Abstract
Methylmalonic aciduria is a rare disorder of organic acid metabolism with limited therapeutic options, resulting in high morbidity and mortality. Positive results from combined liver/kidney transplantation suggest, however, that metabolic sink therapy may be efficacious. Gene therapy offers a more accessible approach for the treatment of methylmalonic aciduria than organ transplantation. Accordingly, we have evaluated a lentiviral vector–mediated gene transfer approach in an in vivo mouse model of methylmalonic aciduria. A mouse model of methylmalonic aciduria (Mut−/−MUTh2) was injected intravenously at 8 weeks of age with a lentiviral vector that expressed a codon-optimized human methylmalonyl coenzyme A mutase transgene, HIV-1SDmEF1αmurSigHutMCM. Untreated Mut−/−MUTh2 and normal mice were used as controls. HIV-1SDmEF1αmurSigHutMCM-treated mice achieved near-normal weight for age, and Western blot analysis demonstrated significant methylmalonyl coenzyme A enzyme expression in their livers. Normalization of liver methylmalonyl coenzyme A enzyme activity in the treated group was associated with a reduction in plasma and urine methylmalonic acid levels, and a reduction in the hepatic methylmalonic acid concentration. Administration of the HIV-1SDmEF1αmurSigHutMCM vector provided significant, although incomplete, biochemical correction of methylmalonic aciduria in a mouse model, suggesting that gene therapy is a potential treatment for this disorder.
Introduction
Propionate metabolism is the major catabolic pathway of essential amino acids such as valine, isoleucine, methionine, odd-chain fatty acids, and the cholesterol side-chain, and for thymine and uracil. Deficiency of the nuclear-encoded mitochondrial enzyme, methylmalonyl coenzyme A mutase (MCM, EC 5.4.99.2), or its cofactor, adenosylcobalamin (AdoCbl) (Horster and Hoffmann, 2004), results in methylmalonic aciduria (MMAuria) (Cox and White, 1962; White and Cox, 1964), characterized by excessively high levels of methylmalonic acid in the blood (100–1000 μM) and urine (1000–10,000 mmol/mol Cr) (Manoli and Venditti, 1993).
The clinical presentations of MMAuria have been well defined, with major manifestations of lethargy, failure to thrive, recurrent vomiting, dehydration, respiratory distress, developmental delay, and coma (Matsui et al., 1983). Severe cases, if untreated, often progress to death. Long-term complications associated with selective organ impairment such as chronic renal failure (Baumgarter and Viardot, 1995), cardiomyopathy (Massoud and Leonard, 1993), and pancreatitis (Kahler et al., 1994) have been recognized.
Newborn screening by mass spectrometry suggests an incidence of between 1:80,000 (Coulombe et al., 1981; Sniderman et al., 1999; Chace et al., 2001) and 1:169,000 (Deodato et al., 2006). Treatments have been introduced for MMAuria based on the biochemical understanding of the disease. Conventional management involves strict dietary protein restriction while maintaining a high caloric intake. Pharmacological therapy involves supplementation with L-carnitine to enable removal of excess acylcarntines, and intermittent metronidazole to reduce propionate-producing gut flora. However, the outcomes of such treatment are generally disappointing, with high morbidity and mortality remaining.
Recently, liver and combined liver–kidney transplantations have been attempted with the aim of replacing MCM activity, thus reducing the need for dietary restriction and preventing long-term complications (Mc Guire et al., 2008). To date, 27 cases of organ transplantation have been published, these being liver transplantation (15 out of 27), kidney transplantation (6 out of 27), and combined liver–kidney transplantation (6 out of 27) (Mc Guire et al., 2008). Although this therapeutic option appears to protect the patients against acute metabolic decompensation, and the quality of life is improved, the effectiveness of this treatment is still controversial (Kaplan et al., 2006; Kasahara et al., 2006). Clinical evaluation showed that most of the patients who received organ transplantation suffered from common postoperative sequela such as infection, acute rejection, toxicity of immunosuppressive medication, and risk of neurologic events (Mc Guire et al., 2008). However, a recent report of combined liver–kidney transplantation suggests that stabilization is possible using this approach (Nagarajan et al., 2005).
