Abstract
In obese humans and rodents there is increased expression of the key glucocorticoid (GC) regenerating enzyme, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), in adipose tissue. This increased expression appears to be of pathogenic importance because transgenic mice overexpressing 11β-HSD1 selectively in adipose tissue exhibit a full metabolic syndrome with visceral obesity, dyslipidemia, insulin-resistant diabetes, and hypertension. In this model, while systemic plasma GC levels are unaltered, GC delivery to the liver via the portal vein is increased. 11β-HSD1 is most highly expressed in liver where inhibition or deficiency of its activity improves glucose and lipid homeostasis. To determine the potential contribution of elevated intrahepatic GCs alone toward development of insulin-resistant syndromes we generated transgenic mice expressing increased 11β-HSD1 activity selectively in the liver under transcriptional control of hepatic regulatory sequences derived from the human apoE gene (apoE-HSD1). Transgenic lines with 2- and 5-fold-elevated 11β-HSD1 activity exhibited mild insulin resistance without altered fat depot mass. ApoE-HSD1 transgenic mice exhibited fatty liver and dyslipidemia with increased hepatic lipid synthesis/flux associated with elevated hepatic LXRα and PPARα mRNA levels as well as impaired hepatic lipid clearance. Further, apoE-HSD1 transgenic mice have a marked, transgene-dose-associated hypertension paralleled by incrementally increased liver angiotensinogen expression. These data suggest that elevated hepatic expression of 11β-HSD1 may relate to the pathogenesis of specific fatty liver, insulin-resistant, and hypertensive syndromes without obesity in humans as may occur in, for example, myotonic dystrophy, and possibly, the metabolically obese, normal-weight individual.
In Cushing's syndrome, high circulating glucocorticoid (GC) levels cause visceral obesity, insulin resistance, diabetes mellitus, dyslipidemia, hypertension, and an increased risk of cardiovascular disease (1, 2). The much more prevalent “metabolic syndrome” (insulin resistance, type 2 diabetes, dyslipidemia, and hypertension, typically in association with visceral obesity) has similarities to Cushing's syndrome (1). However, any pathogenic role for GCs in the metabolic syndrome or idiopathic obesity has been unclear because circulating cortisol levels are typically near normal or even low (3). However, recent studies in humans and rodents suggest a role for tissue rather than plasma GC excess in the development of idiopathic obesity and the metabolic syndrome via intracellular steroid reactivation of inert circulating 11-dehydrocorticosterone (cortisone in humans) into active corticosterone (cortisol) by 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1. This enzyme is highly expressed in liver, adipose tissue, and brain (4).
The 2- to 3-fold-increased 11β-HSD1 activity in adipose tissue in obese Zucker rats (5) and in some (6, 7) but not all (8) studies of obese humans may be causal of visceral obesity and its metabolic consequences. Supporting this hypothesis, visceral obesity, hyperlipidemia, insulin resistance, glucose intolerance/diabetes (9), and hypertension (10) are driven in transgenic (TG) mice by overexpression (2- to 3-fold) of 11β-HSD1 selectively in adipose tissue. Notably, as with human metabolic syndrome, circulating plasma corticosterone levels in aP2-HSD1 TG mice are unaltered (9). Conversely, 11β-HSD1 null mice exhibit a protective glycemic, lipid, and lipoprotein profile (11, 12) and show increased expression of hepatic mRNAs encoding regulators of fatty acid beta-oxidation (12). While intraadipose but not systemic corticosterone concentrations are elevated in aP2-HSD1 TG mice, corticosterone delivery to the liver is also increased ≈3-fold via spillover of adipose steroid production into the portal vein. 11β-HSD1 shows highest expression in the liver (13). Hepatic 11β-HSD1 mRNA levels are regulated by diet, gender, and hormones (1, 13–15). Heterogeneity of hepatic 11β-HSD1 activity may be relevant to the development of specific fatty liver, insulin-resistant, and hypertensive syndromes without obesity in humans as may occur in, for example, myotonic dystrophy where marked insulin resistance and dyslipidemia have been shown to occur with elevated hepatic 11β-reduction of cortisone to cortisol in positive correlation with the severity of disease (16). To dissect the role of elevated hepatic 11β-HSD1 in visceral obesity/metabolic syndrome phenotypes, we generated TG mice overexpressing 11β-HSD1 selectively in liver by using the previously characterized hepatic transcriptional control sequences of the human apoE gene promoter and enhancer (17).
Materials and Methods
Construct for Liver Overexpression of 11β-HSD1 in TG Mice. Plasmid (pLIVeGFP) comprising human apoE gene sequences driving expression of enhanced GFP (EGFP) cDNA (kindly provided by J. M. Taylor, Gladstone Institute, University of California, San Francisco) was manipulated for 11β-HSD1 expression in hepatocytes by using standard recombinant DNA techniques. Briefly, eGFP was removed from pLIV by KpnI/MluI digestion and the vector was blunt-end-ligated to the rat 11β-HSD1 cDNA fused in-frame at the C terminus to the influenza virus-derived HA epitope tag by PCR-mediated site-directed mutagenesis. The transgene construct, apoE-HSD1, a 5.1-kb DNA fragment subsequently excised by NotI/partial EcoRI digestion, was prepared for micro-injection by agarose gel electrophoresis, electroelution, and dialysis against 10 mM Tris·HCl/0.1 mM EDTA (pH 7.4) before dilution of DNA to a concentration of 2 ng/μl.
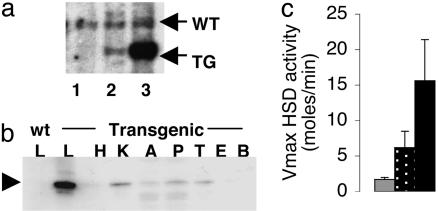
TG and Experimental Animals. Microinjection into the pronuclei of fertilized C57BL/6xCBA/C3H F1 embryos was performed by using standard techniques. G0 offspring were screened by Southern blotting analysis of tail biopsy genomic DNA digested with BamHI and probed with [α-32P]dCTP-labeled rat 11β-HSD1 cDNA to reveal diagnostic restriction fragments as shown in Fig. 1a. TG lines 1066 and 1065, estimated to carry 2–3 and 8–10 copies of the transgene, respectively, were propagated from independent founder animals. F3 C57BL/6J backcross male mice were studied throughout. Mice hemizygous for the transgene (referred to as apoE-HSD1 and/or TG mice) were compared with non-TG littermate controls. Animals were routinely fed standard chow (Special Diet Services, Essex, U.K., product 801190) ad libitum. To address responses to a diet previously optimized for weight gain and insulin resistance, groups of TG and non-TG mice were fed high-fat (HF) diet (58% calories as fat with sucrose, Research Diets D12331) or low-fat (LF) diet (11% calories as fat with cornstarch, Research Diets D12328) as control for 20 weeks from 4 weeks of age. Mice were housed singly for the final 4–5 days before killing between 6 and 9 a.m. or blood sampling for corticosterone by venesection from conscious animals at 8 a.m. or 8 p.m. (nadir and peak of diurnal rhythm), within 1 min of disturbing each cage.
