Abstract
Objectives
1) To characterize vitamin D status at initiation of critical care in surgical ICU patients and 2) to determine whether this vitamin D status is associated with the risk of prolonged hospital length of stay, 90-day readmission, and 90-day mortality.
Design
Prospective cohort study.
Setting
A teaching hospital in Boston, MA.
Patients
Hundred surgical ICU patients.
Interventions
None.
Measurements and Main Results
Mean (± SD) serum total 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels were 17 ± 8 ng/mL and 32 ± 19 pg/mL, respectively. Mean calculated bioavailable 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were 2.5 ± 2.0 ng/mL and 6.6 ± 5.3 pg/mL, respectively. Receiver- operating characteristic curve analysis demonstrated that all of four vitamin D measures predicted the three clinical outcomes; total 25-hydroxyvitamin D was not inferior to the other measures. Median (interquartile range) hospital length of stay was 11 days (8–19 d). Poisson regression analysis, adjusted for biologically plausible covariates, demonstrated an association of total 25-hydroxyvitamin D with hospital length of stay (incident rate ratio per 1 ng/mL, 0.98; 95% CI, 0.97–0.98). The 90-day readmission and mortality rates were 24% and 22%, respectively. Even after adjustment for biologically plausible covariates, there remained significant associations of total 25-hydroxyvitamin D with readmission (odds ratio per 1 ng/mL, 0.84; 95% CI, 0.74–0.95) and mortality (odds ratio per 1 ng/mL, 0.84; 95% CI, 0.73–0.97).
Conclusions
Serum 25-hydroxyvitamin D levels within 24 hours of ICU admission may identify patients at high risk for prolonged hospitalization, readmission, and mortality. Randomized trials are needed to assess whether vitamin D supplementation can improve these clinically relevant outcomes in surgical ICU patients.
Keywords: 25-hydroxyvitamin, bioavailable 25-hydroxyvitamin, intensive care unit, mortality, vitamin D
The prevalence of vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] < 20 ng/mL) (1) exceeds 70% in critically ill patients (2–4). Mounting evidence suggests that lower 25(OH)D levels in the ICU are associated with increased infection rates, prolonged length of stay (LOS), excessive healthcare costs, higher in-hospital mortality, and greater mortality postdischarge from the acute care setting (2, 5–7). Although retrospective analyses have explored the association between 25(OH)D levels from 365 days before to 7 days after the onset of critical illness and these clinically significant outcomes (8, 9), they do not provide a sense of when vitamin D assessments would be most informative in this patient cohort. Furthermore, the current body of knowledge related to vitamin D and critical illness is based largely on mixed ICU cohorts (4, 6–10); however, mean 25(OH)D levels may be quite different in surgical ICU patients as compared with patients in the medical ICU (8, 9).
In the general population, serum 25(OH)D level is a relatively stable biomarker (half-life of 2–3 wk) (11, 12) and serves as an acceptable surrogate for the pool of vitamin D that is available for conversion to 1,25-dihydroxyvitamin D [1,25(OH)2D] to support various metabolic processes (13, 14). However, only 0.03% of 25(OH)D is available in its free form, with approximately 88% bound to vitamin D–binding protein (DBP) and the remainder bound to albumin (15, 16). As binding to albumin is considerably weaker than binding to DBP, free and albumin-bound 25(OH)D is collectively referred to as “bioavailable” 25(OH)D. Previous studies have suggested that bioavailable measures of 25(OH)D correlate better with bone density and measures of mineral metabolism than do total 25(OH)D levels (17).
During critical illness, there is an increased tissue demand for 1,25(OH)2D (18). Altered DBP and albumin levels in the setting of inflammation, fluid shifts, capillary leak, and renal wasting are likely to have a strong influence on the bioavailable 25(OH)D pool (16, 18–20). It is not known whether bioavailable 25(OH)D levels may be more clinically informative in ICU patients, since the majority of existing studies related to vitamin D and critical illness have only focused on total serum 25(OH) D levels (18). Therefore, we prospectively assessed vitamin D status in a cohort of patients admitted to the surgical ICU. Our goal was to characterize total and bioavailable 25(OH)D levels at initiation of care in critically ill surgical patients and to investigate whether vitamin D status was associated with hospital LOS, 90-day readmission, and 90-day mortality.
