Abstract
Imbalance between production and scavenging of superoxide anion results in hypertension by the inactivation of nitric oxide, and the increased oxidative stress from the resultant peroxynitrite that is produced promotes inflammatory processes such as atherosclerosis. Induction of phase 2 proteins promotes oxidant scavenging. We hypothesized that intake of dietary phase 2 protein inducers would ameliorate both hypertension and atherosclerotic changes in the spontaneously hypertensive stroke-prone rat. For 5 days/week for 14 weeks, we fed rats 200 mg/day of dried broccoli sprouts that contained glucoraphanin, which is metabolized into the phase 2 protein-inducer sulforaphane (Group A), sprouts in which most of the glucoraphanin was destroyed (Group B), or no sprouts (Group C). After 14 weeks of treatment, no significant differences were seen between rats in Groups B and C. Rats in Group A had significantly decreased oxidative stress in cardiovascular and kidney tissues, as shown by increased glutathione (GSH) content and decreased oxidized GSH, decreased protein nitrosylation, as well as increased GSH reductase and GSH peroxidase activities. Decreased oxidative stress correlated with better endothelial-dependent relaxation of the aorta and significantly lower (20 mm Hg) blood pressure. Tissues from Groups B and C had considerable numbers of infiltrating activated macrophages, indicative of inflammation, whereas animals in Group A had few detectable infiltrating macrophages. There is interest in dietary phase 2 protein inducers as means of reducing cancer incidence. We conclude that a diet containing phase 2 protein inducers also reduces the risk of developing cardiovascular problems of hypertension and atherosclerosis.
Oxidative stress has been implicated as a causal factor in diseases such as hypertension (1–3) and atherosclerosis (4). Increased production, or decreased scavenging, of oxidants such as superoxide anion can give rise to hypertension by superoxide interacting with nitric oxide, forming peroxynitrite (5–7), thereby decreasing nitric oxide availability for smooth muscle relaxation function. The peroxynitrite formed is a strong oxidant that promotes inflammatory processes by activating transcriptional factor complexes such as NF-κB (8). Such inflammatory processes result in the formation of atherosclerotic lesions (9). In principle, decreasing oxidative stress and associated inflammation should provide the beneficial effect of preventing and/or inhibiting the development of these cardiovascular diseases.
Central in scavenging strong oxidants is the tripeptide glutathione (GSH) (10). Indeed, GSH depletion alone will result in development of severe hypertension (11). We have shown (12) that smooth muscle cells from spontaneously hypertensive rats (SHR), in comparison with those from normotensive rats, have increased oxidative stress that was correlated with lower GSH levels. We have also shown that increasing GSH content in SHR smooth muscle cells greatly decreased the oxidative stress in these cells (12). We decreased oxidative stress by exposing the cells to the phase 2 protein inducer sulforaphane. Sulforaphane (4-methylsulfinylbutyl isothiocyanate) is the most potent phase 2 protein inducer found in our food sources (13). A major action of phase 2 proteins, mainly enzymes, is to inactivate electrophiles and strong oxidants (14); hence, there has been a research interest in dietary phase 2 protein inducers as a means to prevent cancers (15).
Methods
We have argued that dietary phase 2 protein inducers should ameliorate conditions such as hypertension and atherosclerosis (16). The objective of the present research was to test this idea. The animal model chosen was the stroke-prone SHR (SHRsp). This strain develops severe hypertension (17) that is related to increased peroxynitrite formation (6) and develops inflammatory changes in blood vessels (18, 19). We used Sprague–Dawley (SD) rats as the normotensive controls. We chose broccoli sprouts as the source of phase 2 protein inducer because certain cultivars of broccoli have been shown to be high in the glucosinolate (GS) glucoraphanin (Grn) that is metabolized into the phase 2 protein-inducing isothiocyanate sulforaphane (13). GS are β-thioglucoside N-hydroxysulfates present in 16 families of higher plants with the distribution in the family Brassicaceae, particularly the genus Brassica, which is well described in ref. 20. Grn is not available in sufficient quantities to perform dietary experiments. We, therefore, chose to feed animals air-dried broccoli sprouts of the calabrese cultivar that we had determined were high in Grn and low in antinutritive GS. As a placebo control, we fed animals with broccoli sprouts that were frozen, thawed, and dried. This procedure allowed the myrosinase enzyme in the sprouts to access the GS and hydrolyze them (21). The basic difference between the two forms of dried sprouts is the presence of GS in the former and low levels of GS in the latter.
Supporting Information. For additional details on methods, see Supporting Methods, which is published as supporting information on the PNAS web site.
Broccoli Sprout Preparation and GS Measurements. Broccoli seeds, obtained from Mumm's Seeds and Sprouting (Shellbrook, Saskatoon, SK, Canada), were sprouted according to the supplier's instructions and screened for GS content by using HPLC, as outlined in ref. 22. For calibration purposes, a small amount of Grn was obtained from Ian McGregor (Agriculture and Agrifood Canada, Saskatoon Research Station, SK, Canada). The calabrese cultivar was selected because of its major Grn peak and low levels of antinutritive GS. We either air-dried 4-day broccoli sprouts at room temperature for 7 days before feeding the animals, or we froze, thawed, and dried them at 60°C.