The cloning of the gene encoding MCM has allowed both the identification of the causative mutations and the prospect of treating the disorder by gene therapy (Ledley et al., 1990; Raff et al., 1991; Crane et al., 1992). Although the pathology of MMAuria affects many tissues, the fact that the metabolites elevated in the disease are freely diffusible means that the creation of an efficient metabolic sink may be effective in controlling the disease. In the early 1990s, a study using an MCM retroviral vector system successfully demonstrated gene correction of MMAuria in vitro (Sawada and Ledley, 1992). The subsequent development of animal models of MMA has now made possible the testing of different gene therapy strategies in vivo (Peters et al., 2003, 2012; Chandler and Venditti, 2008). Recently, rescue of a murine model of MMAuria using an adenovirus vector that expressed MCM was demonstrated (Chandler and Venditti, 2010). We have demonstrated that MCM introduced via a lentivirus vector can be expressed in vivo and function properly, prolonging the lifespan of the MMAuria mice.
Materials and Methods
MMAuria murine model
The Mut−/−MUTh2 mice used in this study are transgenic for two copies of the human MCM gene on a knockout of the mouse gene and were developed by Dr. Heidi Peters from the Murdoch Children's Research Institute, Metabolic Research Group (Peters et al., 2012). This animal model is biochemically and phenotypically consistent with an intermediate form of MMAuria; they are rescued from neonatal lethality and are significantly smaller than age- and sex-matched littermates. Biochemically, there is a significant increase in MMA concentrations in the blood (500 μM at 8 weeks old) and urine (10-fold increase at 3 days of age). Their mutase enzyme activity, as measured by the propionate incorporation assay in cultured fibroblasts, is 20% of wild-type control. Their survival curve demonstrates that the Mut−/−MUTh2 mouse model encountered a significant mortality rate in the first 6 months, which then plateaus, with further losses paralleling those seen in normal mice (Peters et al., 2012). Based on their data, this mouse model is able to live long enough (∼2 years) to allow therapeutic strategies to be successfully evaluated.
Since the mice injected with HIV-1SDmEF1αhMCM in our initial study at doses of 50 μg p24 equivalent showed no significant improvement in reducing the MMA level in treated animals (data not shown), we increased the dose to an average of 130 μg p24 equivalent in a total volume of 300 μl. Six affected mice (Mut−/−MUTh2), 3 males and 3 females, were treated with approximately 130 μg p24 equivalent of HIV-1SDmEF1αmurSigHutMCM (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum) virus at the age of 8 weeks (Supplementary Table S1), provided that they had achieved a minimum body weight of 11 g at the time of treatment. Previous observations suggested that mice of this body weight were more resilient to the treatment regimen.
The mice were pretreated with 10% dextrose and intralipid before the virus delivery to stabilize their metabolic condition and to limit the likelihood of acute metabolic decompensation. Blood samples were collected before treatment, at 1 week, and 1, 3, 5, and 6 months after treatment, and urine samples were collected on a weekly basis. The brain, liver, kidneys, and spleen were removed at postmortem examination. These samples were used for metabolic studies, vector copy number measurement, Western blot analysis, and enzyme activity assays.
Ethics approval for these studies was granted by the Animal Ethics Committees of the Women's and Children's Hospital and the Adelaide University. All procedures carried out on these animals were in accordance with the guidance set by these committees and in accordance with the recommendations of National Health and Medical Research Council.
Vector construction
The codon-optimized human MCM coding sequence (murSigHutMCM) was designed and manufactured by GenScript (Piscataway, NJ) as a 5′-ClaI/3′-NdeI, resulting in a sticky-end fragment of 2268 bp that was cloned between the 5′-ClaI/3′-NdeI (also a sticky-end) in pHIV-1SDmEF1αLuciferase (unpublished), placing the human MCM coding sequence under the transcriptional control of the EF1-α gene promoter to give pHIV-1SDmEF1αmurSigHutMCM (Supplementary Fig. S1).
