Abstract
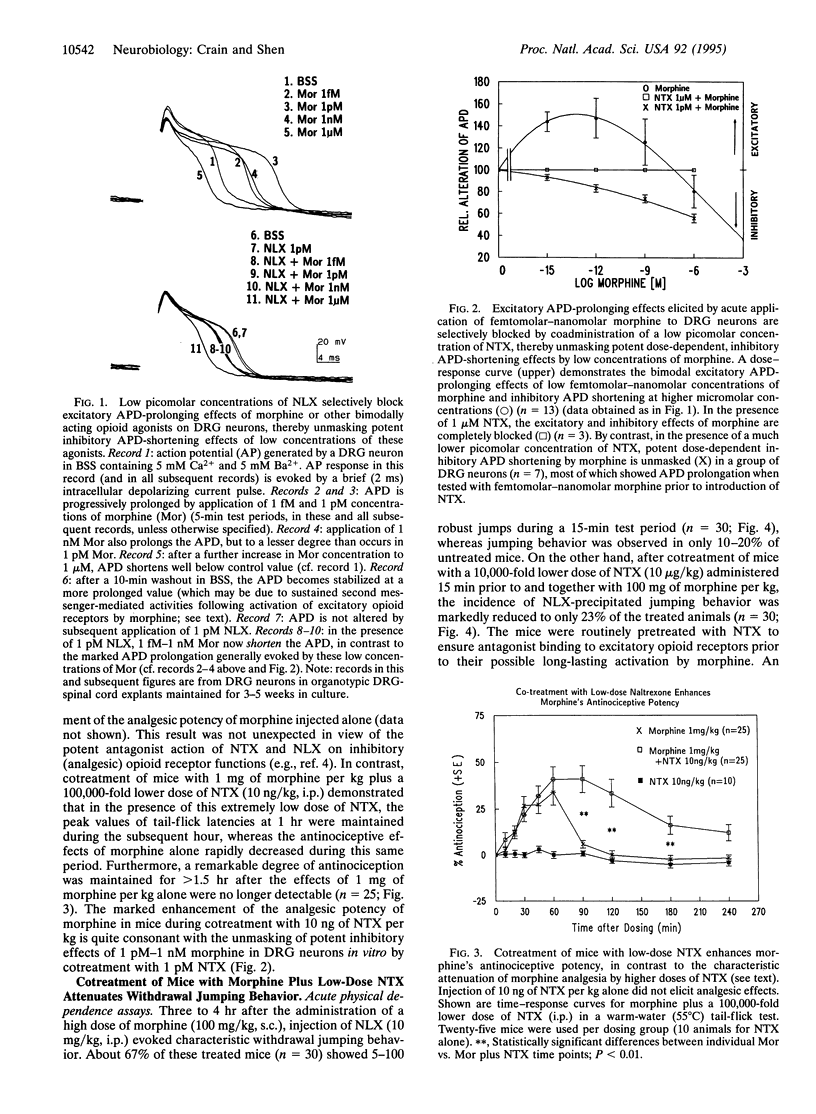
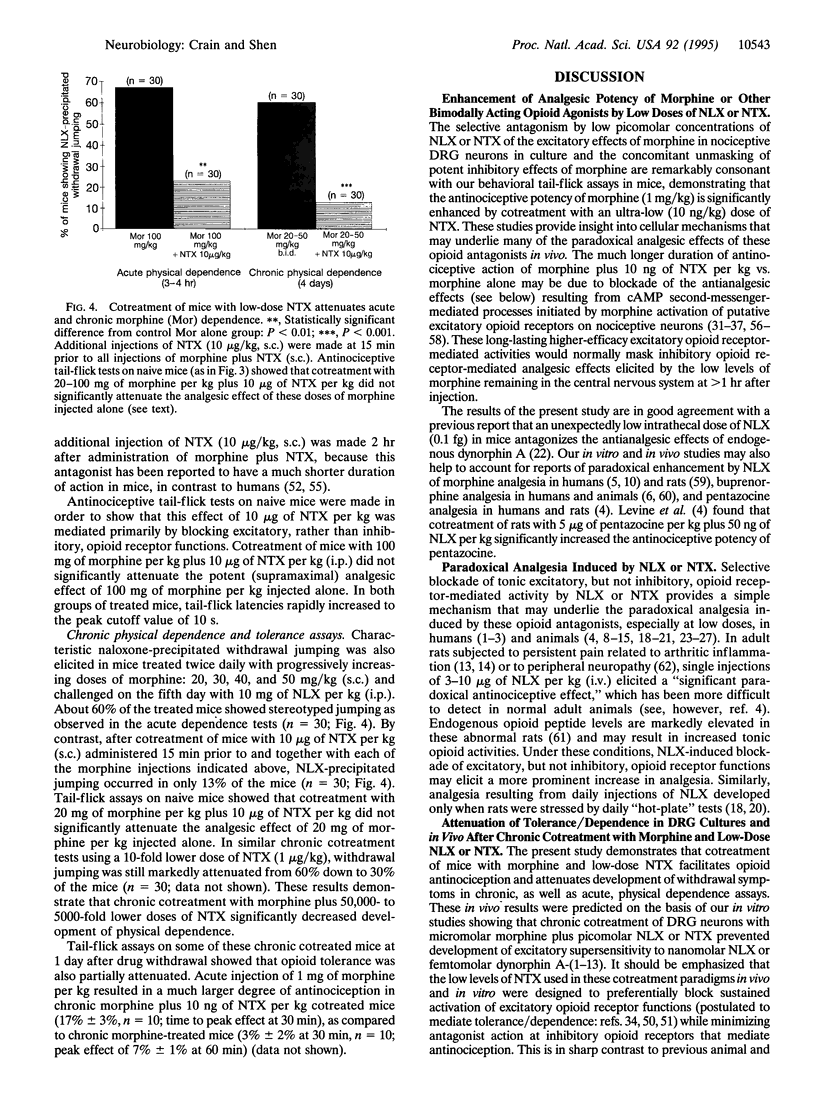
Ultra-low picomolar concentrations of the opioid antagonists naloxone (NLX) and naltrexone (NTX) have remarkably potent antagonist actions on excitatory opioid receptor functions in mouse dorsal root ganglion (DRG) neurons, whereas higher nanomolar concentrations antagonize excitatory and inhibitory opioid functions. Pretreatment of naive nociceptive types of DRG neurons with picomolar concentrations of either antagonist blocks excitatory prolongation of the Ca(2+)-dependent component of the action potential duration (APD) elicited by picomolar-nanomolar morphine and unmasks inhibitory APD shortening. The present study provides a cellular mechanism to account for previous reports that low doses of NLX and NTX paradoxically enhance, instead of attenuate, the analgesic effects of morphine and other opioid agonists. Furthermore, chronic cotreatment of DRG neurons with micromolar morphine plus picomolar NLX or NTX prevents the development of (i) tolerance to the inhibitory APD-shortening effects of high concentrations of morphine and (ii) supersensitivity to the excitatory APD-prolonging effects of nanomolar NLX as well as of ultra-low (femtomolar-picomolar) concentrations of morphine and other opioid agonists. These in vitro studies suggested that ultra-low doses of NLX or NTX that selectively block the excitatory effects of morphine may not only enhance the analgesic potency of morphine and other bimodally acting opioid agonists but also markedly attenuate their dependence liability. Subsequent correlative studies have now demonstrated that cotreatment of mice with morphine plus ultra-low-dose NTX does, in fact, enhance the antinociceptive potency of morphine in tail-flick assays and attenuate development of withdrawal symptoms in chronic, as well as acute, physical dependence assays.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arts K. S., Fujimoto J. M., Crain S. M. Inhibition of the antianalgesic action of dynorphin A in mice by cholera toxin. Pharmacol Biochem Behav. 1993 Nov;46(3):623–629. doi: 10.1016/0091-3057(93)90553-6. [DOI] [PubMed] [Google Scholar]
- Attal N., Kayser V., Jazat F., Guilbaud G. Behavioural evidence for a bidirectional effect of systemic naloxone in a model of experimental neuropathy in the rat. Brain Res. 1989 Aug 14;494(2):276–284. doi: 10.1016/0006-8993(89)90596-9. [DOI] [PubMed] [Google Scholar]
- Bergman S. A., Wynn R. L., Myers D. E., Rudo F. G. Low dose naloxone enhances buprenorphine in a tooth pulp antinociceptive assay. Arch Int Pharmacodyn Ther. 1988 Jan-Feb;291:229–237. [PubMed] [Google Scholar]
- Bhargava H. N. Diversity of agents that modify opioid tolerance, physical dependence, abstinence syndrome, and self-administrative behavior. Pharmacol Rev. 1994 Sep;46(3):293–324. [PubMed] [Google Scholar]
- Bhargava H. N. The effects of naltrexone on the development of physical dependence on morphine. Eur J Pharmacol. 1978 Aug 1;50(3):193–202. doi: 10.1016/0014-2999(78)90351-5. [DOI] [PubMed] [Google Scholar]