MATERIALS AND METHODS
Patients were recruited from two surgical ICUs at the Massachusetts General Hospital (MGH), in Boston, MA. Both of these 18-bed ICUs receive admissions from all surgical services except for cardiac surgery. MGH is a 1,052-bed, teaching hospital and a level-one trauma center, which serves a diverse population in and around Eastern Massachusetts. The Partners Human Research Committee (institutional review board) approved the study protocol, and all subjects were enrolled between December 1, 2012, and January 31, 2013.
Inclusion and Exclusion Criteria
All adult men and women, 18 years old or older, expecting to require at least 48 hours of critical care (as determined by the treating ICU team) were deemed eligible to participate. Informed consent was obtained from subjects if they were oriented to person, place, and time in addition to testing negative on the Confusion Assessment Method for the ICU test (21). Surrogate consent was obtained when subjects did not meet these criteria. Subjects were only included in the study if blood samples could be obtained within 24 hours of admission to the ICU.
Exclusion criteria included a known history of anemia at the time of ICU admission (defined as hematocrit < 25%), current pregnancy or immediate postpartum status, and recent history of vitamin D supplementation more than or equal to 4,000 IU/d. Subjects were also excluded if they were transferred from another ICU, either at MGH or an external facility, since the goal of the study was to ascertain vitamin D status within 24 hours of an initial ICU admission. Patients who had a previous ICU course within 1 year of the current admission were also excluded to minimize confounding related to chronic illness. Finally, patients were excluded if they did not have available a suitable healthcare proxy (when patients were not able to provide consent), if they had a high likelihood of dying within the first 24 hours of ICU admission (as determined by the treating ICU team), or if they refused to participate.
Blood Sample Processing and Biomarker Assays
Following informed consent, fresh blood was acquired from an indwelling arterial catheter and was collected directly into an ethylenediaminetetraacetic acid containing tube (lavender top). The sample was immediately stored on ice and then centrifuged within 30 minutes to separate out plasma. All samples were centrifuged at 2,300 rpm for 15 minutes at a temperature of 4°C. The separated plasma was immediately transferred to polypropylene tubes and stored at −80°C until biomarker testing was ready to be initiated.
Assays were performed at the Harvard Medical School Clinical and Translational Science Award core laboratory at MGH. 25(OH)D (combined D2 and D3) and DBP were measured by enzyme-linked immunoabsorbent assay, using commercially available kits (Abbott Laboratories, Abbott Park, IL and Hycult Biotech, Plymouth Meeting, PA, respectively). Intra- and interassay coefficients of variation (CV) were both less than 10% for 25(OH)D and 5% and 12% for DBP, respectively. 1,25(OH)2D was measured using ultra performance liquid chromatography with tandem mass spectrometry; intra- and interassay CVs were both less than 10%. The MGH core laboratory is a Clinical Laboratory Improvement Amendments—certified facility and uses rigorous methods and continuously updated reference standards for the assessment of hemoglobin, WBC count, glucose, albumin, parathyroid hormone (PTH), calcium, creatinine, and high-sensitivity C-reactive protein (hsCRP).
Calcium measurements were corrected for albumin levels, using the formula (22):
where CCa is corrected calcium, MCa is measured plasma or serum calcium, NAlb is normal albumin = 4 g/dL, and MAlb is measured plasma or serum albumin.
Bioavailable 25(OH)D and 1,25(OH)2D were calculated using previously used formulas (20):
where [Dtotal] is total measured 25(OH)D or 1,25(OH)2D; [Alb] is measured albumin; [DBP] is measured DBP; [DAlb] is concentration of albumin-bound 25(OH)D or 1,25(OH)2D; [DDBP] is concentration of DBP-bound 25(OH)D or 1,25(OH)2D; [Dfree] is concentration of free 25(OH)D or 1,25(OH)2D; [Dbioavailable] is concentration of bioavailable 25(OH)D or 1,25(OH)2D = [Dfree] + [DAlb]; Kalb is affinity constant between 25(OH)D or 1,25(OH)2D and albumin = 6 × 105 M−1 [for 25(OH)D] or 5.4 × 104 M−1 [for 1,25(OH)2D]; and KDBP is affinity constant between 25(OH)D or 1,25(OH)2D and DBP = 7 × 108 M−1 [for 25(OH)D] or 3.7 × 107 M−1 [for 1,25(OH)2D].