The virus was made using our standard packaging plasmids and conditions with virus collected in OptiProSFM (Gibco-BRL, Grand Island, NY) as previously described with some modifications (Koldej et al., 2005). Ion exchange chromatography replaced ultra/diafiltration in the purification procedure.
The cell culture supernatant was pumped through an assembly of a Polydisc AS, 0.45 μM disposable polypropylene filter device (Whatman Inc., Clifton, NJ) and 2× Mustang Q ×75 ion exchange devices (Pall Corp., Pennsylvania, PA) in series using a Watson-Marlow Bredel 323 Dz pump (Wetherill Park, New South Wales, Australia). After all the virus had been loaded, the 0.45 μM filter was removed and the Mustang Q filter assembly washed with 3× 10 ml of PBS (Sigma Chemical Co., St. Louis, MO). The virus was then eluted with 4 ml of 1.5 M NaCl into a sterile 10 ml tube. Four milliliters of 2% (v/v) mouse serum albumin (Sigma Chemical Co.) was immediately added and mixed by gentle pipetting. The virus was then pelleted by ultracentrifugation (20,000 rpm, 4°C for 90 min). The viral pellet was resuspended in 300 μl of 0.9% (w/v) NaCl, 0.1% (w/v) mouse serum albumin. The virus was then aliquoted into sterile tubes and stored at −80°C.
Measurement of MMA in plasma
Blood samples were collected using orbital bleed into heparin-coated capillary tubes and microcentrifuged at 18,300×g for 10 min, and the plasma was decanted and stored at 4°C until analysis. For measurement of MMA in plasma, 10 μl of 15 μM stable isotope-labeled internal standard (2-(2H3-methyl)-malonic acid) (Sigma Chemical Co.) was added to each 10 μl plasma sample (PS), which was then extracted using a modification of the previously described method (Marcell et al., 1985). The extract was derivatized with 1% BSTFA (Grace Davison Discovery Sciences, Victoria, Australia) by heating at 60°C for 30 min, and the TMS ester was quantified using a Perkin Elmer Turbomass GC/MS. A positive control, MMA level 2 serum toxicology control (Utak Laboratoris Inc., Valencia, CA), was processed in a same way in each batch. Simultaneously, a response ratio sample (RRS) was prepared by evaporating a mixture of 10 μl of 15 μM MMA working solution and 10 μl of 15 μM stable isotope-labeled internal standard (2-(2H3-methyl)-malonic acid), derivatized with 1% BSTFA as described above. Selected ion monitoring at 247 m/z (for MMA) and 250 m/z (for 2-(2H3-methyl)-malonic acid) was performed. The ratio of ions m/z 247/250 for RRS (typically 1.0), PS, and the control sample was calculated. The PS ratio divided by the RRS ratio and multiplied by 1.5 followed by a dilution factor of 10 gives the concentration of MMA acid in μM. Results were determined to be valid if the concentration of the control sample was 1.3 μM±10%.
Measurement of MMA concentration in urine
Urine was collected from each mouse in individual metabolic cages (Hatteras Instruments, Inc., Cary, NC) overnight, with food and water available ad lib. A 100 μl−1 ml sample was analyzed for creatinine, and a volume equivalent to 250 μg creatinine was used for analysis. Ten microliters of 3,3-dimethylglutaric acid (Sigma Chemical Co.) was added to each sample as an internal standard. One milliliter of water was then added and the sample acidified to pH<2, as measured by Universal pH–indicator strips (Merck, Kilsyth, Victoria, Australia), with 6 M hydrochloric acid. Subsequently, the mixture was saturated with 1.5 g of sodium hydroxide. The sample was then extracted using a modification of the method as previously described (Tanaka et al., 1980). The extract was derivatized with 50 μl of BSTFA/1% TCMS (Selectra-Sil, UCT Inc., Bristol, PA) at 60°C for 30 min. Ten microliters of the derivatized sample was diluted with 100 μl of a pyridine (Ajax Chemical, Sydney, Australia) and iso-octane (Allied Signal Inc., Muskegon, MI) mixture (1:99) before injection into a Hewlett Packard (HP 6890 series) gas chromatograph with mass spectrometry detector. A standard curve of MMA was used to quantify the organic acid level in the urine sample.
