reflect gene ontology terms with a greater number of genes meeting the criterion than is expected by chance, whereas negative z-scores indicate gene ontology terms with a lower number of genes meeting the criterion than expected by chance. A z-score close to zero indicates that the number of genes meeting the criterion approximates the anticipated number.17 In this table, z-scores with values > 2.0 are reported for selected ontologies with ≥4 genes. Genes, number of genes up- or downregulated in primary (Pri) and immortalized (Imm) human meibomian gland epithelial cells after treatment with serum; z-score, significant score for the up- and downregulated genes in the serum-treated cells.
Table 4.
Influence of Serum on Biological Process Ontologies in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Ontology |
Undifferentiated vs. Serum |
SFM vs. Serum |
||||||
|
Pri Genes |
Pri
z-Score |
Imm Genes |
Imm
z-Score |
Pri Genes |
Pri
z-Score |
Imm Genes |
Imm
z-Score |
|
| Upregulation | ||||||||
| Anatomical structure development | 349 | 2.64 | 334 | 3.35 | 256 | 4.28 | 291 | 4.4 |
| Apoptosis | 195 | 6 | 184 | 6.13 | 132 | 5.21 | 159 | 6.43 |
| Apoptotic mitochondrial changes | 11 | 3.68 | 9 | 2.89 | 10 | 4.69 | 8 | 2.97 |
| Cell adhesion | 117 | 4.49 | 96 | 2.78 | 97 | 6.52 | 103 | 5.88 |
| Cell death | 209 | 5.85 | 199 | 6.17 | 142 | 5.16 | 166 | 5.88 |
| Cell differentiation | 237 | 2.25 | 223 | 2.49 | 162 | 2.38 | 195 | 3.38 |
| Cell migration | 87 | 4.13 | 76 | 3.31 | 62 | 4.11 | 71 | 4.4 |
| Cell morphogenesis | 86 | 2.81 | 75 | 2.07 | 62 | 3.11 | 73 | 3.64 |
| Cellular response to stimulus | 500 | 4.25 | 446 | 3.07 | 337 | 3.99 | 387 | 4.31 |
| Epidermis development | 42 | 4.72 | 46 | 6.23 | 28 | 3.86 | 27 | 2.82 |
| Epithelium development | 65 | 4.3 | 61 | 4.28 | 46 | 4.09 | 49 | 3.67 |
| Induction of apoptosis | 60 | 3.99 | 59 | 4.47 | 36 | 2.44 | 54 | 5.16 |
| Induction of programmed cell death | 60 | 3.95 | 59 | 4.43 | 37 | 2.62 | 54 | 5.11 |
| Organ morphogenesis | 76 | 2.24 | 75 | 2.84 | 53 | 2.29 | 72 | 4.24 |
| Programmed cell death | 196 | 5.99 | 185 | 6.12 | 134 | 5.36 | 160 | 6.45 |
| Proteolysis | 132 | 5.22 | 130 | 5.98 | 87 | 4.18 | 108 | 5.59 |
| Signal transduction | 430 | 4.31 | 391 | 3.71 | 294 | 4.3 | 328 | 3.95 |
| Signaling | 453 | 3.21 | 412 | 2.65 | 314 | 3.77 | 347 | 3.11 |
| Tissue development | 136 | 5.27 | 128 | 5.33 | 98 | 5.39 | 111 | 5.61 |
| Vesicle-mediated transport | 115 | 4.36 | 103 | 3.76 | 77 | 3.67 | 80 | 2.79 |
| Wound healing | 84 | 3.67 | 71 | 2.55 | 59 | 3.56 | 75 | 5.01 |
| Downregulation | ||||||||
| Cell cycle | 209 | 7.95 | 327 | 15.98 | 124 | 2.54 | 84 | 2.34 |
| Cell cycle checkpoint | 56 | 7.04 | 77 | 9.92 | 28 | 2.25 | 22 | 2.85 |
| Cell cycle phase | 154 | 9.09 | 248 | 17.46 | 79 | 2.22 | 54 | 2.11 |
| Cell cycle process | 168 | 8 | 275 | 16.69 | 95 | 2.37 | 64 | 2.12 |
| Cell division | 79 | 6.01 | 139 | 13.58 | 47 | 2.47 | 37 | 3.32 |
| Gene expression | 505 | 9.8 | 555 | 8.19 | 412 | 9.61 | 226 | 3.75 |
| M phase | 92 | 6.38 | 172 | 15.94 | 52 | 2.12 | 37 | 2.26 |
| M phase of mitotic cell cycle | 78 | 7.33 | 143 | 16.41 | 40 | 2.2 | 29 | 2.39 |
| Mitosis | 76 | 7.31 | 139 | 16.26 | 39 | 2.23 | 29 | 2.59 |
| Mitotic cell cycle | 151 | 10.08 | 233 | 17.53 | 76 | 2.72 | 50 | 2.17 |
| NcRNA metabolic process | 93 | 12.6 | 96 | 11.27 | 71 | 10.55 | 32 | 4.49 |
| NcRNA processing | 66 | 10.51 | 68 | 9.37 | 54 | 9.73 | 25 | 4.45 |
| Nuclear division | 76 | 7.31 | 139 | 16.26 | 39 | 2.23 | 29 | 2.59 |
| Nuclear export | 39 | 9.3 | 30 | 5.31 | 30 | 7.88 | 14 | 3.76 |
| Ribonucleoprotein complex assembly | 33 | 8.13 | 30 | 6.03 | 19 | 4.44 | 10 | 2.38 |
| Ribonucleoprotein complex biogenesis | 91 | 15.81 | 80 | 11.5 | 70 | 13.43 | 27 | 4.84 |
| Ribonucleoprotein complex subunit organization | 34 | 8.06 | 31 | 5.97 | 20 | 4.52 | 11 | 2.63 |
| Ribonucleotide metabolic process | 18 | 4.82 | 17 | 3.63 | 58 | 3.28 | 36 | 2.17 |
| Ribosome biogenesis | 69 | 15.54 | 60 | 11.38 | 61 | 15.71 | 20 | 4.84 |
| RNA processing | 172 | 13.95 | 180 | 12.43 | 126 | 10.82 | 52 | 3.11 |
| rRNA metabolic process | 47 | 11.72 | 44 | 9.39 | 41 | 11.7 | 17 | 4.98 |
| rRNA processing | 46 | 11.88 | 43 | 9.53 | 40 | 11.8 | 17 | 5.24 |
| Sterol biosynthetic process | 19 | 6.39 | 19 | 5.52 | 9 | 2.52 | 10 | 4.56 |
| Translation | 180 | 21.45 | 157 | 15.28 | 151 | 20.43 | 67 | 9.34 |
| Translational elongation | 75 | 19.97 | 55 | 12.1 | 77 | 23.76 | 32 | 11.05 |
| Translational initiation | 27 | 7.6 | 26 | 6.21 | 23 | 7.41 | 12 | 4.27 |
| Translational termination | 68 | 19.2 | 52 | 12.31 | 74 | 24.37 | 31 | 11.52 |
Specific ontologies were selected after analyses of nontransformed data. Data are reported for designated ontologies with ≥8 genes.