Phase 2 enzyme-inducing activity was measured by using a bioassay, as outlined in ref. 13, for the two broccoli sprout preparations.
Animals and Tissue Preparation. We used male SHRsp (originally obtained from the National Institutes of Health, Bethesda, and maintained by the University of Saskatchewan Animal Facilities) and male SD rats (Charles River Breeding Laboratories, St. Constant, Quebec). All animals were treated in accordance with the Canadian Council on Animal Care Standards. We placed 5-week-old rats on AIN-93 defined diet (23), containing no synthetic antioxidants, and with an 18% casein-based protein content (Dyets, Bethlehem, PA). Each strain was divided into three groups (n = 8–10 rats per group) and treated for 14 weeks as follows: control diet (CT), AIN93 diet only; Grn+, AIN93 diet plus 200 mg of air-dried broccoli sprouts; Grn-, AIN93 diet plus 200 mg of broccoli sprouts in which GS were destroyed. Animals were housed individually, and 5 days each morning during the working week, the Grn+ and Grn- groups were fed 200 mg of the appropriate dried sprouts. Systolic blood pressures were measured biweekly by using an indirect tail-cuff plethysmography as described (24).
At the end of study, anesthetized animals were perfused with buffered saline, and aorta, carotid artery, heart, and kidneys were removed. Some aorta tissues were used immediately for isometric tension studies, and the remaining tissues were frozen in liquid nitrogen. The frozen tissues were ground in the frozen state, and homogenized tissue powders were used for GSH analyses, enzyme assays, and Western blotting.
Rats used for immunohistochemical studies were further perfused with a solution containing 4% paraformaldehyde. Aorta, carotid artery, heart, and kidney were dissected out, kept in same fixative overnight, frozen, cut into sections (10–12 μm), collected on gelatin-chrome alum-coated slides, and stored at -20°C until further processing.
Isometric Tension Studies. The thoracic aortae were cut into 2- to 3-mm rings and suspended under 1.0 g of tension in a 10-ml organ bath chamber filled with Krebs' saline solution aerated with 95%O2/5%CO2. Relaxation properties were measured as described (25).
Measurements of GSH, Oxidized GSH (GSSG), and Enzyme Activities. GSH and oxidized GSH content were determined by using the HPLC method described in refs. 26 and 27. The activities of GSH peroxidase (GPx), GSH reductase (GRed), and GST were measured as described (12). NQ01, NAD(P)H quinone reductase (QR) activity was measured as outlined in ref. 28. Protein content was determined by using the bicinchoninic acid procedure with BSA as reference (29).
Immunohistochemistry. Frozen sections were immunostained by using primary Abs and either Cy3-fluorescence (Jackson ImmunoResearch) or the avidin–biotin–peroxidase (Vector Laboratories) detection systems. Primary Abs used were ED-1 (Serotec), marker for activated macrophages, and anti-NF-κB p65 (Transduction Laboratories, Lexington, KY) that recognized the nuclear localization signal on p65. For quantification of inflammation in the kidney medulla, ED-1-positive cells were counted in 15 high-power microscopic fields (×400) per section, with six sections randomly selected per animal (n = 5 in each group).
Western Blot Analysis. Proteins were separated on polyacrylamide gels by using a minivertical electrophoresis system (Bio-Rad) and transblotted onto polyvinyldifluoridene membranes, and membranes were then incubated with the appropriate primary Abs, followed by incubation with a horseradish peroxidase-conjugated secondary Ab (Bio-Rad) as done before (12). Proteins were visualized by using Chemiluminescent substrate kit (Amersham Biosciences) and quantified by using the un-scan-it gel automated digitizing system (version 5.1, Silk Scientific, Orem, UT). The membrane was reprobed with anti-β-actin (Sigma). Quantification was relative to the β-actin. For quantification of nitrosylated proteins, a mouse IgG mAb (Upstate Biotechnology, Lake Placid, NY) was used. For quantification of nuclearly localized NF-κB p65, nuclear extracts were obtained by using the NE-PER nuclear and cytoplasmic extraction kit (Pierce) as described (27).
Data Analysis. Data are expressed as means ± SEM and analyzed by using Student's t test or ANOVA in conjunction with the Newman–Keuls test where applicable. Differences between groups were considered statistically significant at P < 0.05.