Assessment of MMA concentration in liver
The MMA concentration in the liver of each animal was evaluated using homogenized liver samples. For measurement of MMA in the liver, 3.2 (l of homogenized liver sample was spotted onto filter paper (Guthrie card) and allowed to dry. A 3-mm-diameter sample was then punched from the card and placed in a 5 ml glass tube, and 5 (l of 15 (M of 2-(2H3-methyl)-malonic acid (Cambridge Isotope Laboratories Inc., Andover, MA) added as a stable isotope-labeled internal standard. The sample was then extracted with 200 (l of solution that consisted of 99.5% (v/v) acetonitrile/H2O (at a ratio of 1:1) and 0.5% (v/v) formic acid at room temperature for 15 min with shaking. The sample was then transferred into a 96-well plate and the MMA level was quantified using MS mass spectrometry according to the method as previously described (la Marca et al., 2007).
Real-time polymerase chain reaction analysis for gene vector copy number
Vector copy number was determined by real-time polymerase chain reaction (PCR) analysis as previously described with some minor modifications (Anson and Fletcher, 2007). DNA from an NIH3T3-derived cell line and containing a single copy of the lentiviral vector was used to provide an absolute standard for copy number. All samples were analyzed in triplicate.
MCM enzyme assay
Homogenized liver samples were lysed by sonication at 25 W for 20 min at 0°C and then microcentrifuged at 4200×g for 10 min at 4°C, and the supernatant was decanted and assayed for MCM enzyme activity by a modification of the method as previously described (Kikuchi et al., 1989). The reaction mixture (final volume 90 μl) contained 0.2 M Tris-sulfate pH 7.5, 0.8 mM methylmalonyl coenzyme A (Sigma Chemical Co.), cell lysate (∼100 μg total protein), and 130 μM AdoCbl (Fluka Chemie GmbH, Buchs, Switzerland). The reaction mixture without the methylmalonyl coenzyme A was preincubated for 10 min at 37°C before initiating the reaction by addition of the methylmalonyl coenzyme A. A 45 μl aliquot was then immediately removed from the mixture and the reaction terminated by the addition of 7.3 μl of 24% (w/v) trichloroacetic acid (zero time point sample). The remainder of the reaction was incubated for 15 min at 37°C and then terminated as stated above. The samples were centrifuged at 16,900×g by microcentrifugation for 10 min, and the supernatant was decanted to a fresh tube and neutralized with 7.5 μl of 1 M Tris-base. Twenty microliters of the final supernatant was then used for high-performance liquid chromatography (HPLC) analysis.
The HPLC system consisted of a G1379A degasser, a G1312A Bin Pump, and a G1328 manual injector (Agilent Technologies Australia Pty Ltd., Mulgrave, Victoria, Australia). An Alltech Alltima C18-LL HPLC column (5 μM, 150×2.1 mm; Grace Davison Discovery Sciences, Victoria, Australia) was equilibrated with 100% solvent A (50 mM KH2PO4, pH 3.5) for 20 min before injecting the sample. Once injected, solvent A was gradually mixed with a linear gradient of solvent B, 100% (v/v) methanol, over 5 min to the desired ratio of 80:20 for subsequent analysis. A flow rate of 0.5 ml/min was used for assaying samples. The coenzyme A derivatives were detected at 254 nm using a G315B DAD UV detector (Agilent Technologies Australia Pty Ltd.). Methylmalonyl coenzyme A and succinyl coenzyme A peaks were detected at approximately 11 and 13 min, respectively.