Table 5.
Impact of Serum on Molecular Function Ontologies in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Ontology |
Undifferentiated vs. Serum |
SFM vs. Serum |
||||||
|
Pri Genes |
Pri z-Score |
Imm Genes |
Imm z-Score |
Pri Genes |
Pri z-Score |
Imm Genes |
Imm z-Score |
|
| Upregulation | ||||||||
| Cysteine-type endopeptidase activity | 13 | 2.74 | 12 | 2.57 | 9 | 2.46 | 13 | 3.9 |
| Cytoskeletal protein binding | 70 | 2.76 | 62 | 2.13 | 58 | 4.49 | 56 | 2.99 |
| Enzyme binding | 94 | 2.92 | 93 | 3.49 | 68 | 3.42 | 72 | 2.75 |
| Growth factor activity | 23 | 3.8 | 17 | 2.19 | 23 | 6.15 | 21 | 2.78 |
| Peptidase activity | 78 | 3.38 | 84 | 4.91 | 54 | 3.29 | 68 | 4.5 |
| Downregulation | ||||||||
| DNA-dependent ATPase activity | 16 | 3.38 | 25 | 5.94 | 12 | 2.73 | 8 | 2.17 |
| Electron carrier activity | 31 | 3.28 | 31 | 2.29 | 24 | 2.8 | 16 | 2.21 |
| GTP binding | 60 | 3.67 | 64 | 2.92 | 49 | 3.61 | 29 | 2 |
| GTPase activity | 44 | 4.76 | 41 | 2.95 | 29 | 2.81 | 23 | 3.32 |
| Ligase activity | 64 | 2.46 | 83 | 3.77 | 57 | 3.36 | 37 | 2.43 |
| mRNA binding | 25 | 6.13 | 24 | 4.84 | 21 | 5.96 | 9 | 2.28 |
| Nucleic acid binding | 437 | 7.33 | 492 | 6.55 | 351 | 7.1 | 211 | 3.51 |
| Nucleotide binding | 345 | 8.25 | 389 | 7.76 | 250 | 5.57 | 150 | 2.67 |
| Purine nucleotide binding | 257 | 4.97 | 310 | 5.94 | 191 | 3.46 | 126 | 2.52 |
| Purine ribonucleoside triphosphate binding | 253 | 5.06 | 309 | 6.32 | 187 | 3.44 | 126 | 2.8 |
| Purine ribonucleotide binding | 255 | 4.93 | 309 | 6 | 189 | 3.38 | 126 | 2.6 |
| Ribonucleotide binding | 255 | 4.93 | 309 | 6 | 189 | 3.38 | 126 | 2.6 |
| RNA binding | 251 | 19.9 | 223 | 13.73 | 198 | 17.62 | 89 | 7.18 |
| Structural constituent of ribosome | 109 | 24.09 | 86 | 16.09 | 99 | 25.05 | 38 | 10.23 |
| Structure-specific DNA binding | 36 | 3.92 | 44 | 4.63 | 32 | 4.46 | 18 | 2.47 |
| Transferase activity, transferring alkyl or aryl (other than methyl) groups | 12 | 3.17 | 12 | 2.54 | 13 | 4.65 | 7 | 2.67 |
| Translation factor activity, nucleic acid binding | 33 | 8.51 | 32 | 7.05 | 25 | 7.12 | 12 | 3.46 |
Designated ontologies were chosen after analyses of nontransformed data. Data are reported for selected ontologies with ≥7 genes.
Table 6.