Clinical Data Collection
Partners Healthcare electronic medical records system was used to obtain baseline information related to 1) age, 2) sex, 3) race, 4) body mass index (BMI), 5) Acute Physiology and Chronic Health Evaluation II (APACHE II) score, 6) type of surgical patient (e.g., thoracic, vascular, and urologic), and 7) vitamin D supplementation status. Admission laboratory results also abstracted from the electronic medical records system included 1) hemoglobin level, 2) WBC count, and 3) serum glucose level. Total body fluid balance (TBB) from admission to the time of study-related blood sample acquisition was calculated from the ICU bedside care flow sheet for each subject. ICU LOS, hospital LOS, and 90-day readmission rate were estimated from individual electronic records. The 90-day mortality was estimated by cross- referencing each record with the Social Security Death Index Master File.
Statistical Analysis
Descriptive statistics were tabulated in aggregate as subjects with 25(OH)D levels less than 20 ng/mL versus those with levels more than or equal to 20 ng/mL. Continuous data were reported as means ± SDs or medians with interquartile ranges (IQRs). Comparison of characteristics was performed using t tests and Mann-Whitney analysis for normally-distributed variables and nonparametric variables, respectively. Categorical values were expressed as proportions and compared using chi-square test.
We performed receiver-operating characteristic (ROC) curve analysis to evaluate the predictive value of total 25(OH) D, bioavailable 25(OH)D, total 1,25(OH)2D, and bioavailable 1,25(OH)2D for risk of prolonged hospital LOS, 90-day readmission, and 90-day mortality. All outcomes were dichotomous variables. Prolonged hospital LOS was defined as a value greater than the median (vs ≤ median), whereas readmission and mortality were binary values (yes/no).
Since ICU and hospital LOS are typically discrete variables with skewed distributions (2, 3), which assume values more than or equal to 1, zero-truncated Poisson regression analysis was used to model the relationship between total 25(OH) D and LOS, while controlling for biologically plausible covariates. In this overparameterized approach, we controlled for age, sex, BMI, APACHE II score, TBB, DBP, albumin, PTH, calcium, and hsCRP. To test the robustness of the biologically plausible model, we then performed stepwise Poisson regression using forward and backward selection methods. Variables considered for entry or removal in the stepwise approach included age, sex, race, BMI, APACHE II score, patient type, TBB, hemoglobin, WBC, glucose, total 25(OH)D, DBP, albumin, PTH, calcium, creatinine, and hsCRP. Subjects who did not survive beyond 72 hours post ICU admission were excluded from the “at-risk” group for ICU and hospital LOS assessments. Results are reported as incident rate ratios (IRRs) with 95% CIs.
To test for the association between total 25(OH)D levels and the risk of 90-day readmission, we first performed multivariable logistic regression analyses to control for biologically plausible covariates. In this overparameterized approach, we controlled for age, sex, BMI, APACHE II, TBB, DBP, albumin, PTH, calcium, and hsCRP. To test the robustness of the biologically plausible model, we then performed stepwise logistic regression using forward and backward selection methods. Variables considered for entry or removal in the stepwise approach included age, sex, race, BMI, APACHE II, patient type, TBB, hemoglobin, WBC, glucose, total 25(OH)D, DBP, albumin, PTH, calcium, creatinine, and hsCRP. Similar regression models were built to test for the association of total 25(OH) D with the risk of 90-day mortality. In all logistic regression models, 25(OH)D levels were treated as a continuous variable, whereas 90-day readmission and mortality rate were treated as binary variables. Subjects who did not survive their initial ICU admission were not included in the at-risk group for readmissions. Results are reported as odds ratios (ORs) with 95% CIs.
Published data specific to ICU patients within the Partners Healthcare network suggested that the mean 25(OH)D in mixed ICU cohorts is between 18 (± 14) and 26 (± 15) ng/mL (8, 9). Mortality rate in this cohort was between 13% and 35%. With recruitment of 100 patients into the study, we estimated that the probability was 80% that the study would detect a relationship between total 25(OH)D and 90-day mortality at a two-sided 0.05 significance level if the true change in 90-day mortality was 0.15% per unit change in 25(OH)D. This is based on the assumption that the SD of total 25(OH)D in the study cohort is 14 ng/mL and the SD of 90-day mortality is 7% (8, 9). All analyses were performed in STATA 12.0 (StataCorp LP, College Station, TX). A two-tailed p value of less than 0.05 or 95% CI that did not span a value of 1 was considered to be significant for all analyses.