Results
Broccoli Sprout Preparations. Our ability to deliver Grn, the GS precursor of the powerful phase 2 protein inducer sulforaphane, was limited by the unavailability of pure Grn in adequate quantities for chronic animal experiments. Young broccoli sprouts grown from suitable seeds are an extremely concentrated source of Grn (13). Slow air-drying of broccoli sprouts at room temperature for 7 days provides a dry product that is avidly consumed by rats, and was used for the Grn+ experiments. HPLC analyses of the desulfoglucosinolates obtained from extracts showed nine major GS peaks, of which seven were identified (see Fig. 7A, which is published as supporting information on the PNAS web site). Estimates of the relative amounts of GS by peak integration were as follows: glucoiberin, 3-(methylsulfinyl)propyl-GS, 13.4%; Grn, 4-(methylsulfinyl)butyl-GS, 35.1%; glucobrassicanapin, 4-pentenyl-GS, 8.0%; and gluconasturtiin, 2-phenethyl-GS, 14.1%. The antinutritive GS progoitrin (2-hydroxy-3-butenyl-GS, 2.8%) and sinigrin (2-propenyl-GS, 1.0%) were only minor components, as were the two unknowns (6.53% and 3.3%). By using desulfoglucoraphanin that we had collected by using HPLC as a reference, we estimated that these sprouts contained ≈12 μmol of Grn per gram of dry weight. In contrast, no GS were detected in extracts of broccoli sprouts that were frozen, thawed, and then dried at 60°C. The latter preparations were used for the Grn- diets (see Fig. 7B).
To characterize these dried sprout preparations further, bioassays of the inducer potencies of extracts were carried out by described methods (13, 28). The Grn+ preparation treated with purified myrosinase contained 909,000 units of inducer activity per gram (equivalent to the inducer activity of 27.3 μmol of sulforaphane per gram). No inducer activity was detected in the absence of added myrosinase, indicating that little, if any, GS hydrolysis had occurred during the drying at room temperature. Because sulforaphane has ≈10 times greater inducer activity than other isothiocyanates (30) and Grn is the predominant GS in the preparation, one would have expected a better correlation between Grn quantification and sulforaphane-equivalents inducer activity. We now know that this discrepancy is due to suboptimal extraction of GS from our preparations during the analytical procedure (31).
Extracts of the Grn- preparations, which contained no detectable GS by the desulfoglucosinolate HPLC method, had phase 2 protein inducer activities of 16,700 and 76, 900 units per gram, before and after myrosinase treatment, respectively. These values correspond to the inducer equivalent of 0.5 and 2.31 μmol sulforaphane, respectively. The Grn- preparation therefore contains ≈ 8.4% of the inducer activity of the Grn+ preparation, and there is, thus, a more than one order of magnitude difference in the GS content of these preparations. We conclude that the freezing, thawing, and drying at 60°C released the endogenous plant myrosinase, which hydrolyzed most of the GS to isothiocyanates, which were then inactivated probably by binding to proteins and other intracellular components. The Grn- preparation is, therefore, an appropriate control for the Grn+ preparation.
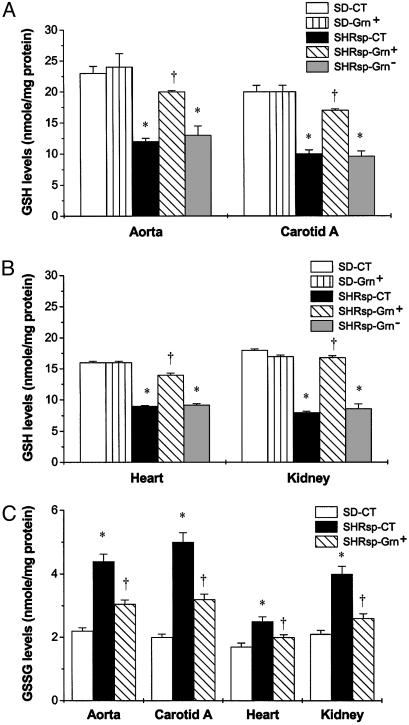
Up-Regulation of GSH Levels and Down-Regulation of Oxidative Stress by Broccoli Sprout Consumption in SHRsp. In rats on CT, the basal levels of GSH in blood vessels, heart, and kidney were significantly lower in SHRsp than in SD (Fig. 1 A and B). Although the Grn+ diet had no effect on GSH levels in SD tissues, it resulted in significant increases (≈1.5- to 2-fold) in SHRsp tissues (Fig. 1 A and B). The Grn- diet had no effect on GSH levels in the tissues of SHRsp. In contrast, GSSG levels were higher in these tissues in SHRsp than SD rats on CT (Fig. 1C). The Grn+ diet caused a significant decrease in tissue GSSG in SHRsp (Fig. 1C). The increase in GSH and decrease in GSSG caused by the Grn+ diet indicates a decrease in tissue oxidative stress. Congruent with increased oxidative stress was the observation that SHRsp kidney tissues had a prominent 45-kDa nitrosylated protein band (see Fig. 8, which is published as supporting information on the PNAS web site). SHRsp on Grn+ diet had a 37% reduction in this nitrosylated protein compared with rats on control and Grn- diets.
Fig. 1.
Effect of Grn+ and Grn- diets compared with CT on GSH and GSSG in cardiovascular and kidney tissues of 19-week-old SHRsp and SD rats, n = 3–4 rats per group. Grn+ diet had no effect compared with CT in SD rats (A and B). SHRsp-CT and SHRsp-Grn- had significantly lower levels of GSH (A and B). SHRsp-CT had significantly higher levels of GSSG (C) in tissues examined compared with SD rats. When placed on the Grn+ diet, SHRsp rats demonstrated significant increases in GSH levels in all tissues examined (A and B). A Grn+ diet also decreased GSSG levels significantly in cardiovascular and kidney tissues in SHRsp (C). *, P < 0.05, for SHPsp controls or SHRsp-Grn- versus SD-CT and SD-Grn+; †, P < 0.05, for SHRsp-Grn+ versus SHRsp-Grn-.