Serum-Induced Alteration of Genes Related to Tissue Development in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Accession # |
Gene |
Ratio |
Ontology |
| Upregulation | |||
| NM_000598 | Insulin-like growth factor binding protein 3 | 54.2, 33.8, 41.3, 20.8 | Regulation of cell growth |
| NM_005046 | Kallikrein-related peptidase 7 | 52.5, 80.9, 4.5, 2.9 | Epidermis development |
| NM_001878 | Cellular retinoic acid binding protein 2 | 44.8, 20.9, 2.0, 3.8 | Epidermis development |
| NM_000900 | Matrix Gla protein | 39.2, 3.5, 33.2, 4.8 | Protein complex assembly |
| NM_001323 | Cystatin E/M | 31.3, 59.2, 3.2, 5.6 | Epidermis development |
| NM_002167 | Inhibitor of DNA binding 3 | 21.5, 28.5, 3.3, 5.5 | Negative regulation of transcription |
| NM_021229 | Netrin 4 | 21.1, 4.2, 6.3, 3.7 | Neuron remodeling |
| NM_172037 | Retinol dehydrogenase 10 | 18.0, 5.2, 8.4, 3.3 | Organ morphogenesis |
| NM_019554 | S100 calcium binding protein A4 | 17.5, 20.8, 8.4, 3.5 | Cell differentiation |
| NM_002166 | Inhibitor of DNA binding 2 | 14.9, 36.8, 5.3, 3.1 | Negative regulation of transcription |
| NM_004936 | Cyclin-dependent kinase inhibitor 2B | 14.4, 15.9, 5.0, 3.7 | Regulation of cyclin-dependent protein kinase activity |
| NM_001901 | Connective tissue growth factor | 14.0, 9.2, 4.6, 10.5 | Angiogenesis |
| NM_001453 | Forkhead box C1 | 11.4, 8.8, 3.2, 3.1 | Blood vessel development |
| NM_000393 | Collagen, type V, α2 | 7.6, 3.9, 2.5, 8.8 | Axon guidance |
| NM_001955 | Endothelin 1 | 6.1, 4.1, 2.0, 4.1 | Prostaglandin biosynthetic process |
| NM_001018004 | Tropomyosin 1α | 4.6, 8.4, 2.1, 6.0 | Cellular component movement |
| NM_000693 | Aldehyde dehydrogenase 1 family, member A3 | 2.9, 2.5, 3.1, 3.5 | Retinoic acid biosynthetic process |
| NM_000362 | TIMP metallopeptidase inhibitor 3 | 2.2, 4.6, 2.8, 2.7 | Central nervous system development |
| NM_001039348 | EGF-containing fibulin-like extracellular matrix protein 1 | 2.1, 2.4, 1.9, 12.6 | Epidermal growth factor receptor signaling pathway |
| NM_000963 | Prostaglandin-endoperoxide synthase 2 | 2.0, 7.2, 5.6, 13.7 | Prostaglandin biosynthetic process |
| Downregulation | |||
| NM_003068 | Snail homolog 2 | 5.6, 4.2, 3.1, 2.2 | Negative regulation of transcription |
| NM_005213 | Cystatin A | 5.2, 3.2, 4.3, 16.9 | Negative regulation of peptidase activity |
| NM_000575 | Interleukin 1α | 4.5, 3.6, 2.2, 2.6 | Inflammatory response |
| NM_033260 | Forkhead box Q1 | 4.2, 5.2, 2.5, 3.7 | DNA fragmentation involved in apoptotic nuclear change |
| NM_006142 | Stratifin | 4.1, 2.3, 2.4, 2.0 | Regulation of cyclin-dependent protein kinase activity |
| NM_000067 | Carbonic anhydrase II | 4.0, 2.6, 13.3, 7.3 | 1-Carbon metabolic process |
| NM_004429 | Ephrin-B1 | 4.0, 2.9, 2.2, 2.2 | Neural crest cell migration |
Relative ratios were calculated by comparing the degree of gene expression in primary and immortalized meibomian gland epithelial cells exposed to serum, compared to the undifferentiated or SFM control groups. The ratios, left to right, are from the following comparisons: primary cells, serum versus undifferentiated; immortalized cells, serum versus undifferentiated; primary cells, serum versus SFM; immortalized cells, serum versus SFM.
Table 7.
Effect of Serum on KEGG Pathways in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Ontology |
Undifferentiated vs. Serum |
SFM vs. Serum |
||||||
|
Pri Genes |
Pri z-Score |
Imm Genes |
Imm z-Score |
Pri Genes |
Pri z-Score |
Imm Genes |
Imm z-Score |
|
| Upregulation | ||||||||
| Lysosome | 35 | 6.5 | 31 | 5.96 | 21 | 4.29 | 17 | 2.29 |
| p53 signaling pathway | 18 | 4.15 | 19 | 5.05 | 12 | 3.26 | 16 | 4.51 |
| Phagosome | 37 | 5.54 | 31 | 4.57 | 25 | 4.45 | 26 | 4.02 |
| Downregulation | ||||||||
| Aminoacyl-tRNA biosynthesis | 12 | 3.1 | 17 | 4.96 | 11 | 3.58 | 7 | 2.82 |
| Cell cycle | 36 | 5.3 | 51 | 8.51 | 24 | 3.43 | 21 | 4.83 |
| Ribosome | 69 | 18.22 | 50 | 11.32 | 74 | 23.08 | 31 | 11.08 |
| RNA transport | 51 | 7.58 | 57 | 8.19 | 40 | 6.71 | 17 | 2.45 |
| Steroid biosynthesis | 10 | 5.04 | 10 | 4.66 | 6 | 3.05 | 6 | 4.43 |
Selected ontologies were chosen after analyses of nontransformed data. Data are reported for designated ontologies with ≥6 genes.
The influence of serum on genes associated with cell differentiation also was quite evident from zsc analyses of biological process, molecular function, and cellular component ontologies in human meibomian gland cells. Compared to undifferentiated or SFM controls, serum induced a significant increase in ontologies related to the endoplasmic reticulum, Golgi apparatus, lysosomes, vesicles, growth factor activity, cell differentiation, epithelium development, and vesicle-mediated transport (Tables 3–5). Conversely, serum also caused a significant decrease in the expression of genes linked mitochondria, ribosomes, cell cycle, mitosis and translation (Tables 3–5). Examples of gene activities in the tissue development ontology that were significantly altered by serum are shown in Table 6.
These differentiative effects were duplicated in another series of experiments, which involved the exposure of immortalized human meibomian gland epithelial cells (n = 3 wells/condition) to serum-containing or serum-free media for 4 days. Serum treatment significantly (P < 0.05) increased ontologies and pathways linked to cytoplasmic vesicles (zsc = 4.7), epithelium development (zsc = 3.3), Golgi apparatus (zsc = 8.4), insulin signaling pathway (zsc = 2.1), lysosomes (zsc = 7.2), vesicle-mediated transport (zsc = 7.0), and autophagy (zsc = 4.0), and decreased those associated with cell cycle (zsc = 16.2), focal adhesion (zsc = 3.6), mitosis (zsc = 15.0), mitochondrion (zsc = 10.0), ribosomes (zsc = 7.1), and translational elongation (zsc = 4.2).
The differentiating impact of serum on primary and immortalized human meibomian gland epithelial cells also was demonstrated by the analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. As shown in Table 7, serum enhanced the activity of lysosome, phagosome and p53 signaling pathways, and decreased those associated with cell cycle and ribosomes.
To verify in part the Illumina BeadChip results, selected genes were analyzed by qPCR. This experimental approach confirmed the regulatory effects of serum on lipocalin 2, claudin 1, ornithine decarboxylase 1, and acetyl-coenzyme A acetyltransferase 2 (Table 8).
Table 8.