RESULTS
We screened 236 consecutive patients during the study period, and 136 patients were excluded for the following reasons: four patients (or their healthcare proxies) did not agree to study participation, four patients were either pregnant or immediately postpartum, six patients had received high-dose vitamin D supplementation (50,000 IU/wk) within 1 month of ICU admission, 12 patients had significant baseline history of anemia, 27 patients were not expected to survive more than 24 hours, and 83 patients were expected to require postoperative hemodynamic or respiratory monitoring for less than 48 hours.
Baseline characteristics of the remaining 100 patients are shown in Table 1. The average (mean ± SD) age was 65 ± 16 years. Majority of subjects were women (58%) and white (91%). Overall BMI was 29.5 ± 7.6 kg/m2 and APACHE II score was 18 ± 9. Mean 25(OH)D level for the study cohort was 18 ± 8 ng/ mL. However, 18% of patients had 25(OH)D levels between 0 and 9.9 ng/mL, 45% had levels between 10 and 19.9 ng/mL, and 30% had levels between 20 and 29.9 ng/mL. The average DBP and albumin levels in the cohort were 167 ± 72 μg/mL and 2.9 ± 0.5 g/ dL, respectively. Mean calcium, PTH, and creatinine levels were 9.1 ± 0.7 mg/dL, 70 ± 59 pg/mL, and 1.4 ± 1.3 mg/dL, respectively. Median (IQR) LOS in the ICU was 5 days (IQR, 4–9 d) and in the hospital was 11 days (IQR, 8–19 d). The 90-day readmission rate was 24%, whereas 90-day mortality rate was 22%.
TABLE 1.
Demographic Factors, Baseline Clinical Information, and Major Clinical Outcomes Among Patients in the Surgical ICU, According to Vitamin D Status at Initiation of Care (n = 100)
| Variable | 25(OH)D < 20 ng/mL (n = 63) | 25(OH) ≥ 20 ng/mL (n = 37) | p |
|---|---|---|---|
| Age (yr) | 64 ± 16 | 67 ± 16 | 0.37 |
| Sex (%) | |||
| Female | 39 | 19 | 0.31 |
| Male | 24 | 18 | |
| Race (%) | |||
| White | 56 | 35 | 0.34 |
| Nonwhite | 7 | 2 | |
| Body mass index (kg/m2) | 30.1 ± 8.2 | 29.4 ± 8.3 | 0.55 |
| Acute Physiology and Chronic Health Evaluation II | 18 ± 16 | 16 ± 7 | 0.17 |
| Patient type (%) | |||
| Trauma | 10 | 6 | 0.06 |
| Vascular | 10 | 8 | |
| Gynecological | 4 | — | |
| Thoracic | 6 | 4 | |
| General | 19 | 13 | |
| Neurosurgery | 6 | 5 | |
| Urology | — | 2 | |
| Transplant | 1 | 6 | |
| Total body fluid balance (mL) | 2,428 ± 1,758 | 1,854 ± 1,283 | 0.06 |
| Hemoglobin (g/dL) | 10 ± 2 | 11 ± 2 | 0.17 |
| WBC count (× 109/L) | 12 ± 6 | 12 ± 5 | 0.66 |
| Glucose (mg/dL) | 152 ± 40 | 145 ± 40 | 0.40 |
| 25(OH)D (ng/mL) | 13 ± 4 | 26 ± 6 | < 0.001 |
| Bioavailable 25(OH)D (ng/mL) | 1.8 ± 1.2 | 3.7 ± 2.5 | < 0.001 |
| 1,25(OH)2D (pg/mL) | 26 ± 16 | 39 ± 30 | 0.03 |
| Bioavailable 1,25(OH)2D (pg/mL) | 5.7 ± 3.8 | 8.1 ± 7.0 | 0.06 |
| Vitamin D–binding protein (μg/mL) | 161 ± 64 | 177 ± 85 | 0.33 |
| Albumin (g/dL) | 3.0 ± 0.5 | 2.9 ± 0.4 | 0.28 |
| Parathyroid hormone (pg/mL) | 82 ± 69 | 49 ± 28 | 0.001 |
| Calcium (mg/dL) | 9.1 ± 0.8 | 9.2 ± 0.6 | 0.48 |
| Creatinine (mg/dL) | 1.5 ± 1.5 | 1.3 ± 1.1 | 0.45 |
| High-sensitivity C-reactive protein (mg/L) | 156 ± 103 | 100 ± 94 | 0.006 |
| ICU LOS (d) | 6 (IQR, 4–9) | 5 (IQR, 4–10) | 0.33 |
| Hospital LOS (d) | 12 (IQR, 8–25) | 10 (IQR, 6–15) | 0.001 |
| 90-day readmission (%) | |||
| ICU readmission | 19 | 2 | 0.001 |
| No ICU readmission | 35 | 34 | |
| 90-day mortality (%) | |||
| Deceased | 19 | 3 | 0.01 |
| Alive | 44 | 34 | |
25(OH)D = 25-hydroxyvitamin D, 1,25(OH)2D = 1,25-dihydroxyvitamin D, LOS = length of stay, IQR = interquartile range.