Western blot
A 10 μl sample containing 25 μg of clarified liver lysate was mixed with an equal volume of 2× Fairbanks buffer containing 2% (w/v) dithiothreitol (Bio-Rad Laboratories, Inc., New South Wales, Australia), and incubated at 100°C before loading onto a 10% (w/v) polyacrylamide minigel. A benchmark prestained protein standard (Life Technologies Corporation, Invitrogen, Victoria, Australia) (10 μl/well) was also loaded. The gel was run and then electroblotted onto a nitrocellulose or PVDF membrane using standard procedures. The membrane was then simultaneously probed with an antihuman methylmalonyl coenzyme A antibody (ab67869) (Abcam plc; Cambridge Science Park, Cambridge, United Kingdom) and a mouse anti-beta actin monoclonal (ab8226) (Abcam plc; Cambridge Science Park), diluted 1 in 750 and 1 in 500, respectively. Detection was using an HRP-conjugated sheep antimouse serum (AP326P; Chemicon, Merck Millipore, Bilerica, MA), diluted 1 in 1500, and visualization was with 3′3′5′5′-tetramethylbenzidine (Sigma Chemical Co.).
A freeware analysis program, ImageJ v1.45 (http://imagej.nih.gov/ij/download.html), was used to analyze the band intensity to allow quantification.
Statistical analysis
p-Values were considered significant in all cases if the values were less than 0.05. The statistical analysis of bodyweight was done by comparing the linear regression (R2) between the treatment groups using SAS software (SAS Institute Inc., Cary, NC). The comparison of plasma and urinary MMA levels between treatment groups was determined by calculating area-under-the-curve (AUC) values of plasma and urine MMA concentration of individual mice over a period of designated time points. The data points of individual mice were integrated by calculating the AUC using trapezoidal rule. It should be noted that the integration of HIV-1SDmEF1αmurSigHutMCM-treated group was started from 1 week after treatment until 6 months posttreatment or until death for deceased mice, whereby the pretreatment values were included in the integration for both the normal and untreated groups. The average of total AUC for treatment groups were then calculated followed by the use of one-way ANOVA to determine the differences in plasma and urine MMA levels between the treatment groups. In all cases, unless specified, all other statistical analyses were conducted using one-way ANOVA with a post hoc Tukey's test to determine significance.
Results
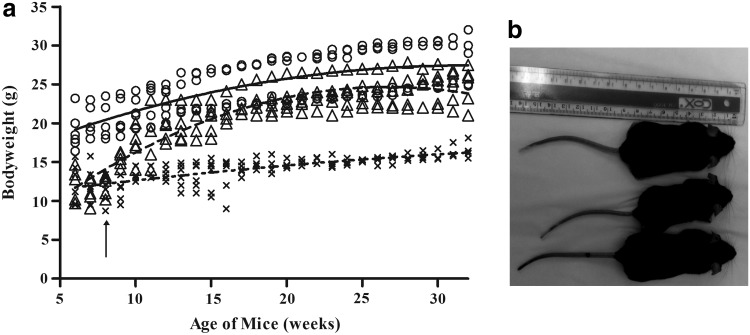
A total of 6 Mut−/−MUTh2 mice were treated with lentivirus at 8 weeks of age, with the virus delivery well tolerated in 5 mice. One treated mouse (M6) died 2 weeks postinjection. Mice were maintained in a humidicrib at 29°C for a minimum of 2 days posttreatment, with a supply of sunflower seeds and moistened food until they were recovered. Treated mice progressively gained body weight from 1 week after treatment, and by the age of approximately 120 days, 2 months after treatment, their body weight was within the normal range (Fig. 1a). At the end of the study, the average weight of the treated group was significantly heavier (with a mean±standard error of the mean [SEM] of 25±1 g) than the untreated group (with a mean±SEM of 17±1 g) and was approximately 90% of that of normal mice. Importantly, they were otherwise difficult to distinguish physically from age-matched normal controls (Fig. 1b).
FIG. 1.