Confirmation of Selected BeadChip Gene Expression Results
|
Gene |
Cell |
SFM BeadChip |
SFM qPCR |
Serum BeadChip |
Serum qPCR |
| Upregulation | |||||
| LCN2 | Primary | 6.2 | 7.5 | 72.0 | 161.9 |
| LCN2 | Imm | 22.6 | 34.0 | 21.7 | 40.3 |
| CLDN1 | Primary | 15.1 | 13.9 | 10.2 | 11.5 |
| CLDN1 | Imm | 8.1 | 9.0 | 8.6 | 9.8 |
| Downregulation | |||||
| ODC1 | Primary | 7.8 | 12.1 | 21.9 | 19.7 |
| ODC1 | Imm | 4.5 | 5.9 | 6.9 | 14.6 |
| ACAT2 | Primary | 10.3 | 15.4 | 12.4 | 17.4 |
| ACAT2 | Imm | 4.3 | 3.4 | 7.1 | 9.5 |
The expression of selected genes, that were up- or downregulated by serum exposure in human meibomian gland epithelial cells according to Illumina BeadChip analyses, were reevaluated with qPCR procedures. Numbers represent the relative increase or decrease of gene expression, compared to control values. LCN2, lipocalin 2; CLDN1, claudin 1; ODC1, ornithine decarboxylase 1; ACAT2, acetyl-coenzyme A acetyltransferase 2.
Influence of Serum on Neutral and Polar Lipid Expression in Human Meibomian Gland Epithelial Cells
To examine whether serum-induced differentiation is paralleled by changes in the lipid profile of primary and immortalized human meibomian gland epithelial cells, we analyzed the neutral and polar lipid content in cells after the 2-week culture in SFM or serum-containing medium.
Our results showed that serum exerts quantitative and qualitative alterations in neutral and polar lipid patterns of human meibomian gland epithelial cells. In addition, the nature of the serum-related response was similar in primary and immortalized cells. Serum exposure was associated with a significant (P < 0.05, 1-tail) 34% increase in cholesterol levels (primary [P] + immortalized [I] SFM = 189.1 ± 24.5 ng, P + I serum = 253.4 ± 39.9 ng). Similarly, serum treatment led up to 34-fold differences in the amount of various fatty acids in wax and cholesterol esters (Table 9).
Table 9.
Influence of Serum on Wax and Cholesterol Ester Fatty Acid Expression in Primary and Immortalized Human Meibomian Gland Epithelial Cells
|
Fatty Acid |
Serum-Free Media |
Serum-Containing Media |
||
|
Primary Cells |
Immortalized Cells |
Primary Cells |
Immortalized Cells |
|
| Wax ester | ||||
| 33:1 | 587.0, 476.6 | 707.2, 897.2 | 166.7, 115.8 | 291.8, 236.1 |
| 41:3 | 58.0, 41.9 | 24.3, 25.2 | 162.9, 92.4 | 78.7, 121.5 |
| 42:2 | 56.4, 84.2 | 37.7, 67.2 | 234.5, 205.0 | 291.4, 292.7 |
| Cholesterol ester | ||||
| 20:3 | 89.0, 216.2 | 166.2, 176.8 | 854.7, 1023.9 | 818.1, 1216.9 |
| 20:4* | 70.4, 364.5* | 220.6, 470.3* | 744.7, 1152.0* | 887.9, 1192.5* |
| 22:4 | 1.0, 8.5 | 6.8, 5.7 | 148.0, 173.7 | 154.8, 279.6 |
Primary and immortalized human meibomian gland epithelial cells (n = 2 samples/condition) were cultured serum-free or serum-containing media and then processed for the isolation of lipids and the analysis of fatty acids in wax and cholesterol esters. The lipid weights (mg) of each sample were: primary cells, serum-free = 2.6 and 1.5; immortalized cells, serum-free = 2.4 and 3.4; primary cells, serum-containing = 2.8 and 1.8; immortalized cells, serum-containing = 2.7 and 3.2. The numbers in the “Fatty Acid” column, such as 33:1, take the form C:D, where C is the number of carbon atoms in the fatty acids (i.e., 33) and D is the number of double bonds (i.e., 1). The numbers in the cell columns represent the amount (pg) of designated wax and cholesterol ester fatty acids in each sample/condition.
The fatty acid (20:4) reflects arachidonic acid.
The response to serum-induced differentiation appeared to be most pronounced in cellular phospholipids. As shown in Table 10, primary and immortalized human meibomian gland epithelial cells contained many different phospholipids, including phosphatidylcholine, sphingomyelin, lysophosphatidylethanolamine, lysophosphatidylserine, phosphatidylserine, phosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, phosphatidic acid, phosphatidylglycerol, lysophosphatidylcholine, lysophosphatidylinositol, and phosphatidylinositol. Phosphatidylcholine was expressed at the highest level (i.e., 47%–57% of total measured phospholipids). Culture of cells in serum resulted in numerous alterations in the type and amount of fatty acids in most of the phospholipid species. Two very consistent changes were the often dramatically increased levels (e.g., up to 109-fold greater) of polyunsaturated fatty acids, as well as arachidonic acid, in differentiated cells (Table 11).
Table 10.
Phospholipid Expression in Human Primary and Immortalized Meibomian Gland Epithelial Cells In Vitro
|
Phospholipid |
Serum-Free Media |
Serum-Containing Media |
||
|
Primary Cells |
Immortalized Cells |
Primary Cells |
Immortalized Cells |
|
| Phosphati-dylcholine | 57.00, 52.35 | 49.43, 43.93 | 55.97, 50.63 | 47.04, 44.97 |
| Sphingomyelin | 19.93, 16.91 | 20.55, 20.47 | 13.49, 15.45 | 18.47, 17.95 |
| Lysophosphati-dylethanolamine | 11.24, 13.16 | 11.98, 15.48 | 14.13, 15.30 | 14.77, 16.89 |
| Lysophospha-tidylserine | 3.70, 5.01 | 5.61, 7.03 | 6.45, 6.76 | 7.24, 7.97 |
| Phosphatidyl-serine | 2.39, 4.69 | 4.17, 4.53 | 4.05, 5.27 | 5.47, 4.91 |
| Phosphatidyl-ethanolamine | 2.47, 3.63 | 3.29, 3.05 | 2.59, 3.60 | 3.02, 2.68 |
| Lysophosphati-dylglycerol | 1.55, 2.26 | 2.46, 2.55 | 1.94, 1.29 | 1.51, 2.67 |
| Lysophosphatidic acid | 1.27, 1.17 | 1.41, 1.46 | 0.91, 1.12 | 1.57, 1.22 |
| Phosphatidic acid | 0.41, 0.70 | 0.97, 1.31 | 0.41, 0.51 | 0.84, 0.63 |
| Phosphati-dylglycerol | 0.05, 0.13 | 0.13, 0.19 | 0.08, 0.07 | 0.07, 0.10 |
Primary and immortalized human meibomian gland epithelial cells (n = 2 samples/condition) were cultured serum-free or containing media and then processed for the analysis of phospholipids. The numbers represent the percentage expression of a given phospholipid in the total phospholipid amount of each sample/condition. The total amount was determined by calculating and adding the picogram quantities of phospholipids shown in this table. Lysophosphatidylcholine, lysophosphatidylinositol, and phosphatidylinositol also were identified in the cellular samples. However, without internal standards for these species, their picogram amounts could not be calculated.