Dashes indicate there were no patients of this type for the dichotomized 25(OH)D level.
Data presented as mean ± SD, median (IQR), or percentage.
Boldface values indicate statistical significance.
ROC Curve Analysis
ROC curve analysis demonstrated that total 25(OH)D, bioavailable 25(OH)D, 1,25(OH)2D, and bioavailable 1,25(OH)2D were all significant predictors of prolonged LOS (not shown), 90-day readmission (not shown), and 90-day mortality (Fig. 1). However, no difference between the areas under the curve (AUCs) (Table 2) was observed, suggesting—at least within the limitations of the relatively small sample—that total 25(OH)D was similar (not inferior) to bioavailable 25(OH)D, 1,25(OH)2D, and bioavailable 1,25(OH)2D as a predictor of all three clinical outcomes.
Figure 1.
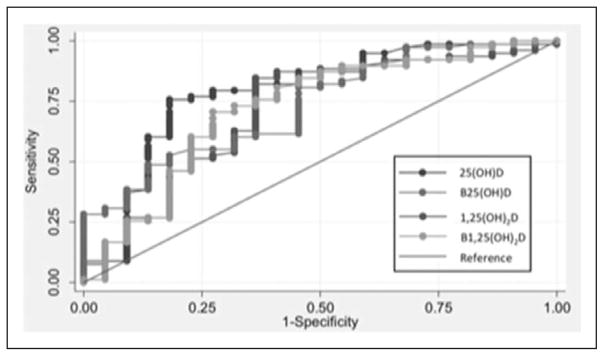
Receiver-operating characteristic curves comparing 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), bioavailable 25(OH)D, and bioavailable 1,25(OH)2D as predictors of 90-day mortality.
Table 2.
Comparison of Areas Under the Receiver-Operating Characteristic Curves for Vitamin D Measures as Predictors of Prolonged Hospital Length of Stay, 90-Day Readmission, and 90-Day Mortality Among Surgical ICU Patients (n = 100)
| Variable | AUC Hospital Length of Stay | p | AUC 90-Day Readmission | p | AUC 90-Day Mortality | p |
|---|---|---|---|---|---|---|
| Total 25(OH)D | 0.61 (0.50–0.73) | 0.48 | 0.71 (0.58–0.83) | 0.07 | 0.79 (0.67–0.91) | 0.32 |
| Bioavailable 25(OH)D | 0.57 (0.45–0.68) | 0.52 (0.37–0.66) | 0.72 (0.58–0.85) | |||
| Total 1,25(OH)2D | 0.61 (0.49–0.72) | 0.69 (0.56–0.81) | 0.73 (0.62–0.85) | |||
| Bioavailable 1,25(OH)2D | 0.60 (0.48–0.71) | 0.56 (0.41–0.71) | 0.72 (0.59–0.86) |
AUC = area under the curve, 25(OH)D = 25-hydroxyvitamin D, 1,25(OH)2D = 1,25-dihydroxyvitamin D.
Results presented as AUC (95% CI). Chi-square test was used to compare AUCs.