- Brase D. A., Iwamoto E. T., Loh H. H., Way E. L. Reinitiation of sensitivity to naloxone by a single narcotic injection in postaddicted mice. J Pharmacol Exp Ther. 1976 May;197(2):317–325. [PubMed] [Google Scholar]
- Buchsbaum M. S., Davis G. C., Bunney W. E., Jr Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature. 1977 Dec 15;270(5638):620–622. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- Cappell H., Poulos C. X., Lê A. D. Enhancement of naloxone-induced analgesia by pretreatment with morphine. Pharmacol Biochem Behav. 1989 Oct;34(2):425–427. doi: 10.1016/0091-3057(89)90337-7. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A., Crain S. M. Maturation of opioid sensitivity of fetal mouse dorsal root ganglion neuron perikarya in organotypic cultures: regulation by spinal cord. Neuroscience. 1986 Apr;17(4):1181–1198. doi: 10.1016/0306-4522(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. After GM1 ganglioside treatment of sensory neurons naloxone paradoxically prolongs the action potential but still antagonizes opioid inhibition. J Pharmacol Exp Ther. 1992 Jan;260(1):182–186. [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. After chronic opioid exposure sensory neurons become supersensitive to the excitatory effects of opioid agonists and antagonists as occurs after acute elevation of GM1 ganglioside. Brain Res. 1992 Mar 13;575(1):13–24. doi: 10.1016/0006-8993(92)90417-8. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Shen K. F., Chalazonitis A. Opioids excite rather than inhibit sensory neurons after chronic opioid exposure of spinal cord-ganglion cultures. Brain Res. 1988 Jul 5;455(1):99–109. doi: 10.1016/0006-8993(88)90118-7. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990 Feb;11(2):77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Cruciani R. A., Dvorkin B., Morris S. A., Crain S. M., Makman M. H. Direct coupling of opioid receptors to both stimulatory and inhibitory guanine nucleotide-binding proteins in F-11 neuroblastoma-sensory neuron hybrid cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3019–3023. doi: 10.1073/pnas.90.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson A. H., Le Bars D., Besson J. M. Endogenous opiates and nociception: a possible functional role in both pain inhibition and detection as revealed by intrathecal naloxone. Neurosci Lett. 1981 Jul 2;24(2):161–164. doi: 10.1016/0304-3940(81)90241-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto J. M., Rady J. J. Intracerebroventricular physostigmine-induced analgesia: enhancement by naloxone, beta-funaltrexamine and nor-binaltorphimine and antagonism by dynorphin A (1-17). J Pharmacol Exp Ther. 1989 Dec;251(3):1045–1052. [PubMed] [Google Scholar]
- Gillman M. A., Lichigjeld F. J. Naloxone analgesia: an update. Int J Neurosci. 1989 Oct;48(3-4):321–324. doi: 10.3109/00207458909002178. [DOI] [PubMed] [Google Scholar]