Table 11.
Effect of Serum Exposure on Phospholipid Fatty Acid Content in Human Primary and Immortalized Meibomian Gland Epithelial Cells
|
Phospholipid |
Serum-Free Media |
Serum-Containing Media |
||
|
Primary Cells |
Immortalized Cells |
Primary Cells |
Immortalized Cells |
|
| Phosphatidylcholine | ||||
| 38:2 | 2480, 2976 | 2798, 3794 | 1,795, 828.2 | 1,687, 1,168 |
| 38:4* | 567.2, 1206* | 699.4, 647.2* | 14,642, 9,083* | 5,157, 5,304* |
| 38:5 | 277.4, 629.3 | 338.3, 292.5 | 10,131, 6,107 | 4,206, 4,535 |
| 38:6 | 97.2, 156.1 | 66.0, 59.9 | 2,565, 1,674 | 1,169, 1,115 |
| Lysophosphatidylethanolamine | ||||
| 20:4* | 2155, 5363* | 2192, 3504* | 19,861, 12,622* | 11,603, 15,859* |
| 22:2 | 204.2, 386.6 | 364.0, 1091 | 75.6, 45.7 | 104.5, 77.5 |
| 22:4 | 312.6, 811.0 | 585.6, 1457 | 3,154, 2,254 | 2,220, 3,016 |
| 22:6 | 456.0, 1080 | 479.1, 916.8 | 21,845, 16,015 | 12,697, 14,728 |
| Lysophosphatidylserine | ||||
| 22:5 | 193.3, 521.9 | 240.6, 481.3 | 2,080, 1,383 | 1,449, 1,585 |
| 22:6 | 75.8, 222.9 | 103.6, 198.4 | 3,872, 2,435 | 2,111, 2,756 |
| Phosphatidylserine | ||||
| 40:6 | 56.7, 235.0 | 107.5, 160.8 | 2,836, 2,301 | 1,866, 1,897 |
| 40:7 | 17.8, 51.3 | 66.7, 25.0 | 221.3, 213.1 | 164.3, 131.4 |
| 42:9 | 0.4, 1.8 | 0.5, 0.8 | 118.8, 104.9 | 81.9, 79.6 |
| Phosphatidylethanolamine | ||||
| 38:2 | 312.8, 569.5 | 440.2, 618.0 | 167.0, 142.5 | 203.1, 118.4 |
| 38:4* | 330.0, 975.4* | 365.8, 404.6* | 2,970, 2,792* | 1,810, 1,753* |
| 38:6 | 30.7, 73.2 | 26.1, 26.9 | 551.7, 571.7 | 329.5, 321.0 |
| 40:7 | 17.0, 49.9 | 35.3, 35.3 | 925.7, 898.9 | 685.5, 616.5 |
| Lysophosphatidylglycerol | ||||
| 20:2 | 212.4, 823.6 | 585.1, 973.0 | 3,608, 1,574 | 1,550, 3,348 |
| 20:4* | 39.2, 131.8* | 119.3, 155.1* | 642.5, 277.9* | 232.3, 569.1* |
| 22:5 | 21.8, 85.0 | 24.7, 39.6 | 530.4, 205.6 | 232.2, 490.5 |
| 22:6 | 19.3, 75.2 | 18.8, 37.2 | 2,261, 1,029 | 970.4, 2,117 |
| Phosphatidylglycerol | ||||
| 40:7 | 0.6, 4.8 | 1.7, 3.9 | 117.1, 53.6 | 51.7, 97.0 |
Primary and immortalized human meibomian gland epithelial cells (n = 2 samples/condition) were cultured serum-free or serum-containing media and then processed for the analysis of fatty acids in the phospholipids. The numbers in the cell columns equal the amount (pg) of selected phospholipid fatty acids in each sample/condition.
The fatty acids represent (20:4), or may contain (38:4), arachidonic acid.
All our neutral and polar lipid raw data are shown in the Supplementary Tables.
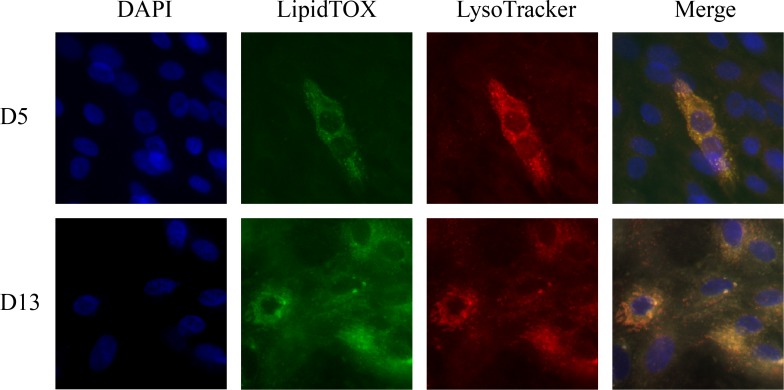
The exposure of human meibomian gland epithelial cells to serum, as we previously discovered,11 was associated with the acquisition of lipid-containing vesicles. As demonstrated in the Figure, much of this lipid accumulation appeared to occur within lysosomes.
Figure.