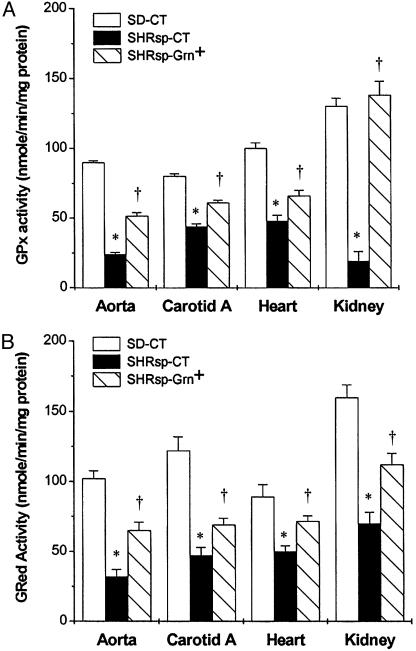
GSH-Related Enzyme and NQ01 NAD(P)H QR Activities. Basal activities of GPx (Fig. 2A) and GRed (Fig. 2B) were significantly lower in aorta, carotid artery, heart, and kidney from SHRsp-CT than from SD-CT. In animals on the Grn+ diet, the enzyme activities of GPx and GRed were significantly higher than in SHRsp-CT (Fig. 2 A and B). There were no significant differences in GST in the cardiovascular or kidney tissues among the various experimental groups (data not presented). Because cardiovascular and kidney tissues were completely used in the other assays, QR activity was measured in liver. The activity was low in the male SHRsp, and the Grn+ diet did not affect the activity (14.6 ± 0.9 units in Grn+ and 14.5 ± 2.1 units in Grn-). In contrast, SD values were significantly higher in animals on Grn+ diet (96.6 ± 9.2 units) than on Grn- diet (60.8 ± 14 units).
Fig. 2.
GPx and GRed activities in cardiovascular and kidney tissues of 19-week-old SHRsp and SD rats; n = 3–4 per group. GPx (A) and GRed (B) activities are significantly lower in all tissues examined in SHRsp on CT compared with SD rats on CT. SHRsp fed Grn+ diet had significantly increased GPx and GRed activities. *, P < 0.05, for SHRsp-CT versus SD-CT; †, P < 0.05, for SHPsp-Grn+ versus SHRsp-CT.
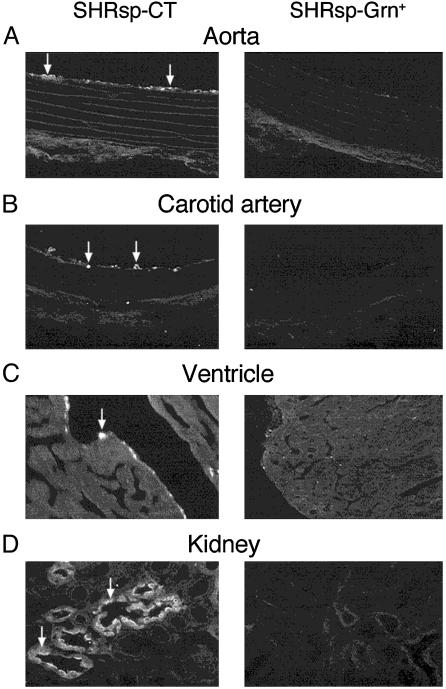
Decrease of Macrophage Infiltration by Broccoli Sprout Consumption in SHRsp. In SHRsp-CT, activated macrophages could be identified in the inner intimal layers of the aorta, carotid artery, and endocardium of the heart, as well as in the kidney medullary interstitium and tubules (Fig. 3), indicating an inflammatory state in these structures. Few activated macrophages were seen in the cardiovascular system in SHRsp animals on Grn+ diet (Fig. 3 A–C). There were also significantly fewer (P = 0.026) activated macrophages in the kidney medulla of SHRsp on Grn+ diet (3.4 ± 0.9 cells per microscope field) than in SHRsp on Grn- diet (7.8 ± 1.0 cells per microscope field). Activated macrophages were not detectable in these tissues in the SD rats.
Fig. 3.
Representative micrographs demonstrating localization of activated macrophages (arrows indicate bright fluorescent cells) in the inner tissue linings of the aorta (A), carotid artery (B), and ventricle of the heart (C), as well as in the medulla of the kidney (D) in 19 week-old SHRsp on CT or Grn+ diet. Few such activated macrophages are present in tissues of animals on Grn+ diet.