Outcomes
Although we found no significant association between total 25(OH)D and ICU LOS in an overparameterized biologically plausible zero-truncated Poisson regression model (IRR per 1 ng/mL, 0.97; 95% CI, 0.93–1.07), total 25(OH)D level within 24 hours of ICU admission was found to be inversely associated with hospital LOS (IRR per 1 ng/mL, 0.98; 95% CI, 0.97–0.98). APACHE II score, albumin, and calcium levels were also found to be significantly associated with hospital LOS in this model (Table 3). In the stepwise regression analysis for hospital LOS, the only variables retained in the final model were total 25(OH) D level (OR per 1 ng/mL, 0.98; 95% CI, 0.97–0.98), APACHE II score, albumin, and calcium.
Table 3.
Adjusted Risk Ratios for the Association of 25-Hydroxyvitamin D With Hospital Length of Stay, 90-Day Readmission, and 90-Day Mortality in Surgical ICU Patients (n = 100)
| Variable | Biological Model | Stepwise Model |
|---|---|---|
| Hospital length of stay | ||
| APACHE II | 1.03 (1.02–1.03) | 1.03 (1.02–1.03) |
| Total 25(OH)D | 0.97 (0.97–0.98) | 0.98 (0.97–0.98) |
| Albumin | 0.84 (0.74–0.95) | 0.83 (0.74–0.94) |
| Calcium | 0.82 (0.75–0.90) | 0.79 (0.73–0.86) |
| 90-day readmission | ||
| APACHE II | 1.17 (1.08–1.26) | 1.04 (0.98–1.11) |
| Total 25(OH)D | 0.85 (0.77–0.95) | 0.84 (0.74–0.95) |
| 90-day mortality | ||
| APACHE II | 1.19 (1.09–1.31) | 1.17 (1.08–1.26) |
| Total 25(OH)D | 0.84 (0.73–0.97) | 0.85 (0.77–0.95) |
APACHE II = Acute Physiology and Chronic Health Evaluation II, 25(OH)D = 25-hydroxyvitamin D.
Results for length of stay are presented as incident rate ratio (95% CI).
Results for 90-day mortality and readmission are presented as odds ratio (95% CI).
In an overparameterized biologically plausible multivariable regression model, total 25(OH)D level within 24 hours of ICU admission was found to be inversely associated with the risk of 90-day readmission (OR per 1 ng/mL, 0.84; 95% CI, 0.74–0.95). APACHE II was not found to be significantly associated with the risk of readmission in this model (Table 3). In the stepwise regression analysis for 90-day readmission, the only variables retained in the final model were total 25(OH)D level (OR per 1 ng/mL, 0.85; 95% CI, 0.77–0.95) and APACHE II score.
Similarly, in an overparameterized biologically plausible multivariable regression model, total 25(OH)D level within 24 hours of ICU admission was found to be inversely associated with the risk of 90-day mortality (OR per 1 ng/mL, 0.83; 95% CI, 0.73–0.97). APACHE II score was also found to be significantly associated with mortality in this model (Table 3). In the stepwise regression analysis for 90-day mortality, the only variables retained in the final model were total 25(OH)D level (OR per 1 ng/mL, 0.85; 95% CI, 0.77–0.95) and APACHE II score.
DISCUSSION
In this study, we characterized the vitamin D status of surgical ICU patients within 24 hours of initiating critical care. We determined that total 25(OH)D, bioavailable 25(OH)D, total 1,25(OH)2D, and bioavailable 1,25(OH)2D levels were all predictive of clinically important outcomes. However, no one particular biomarker was found to be a superior predictor of hospital LOS, 90-day readmission, and 90-day mortality when compared with each other. Since our research design was observational, and not interventional, we caution that the causal nature of the relationship between vitamin D status and such clinical outcomes cannot be inferred from this study.
Evidence regarding the potential importance of vitamin D status during critical illness continues to grow (18) and suggests a central role for intracellular 1,25(OH)2D levels in promoting favorable cellular processes (1, 16). Although free 25(OH)D is most readily available for conversion into 1,25(OH)2D (16), less than 1% of total vitamin D circulates in this form (20). As such, the albumin-bound component of total 25(OH)D can be easily mobilized in times of need and provides a larger pool of bioavailable 25(OH)D to maintain optimal vitamin D-mediated autocrine and paracrine activity (20). By contrast, the 25(OH)D-DBP complex is very stable, with an affinity of binding several orders of magnitude greater than that of 25(OH)D to albumin (20). 25(OH)D bound to DBP is typically involved in the regulation of gene expression, requiring intracellular enzymatic cleavage of the 25(OH)D, and thus, it is thought to have limited biological activity during acute stress (20, 23). Therefore, it has been hypothesized that assessment of the bioavailable forms of vitamin D may have greater predictive value for important ICU-related outcomes when compared with total serum 25(OH)D (20).