Lipid accumulation in immortalized human meibomian gland epithelial cells. Cells were cultured in serum for 5 or 13 days, and then processed for the identification of neutral lipids with LipidTOX, lysosomes with LysoTracker Red DND-99, and nuclei with DAPI. The figures were overlaid with Adobe Photoshop (Adobe Systems, San Jose, CA, USA) to create the “Merge” figure.
Discussion
Our results supported our hypothesis that serum stimulates the differentiation of human meibomian gland epithelial cells in vitro. This serum-induced effect is associated with a significant increase in the expression of genes linked to cell differentiation, epithelium development, the endoplasmic reticulum, Golgi apparatus, vesicles, and lysosomes, and a significant decrease in gene activity related to the cell cycle, mitochondria, ribosomes, and translation. These cellular responses are accompanied by an accumulation of lipid-containing organelles (e.g., lysosomes), as well as alterations in the fatty acid content of neutral and polar lipids. Of particular importance, our results showed that the molecular and biochemical changes of immortalized human meibomian gland epithelial cells during differentiation are similar to those of primary cells. Overall, our findings indicated that immortalized human meibomian gland epithelial cells may serve as an ideal preclinical model to identify factors that control cell differentiation in vitro.
Our hypothesis that exposure of human meibomian gland epithelial cells to serum would induce differentiation, compared to proliferation, was prompted by several considerations. First, serum has been shown to promote the differentiation of other epithelial cell types, including those from human foreskin, mammary, bronchial, and corneal tissues.18–23 This developmental process is associated with an upregulation of genes related to cell differentiation and tissue development, and a downregulation of genes linked to cell cycle and cell division.24,25 Second, serum is known to reduce or inhibit the proliferation of many types of epithelial cells, including those from the trachea, bronchus, mammary gland, urothelium, conjunctiva, and oral mucosa.18–20,26–28 We also recently have found that serum decreases the proliferation of primary and immortalized human meibomian gland epithelial cells, compared to that of cells cultured in SFM.10,11 Third, we have discovered that serum treatment increases the accumulation of neutral lipids in immortalized human meibomian gland epithelial cells.10,11 This response is characteristic of differentiation in other sebaceous gland epithelial cells,11,29,30 as well as in adipocytes.31,32 Of interest, we observed in the present study that lipid accumulation in differentiated meibomian gland epithelial cells appears to occur, at least in part, in lysosomes.
The serum-induced differentiation of human meibomian gland epithelial cells is associated not only with an increased expression of tissue development genes, but also those associated with anti-inflammatory and antibacterial activities. For example, serum upregulates the gene encoding secretoglobin, family 1A, member 1 (i.e., uteroglobin), which suppresses inflammation,33 and downregulates the gene for interleukin 1α, a proinflammatory cytokine. In addition, serum enhances the expression of genes for phospholipase A2, group XVI, and CCL28. Phospholipase A2 kills gram-positive bacteria and is a key bactericide in human tears.34 Also, CCL28 has antimicrobial activity against gram-positive and gram-negative bacteria, and Candida albicans.35 These transcripts, if translated, could contribute to the absence of inflammation and bacterial invasion within the meibomian gland in obstructive MGD.14,36–38
It also is of interest that CCL28 attracts IgA-positive cells to mucosal tissues.39 Given that serum stimulates pIgR gene expression in primary human meibomian gland epithelial cells, it is possible that these differentiated cells could have a role in transporting polymeric IgA antibodies to the ocular surface and protecting against microbial infection. In support of this concept, we previously have identified IgA α chain in human meibomian gland secretions,40 and others have shown that sebaceous glands can secrete IgA.41
A particularly intriguing finding was the observation that serum upregulates the expression of genes encoding netrin 4 and collagen, type V, α2. These proteins are involved with neuron remodeling and axon guidance, and, if translated and secreted in vivo during cellular differentiation, could possibly influence meibomian gland innervation. The meibomian gland is the only sebaceous gland that is heavily innervated,5 and human meibomian gland epithelial cells are responsive to neurotransmitters.42 However, essentially nothing is known about the control of meibomian gland innervation in vivo.
We discovered that the process of differentiation is associated with significant alterations in the amount of cholesterol, as well as the fatty acid profiles of wax esters, cholesterol esters and phospholipids, in both primary and immortalized human meibomian gland epithelial cells. That such a response can occur is not surprising, given that meibomian glands contain the transcripts necessary for all the key lipogenic enzymes involved in cholesterol and fatty acid synthesis.43,44 Further, the expression of these enzymatic mRNAs is known to be significantly increased by factors (e.g., androgens) that promote differentiated functions in meibomian gland epithelial cells.43–46
It is possible that the quantitative and qualitative changes in fatty acid levels during cellular differentiation might be influenced by the relative activity of several enzymes. These include: phospholipase A2, group XVI, which catalyzes the release of fatty acids from phosphatidylcholine and phosphatidylethanolamine33; secretoglobin, family 1A, member 1, which may inhibit phospholipase A233; and prostaglandin-endoperoxide synthase 1 and 2, also known as cyclooxygenase (COX) 1 and 2, which convert free arachidonic acid after its release from membrane phospholipids by phospholipase A2 to prostaglandin H2.33 The gene expression for these enzymes is significantly altered during serum-stimulated differentiation of human meibomian gland epithelial cells.
Two of the most striking changes during cellular differentiation were the increases in arachidonic acid content in, and polyunsaturation of, phospholipid fatty acids. If released by the action of phospholipase A2, arachidonic acid could serve as the precursor of many biologically active products (i.e., eicosanoids) that modulate epithelial cell growth and differentiation.47,48 Arachidonic acid also may be metabolized by 5-lipoxygenase, ultimately yielding leukotriene B4 (LTB4).47–49 The LTB4 is secreted by human meibomian gland epithelial cells in response to bacterial toxin exposure50 and theoretically could promote ocular surface inflammation. It remains to be determined whether these changes in arachidonic acid levels also occur in vivo during differentiation. It is possible that they reflect increased arachidonic acid uptake from the serum-containing media in vitro.51–53 As concerns the increased polyunsaturation, it is of interest that a decreased unsaturation of nonpolar fatty acids has been associated with MGD.54–56
The human meibomian gland epithelial cell lipids detected in our study do not reflect the levels of neutral and polar lipids typically found in human meibum.2,57–60 The reason is that we analyzed cellular extracts, not meibum, and the lipid components of these products are not necessarily the same. To explain, the meibomian gland secretes through a holocrine process, which involves disintegration of the whole cell and secretion of the entire cell components into the lateral ductile and, ultimately, the central and terminal ducts.1,5–9 This would include the membranes, which are enriched in phospholipids. However, ductal cells, as in other exocrine glands, often alter the content of their luminal secretions. Such ductal activity also has been proposed for sebaceous glands.61 More specifically, Thiboutot speculated that phospholipids are recycled in sebaceous gland ducts after holocrine secretion, resulting in the delivery of predominantly neutral lipids to the skin.61 This recycling process also may occur in the meibomian gland, which would explain, for example, why meibum is depleted of phospholipids.2,57–59 Further research is required to determine the precise role of ductal cells in modulating the lipid content in the human meibomian gland.