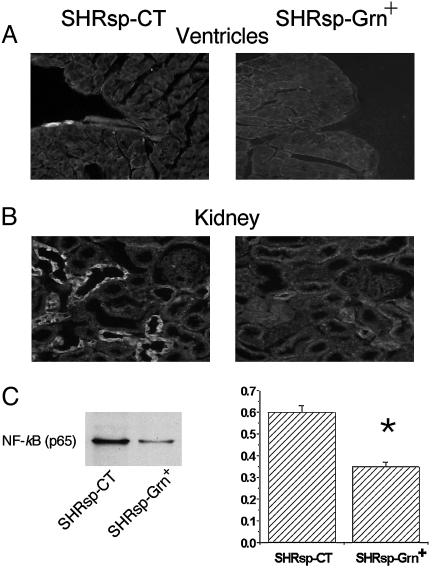
Decreased Activation of Nf-κB by Broccoli Sprout Consumption in SHRsp. Inflammation is associated with activation of the transcriptional factor complex NF-κB (32). In SHRsp on CT, nuclei containing NF-κB p65 were seen in the endocardium of heart (Fig. 4A) and tubules of the kidney medulla (Fig. 4B). Few cells expressed nuclearly localized NF-κB p65 in these organs in SHRsp on Grn+ diet. Western blot analysis demonstrated that nuclearly localized NF-κB p65 was significantly decreased by 40% in kidneys from SHRsp-Grn+ compared with SHRsp-CT.
Fig. 4.
Micrographs (A and B) and Western blot (C) showing the localization of activated NF-κB in SHRsp fed CT or Grn+ diet in heart ventricle (A) and kidney medulla (B and C) of 19-week-old animals. Positive nuclear NF-κB p65 signal was observed in ventricular endothelium and in the tubules of the kidney of SHRsp-CT with few positive cells seen in the SHRsp-Grn+ group. (C) Left shows a representative Western blot of nuclearly localized NF-κB p65 is shown on the left, whereas Right quantification of NF-κB p65 relative to β-actin (y axis depicts relative units). Data taken from kidney tissues of three animals per group. *, P < 0.05, for SHRsp-Grn+ versus SHRsp-CT.
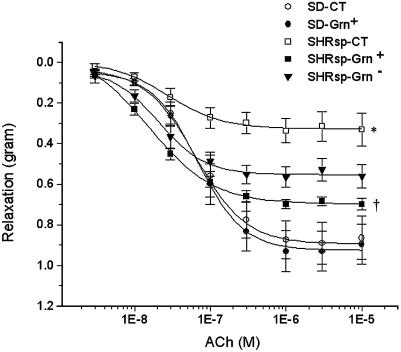
Prevention of Vascular Endothelium Dysfunction and Hypertension by Broccoli Sprout Consumption in SHRsp. Endothelial nitric oxide function was examined by precontracting aortic rings with phenylephrine, followed by exposure to acetylcholine (Fig. 5A). There was a concentration dependent decrease in tension in SD rats. There were no differences in relaxation with SD rats on CT, compared with Grn+ diet. In both cases there was an ≈0.9-g relaxation force. SHRsp-CT had a maximal relaxation of ≈0.33 g in response to acetylcholine, whereas rats on Grn+ diet had a significantly better mean relaxation of 0.70 g. SHRsp-Grn- had a mean relaxation of 0.57 g, which was significantly different from SHRsp-CT but only borderline significantly different (P = 0.0538) from SHRsp-Grn+.
Fig. 5.
Aortic-relaxation function in SD rats on CT; SD on Grn+ diet; and SHRsp on CT, Grn+ diet, and Grn- diet (n = 9 per group). Aortic rings were precontracted with phenylephrine, followed by administration of acetylcholine (ACh), and decrease in tension was measured. Diet had no effect on relaxation properties in SD rats. SHRsp-CT had significantly poorer relaxation responses to ACh than either of the SD groups. SHRsp-Grn+ had significantly better relaxation response to ACh than SHRsp-CT, whereas SHRsp-Grn- had an intermediate response. *, P < 0.001, for SHRsp-Grn- versus SD-CT and SD-Grn+. †, P < 0.001, for SHRsp-Grn+ versus SHRsp-CT.
The ability of the nitric oxide donor sodium nitroprusside to cause a decrease in tension in aortic rings that were precontracted with phenylephrine was examined (see Fig. 9, which is published as supporting information on the PNAS web site). There was a dose-dependent decrease in tension in SD rats with a maximal decrease in tension of ≈0.98 g of force. The aortic rings obtained from all SHRsp diet groups had a significant impairment in relaxation in response to SNP with a maximal decrease in tension of ≈0.6 g.
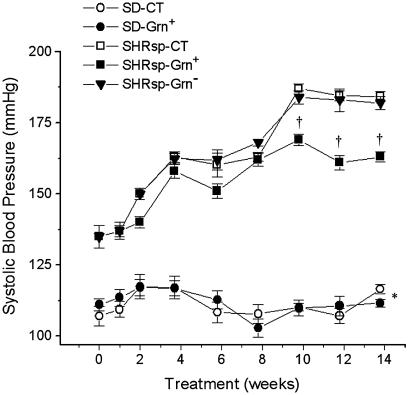
Systolic blood pressure was significantly lower in SD rats compared with SHRsp and did not change over the course of the experiment, regardless of diet. In SHRsp, the systolic blood pressure was 135 ± 4.6-mm Hg at the beginning of the dietary intervention (Fig. 6). This increased to 163 ± 1.8-mm Hg after 8 weeks on Grn+ and showed no further increase to the end of study. In contrast, blood pressures of SHRsp-CT and SHRsp-Grn- were significantly higher (184 ± 1.8-mm and 182 ± 2.3-mm Hg, respectively) by the end of the study.