Fig. 1.
ApoE-HSD1 TG mice. (a) ApoE-HSD1 mice propagated from TG founder males 1066 and 1065 bearing a single insertion of transgene genotyped by Southern blot. Lane 1, non-TG; lane 2, 1066 TG; lane 3, 1065 TG. (b) Expression of epitope-tagged 11β-HSD1 (arrowhead) in tissue extracts (L, liver; H, heart; K, kidney; A, adipose; P, pancreas; T, testes; E, epididymis; B, brain) determined by anti-HA immunoblot. (c) In liver, Vmax for 11β-HSD1 activity in 1066 TG (stippled bar) and 1065 TG (black bar) increased 2- and 5-fold above non-TG controls (gray bar), respectively.
Blood Analysis. Plasma corticosterone levels were determined by radio immunoassay as described (18). Plasma glucose was assayed by using the Trinder kit (Sigma). Serum or plasma insulin was measured by using the Ultra Sensitive Insulin ELISA kit (Crystal Chem, Downers Grove, IL). Serum lipids and apolipoproteins were assayed as described (12). Serum lipids pooled from groups of male TG and non-TG fed either HF or control LF diet (as above) were fractionated relative to lipoprotein size and assayed as described (12).
Recording of Blood Pressure. Radio-telemetric devices (TA11PAC20, Datasciences, Minneapolis) were implanted under anesthesia into the carotid artery of adult male TG and non-TG littermate mice aged 8–10 months for recording of activity and blood pressure by using dataquest art Version 2.3 (Datasciences). The data presented (6 h moving average per individual animal; mean ± SEM per group) are from recordings taken over 3 consecutive days between 6 and 18 days postsurgery.
Analysis of Transgene Expression by Immunoblotting. Tissues were homogenized in 10 vol of 250 mM sucrose/10 mM triethanolamine (pH 7.6), and protein concentration was determined by photometric assay (BCA kit, Pierce). Total proteins (40–80 μg) were resolved by SDS/PAGE minigel and electroblotted onto poly(vinylidene difluoride) membrane (Hybond-P) and processed according to the manufacturer's instructions (Amersham Biosciences) with anti-HA antibody (rabbit polyclonal F7, Santa Cruz Biotechnology) diluted 1:1,000, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Diagnostics Scotland, Edinburgh) diluted 1:1,000. HRP signal was visualized by using color developer TMB (3,3′,5,5′-tetramethylbenzidine, Promega).
11β-HSD1 Enzyme Activity. 11β-HSD activity in liver was assayed after homogenization as described (13). The reaction included 0.1 mg/ml protein, 25 nM tritiated corticosterone, and an excess (2 μM) of the 11β-HSD1-specific cofactor NADP together with unlabeled corticosterone from 0.5 to 20 μM to allow assay within the linear range for accurate determination of Vmax and Km. After a 10-min incubation, steroids were extracted with ethyl acetate, separated by TLC, identified by migration in comparison to standards, and quantified with a phosphorimager tritium screen (Fuji). Identity of corticosterone and 11-DHC were confirmed by HPLC analysis as described (12).
RNA Extraction and Gene Expression Analysis: RNA Preparation and Northern Blotting. The procedure and all probes were as described (12), except CYP7a, which was amplified from mouse liver cDNA by PCR with oligonucleotide primers as described (19). Remaining probes were generated by using the following primers (5′ to 3′; up/downstream pairs): LXRα, CAG TGT CTT GGT AAT GTC CAG G and GCC TGT TAC ACT GTT GCT GG; RXRα/β,TTA CCA ACA TCT GTC AAG CAG C and TAG GTG GCT TGA TGT GGT GC; FXR, CGA AGA AGC ATT ACC AAG AAC G and TCT GTC TGG AGA GAG GAT GAC G; ABCA1, GTC AGT CAC ATA GAG GAA TG and GGT ATG CCA ATA ACT ACT GG.
Gene expression was quantified in arbitrary units from phosphorimages as described (12) at an exposure appropriate for the intensity of the signal for a given probe relative to RNA loading by using a probe for U1 small nuclear rRNA. U1 levels do not change with genotype or the dietary manipulation described as tested by reference to 18S and 28S rRNA levels.
Tissue Biochemistry: Hepatic Lipid Content. tissues were thoroughly homogenized in 4–10 vol of isopropanol for 2 × 20 seconds at 30 Hz in Mixer Mill 301 (Retsch) and cleared of debris by centrifugation (>10,000 × g), and 5 or 2.5 μl of the extract was assayed manually by using kits for determination of triglyceride (Sigma) or total cholesterol (Wako Biochemicals, Osaka) concentration. Measurements from livers of non-TG mice were comparable to those published for wild-type mice (20). Oil Red O staining of tissue sections was performed as described (12).
Statistics. Data are expressed as mean ± SEM and were analyzed by one- or two-way ANOVA with genotype and/or diet for most parameters, or by repeated-measures ANOVA with genotype and time for glucose tolerance test and radio-telemetry mean arterial blood pressure data.
Results
Hepatic Overexpression of Active 11β-HSD1 in TG Mice. Two lines of mice, 1066 and 1065, estimated to carry 2–3 and 8–10 copies of the apoE-HSD1 transgene, respectively, were propagated for further study (Fig. 1a). Assay of transgene expression by anti-HA immunoblotting revealed overexpression of epitope-tagged 11β-HSD1 in liver (Fig. 1b). Some expression of transgene product was detected in kidney. Assay of 11β-HSD1 activity in kidney and adipose tissue revealed no significant difference between TG and non-TG groups (data not shown). Kinetic analyses of liver 11β-HSD activity, however, indicated a 2-fold increase in Vmax for TG mice from line 1066 and a 5-fold increase in line 1065 (Fig. 1c) with unaltered affinity of the enzyme for its substrate (Km corticosterone; TG 10.1 ± 3.3 μM, non-TG 5.7 ± 1.8 μM; not significant), confirming that overexpressed 11β-HSD1 was unaffected by the HA tag.