Although it was somewhat counterintuitive, we did not observe any advantage to the use of bioavailable 25(OH) D, total 1,25(OH)2D, or bioavailable 1,25(OH)2D levels over total 25(OH)D to predict the risk of prolonged LOS, 90-day readmission, or 90-day mortality in our surgical ICU cohort. Whilst the study sample size may be a limitation, the relatively conservative 95% CIs of the AUCs generated from our ROC curve analysis do not suggest that a larger cohort would materially change this result (Table 2). Since calculations for bioavailable 25(OH)D depend on DBP and albumin concentrations, the current study findings may highlight an important difference between medical and surgical patients. The burden of chronic disease is generally more pronounced in medical ICU patients (8, 9), as is the typical duration of critical illness (2, 3), which may be associated with greater derangements in DBP and albumin levels not only at the time of admission but also through the course of ICU care. As such, a comparison of total versus bioavailable 25(OH)D as a predictor of prolonged LOS, 90-day readmission, and 90-day mortality in medical ICU patients may yield findings different from the present study, and this merits further study. Furthermore, the assessment of bioavailable 25(OH)D levels in this study was based on formulae derived from noncritically ill subjects. It is possible that the binding characteristics of 25(OH)D to albumin and DBP differ in the setting of critical illness; direct measurements of bound and unbound 25(OH)D, if technically feasible, in this population may provide additional insight.
The tight regulation of serum 1,25(OH)2D is also noteworthy since it is known that measured levels may remain normal or even elevated despite low 25(OH)D levels (16, 17). The half- life of 1,25(OH)2D is only a few hours, and small changes in levels may be difficult to assess due to its presence in picomolar concentrations (16, 17). Although it remains unclear whether total or bioavailable 1,25(OH)2D levels change dramatically over the course of critical illness, it is biologically plausible that as compensatory mechanisms are exhausted, significant changes in 1,25(OH)2D levels may be observed independent of any changes in total or bioavailable 25(OH) D levels. Such derangements over the acute phase of critical illness may also improve risk stratification for LOS, readmissions, and mortality.
The current study was underpowered to adequately investigate the association of vitamin D status with ICU LOS. In particular, the availability of information related to bioavailable vitamin D metabolites was a limitation in our present sample size calculation. The results of our current study provide initial data that will be helpful in performing adequately powered studies in the future. Additionally, measured LOS and readmission rates may not be accurate if subjects were admitted to another facility. It is unlikely that such an event would not be logged in our electronic medical records system; however, it may be possible if patients traveled away from the surrounding area within 90 days of discharge from MGH. We selected a 90-day follow-up period based on historically low 30- and 60-day readmission and mortality rates in the MGH surgical ICUs. The use of these earlier cutpoints (instead of 90 d) would have likely resulted in an underpowered study, which in turn may have depicted a misleading association (or lack of association) of vitamin D status with LOS, readmission, and mortality.
Although our findings present compelling evidence in support of the association between admission vitamin D status and clinically important outcomes in surgical ICU patients, it is important to discuss potential limitations. Observational cohort studies such as this cannot establish causation, but they can highlight the existence or absence of associations and thereby direct future research. Furthermore, observational studies may be limited by the lack of a randomly distributed exposure. Despite adjustment for multiple potential confounders, there may still be residual confounding that contributes to the observed differences in outcomes. Specifically, vitamin D status may simply be a reflection of the overall health of patients, for which we were unable to fully adjust. We were also unable to adjust for immobilization, lack of sun exposure, and vitamin D supplementation upon discharge from the hospital. It is important to note that we did not include vitamin D supplementation status in any of our models since only 9% of patients were documented to be taking between 1,000 and 2,000 IU of either ergocalciferol or cholecalciferol prior to ICU admission. Although changes in vitamin D status may be observed within hours of exposure to acute stress (18), our samples were obtained within 24 hours of admission to the surgical ICU and may not reflect true vitamin D status at the outset of critical illness. Although we included known causes for this altered vitamin D status in our models (inflammatory response, fluid loading, and renal wasting of albumin as well as DBP), there may be other factors that we were unable to adjust for in our multivariable analysis. In addition, we used admission glucose levels as a proxy for diabetes mellitus or prediabetes. Future studies should consider the use of hemoglobin A1c levels to better characterize the presence of these disease states. Although we provided a comprehensive view of overall calcium regulation (total serum calcium, albumin, 25(OH)D, 1,25(OH)2D, PTH, and C-reactive protein) in our study cohort, we did not assess ionized calcium levels, which have been shown to be more accurate than albumincorrected total serum calcium levels during critical illness (24). These issues, and our exclusion of patients with a known history of anemia and those unlikely to survive beyond 24 hours after ICU admission, may decrease the generalizability of our results to all critically ill surgical ICU patients.