Fig. 6.
Systolic blood pressures over time in SD on CT; SD on Grn+ diet; and SHRsp on CT, Grn+ diet, and Grn- diet (n = 9 per group). No change in blood pressure is seen in SD over the 14-week period after weaning. Blood pressures progressively rise in all SHRsp groups for the first 8 weeks on the postweaning diet and are significantly higher than in SD rats. From 8 to 10 weeks, blood pressures rise further in SHRsp-CT and SHR-Grn- and then plateau. From 10 weeks of the dietary intervention onwards, blood pressure is significantly lower in SHRsp-Grn+ than in the two other SHRsp dietary groups. *, P < 0.05, for SD diet groups versus SHRsp diet groups; †, P < 0.05, for SHRsp-Grn+ vs. SHRsp-CT and SHRsp-Grn-.
Discussion
SHRsp fed a Grn-containing diet had a major improvement in the cardiovascular system, as demonstrated by decrease in the rise in blood pressure and inflammation. Unlike the normotensive SD rats for which blood pressures remained constant during the experimental period, blood pressures in all three diet groups of SHRsp increased progressively until the animals were 13 weeks of age. Thereafter, animals on the Grn+ diet had significantly lower blood pressures than in the two other diet groups.
The hypothesis initiating this research was that animals on a Grn+ diet would have decreased oxidative stress. This hypothesis was upheld. An index of oxidative stress is the GSH/GSSG ratio. Normotensive SD rats on control or Grn+ diets had ratios in the different tissues ranging from 17.1 to 20.9, SHRsp-CT had ratios ranging from 4.0 to 7.2, and SHRsp-Grn+ had significantly higher ratios ranging from 10.6 to 14.0. A decrease in oxidative stress with Grn+ is supported also by the increase in GPx activity in different tissues in SHRsp-Grn+. GPx is an enzyme that is susceptible to oxidative inactivation (33). Also in support of our hypothesis is the decrease in quantity of the 45-kDa nitrosylated protein band in kidney tissue.
Higher oxidative stress in blood vessels should result in poorer endothelial function. Indeed, this effect is what we see with SHRsp on CT, as determined by ability of acetylcholine to cause only a 37% relaxation in phenylephrine-constricted aortae when compared with ability of SD aortae to relax. SHRsp on Grn+ diet had a response that was 78% of that of SD. Interestingly, SHRsp on Grn- diet had an endothelial cell response that was considerably better (68% of normotensive animals) than SHRsp on CT, but this better endothelial response did not translate into lower blood pressures.
One source of oxidative stress is increased expression and activity of NAD(P)H oxidase that produces superoxide anion radicals (34). The expression of NAD(P)H oxidase proteins increases greatly after 15 weeks of age in both SHRsp and related SHR strain (35, 36). Inhibition of the activity of NAD(P)H oxidase has been shown to improve endothelial function (37). Hence, possibly what the Grn+ diet may be promoting is scavenging of such increased production of superoxide anion in the older SHRsp. It is known that endothelial nitric oxide synthase is also down-regulated in SHRsp and that inhibiting advanced glycation endproduct formation increases endothelial nitric oxide synthase expression (38). It is possible that the decreased vascular oxidative stress seen in our study also promoted endothelial nitric oxide synthase expression. The decrease of 11% in systolic blood pressure in SHRsp-Grn+ compared with SHRsp-CT is similar to the decrease in SHRsp blood pressure seen when oxidative stress was minimized by feeding rats a pharmacological inhibitor of advanced glycation endproduct formation (38).
Higher oxidative stress should also increase activation of NF-κB promoting inflammatory responses. Indeed, we have shown that there is an increase in the activation of NF-κB in numerous tissues in SHRsp compared with SD rats and that a Grn+ diet decreases NF-κB activation. Decreased NF-κB activation correlated with decreased tissue infiltration of activated macrophages. Increased strong oxidant production in blood vessels and NF-κB activation plus infiltration of activated macrophages into tissues such as kidney is also seen in rats made hypertensive with deoxycorticosterone acetate (DOCA) (39). As with our study, DOCA rats exhibited infiltration of kidney tubules by activated macrophages. DOCA rats treated long-term with the synthetic antioxidants pyrrolidinedithiocarbamate or 4-hydroxy-2,2,6,6-tetramethyl piperidinoxyl have a decreased NF-κB activation and activated macrophage infiltration in kidneys (39). In these animals, the long-term administration of antioxidants also inhibited the rise in blood pressure.