- Gillman M. A., Lichtigfeld F. J. A comparison of the effects of morphine sulphate and nitrous oxide analgesia on chronic pain states in man. J Neurol Sci. 1981 Jan;49(1):41–45. doi: 10.1016/0022-510x(81)90186-6. [DOI] [PubMed] [Google Scholar]
- Gillman M. A., Lichtigfeld F. J. A pharmacological overview of opioid mechanisms mediating analgesia and hyperalgesia. Neurol Res. 1985 Sep;7(3):106–119. doi: 10.1080/01616412.1985.11739709. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. P., Brogden R. N. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988 Mar;35(3):192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Greeley J. D., Lê A. D., Poulos C. X., Cappell H. "Paradoxical" analgesia induced by naloxone and naltrexone. Psychopharmacology (Berl) 1988;96(1):36–39. doi: 10.1007/BF02431530. [DOI] [PubMed] [Google Scholar]
- Hamann S. R., Martin W. R. Hyperalgesic and analgesic actions of morphine, U50-488, naltrexone, and (-)-lobeline in the rat brainstem. Pharmacol Biochem Behav. 1994 Jan;47(1):197–201. doi: 10.1016/0091-3057(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Hamann S. R., Martin W. R. Opioid and nicotinic analgesic and hyperalgesic loci in the rat brain stem. J Pharmacol Exp Ther. 1992 May;261(2):707–715. [PubMed] [Google Scholar]
- Holmes B. B., Fujimoto J. M. Inhibiting a spinal dynorphin A component enhances intrathecal morphine antinociception in mice. Anesth Analg. 1993 Dec;77(6):1166–1173. doi: 10.1213/00000539-199312000-00015. [DOI] [PubMed] [Google Scholar]
- Horan P. J., Mattia A., Bilsky E. J., Weber S., Davis T. P., Yamamura H. I., Malatynska E., Appleyard S. M., Slaninova J., Misicka A. Antinociceptive profile of biphalin, a dimeric enkephalin analog. J Pharmacol Exp Ther. 1993 Jun;265(3):1446–1454. [PubMed] [Google Scholar]
- Kayser V., Besson J. M., Guilbaud G. Analgesia produced by low doses of the opiate antagonist naloxone in arthritic rats is reduced in morphine-tolerant animals. Brain Res. 1986 Apr 16;371(1):37–41. doi: 10.1016/0006-8993(86)90807-3. [DOI] [PubMed] [Google Scholar]
- Kayser V., Guilbaud G. Dose-dependent analgesic and hyperalgesic effects of systemic naloxone in arthritic rats. Brain Res. 1981 Dec 7;226(1-2):344–348. doi: 10.1016/0006-8993(81)91110-0. [DOI] [PubMed] [Google Scholar]
- Kiyatkin E. A. Morphine: some puzzles of well-known substance. Int J Neurosci. 1989 Apr;45(3-4):231–246. doi: 10.3109/00207458908986236. [DOI] [PubMed] [Google Scholar]
- Lasagna L. Drug interaction in the field of analgesic drugs. Proc R Soc Med. 1965 Nov;58(11 Pt 2):978–983. doi: 10.1177/003591576505811P207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Gordon N. C., Fields H. L. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979 Apr 19;278(5706):740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Gordon N. C. Method of administration determines the effect of naloxone on pain. Brain Res. 1986 Feb 19;365(2):377–378. doi: 10.1016/0006-8993(86)91653-7. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Gordon N. C., Taiwo Y. O., Coderre T. J. Potentiation of pentazocine analgesia by low-dose naloxone. J Clin Invest. 1988 Nov;82(5):1574–1577. doi: 10.1172/JCI113768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. R., Jasinski D. R., Mansky P. A. Naltrexone, an antagonist for the treatment of heroin dependence. Effects in man. Arch Gen Psychiatry. 1973 Jun;28(6):784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvini S., Hamann S. R., Martin W. R. Pharmacologic characteristics of a medullary hyperalgesic center. J Pharmacol Exp Ther. 1993 Apr;265(1):286–293. [PubMed] [Google Scholar]
- Ritter A. M., Mendell L. M. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992 Dec;68(6):2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Schmidt J. F., Chraemmer-Jørgensen B., Pedersen J. E., Risbo A. Postoperative pain relief with naloxone. Severe respiratory depression and pain after high dose buprenorphine. Anaesthesia. 1985 Jun;40(6):583–586. doi: 10.1111/j.1365-2044.1985.tb10903.x. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Antagonists at excitatory opioid receptors on sensory neurons in culture increase potency and specificity of opiate analgesics and attenuate development of tolerance/dependence. Brain Res. 1994 Feb 14;636(2):286–297. doi: 10.1016/0006-8993(94)91028-6. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Cholera toxin-A subunit blocks opioid excitatory effects on sensory neuron action potentials indicating mediation by Gs-linked opioid receptors. Brain Res. 1990 Aug 20;525(2):225–231. doi: 10.1016/0006-8993(90)90868-c. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Cholera toxin-B subunit blocks excitatory effects of opioids on sensory neuron action potentials indicating that GM1 ganglioside may regulate Gs-linked opioid receptor functions. Brain Res. 1990 Oct 29;531(1-2):1–7. doi: 10.1016/0006-8993(90)90751-v. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Chronic selective activation of excitatory opioid receptor functions in sensory neurons results in opioid 'dependence' without tolerance. Brain Res. 1992 Nov 27;597(1):74–83. doi: 10.1016/0006-8993(92)91507-b. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Dual opioid modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Res. 1989 Jul 10;491(2):227–242. doi: 10.1016/0006-8993(89)90059-0. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M., Ledeen R. W. Brief treatment of sensory ganglion neurons with GM1 ganglioside enhances the efficacy of opioid excitatory effects on the action potential. Brain Res. 1991 Sep 13;559(1):130–138. doi: 10.1016/0006-8993(91)90295-7. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Nerve growth factor rapidly prolongs the action potential of mature sensory ganglion neurons in culture, and this effect requires activation of Gs-coupled excitatory kappa-opioid receptors on these cells. J Neurosci. 1994 Sep;14(9):5570–5579. doi: 10.1523/JNEUROSCI.14-09-05570.