(a) Body weight of individual mice from normal ( ), untreated (
), untreated ( ), and HIV-1SDmEF1αmurSigHutMCM-treated (
), and HIV-1SDmEF1αmurSigHutMCM-treated ( ) groups. The arrow indicates the treatment time point at 8 weeks of age. The curved line represents the fit of curves (R2) for each group, the solid line indicates the wild-type group, and the dashed line and dotted dash line indicate the HIV-1SDmEF1αmurSigHutMCM-treated and untreated groups, respectively. The statistical analysis on the fit of curves shows that the mice in the normal group weigh significantly (p<0.001) more on average than the HIV-1SDmEF1αmurSigHutMCM-treated and untreated groups. The HIV-1SDmEF1αmurSigHutMCM-treated group weighed significantly (p<0.001) more on average than the untreated group. In addition, the untreated group weighed significantly lower (p<0.001) over time compared with the other two groups. (b) Normal (top), untreated (middle), and HIV-1SDmEF1αmurSigHutMCM-treated mice (bottom) are shown at 6 months after treatment. Compared with an untreated control mouse (middle), the lentiviral vector (LV)–treated mouse shows an increase in body size and is indistinguishable from normal. In contrast, an age-matched untreated Mut−/−MUTh2 mouse is obviously smaller than normal. Other than the difference in size, there are no other overt differences in appearance in this model. MCM, methylmalonyl coenzyme A mutase.
) groups. The arrow indicates the treatment time point at 8 weeks of age. The curved line represents the fit of curves (R2) for each group, the solid line indicates the wild-type group, and the dashed line and dotted dash line indicate the HIV-1SDmEF1αmurSigHutMCM-treated and untreated groups, respectively. The statistical analysis on the fit of curves shows that the mice in the normal group weigh significantly (p<0.001) more on average than the HIV-1SDmEF1αmurSigHutMCM-treated and untreated groups. The HIV-1SDmEF1αmurSigHutMCM-treated group weighed significantly (p<0.001) more on average than the untreated group. In addition, the untreated group weighed significantly lower (p<0.001) over time compared with the other two groups. (b) Normal (top), untreated (middle), and HIV-1SDmEF1αmurSigHutMCM-treated mice (bottom) are shown at 6 months after treatment. Compared with an untreated control mouse (middle), the lentiviral vector (LV)–treated mouse shows an increase in body size and is indistinguishable from normal. In contrast, an age-matched untreated Mut−/−MUTh2 mouse is obviously smaller than normal. Other than the difference in size, there are no other overt differences in appearance in this model. MCM, methylmalonyl coenzyme A mutase.
Vector copy number measurement by quantitative PCR
Quantitative real-time PCR demonstrated high levels of vector in the liver (3.2±1.8 copies per cell), followed by spleen (0.9±0.5 copies per cell) (Supplementary Table S2). However, these results were skewed by one mouse (M1) having a much higher copy number (10.4 and 2.8 in the liver and spleen, respectively) than the others (Supplementary Table S1). Vector copy number was extremely low in the kidney, at 0.01±0.01 copy per cell. As expected, no copies of the vector were detected in either normal control or untreated Mut−/−MUTh2 mice in all tissues tested (Supplementary Table S2).
Western blot analysis
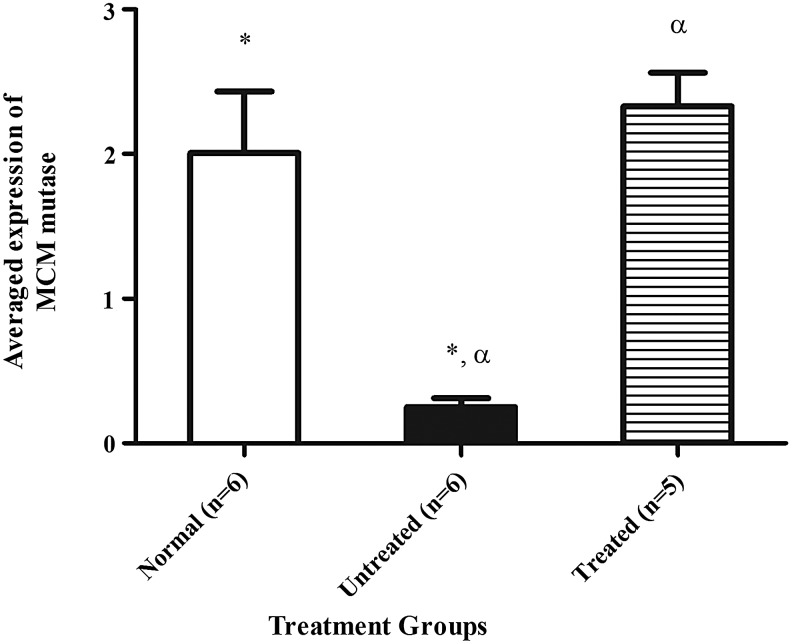
Western blot analysis of liver extracts was performed to evaluate the expression of MCM protein 6 months posttreatment. Using an extract of HEK293T cells as a positive control for (human) MCM, mean averaged expression±SEM of 2±0.4 and 0.3±0.1 was for normal control and untreated Mut−/−MUTh2 mice, respectively, after normalization to loading control (β-actin). The treated mice demonstrated mean averaged expression±SEM of 2.3±0.2, not significantly different from the normal controls, consistent with almost complete, if not complete, restoration of MCM protein levels in the liver of the treated mice (Fig. 2).