Are the effects seen in SHRsp fed Grn+ diet due to increased expression of phase 2 proteins? Because of the centrality of GSH- and GSH-using enzymes in the antioxidant defense (10), we focused on this system. The rate-limiting enzyme for GSH synthesis, γ-glutamyl-cysteine ligase, belongs to the phase 2 gene synexpression group (40). That Grn+ diet had no effect on GSH levels in SD rats does not necessarily mean that γ-glutamyl-cysteine ligase was not induced because there is feedback inhibition of γ-glutamyl-cysteine ligase activity by GSH (41). That the increased GSH/GSSG ratio may be due to induction of phase 2 proteins is supported by the observation that another phase 2 enzyme, GRed (42), is also up-regulated. However, QR activity was not induced in liver tissues in the SHR-Grn+ compared with SHRsp-CT, although we did demonstrate an increase in hepatic QR activity in the SD-Grn+ compared with SD-CT. Our findings are supportive of the idea that one consequence of the Grn+ diet is an up-regulation of phase 2 protein gene expression.
Our results show that intake of broccoli sprouts high in Grn, whose metabolite sulforaphane is a potent phase 2 protein inducer, decreased oxidative stress and inflammation in kidneys and the cardiovascular system. The promotion of cardiovascular function is similar to that seen with long-term consumption of pharmacological antioxidants. In the present study, two doses were examined, 0.5 and 5.5 μmol sulforaphane equivalents per rat per day. An effect on oxidative stress parameters and blood pressure was seen only with the higher dose, although some positive effects on endothelial function was seen with the lower dose. It may be true that even more profound effects might be seen with higher intake of Grn. Whether the positive effects seen are due to induction of phase 2 proteins is not yet clear, and it may be true that Grn affects the GSH-related antioxidant system in some other way.
In 1995, the direct costs of hypertension to the health care system in the United States of America was almost 20 billion dollars (43). Our results show that even a modest change in diet has the potential to have a major impact on health and reduce health care costs significantly.
Supplementary Material
Acknowledgments
We thank Arlene Drimmie, Anita Quon, Koleen Safiniuk, Connie Wong, and Yi Qian Wang for their excellent technical assistance. We especially thank Drs. J. W. Fahey and P. Talalay (The Johns Hopkins University School of Medicine, Baltimore) for their analyses of phase 2 enzyme-inducing activity in our sprout preparations, and Dr. Albena Dinkova-Kostova for advice on the QR assay. This work was supported by the Saskatchewan Agriculture Development Fund.
Abbreviations: CT, control diet; GPx, GSH peroxidase; GRed, GSH reductase; Grn, glucoraphanin; GS, glucosinolate; GSH, glutathione; GSSG, oxidized GSH; QR, quinone reductase; SD, Sprague–Dawley; SHR, spontaneously hypertensive rats; SHRsp, stroke-prone SHR.
References
- 1.Russo, C., Olivieri, O., Girelli, D., Faccini, G., Zenari, M. L., Lombardi, S. & Corrocher, R. (1998) J. Hypertens. 16, 1267-1271. [DOI] [PubMed] [Google Scholar]
- 2.Cai, H. & Harrison, D. G. (2000) Circ. Res. 87, 840-844. [DOI] [PubMed] [Google Scholar]
- 3.Berry, C., Brosnan, M. J., Fennell, J., Hamilton, C. A. & Dominiczak, A. F. (2001) Curr. Opin. Nephrol. Hypertens. 10, 247-255. [DOI] [PubMed] [Google Scholar]
- 4.Kunsch, C. & Medford, R. M. (1999) Circ. Res. 85, 753-766. [DOI] [PubMed] [Google Scholar]
- 5.Taddei, S., Virdis, A., Ghiadoni, L., Salvetti, G. & Salvetti, A. (2000) J. Nephrol. 13, 205-210. [PubMed] [Google Scholar]
- 6.Ma, X. L., Gao, F., Nelson, A. H., Lopez, B. L., Christopher, T. A., Yue, T. L. & Barone, F. C. (2001) J. Pharmacol. Exp. Ther. 298, 879-885. [PubMed] [Google Scholar]
- 7.Zalba, G., Beaumont, F. J., San Jose, G., Fortuno, A., Fortuno, M. A. & Diez, J. (2001) Nephrol. Dial. Transplant. 16, 2-5. [DOI] [PubMed] [Google Scholar]
- 8.Cooke, C. L. & Davidge, S. T. (2002) Am. J. Physiol. 282, C395-C402. [DOI] [PubMed] [Google Scholar]