ApoE-HSD1 TG Mice Show Normal Circulating Corticosterone Levels. Circulating corticosterone levels, measured in the highest expressing line, were unaltered (8 a.m.: non-TG 32 ± 10 nmol/liter, TG 38 ± 12 nmol/liter; 8 p.m.: non-TG 173 ± 21 nmol/liter, TG 176 ± 54 nmol/liter), indicating a normal plasma corticosterone diurnal rhythm despite elevated hepatic regeneration. Chronically increased tissue regeneration of corticosterone in liver might cause down-regulation of GC receptors (GR) (21). Hepatic GR and its mRNA show a diurnal variation (22), but at both morning and evening time points apoE-HSD1 TG mouse hepatic GR mRNA levels were at or above wild-type levels [Northern blot data (mean ± SEM) expressed as a percentage relative to control morning values: (a.m.) non-TG 100 ± 14, 1065 TG 236 ± 64, 1066 TG 237 ± 32, (p.m.) non-TG 261 ± 21, 1065 TG 258 ± 19; n = 6–9].
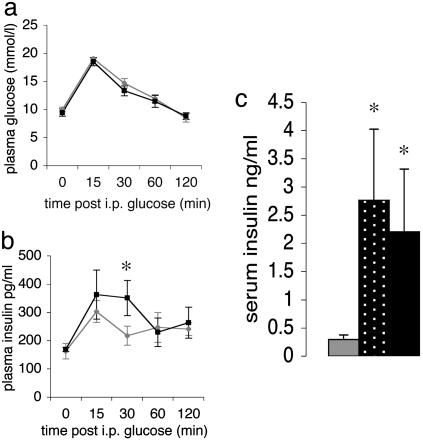
ApoE-HSD1 TG Mice Display Normal Glucose Tolerance, Body Weight, and Fat Depots but Develop Hyperinsulinemia. In contrast to mice TG for aP2-HSD1, adult male apoE-HSD1 TG mice aged 24 weeks expressing the highest level of 11β-HSD1 activity in liver exhibited no significant differences in body weight, fat-depot mass, or organ-to-body-weight ratio compared to non-TG littermates. On high fat diet, apoE-HSD1 mice gained equal weight with unaltered adipose distribution compared to controls (Table 1). At 18 weeks of age, apoE-HSD1 mice expressing 5-fold-increased enzyme activity had entirely normal fasting plasma glucose levels and glucose tolerance (Fig. 2a) but showed elevated 30-min post-IP glucose bolus plasma insulin levels (Fig. 2b), and by 24 weeks of age, fasting insulin levels were elevated in TGs from both apoE-HSD1 lines, suggesting the development of modest insulin resistance (Fig. 2c).
Table 1. Morphometric and lipid parameters.
| Diet fed | 1065 TG | Non-TG | |
|---|---|---|---|
| Body weight, g | LF | 30.3 ± 1.2 | 32.2 ± 0.9 |
| HF | 40.2 ± 3.5 | 44.4 ± 2.3 | |
| Weight ratio (×103) | |||
| Liver | LF | 49 ± 1.3 | 49 ± 2.1 |
| HF | 52 ± 0.9 | 49 ± 1.0 | |
| Kidney | LF | 7 ± 0.3 | 7 ± 0.1 |
| HF | 6 ± 0.3 | 6 ± 0.3 | |
| Mesenteric fat | LF | 11 ± 1.3 | 10 ± 0.5 |
| HF | 19 ± 3.6 | 25 ± 2.0 | |
| Epididymal fat | LF | 19 ± 2.2 | 22 ± 2.3 |
| HF | 41 ± 5.9 | 51 ± 4.2 | |
| Inguinal fat | LF | 14 ± 1.1 | 13 ± 1.7 |
| HF | 24 ± 5.5 | 32 ± 4.2 | |
| Liver | |||
| Triglycerides, mg/g of liver | LF | 15.80 ± 1.57* | 11.24 ± 0.73 |
| HF | 59.6 ± 18.68 | 70.38 ± 23.85 | |
| Total cholesterol, mg/g of liver | LF | 0.65 ± 0.03 | 0.62 ± 0.03 |
| HF | 0.98 ± 0.03 | 0.93 ± 0.02 | |
| Serum | |||
| NEFA, nmol/liter | LF | 0.73 ± 0.05* | 0.56 ± 0.02 |
| HF | 1.17 ± 0.12 | 1.21 ± 0.11 | |
| Triglycerides, mg/dl | LF | 183 ± 23 | 171 ± 17 |
| HF | 252 ± 37 | 299 ± 47 | |
| Total cholesterol, mg/dl | LF | 143 ± 6 | 130 ± 7 |
| HF | 205 ± 11 | 221 ± 24 | |
| HDL-cholesterol, mg/dl | LF | 107 ± 2 | 93 ± 8 |
| HF | 69 ± 14 | 86 ± 12 |
ApoE-HSD1 TG and non-TG mice aged 24 weeks fed as indicated (n = 4-8). ANOVA, *, P < 0.05 with genotype and LF diet.
Fig. 2.
Glucose homeostasis. IPGTT of chow-fed mice aged 18 weeks. Plasma glucose (a) and insulin (b) in apoE-HSD1 TG mice (black) and controls (gray) are shown. (c) Twenty-four-hour fasting plasma insulin levels in male mice aged 24 weeks [non-TG (n = 12), gray bar; 1066 TG (n = 6), stippled bar; 1065 TG (n = 6), black bar; ANOVA; mean ± SEM; *, P < 0.05 with respect to genotype].