CONCLUSION
We confirm previous studies, which have suggested that total 25(OH)D levels on ICU admission are inversely associated with the risk of mortality in the postacute care setting (2, 3, 5, 9). In addition, we present novel data that better characterize vitamin D status at the initiation of care in surgical ICU patients. Further prospective studies are needed to validate our findings, to report whether vitamin D status changes significantly over the course of critical illness, to assess the potential benefit of optimizing vitamin D status in the surgical ICU, and to identify mechanisms by which vitamin D sufficiency may confer survival benefit from critical illness.
Acknowledgments
Dr. Quraishi received grant support from the National Institutes of Health (NIH) (5T32GM007592-33 and UL1 RR025758) and Harvard Medical School and received support for article research from NIH. His institution received grant support from the NIH, Massachusetts General Hospital, and Harvard Medical School. Dr. Bhan received support for article research from NIH (K23 DK081677). His institution received grant support from NIH. Dr. Camargo Jr received support for article research from NIH (grants R01 AI093723 and U01 AI087881). His institution received grant support from NIH.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
For information regarding this article, squraishi@partners.org
References
- 1.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:769–781. doi: 10.1016/j.beem.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Venkatram S, Chilimuri S, Adrish M, et al. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn L, Zimmerman LH, McNorton K, et al. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg. 2012;203:379–382. doi: 10.1016/j.amjsurg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: Results from a prospective observational study. Intensive Care Med. 2010;36:1609–1611. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 5.Matthews LR, Ahmed Y, Wilson KL, et al. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair P, Lee P, Reynolds C, et al. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 7.McKinney JD, Bailey BA, Garrett LH, et al. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12:208–211. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25- hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 12.Lee P. How deficient are vitamin D deficient critically ill patients? Crit Care. 2011;15:154. doi: 10.1186/cc10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society: Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 15.Lai JK, Lucas RM, Clements MS, et al. Assessing vitamin D status: Pitfalls for the unwary. Mol Nutr Food Res. 2010;54:1062–1071. doi: 10.1002/mnfr.200900468. [DOI] [PubMed] [Google Scholar]
- 16.Amrein K, Venkatesh B. Vitamin D and the critically ill patient. Curr Opin Clin Nutr Metab Care. 2012;15:188–193. doi: 10.1097/MCO.0b013e32834f0027. [DOI] [PubMed] [Google Scholar]
- 17.Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15:625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. 2010;14:R216. doi: 10.1186/cc9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leaf DE, Waikar SS, Wolf M, et al. Dysregulated mineral metabolism in patients with acute kidney injury and risk of adverse outcomes. Clin Endocrinol (Oxf) 2013;79:491–498. doi: 10.1111/cen.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusmao-Flores D, Salluh JI, Chalhub RA, et al. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the diagnosis of delirium: A systematic review and meta-analysis of clinical studies. Crit Care. 2012;16:R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain A, Bhayana S, Vlasschaert M, et al. A formula to predict corrected calcium in haemodialysis patients. Nephrol Dial Transplant. 2008;23:2884–2888. doi: 10.1093/ndt/gfn186. [DOI] [PubMed] [Google Scholar]
- 23.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Byrnes MC, Huynh K, Helmer SD, et al. A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Am J Surg. 2005;189:310–314. doi: 10.1016/j.amjsurg.2004.11.017. [DOI] [PubMed] [Google Scholar]