- 9.Lusis, A. J. (2000) Nature 407, 233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juurlink, B. H. J. (1999) Neurotox. Res. 1, 119-140. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri, N. D., Wang, X. Q., Oveisi, F. & Rad, B. (2000) Hypertension 36, 142-146. [DOI] [PubMed] [Google Scholar]
- 12.Wu, L. & Juurlink, B. H. (2001) J. Hypertens. 19, 1819-1825. [DOI] [PubMed] [Google Scholar]
- 13.Fahey, J. W., Zhang, Y. & Talalay, P. (1997) Proc. Natl. Acad. Sci. USA 94, 10367-10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prestera, T., Holtzclaw, W. D., Zhang, Y. & Talalay, P. (1993) Proc. Natl. Acad. Sci. USA 90, 2965-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talalay, P. & Fahey, J. W. (2001) J. Nutr. 131, 3027s-3033s. [DOI] [PubMed] [Google Scholar]
- 16.Juurlink, B. H. J. (2001) Can. J. Physiol. Pharmacol. 79, 266-282. [PubMed] [Google Scholar]
- 17.Nagaoka, A., Iwatsuka, H., Suzuoki, Z. & Okamoto, K. (1976) Am. J. Physiol. 230, 1354-1359. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y., Jacobowitz, D. M., Barone, F., Mccarron, R., Spatz, M., Feuerstein, G., Hallenbeck, J. M. & Siren, A. L. (1994) J. Cereb. Blood Flow Metab. 14, 348-352. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., Liu, T., Mccarron, R. M., Spatz, M., Feuerstein, G., Hallenbeck, J. M. & Siren, A. L. (1996) Am. J. Physiol. 270, H2125-H2131. [DOI] [PubMed] [Google Scholar]
- 20.Fahey, J. W., Zalcmann, A. T. & Talalay, P. (2001) Phytochemistry 56, 5-51. [DOI] [PubMed] [Google Scholar]
- 21.Wittstock, U. & Halkier, B. A. (2002) Trends Plant. Sci. 7, 263-270. [DOI] [PubMed] [Google Scholar]
- 22.Minchinton, I., Sang, J., Burke, D. & Truscott, R. J. (1982) J. Chromatogr. 247, 141-148. [Google Scholar]
- 23.Reeves, P. G., Nielsen, F. H. & Fahey, G. C., Jr. (1993) J. Nutr. 123, 1939-1951. [DOI] [PubMed] [Google Scholar]
- 24.Wang, R., Wang, Z., Wu, L., Hanna, S. T. & Peterson-Wakeman, R. (2001) Diabetes 50, 166-174. [DOI] [PubMed] [Google Scholar]
- 25.Wu, L., Wang, Z. & Wang, R. (2000) Can. J. Physiol. Pharmacol. 78, 696-707. [PubMed] [Google Scholar]
- 26.Kamencic, H., Griebel, R. W., Lyon, A., Paterson, P. G. & Juurlink, B. H. J. (2001) FASEB J. 15, 243-250. [DOI] [PubMed] [Google Scholar]
- 27.Wu, L. & Juurlink, B. H. (2002) Hypertension 39, 809-814. [DOI] [PubMed] [Google Scholar]
- 28.Prochaska, H. J. & Santamaria, A. B. (1988) Anal. Biochem. 169, 328-336. [DOI] [PubMed] [Google Scholar]
- 29.Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J. & Klenk, D. C. (1985) Anal. Biochem. 150, 76-85. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y. & Talalay, P. (1998) Cancer Res. 58, 4632-4639. [PubMed] [Google Scholar]
- 31.Powell, E. E. (2003) PhD Dissertation (Univ. of Saskatchewan, Saskatoon, SK, Canada).
- 32.Christman, J. W., Lancaster, L. H. & Blackwell, T. S. (1998) Intensive Care Med. 24, 1131-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabatabaie, T. & Floyd, R. A. (1994) Arch. Biochem. Biophys. 314, 112-119. [DOI] [PubMed] [Google Scholar]
- 34.Zalba, G., San Jose, G., Moreno, M. U., Fortuno, M. A., Fortuno, A., Beaumont, F. J. & Diez, J. (2001) Hypertension 38, 1395-1399. [DOI] [PubMed] [Google Scholar]
- 35.Zalba, G., Beaumont, F. J., San Jose, G., Fortuno, A., Fortuno, M. A., Etayo, J. C. & Diez, J. (2000) Hypertension 35, 1055-1061. [DOI] [PubMed] [Google Scholar]
- 36.Morawietz, H., Weber, M., Rueckschloss, U., Lauer, N., Hacker, A. & Kojda, G. (2001) Biochem. Biophys. Res. Commun. 285, 1130-1135. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton, C. A., Brosnan, M. J., Al-Benna, S., Berg, G. & Dominiczak, A. F. (2002) Hypertension 40, 755-762. [DOI] [PubMed] [Google Scholar]
- 38.Mizutani, K., Ikeda, K., Tsuda, K. & Yamori, Y. (2002) J. Hypertens. 20, 1607-1614. [DOI] [PubMed] [Google Scholar]
- 39.Beswick, R. A., Zhang, H., Marable, D., Catravas, J. D., Hill, W. D. & Webb, R. C. (2001) Hypertension 37, 781-786. [DOI] [PubMed] [Google Scholar]
- 40.Wild, A. C. & Mulcahy, R. T. (2000) Free Radical Res. 32, 281-301. [DOI] [PubMed] [Google Scholar]
- 41.Deneke, S. M. & Fanburg, B. L. (1989) Am. J. Physiol. 257, L163-L173. [DOI] [PubMed] [Google Scholar]
- 42.Li, J. & Johnson, J. A. (2002) Physiol. Genomics 9, 137-144. [DOI] [PubMed] [Google Scholar]
- 43.Ambrosioni, W. (2001) J. Hypertens. 19, S33-S40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.