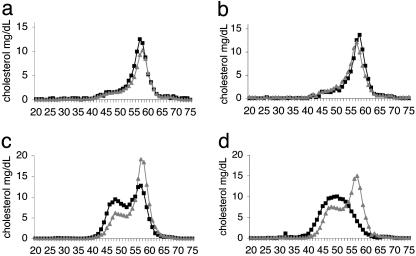
Dyslipidemia in apoE-HSD1 TG Mice. Histopathological staining of liver with Oil Red O suggested an increase in hepatic fat content in apoE-HSD1 mice, and biochemical analyses confirmed the presence of significantly increased intrahepatic triglyceride levels in apoE-HSD1 mice fed LF diet together with an increase in circulating nonesterified fatty acids (NEFA) in this group (Table 1). Serum lipid levels and the cholesterol distribution among lipoproteins did not differ between LF-diet-fed apoE-HSD1 and control groups (Table 1 and Fig. 3 a and b). HF diet feeding increased serum cholesterol and triglycerides in all animals (Table 1) and led to changes in cholesterol distribution between apoE-HSD1 TG and non-TG mice (Fig. 3 c and d). In HF-diet-fed non-TG mice, the elution of expected HDL-associated cholesterol (fractions 55–60) was preceded by another peak (fractions 45–55) coincident with the expected elution of low density lipoproteins and/or differentially sized lipoprotein particles as reported (23). In HF-diet-fed apoE-HSD1 TG mice, the HDL-associated cholesterol peak was apparently reduced and the presence of earlier-eluting particles was more marked, suggesting altered lipoprotein metabolism in the presence of elevated intrahepatic GC regeneration with HF dietary challenge. The degree of divergence of the cholesterol-associated lipoprotein profile was most marked in the line with the greatest transgene overexpression (Fig. 3d). Although a significant difference in HDL cholesterol levels between groups was not detected (Table 1), circulating levels of the major lipoprotein component of HDL-cholesterol, apoAI, were reduced by 40% (1065 TG 126 ± 26 mg/dl, non-TG 220 ± 12 mg/dl; ANOVA, P < 0.01), whereas apoB levels remained similar (1065 TG 54 ± 6 mg/dl, non-TG 62 ± 4 mg/dl), suggesting, therefore, that larger lipoprotein particles are generated in apoE-HSD1 TG mice upon HF diet feeding.
Fig. 3.
Cholesterol lipoprotein distribution profiles. FPLC fractionation of pooled serum from apoE-HSD1 TG mice (black squares) and non-TG littermates [gray triangles; n = 5–7 per group, except 1066 non-TG (n = 2) because of unexpected deaths] with cholesterol content (mg/dl; y axis) plotted for each fraction (x axis). VLDL-, IDL+LDL-, and HDL-associated cholesterol fractions usually elute in fractions 30–35, 45–55, and 55–60, respectively. Cholesterol profiles from animals fed control LF diet (a and b) or HF diet (c and d) from line 1066 (a and c) and 1065 (b and d) are shown.
Hepatic Gene Expression: Altered Lipid Homeostasis. LF diet. To address the possible molecular mechanisms of the dyslipidemia in apoE-HSD1 mice, mRNAs encoding major determinants of hepatic lipid metabolism were quantified by Northern blot analyses (Table 2). On control diet, apoE-HSD1 mice had highly significantly increased expression of mRNA encoding the key limiting enzyme of hepatic triglyceride synthesis, fatty acid synthase (FAS), as well as a trend toward increased glycerol phosphate acyltransferase (GPAT) transcripts (6.6 kb, P = 0.16; 3 kb, P = 0.07), suggesting activation of glycerolipogenesis. Given the elevated FAS expression, it is perhaps surprising that mRNAs for the major transcriptional regulators of the lipogenic and cholesterogenic pathways, serum response element binding protein (SREBP) 1c (24) and SREBP-2 (20), were not induced in apoE-HSD1 mice. In contrast, LXRα, which regulates FAS transcription directly (25), was significantly increased in livers of apoE-HSD1 mice. This appeared to be a specific change because expression of mRNAs encoding other related nuclear receptors, RXRα, RXRβ, and FXR, were unaltered.
Table 2. Hepatic gene expression.
| LF feeding
|
HF feeding
|
|||
|---|---|---|---|---|
| Non-TG | 1065 TG | Non-TG | 1065 TG | |
| Gluconeogenesis | ||||
| PEPCK | 100 ± 20 | 136 ± 35 | 208 ± 23†† | 214 ± 40 |
| Lipogenesis | ||||
| FAS | 100 ± 22 | 247 ± 33*** | 223 ± 16†† | 187 ± 40 |
| GPAT (6.6 kb) | 100 ± 17 | 144 ± 24 | 233 ± 15†† | 100 ± 23 |
| GPAT (3.0 kb) | 100 ± 12 | 160 ± 27 | 413 ± 36††† | 270 ± 61†† |
| SREBP1c | 100 ± 14 | 121 ± 17 | 153 ± 9†† | 116 ± 23 |
| LXRα | 100 ± 3 | 141 ± 5** | 180 ± 6†† | 115 ± 8 |
| Lipid oxidation | ||||
| mCPT1 | 100 ± 11 | 155 ± 10** | 333 ± 22††† | 272 ± 33†† |
| PPARα | 100 ± 15 | 148 ± 14* | 352 ± 23††† | 263 ± 35†† |
| Cholesterol | ||||
| HMG CoA R | 100 ± 22 | 155 ± 34 | 159 ± 14 | 116 ± 9 |
| SREBP2 | 100 ± 9 | 95 ± 5 | 190 ± 11††† | 101 ± 4 |
| Bile acids | ||||
| CYP7a (7.5 kb) | 100 ± 12 | 226 ± 53* | 241 ± 46††† | 211 ± 47† |
| CYP7a (4.5 kb) | 100 ± 19 | 212 ± 57 | 124 ± 65 | 115 ± 26 |
| FXR | 100 ± 8 | 95.6 ± 11 | 213 ± 12††† | 141 ± 13†† |
| RXRα | 100 ± 5 | 115.1 ± 7 | 144 ± 11†† | 113 ± 7 |
| RXRβ | 100 ± 18 | 104 ± 11 | 230 ± 14††† | 169 ± 25† |
| Lipoprotein metabolism | ||||
| ApoAl | 100 ± 1 | 94 ± 11 | 137 ± 13† | 113 ± 10 |
| ABCA1 | 100 ± 11 | 56 ± 10** | 46 ± 14†† | 52 ± 22 |
| LDL receptor | 100 ± 17 | 120 ± 11 | 181 ± 17†† | 125 ± 17 |
| Hepatic lipase | 100 ± 14 | 97 ± 16 | 155 ± 18†† | 84 ± 9 |
Northern blot analysis of apoE-HSD1 TG and control mice. Data (mean ± SEM) are summarized per group (n = 5—7) and expressed as a percentage relative to control LF fed non-TG (100%). ANOVA, *, P < 0.05; **, P < 0.01; and ***, P < 0.001 with genotype and LF diet; †, P < 0.05; ††, P < 0.01; and †††, P < 0.001 with genotype and HF diet.