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Specific N- or C-terminus modified dynorphin and beta-endorphin peptides can selectively block excitatory opioid receptor functions in sensory neurons and unmask potent inhibitory effects of opioid agonists. Brain Res. 1995 Feb 27;673(1):30–38. doi: 10.1016/0006-8993(94)01380-z. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Sato J., Takemori A. E. Maintenance of morphine dependence by naloxone in acutely dependent mice. J Pharmacol Exp Ther. 1990 Sep;254(3):841–846. [PubMed] [Google Scholar]
- Taiwo Y. O., Basbaum A. I., Perry F., Levine J. D. Paradoxical analgesia produced by low doses of the opiate-antagonist naloxone is mediated by interaction at a site with characteristics of the delta opioid receptor. J Pharmacol Exp Ther. 1989 Apr;249(1):97–100. [PubMed] [Google Scholar]
- Ueda H., Fukushima N., Kitao T., Ge M., Takagi H. Low doses of naloxone produce analgesia in the mouse brain by blocking presynaptic autoinhibition of enkephalin release. Neurosci Lett. 1986 Apr 24;65(3):247–252. doi: 10.1016/0304-3940(86)90269-7. [DOI] [PubMed] [Google Scholar]
- Vaccarino A. L., Tasker R. A., Melzack R. Analgesia produced by normal doses of opioid antagonists alone and in combination with morphine. Pain. 1989 Jan;36(1):103–109. doi: 10.1016/0304-3959(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Vaccarino A. L., Tasker R. A., Melzack R. Systemic administration of naloxone produces analgesia in BALB/c mice in the formalin pain test. Neurosci Lett. 1988 Jan 11;84(1):103–107. doi: 10.1016/0304-3940(88)90345-x. [DOI] [PubMed] [Google Scholar]
- Walker M. J., Lê A. D., Poulos C. X., Cappell H. Chronic selective blockade of mu opioid receptors produces analgesia and augmentation of the effects of a kappa agonist. Brain Res. 1991 Jan 11;538(2):181–186. doi: 10.1016/0006-8993(91)90427-w. [DOI] [PubMed] [Google Scholar]
- Way E. L., Loh H. H. Responsivity to naloxone during morphine dependence. Ann N Y Acad Sci. 1976;281:252–261. doi: 10.1111/j.1749-6632.1976.tb27936.x. [DOI] [PubMed] [Google Scholar]
- Way E. L., Loh H. H., Shen F. H. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969 May;167(1):1–8. [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent action potentials. J Pharmacol Exp Ther. 1983 Nov;227(2):394–402. [PubMed] [Google Scholar]
- Woolf C. J. Analgesia and hyperalgesia produced in the rat by intrathecal naloxone. Brain Res. 1980 May 12;189(2):593–597. doi: 10.1016/0006-8993(80)90375-3. [DOI] [PubMed] [Google Scholar]
- Wu K. M., Martin W. R., Kamerling S. G., Wettstein J. G. Possible medullary kappa hyperalgesic mechanism. I. A new potential role for endogenous opioid peptides in pain perception. Life Sci. 1983 Oct 31;33(18):1831–1838. doi: 10.1016/0024-3205(83)90691-4. [DOI] [PubMed] [Google Scholar]
- Yano I., Takemori A. E. Inhibition by naloxone of tolerance and dependence in mice treated acutely and chronically with morphine. Res Commun Chem Pathol Pharmacol. 1977 Apr;16(4):721–734. [PubMed] [Google Scholar]