FIG. 2.
The densitometric analysis of human MCM protein levels expressed in the treatment groups. ImageJ quantification of band intensity for human MCM after normalization to β-actin. The figure demonstrates MCM protein levels in normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,
), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,  ). The error bars represent SEM. The * and α indicate significant difference compared with the untreated Mut−/−MUTh2 control (p<0.05). No significant difference was observed between the normal and the HIV-1SDmEF1αmurSigHutMCM-treated group. The p-value between groups was calculated by using one-way ANOVA with Tukey's post hoc test. SEM, standard error of the mean.
). The error bars represent SEM. The * and α indicate significant difference compared with the untreated Mut−/−MUTh2 control (p<0.05). No significant difference was observed between the normal and the HIV-1SDmEF1αmurSigHutMCM-treated group. The p-value between groups was calculated by using one-way ANOVA with Tukey's post hoc test. SEM, standard error of the mean.
Determination of hepatic MCM enzyme activity
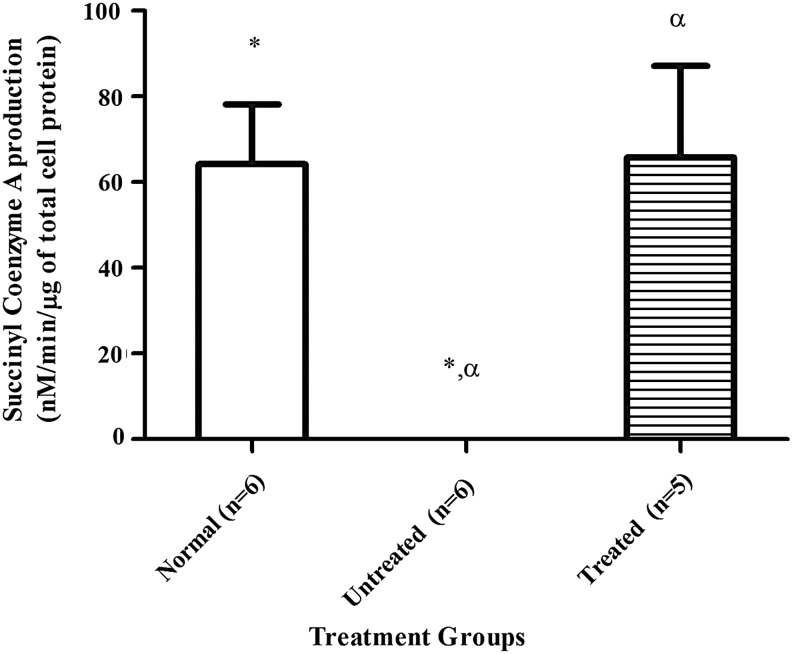
The mean±SEM level of hepatic MCM enzyme activity from the treated group demonstrated an increase of succinyl-coenzyme A production to 66±21 (nM/min/μg of total cell protein), equivalent to the enzyme activity level of the normal control, which was 64±14 (nM/min/μg of total cell protein) (Fig. 3). No enzyme activity was detectable in liver homogenate from the untreated Mut−/−MUTh2 mice.