ApoE-HSD1 mice fed LF control diet also exhibited elevation of mitochondrial carnitine palmitoyltransferase 1 (mCPT-1) expression, suggesting that lipid oxidation may also be stimulated. Indeed, mRNA encoding the major transcriptional regulator of the pathway, PPARα (26), was increased in TG liver, compatible with its known induction by GCs (27). Although a trend for greater levels of HMG-CoA reductase mRNA expression was evident, any increase in cholesterol biosynthesis did not result in increased hepatic cholesterol content in LF-diet-fed TG mice. Although reduced expression of ATP-binding cassette (ABC) protein A1 in LF-dietfed TG mice suggests a possible decrease in cellular cholesterol and phospholipid efflux, marked elevation of the GC-inducible Cyp7a mRNA (28) in liver suggests that bile acid synthesis and thus hepatic cholesterol efflux via this route may be strongly activated, possibly accounting for the normal hepatic cholesterol content. In contrast to the changes in mRNAs of lipid pathways, hepatic phosphoenolpyruvate carboxykinase (PEPCK) mRNA levels, although a classical target for GC induction, were unaltered in apoE-HSD1 mice, suggesting that there is no major activation of the gluconeogenic pathway.
HF diet. With HF diet feeding, control mice showed increased hepatic expression of mRNAs for apoAI and hepatic lipase regulating HDL synthesis and lipoprotein metabolism, as well as the LDL receptor, which contributes to lipid/lipoprotein reuptake (29). Similarly, mRNAs encoding GPAT and regulators of lipid homeostasis SREBP1c, SREBP2, LXRα, RXRα, and RXRβ, as well as the bile acid nuclear receptor FXR, were increased in response to HF diet in control mice, whereas expression of ABCA1 mRNA was decreased. In contrast, the apoE-HSD1 TG mice did not exhibit such changes with HF diet feeding. Other transcripts of liver lipogenic and beta-oxidation pathways (FAS, mCPT1, and PPARα), and PEPCK, were expressed similarly in apoE-HSD1 and control mice in response to HF diet.
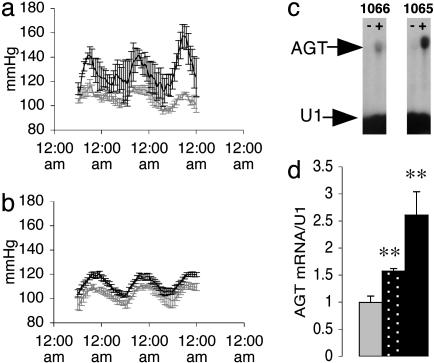
ApoE-HSD1 TG Mice Are Hypertensive. The metabolic syndrome comprises cardiovascular as well as metabolic dysfunction, notably hypertension. To define whether elevated intrahepatic GC regeneration might contribute to the development of hypertension, blood pressure was determined by radio telemetry (Fig. 4 a and b). The apoE-HSD1 mice were hypertensive, particularly during the active period of their circadian rhythm. The level of increase in blood pressure correlated with apoE-HSD1 transgene copy number. Angiotensinogen (AGT), a limiting factor for activation of the renin-angiotensin system (RAS) in mice (30), is GC-inducible and is most highly expressed in liver. Hepatic AGT mRNA was induced in apoE-HSD1 mice and appears incremental with transgene activity (Fig. 4 c and d). Single time point analyses indicated that circulating levels of AGT in control mice were very low with a trend for increased levels in TG mice from line 1065 (TG 51.4 ± 7.5 nmol/liter, non-TG 30.1 ± 11.9 nmol/liter; ANOVA P = 0.16; n = 6/6). Plasma levels of angiotensin II, aldosterone, and renin did not vary significant between apoE-HSD1 TG and non-TG mice [angiotensin II (pmol/liter), TG 268 ± 42, non-TG 211 ± 28 (n = 8/16); aldosterone (pmol/liter), TG 931 ± 206, non-TG 747 ± 120 (n = 11/16); renin concentration (ng/ml/h), TG 789 ± 111, non-TG 865 ± 145 (n = 6/6)], suggesting that the circulating RAS is not chronically activated in apoE-HSD1 mice. Consistent with this finding, no major renal pathology was detected in the TG mice (data not shown).
Fig. 4.
Blood pressure and hepatic AGT mRNA expression. Mean arterial pressure (MAP) (mmHg) measured by radio telemetry in chow-fed apoE-HSD1 TG (black bar) and non-TG control mice (gray bar) in line 1065 (a) (n = 3 TG and 3 non-TG) and line 1066 (b)(n = 3 TG and 6 non-TG). Significant differences in MAP were detected between TGs and control littermates in both lines (1066 TG P < 0.05; 1065 TG P < 0.001). Expression of hepatic AGT mRNA in representative non-TG (-) and TG (+) individuals (c) and values summarized from groups of non-TG (gray bar), 1066 TG (stippled bar), and 1065 TG (black bar) mice (d) are shown. [ANOVA; mean ± SEM; **, P < 0.01 with respect to genotype (n = 6–10 per group)].
Discussion
ApoE-HSD1 TG mice show increased activity for regeneration of intrahepatic GC but have normal corticosterone levels in the systemic circulation. In contrast to the full metabolic syndrome seen in adipose overexpressing aP2-HSD1 TGs (9), the effects of selective liver overexpression are less pronounced, with modest insulin resistance, dyslipidemia, and hypertension, but unaltered adiposity. This attenuated metabolic syndrome, however, parallels the metabolic status of conditions with elevated liver 11β-HSD1 activity, notably myotonic dystrophy (16), and may be relevant to the metabolically obese, normal-weight individual (31), as well as fatty liver syndromes exhibiting insulin resistance, dyslipidemia, and hypertension in the absence of obesity (32). The phenotype of the apoE-HSD1 mice most likely reflects the complex interactions of elevated intrahepatic GC levels, insulin resistance, and secondary alterations in hepatic gene expression.
The elevated levels of insulin detected in response to glucose challenge together with increased fasting insulin levels in older apoE-HSD1 mice suggest that they progressively develop insulin resistance. Elevated hepatic triglycerides, circulating nonesterified fatty acids (NEFA), and a diet-induced dyslipidemic cholesterol lipoprotein profile evident in apoE-HSD1 mice are also associated with Cushing's syndrome and insulin-resistant states. Some of the changes in hepatic gene expression in apoE-HSD1 mice also suggest insulin resistance including elevated basal mCPT-1 (33). This finding might be expected because GCs promote hyperlipidemia and insulin resistance (34, 35) and decrease hepatocyte insulin binding (36). The mechanisms involve both direct effects on target gene expression in the insulin signaling pathway (37) and the alteration of other key transcriptional regulators of lipid homeostasis (38, 39).