Lastly, our study demonstrated that the molecular and biochemical changes of immortalized human meibomian gland epithelial cells during differentiation are similar to those of primary cells. This observation is particularly important, given that other immortalized human ocular surface cells do not necessarily mimic their primary cell counterparts, and that their responses may not be physiologically relevant62,63 Overall, our results indicated that immortalized human meibomian gland epithelial cells may serve as an ideal preclinical model to identify factors that control cell differentiation.
Acknowledgments
The authors express their appreciation to Barbara and James E. Evans (Worcester, Massachusetts, United States) for their technical assistance.
Supported by Grant EY05612 from the National Institutes of Health (Bethesda, Maryland, United States) and grants from Alcon Research, Ltd. (Houston, Texas, USA), the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao & Yang Liu Research Fund.
Disclosure: D.A. Sullivan, Alcon Research, Ltd. (F); Y. Liu, None; W.R. Kam, None; J. Ding, None; K.M. Green, None; S.A. Shaffer, None; M.P. Hatton, None; S. Liu, None
References
- 1. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci. 2011; 52: 1938–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the Subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011; 52: 1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003; 1: 107–126 [DOI] [PubMed] [Google Scholar]
- 4. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011; 52: 1922–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989; 69: 383–416 [DOI] [PubMed] [Google Scholar]
- 6. Sirigu P, Shen RL. Pinto da Silva P. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. Invest Ophthalmol Vis Sci. 1992; 33: 2284–2292 [PubMed] [Google Scholar]
- 7. Mesquita-Guimaraes J, Coimbra A. Holocrine cell lysis in the rat preputial sebaceous gland. Evidence of autophagocytosis during cell involution. Anat Rec. 1976; 186: 49–68 [Google Scholar]
- 8. Mesquita-Guimaraes J, Pignatelli D, Coimbra A. Autophagy during holocrine cell lysis in skin sebaceous glands. J Submicrocs Cytol. 1979; 11: 435–447 [Google Scholar]
- 9. Brandes D, Bertini F, Smith EW. Role of lysosomes in cellular lytic processes. II. Cell death during holocrine secretion in sebaceous cells. Exp Mol Pathol. 1965; 4: 245–265 [DOI] [PubMed] [Google Scholar]
- 10. Liu S, Khandelwal P, Hatton M, Sullivan DA. Culture, immortalization and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 3993–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S, Kam W, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013; 54: 2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chazotte B. Labeling lysosomes in live cells with LysoTracker. Cold Spring Harb Protoc. 2011; 2: pdb.prot5571 [DOI] [PubMed] [Google Scholar]
- 13. Folch J, Lees M. Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226: 497–509 [PubMed] [Google Scholar]
- 14. Liu S, Richards SM, Lo K, Hatton M, Fay AM, Sullivan DA. Changes in gene expression in meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011; 52: 2727–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi L, Reid LH, Jones WD, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nature Biotechnol. 2006; 24: 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature Genet. 2000; 25: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003; 4: R7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wille JJ Jr, Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984; 121: 31–44 [DOI] [PubMed] [Google Scholar]
- 19. Bertolero F, Kaighn ME, Camalier RF, Saffiotti U. Effects of serum and serum-derived factors on growth and differentiation of mouse keratinocytes. In Vitro Cell Dev Biol. 1986; 22: 423–428 [DOI] [PubMed] [Google Scholar]
- 20. Lechner JF, Haugen A, McClendon IA, Shamsuddin AM. Induction of squamous differentiation of normal human bronchial epithelial cells by small amounts of serum. Differentiation. 1984; 25: 229–237 [DOI] [PubMed] [Google Scholar]
- 21. Ang LP, Tan DT, Beuerman RW, Lavker RM. Development of a conjunctival epithelial equivalent with improved proliferative properties using a multistep serum-free culture system. Invest Ophthalmol Vis Sci. 2004; 45: 1789–1795 [DOI] [PubMed] [Google Scholar]
- 22. Hesterberg TW, Maness SC, Iglehart JD, Sanchez JH, Boreiko CJ. Subpopulations of human bronchial epithelial cells in culture respond heterogeneously to 12-O-tetradecanoylphorbol-13-acetate (TPA) and other modulators of differentiation. Carcinogenesis. 1987; 8: 1511–1515 [DOI] [PubMed] [Google Scholar]
- 23. Lu R, Bian F, Zhang X, et al. The beta-catenin/Tcf4/survivin signaling maintains a less differentiated phenotype and high proliferative capacity of human corneal epithelial progenitor cells. Int J Biochem Cell Biol. 2011; 43: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ceder R, Haig Y, Merne M, et al. Differentiation-promoting culture of competent and noncompetent keratinocytes identifies biomarkers for head and neck cancer. Am J Pathol. 2012; 180: 457–472 [DOI] [PubMed] [Google Scholar]
- 25. Taylor JM, Street TL, Hao L, et al. Dynamic and physical clustering of gene expression during epidermal barrier formation in differentiating keratinocytes. PLoS One. 2009; 4: e7651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci U S A. 1984; 81: 5435–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomassen DG, Saffiotti U, Kaighn ME. Clonal proliferation of rat tracheal epithelial cells in serum-free medium and their responses to hormones, growth factors and carcinogens. Carcinogenesis. 1986; 7: 2033–2039 [DOI] [PubMed] [Google Scholar]