The apoE-HSD1 mice showed fat accumulation in the liver, mainly as triglyceride. The association of insulin resistance and fatty liver irrespective of obesity has been noted in patients with nonalcoholic fatty liver disease (32) and has been proposed as an early indicator of primary hepatic insulin resistance preceding more widespread insulin resistance and the full metabolic syndrome (40). Both lipogenesis and lipid oxidation are activated in apoE-HSD1 mice with an apparent net accumulation of lipid in liver and serum. This pattern also occurs in Cushing's Syndrome (41). The key lipogenic enzyme FAS was induced in control-diet-fed apoE-HSD1 mice. FAS is largely regulated by SREBP1c, but neither this nor SREBP2 were elevated in apoE-HSD1 mice. Although posttranslational modifications are important in the activation of SREBPs, an alternative explanation for FAS induction is the elevated hepatic level of LXRα, which may regulate FAS independently of SREBPs (25). Intriguingly, LXRα agonists down-regulate 11β-HSD1, at least in adipose cells and fibroblasts in vitro (42). Whether LXRα is affected by local GC concentrations is not known, but this merits study. Such feedback mechanisms between PPARα and 11β-HSD1 in liver have been proposed (12, 43) and may reflect a primary homeostatic mechanism to maintain liver lipogenic and lipolytic balance under conditions of stress, starvation, and other nutritional challenges.
Elevated levels of PPARα may drive induction of beta-oxidation pathways (mCPT-1) in apoE-HSD1 mice. This induction presumably follows elevated intrahepatic GC levels, which are documented to up-regulate PPARα (27). LXRα- and PPARα-regulated pathways compete through their complex formation with a common heterodimeric RXR partner together with differential recruitment of coactivators and/or corepressors (44, 45). Because at least RXRs appeared to be unaltered in apoE-HSD1 mice, elevated intracellular GC may favor activation of one nuclear receptor pathway over another, leading to fatty liver. This outcome may reflect GC-mediated alteration of ligand availability or interaction with nuclear receptors and/or coactivators (46, 47). Increased expression of GC-inducible CYP7a in apoE-HSD1 TG livers may drive increased bile acid synthesis, contributing to stimulation of LXRα-regulated pathways (and further potentiation of CYP7a mRNA expression) (48, 49), as well as PPARα (50).
The apoE-HSD1 mice distinctly lack glucose intolerance, obesity, or central adiposity. Because these features are found in both the metabolic syndrome and in aP2-HSD1 TG mice, the implication is that they are generated, at least in the latter, specifically by elevated intraadipose GC levels rather than the spillover of adipose steroids into the portal vein and hence to the liver. Indeed, in patients with the metabolic syndrome (6) and in genetically obese rodents (5), liver 11β-HSD1 levels are reduced. In contrast, reduced fasting glucose levels in 11β-HSD1 null mice (11) or with selective inhibition of the enzyme (51) are thought to reflect hepatic insulin sensitization and attenuated induction of GC-responsive gluconeogenic enzymes, notably PEPCK. The lack of fasting hyperglycemia in the apoE-HSD1 mice, although perhaps unexpected, is reinforced by unaltered hepatic PEPCK expression. Although reduced systemic GC levels improve most models of glucose intolerance in the rodent (52, 53), it appears that elevated intrahepatic GC levels alone are insufficient to alter glucose tolerance.
With chronic HF diet feeding, while beta-oxidation pathways remained induced, glycerolipogenesis appeared relatively attenuated (repressed induction of SREBP1c and GPAT mRNA) in apoE-HSD1 mice. Alteration in the cholesterol lipoprotein profile and circulating apoAI levels in apoE-HSD1 mice resembles changes in insulin-resistant syndromes and were accompanied by a failure to induce apoAI, LDL receptor, and hepatic lipase mRNAs in liver. The data suggest a qualitative dyslipidemia in response to HF diet feeding with altered lipoprotein assembly and metabolism, as well as reduced lipoprotein clearance from the circulation with decreases in LDL receptor levels and noncatalytic functions of hepatic lipase (29, 54). Livers of apoE-HSD1 mice under HF-diet challenge also showed attenuated induction of major transcriptional regulators of cholesterol homeostasis including SREBP2 and FXR, as well as LXRα, RXRα, and RXRβ. These adverse consequences precipitated by altered hepatic cholesterol handling in the presence of increased liver GC regeneration by 11β-HSD1 may be relevant to the exacerbation of metabolic disease. Thus, individuals with high hepatic enzyme activity may be predisposed to the development of diet-induced dyslipidemia
The findings in apoE-HSD1 TG mice suggest that increasing intrahepatic GC causes a dose-related increase in blood pressure. GC-mediated activation of AGT gene transcription proportional to GC concentration has been demonstrated in vitro (55). In vivo, single-copy increases in AGT gene dosage in mice (56), and mutations of the AGT gene promoter (57), both amounting to small increases in AGT expression, have been shown to incrementally increase blood pressure. Both aP2-HSD1 and apoE-HSD1 mice show hypertension with parallel increases in AGT gene expression. Chronic activation of the circulating RAS is not evident in apoE-HSD1 mice. However, a grossly elevated RAS (aP2-HSD1 mice) may be misleading. Subtle changes in AGT in apoE-HSD1 mice, which are clearly hypertensive, more closely resemble the AGT TG mice described by Kim et al. (56) that exhibit modestly increasing AGT and stepwise increases in blood pressure with normal compensatory mechanisms intact and an absence of marked renal pathology. The likely mechanism of hypertension in apoE-HSD1 mice remains to be through alteration of the RAS, which now requires detailed physiological study including diurnal measurements of circulating and tissue RAS components. PPARα, recently shown to act as a mediator of dexamethasone-induced insulin resistance and hypertension in mice (39), is also increased in livers of apoE-HSD1 mice and may contribute in part to their hypertensive phenotype. We conclude that tissue specific increases in intracellular GC regeneration by 11β-HSD1 may be a common feature underlying hypertension coincident with distinct profiles of metabolic disease.
Acknowledgments
We thank Gillian Brooker, Morag Meikle, Lynne Ramage, and J. J. Morton for excellent technical assistance. This work is supported by a Wellcome Trust Program Grant (to J.R.S. and J.J.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AGT, Angiotensinogen; FAS, fatty acid synthase; GC, glucocorticoid; LF, low-fat; HF, high-fat; RAS, renin-angiotensin system; SREBP, serum response element binding protein; TG, transgenic.
References
- 1.Seckl, J. R. & Walker, B. R. (2001) Endocrinology 142, 1371-1376. [DOI] [PubMed] [Google Scholar]
- 2.Montague, C. T. & O'Rahilly, S. (2000) Diabetes 49, 883-888. [DOI] [PubMed] [Google Scholar]
- 3.Hautanen, A., Raikkonen, K. & Adlercreutz, H. (1997) J. Intern. Med. 241, 451-461. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson, P. M., Chapman, K. E. & Seckl, J. R. (1999) J. Steroid Biochem. Mol. Biol. 68, 245-250. [DOI] [PubMed] [Google Scholar]
- 5.Livingstone, D. E., Kenyon, C. J. & Walker, B. R. (2000) J. Endocrinol. 167, 533-539. [DOI] [PubMed] [Google Scholar]
- 6.Rask, E., Olsson, T., Soderberg, S., Andrew, R., Livingstone, D. E., Johnson, O. & Walker, B. R. (2001) J. Clin. Endocrinol. Metab. 86, 1418-1421. [DOI] [PubMed] [Google Scholar]
- 7.Paulmyer-Lacroix, O., Boullu, S., Oliver, C., Alessi, M. C. & Grino, M. (2002) J. Clin. Endocrinol. Metab. 87, 2701-2705. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson, J. W., Sinha, B., Bujalska, I., Hewison, M. & Stewart, P. M. (2002) J. Clin. Endocrinol. Metab. 87, 5630-5635. [DOI] [PubMed] [Google Scholar]
- 9.Masuzaki, H., Paterson, J., Shinyama, H., Morton, N. M., Mullins, J. J., Seckl, J. R. & Flier, J. S. (2001) Science 294, 2166-2170. [DOI] [PubMed] [Google Scholar]
- 10.Masuzaki, H., Yamamoto, H., Kenyon, C. J., Elmquist, J. K., Morton, N. M., Paterson, J. M., Shinyama, H., Sharp, M. G., Fleming, S., Mullins, J. J., et al. (2003) J. Clin. Invest. 112, 83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotelevtsev, Y., Holmes, M. C., Burchell, A., Houston, P. M., Schmoll, D., Jamieson, P., Best, R., Brown, R., Edwards, C. R., Seckl, J. R. & Mullins, J. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14924-14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton, N. M., Holmes, M. C., Fievet, C., Staels, B., Tailleux, A., Mullins, J. J. & Seckl, J. R. (2001) J. Biol. Chem. 276, 41293-41300. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson, P. M., Chapman, K. E., Edwards, C. R. & Seckl, J. R. (1995) Endocrinology 136, 4754-4761. [DOI] [PubMed] [Google Scholar]
- 14.Andrew, R., Gale, C. R., Walker, B. R., Seckl, J. R. & Martyn, C. N. (2002) Exp. Clin. Endocrinol. Diabetes 110, 284-290. [DOI] [PubMed] [Google Scholar]
- 15.Rask, E., Walker, B. R., Soderberg, S., Livingstone, D. E., Eliasson, M., Johnson, O., Andrew, R. & Olsson, T. (2002) J. Clin. Endocrinol. Metab. 87, 3330-3336. [DOI] [PubMed] [Google Scholar]
- 16.Johansson, A., Andrew, R., Forsberg, H., Cederquist, K., Walker, B. R. & Olsson, T. (2001) J. Clin. Endocrinol. Metab. 86, 4276-4283. [DOI] [PubMed] [Google Scholar]
- 17.Fan, J., Wang, J., Bensadoun, A., Lauer, S. J., Dang, Q., Mahley, R. W. & Taylor, J. M. (1994) Proc. Natl. Acad. Sci. USA 91, 8724-8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacPhee, I. A., Antoni, F. A. & Mason, D. W. (1989) J. Exp. Med. 169, 431-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu, T. T., Makishima, M., Repa, J. J., Schoonjans, K., Kerr, T. A., Auwerx, J. & Mangelsdorf, D. J. (2000) Mol. Cell 6, 507-515. [DOI] [PubMed] [Google Scholar]
- 20.Shimano, H., Shimomura, I., Hammer, R. E., Herz, J., Goldstein, J. L., Brown, M. S. & Horton, J. D. (1997) J. Clin. Invest. 100, 2115-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinyak, J. E., Dorin, R. I., Hoffman, A. R. & Perlman, A. J. (1987) J. Biol. Chem. 262, 10441-44. [PubMed] [Google Scholar]
- 22.Xu, R. B., Liu, Z. M. & Zhao, Y. (1991) Neuroendocrinology 53, Suppl. 1, 31-36. [DOI] [PubMed] [Google Scholar]
- 23.Peters, J. M., Hennuyer, N., Staels, B., Fruchart, J. C., Fievet, C., Gonzalez, F. J. & Auwerx, J. (1997) J. Biol. Chem. 272, 27307-27312. [DOI] [PubMed] [Google Scholar]
- 24.Shimano, H., Yahagi, N., Amemiya-Kudo, M., Hasty, A. H., Osuga, J., Tamura, Y., Shionoiri, F., Iizuka, Y., Ohashi, K., Harada, K., et al. (1999) J. Biol. Chem. 274, 35832-35839. [DOI] [PubMed] [Google Scholar]
- 25.Joseph, S. B., Laffitte, B. A., Patel, P. H., Watson, M. A., Matsukuma, K. E., Walczak, R., Collins, J. L., Osborne, T. F. & Tontonoz, P. (2002) J. Biol. Chem. 277, 11019-11025. [DOI] [PubMed] [Google Scholar]
- 26.Leone, T. C., Weinheimer, C. J. & Kelly, D. P. (1999) Proc. Natl. Acad. Sci. USA 96, 7473-7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemberger, T., Staels, B., Saladin, R., Desvergne, B., Auwerx, J. & Wahli, W. (1994) J. Biol. Chem. 269, 24527-24530. [PubMed] [Google Scholar]