- 28. Costea DE, Dimba AO, Loro LL, Vintermyr OK, Johannessen AC. The phenotype of in vitro reconstituted normal human oral epithelium is essentially determined by culture medium. J Oral Pathol Med. 2005; 34: 247–252 [DOI] [PubMed] [Google Scholar]
- 29. Ito A, Sakiguchi T, Kitamura K, Akamatsu H, Horio T. Establishment of a tissue culture system for hamster sebaceous gland cells. Dermatology. 1998; 197: 238–244 [DOI] [PubMed] [Google Scholar]
- 30. Xia LQ, Zouboulis C, Detmar M, Mayer-da-Silva A, Stadler R, Orfanos CE. Isolation of human sebaceous glands and cultivation of sebaceous gland-derived cells as an in vitro model. J Invest Dermatol. 1989; 93: 315–321 [PubMed] [Google Scholar]
- 31. Harrison WJ, Bull JJ, Seltmann H, Zouboulis CC, Philpott MP. Expression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytes. J Invest Dermatol. 2007; 127: 1309–1317 [DOI] [PubMed] [Google Scholar]
- 32. Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A. 1978; 75: 6107–6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. GeneCards. Available at: http://www.genecards.org/. Accessed October 2, 2013 [Google Scholar]
- 34. Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998; 66: 2791–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hieshima K, Ohtani H, Shibano M, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003; 170: 1452–1461 [DOI] [PubMed] [Google Scholar]
- 36. Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982; 94: 383–387 [DOI] [PubMed] [Google Scholar]
- 37. Obata H, Horiuchi H, Miyata K, Tsuru T, Machinami R. Histopathological study of the meibomian glands in 72 autopsy cases [in Japanese]. Nippon Ganka Gakkai Zasshi. 1994; 98: 765–771 [PubMed] [Google Scholar]
- 38. Straatsma BR. Cystic degeneration of the meibomian glands. AMA Arch Ophthalmol. 1959; 61: 918–927 [DOI] [PubMed] [Google Scholar]
- 39. Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2006; 25: 5467–5484 [DOI] [PubMed] [Google Scholar]
- 40. Tsai P, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006; 90: 372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Metze D, Jurecka W, Gebhart W, et al. Immunohistochemical demonstration of immunoglobulin A in human sebaceous and sweat glands. J Invest Dermatol. 1989; 92: 13–17 [DOI] [PubMed] [Google Scholar]
- 42. Kam WR, Sullivan DA. Neurotransmitter activation of signaling pathways in human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2011; 52: 8542–8548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp Eye Res. 2006; 83: 291–296 [DOI] [PubMed] [Google Scholar]
- 44. Schirra F, Richards SM, Sullivan DA. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Curr Eye Res. 2007; 32: 393–398 [DOI] [PubMed] [Google Scholar]
- 45. Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009; 10 15; 1553–1572 [PMC free article] [PubMed] [Google Scholar]
- 46. Khandelwal P, Liu S, Sullivan DA. Dihydrotestosterone regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis. 2012; 18: 1055–1067 [PMC free article] [PubMed] [Google Scholar]
- 47. Cabral M, Martín-Venegas R, Moreno JJ. Role of arachidonic acid metabolites on the control of non-differentiated intestinal epithelial cell growth. Int J Biochem Cell Biol. 2013; 45: 1620–1628 [DOI] [PubMed] [Google Scholar]
- 48. Ferrer R, Moreno JJ. Role of eicosanoids on intestinal epithelial homeostasis. Biochem Pharmacol. 2010; 80: 431–438 [DOI] [PubMed] [Google Scholar]
- 49. Jakschik BA, Kuo CG. Characterization of leukotriene A4 and B4 biosynthesis. Prostaglandins. 1983; 25: 767–782 [DOI] [PubMed] [Google Scholar]
- 50. Sahin A, Kam WR. Rahimi Darabad R, Topilow K, Sullivan DA. Regulation of leukotriene B4 secretion by human corneal, conjunctival and meibomian gland epithelial cells. Arch Ophthalmol. 2012; 130: 1013–1018 [DOI] [PubMed] [Google Scholar]
- 51. Pazouki S, Baty JD, Wallace HM, Coleman CS. Utilization of extracellular lipids by HT29/219 cancer cells in culture. Lipids. 1992; 27: 827–834 [DOI] [PubMed] [Google Scholar]
- 52. Duval D, Huneau JF, Homo-Delarche F. Effect of serum on the metabolism of exogenous arachidonic acid by phagocytic cells of the mouse thymic reticulum. Prostaglandins Leukot Med. 1986; 23: 67–83 [DOI] [PubMed] [Google Scholar]
- 53. Yorek MA, Figard PH, Kaduce TL, Spector AA. A comparison of lipid metabolism in two human retinoblastoma cell lines. Invest Ophthalmol Vis Sci. 1985; 26: 1148–1154 [PubMed] [Google Scholar]
- 54. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991; 32: 2272–2280 [PubMed] [Google Scholar]
- 55. Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993; 34: 3515–3521 [PubMed] [Google Scholar]
- 56. Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000; 19: 72–74 [DOI] [PubMed] [Google Scholar]
- 57. Brown SH, Kunnen CM, Duchoslav E, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013; 54: 7417–7424 [DOI] [PubMed] [Google Scholar]
- 58. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass spectrometric analysis and structural elucidation. J Lipid Res. 2007; 48: 2220–2235 [DOI] [PubMed] [Google Scholar]
- 59. Lam SM, Tong L, Yong SS, et al. Meibum lipid composition in Asians with dry eye disease. PLoS One. 2011; 6: e24339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saville JT, Zhao Z, Willcox MD, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp Eye Res. 2011; 92: 238–240 [DOI] [PubMed] [Google Scholar]
- 61. Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004; 123: 1–12 [DOI] [PubMed] [Google Scholar]
- 62. Suzuki T, Sullivan DA. Estrogen stimulation of proinflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea. 2005; 24: 1004–1009 [DOI] [PubMed] [Google Scholar]
- 63. Suzuki T, Sullivan DA. Comparative effects of estrogen on matrix metalloproteinases and cytokines in immortalized and primary human corneal epithelial cell cultures. Cornea. 2006; 25: 454–459 [DOI] [PubMed] [Google Scholar]