- 28.Pandak, W. M., Heuman, D. M., Redford, K., Stravitz, R. T., Chiang, J. Y., Hylemon, P. B. & Vlahcevic, Z. R. (1997) J. Lipid Res. 38, 2483-2491. [PubMed] [Google Scholar]
- 29.Castro Cabezas, M., Halkes, C. J. & Erkelens, D. W. (2001) Nutr. Metab. Cardiovasc. Dis. 11, 134-142. [PubMed] [Google Scholar]
- 30.Smithies, O. & Kim, H. S. (1994) Proc. Natl. Acad. Sci. USA 91, 3612-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman, N., Chisholm, D., Pi-Sunyer, X. & Schneider, S. (1998) Diabetes 47, 699-713. [DOI] [PubMed] [Google Scholar]
- 32.Marchesini, G., Brizi, M., Bianchi, G., Tomassetti, S., Bugianesi, E., Lenzi, M., McCullough, A. J., Natale, S., Forlani, G. & Melchionda, N. (2001) Diabetes 50, 1844-1850. [DOI] [PubMed] [Google Scholar]
- 33.Bremer, J. (2001) Prog. Lipid Res. 40, 231-268. [DOI] [PubMed] [Google Scholar]
- 34.Giorgino, F., Almahfouz, A., Goodyear, L. J. & Smith, R. J. (1993) J. Clin. Invest. 91, 2020-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart, P. M., Toogood, A. A. & Tomlinson, J. W. (2001) Horm. Res. 56, Suppl. 1, 1-6. [DOI] [PubMed] [Google Scholar]
- 36.Olefsky, J. M., Johnson, J., Liu, F., Jen, P. & Reaven, G. M. (1975) Metabolism 24, 517-527. [DOI] [PubMed] [Google Scholar]
- 37.Saad, M. J., Folli, F. & Kahn, C. R. (1995) Endocrinology 136, 1579-1588. [DOI] [PubMed] [Google Scholar]
- 38.Yoon, J. C., Puigserver, P., Chen, G., Donovan, J., Wu, Z., Rhee, J., Adelmant, G., Stafford, J., Kahn, C. R., Granner, D. K., et al. (2001) Nature 413, 131-138. [DOI] [PubMed] [Google Scholar]
- 39.Bernal-Mizrachi, C., Weng, S., Feng, C., Finck, B. N., Knutsen, R. H., Leone, T. C., Coleman, T., Mecham, R. P., Kelly, D. P. & Semenkovich, C. F. (2003) Nat. Med. 9, 1069-1075. [DOI] [PubMed] [Google Scholar]
- 40.McGarry, J. D. (1992) Science 258, 766-770. [DOI] [PubMed] [Google Scholar]
- 41.Taskinen, M. R., Nikkila, E. A., Pelkonen, R. & Sane, T. (1983) J. Clin. Endocrinol. Metab. 57, 619-626. [DOI] [PubMed] [Google Scholar]
- 42.Stulnig, T. M., Oppermann, U., Steffensen, K. R., Schuster, G. U. & Gustafsson, J. A. (2002) Diabetes 51, 2426-2433. [DOI] [PubMed] [Google Scholar]
- 43.Hermanowski-Vosatka, A., Gerhold, D., Mundt, S. S., Loving, V. A., Lu, M., Chen, Y., Elbrecht, A., Wu, M., Doebber, T., Kelly, L., et al. (2000) Biochem. Biophys. Res. Commun. 279, 330-336. [DOI] [PubMed] [Google Scholar]
- 44.Ide, T., Shimano, H., Yoshikawa, T., Yahagi, N., Amemiya-Kudo, M., Matsuzaka, T., Nakakuki, M., Yatoh, S., Iizuka, Y., Tomita, S., et al. (2003) Mol. Endocrinol. 17, 1255-1267. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa, T., Ide, T., Shimano, H., Yahagi, N., Amemiya-Kudo, M., Matsuzaka, T., Yatoh, S., Kitamine, T., Okazaki, H., Tamura, Y., et al. (2003) Mol. Endocrinol. 17, 1240-1254. [DOI] [PubMed] [Google Scholar]
- 46.Kurihara, I., Shibata, H., Suzuki, T., Ando, T., Kobayashi, S., Hayashi, M., Saito, I. & Saruta, T. (2002) Mol. Cell. Endocrinol. 189, 181-189. [DOI] [PubMed] [Google Scholar]
- 47.Li, X., Wong, J., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (2003) Mol. Cell. Biol. 23, 3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song, C. & Liao, S. (2000) Endocrinology 141, 4180-4184. [DOI] [PubMed] [Google Scholar]
- 49.Peet, D. J., Turley, S. D., Ma, W., Janowski, B. A., Lobaccaro, J. M., Hammer, R. E. & Mangelsdorf, D. J. (1998) Cell 93, 693-704. [DOI] [PubMed] [Google Scholar]
- 50.Pineda Torra, I., Claudel, T., Duval, C., Kosykh, V., Fruchart, J. C. & Staels, B. (2003) Mol. Endocrinol. 17, 259-272. [DOI] [PubMed] [Google Scholar]
- 51.Alberts, P., Engblom, L., Edling, N., Forsgren, M., Klingstrom, G., Larsson, C., Ronquist-Nii, Y., Ohman, B. & Abrahmsen, L. (2002) Diabetologia 45, 1528-1532. [DOI] [PubMed] [Google Scholar]
- 52.Fletcher, J. M. (1986) Biochem. J. 238, 459-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman, M. R., Horwitz, B. A. & Stern, J. S. (1986) Am. J. Physiol. 250, R595-R607. [DOI] [PubMed] [Google Scholar]
- 54.Zambon, A., Deeb, S. S., Pauletto, P., Crepaldi, G. & Brunzell, J. D. (2003) Curr. Opin. Lipidol. 14, 179-189. [DOI] [PubMed] [Google Scholar]
- 55.Chang, E. & Perlman, A. J. (1987) Endocrinology 121, 513-519. [DOI] [PubMed] [Google Scholar]
- 56.Kim, H. S., Krege, J. H., Kluckman, K. D., Hagaman, J. R., Hodgin, J. B., Best, C. F., Jennette, J. C., Coffman, T. M., Maeda, N. & Smithies, O. (1995) Proc. Natl. Acad. Sci. USA 92, 2735-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain, S., Tang, X., Narayanan, C. S., Agarwal, Y., Peterson, S. M., Brown, C. D., Ott, J. & Kumar, A. (2002) J. Biol. Chem. 277, 36889-36896. [DOI] [PubMed] [Google Scholar]