FIG. 3.
The MCM enzyme activity in liver extracts. Hepatic enzyme activity from normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,
), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,  ). The HIV-1SDmEF1αmurSigHutMCM-treated mice had a mean±SEM of 66±21 (nM/min/μg of total hepatocyte protein) and is significantly different (p<0.05) compared with the Mut−/−MUTh2 untreated group (undetectable). The error bars represent SEM. The * and α indicate significant difference between the treatment groups (normal vs. untreated) and (treated vs. untreated), respectively. The p-value between groups was calculated using one-way ANOVA with Tukey's post hoc test.
). The HIV-1SDmEF1αmurSigHutMCM-treated mice had a mean±SEM of 66±21 (nM/min/μg of total hepatocyte protein) and is significantly different (p<0.05) compared with the Mut−/−MUTh2 untreated group (undetectable). The error bars represent SEM. The * and α indicate significant difference between the treatment groups (normal vs. untreated) and (treated vs. untreated), respectively. The p-value between groups was calculated using one-way ANOVA with Tukey's post hoc test.
We were unable to correlate the vector copy number and the MCM activity, although a high vector copy number was observed in one of the HIV-1SDmEF1αmurSigHutMCM-treated mice, M1, with a vector copy number of 10.4 and the MCM enzyme activity (111 nM/min/μg of total cell protein) indicated only slightly higher enzyme activity than the other treated mice (Supplementary Table S1).
Plasma MMA measurement
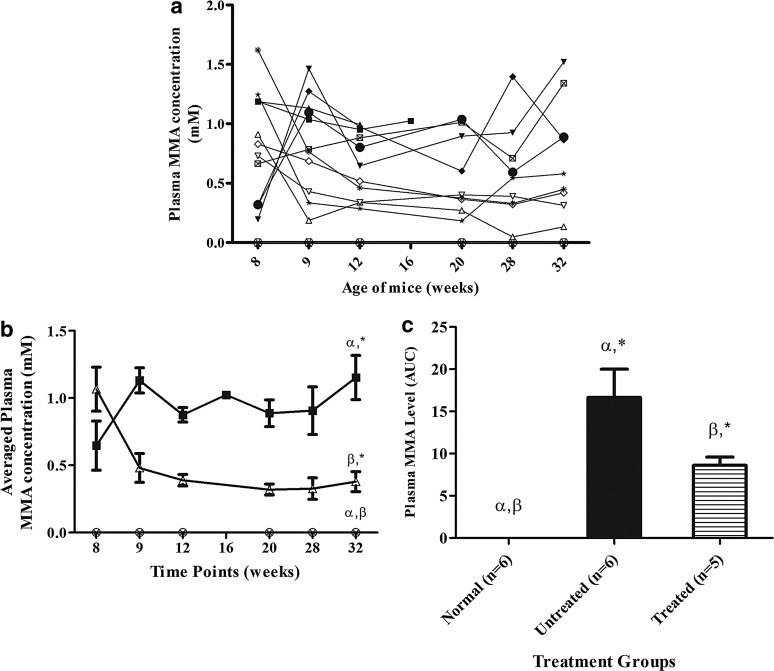
All surviving treated mice demonstrated a significant decrease in plasma MMA levels 1 week posttreatment. Thereafter, the plasma MMA levels decreased gradually, with the exception of one mouse (M2), which had a slight elevation at 28 weeks of age (Fig. 4a). The mean±SEM plasma MMA concentration of treated mice at the end of experiment was 0.38±0.074 mM (Fig. 4b). Untreated Mut−/−MUTh2 mice showed continued MMA accumulation in plasma and had a mean±SEM plasma MMA concentration of 1.15±0.16 mM at 6 months (Fig. 4b). Normal mice had a mean plasma MMA level of 0.1 μM at the end of the experiment.
FIG. 4.
(a) The plasma MMA concentrations of individual mice over time. The Mut−/−MUTh2 mice from untreated group are UnT1 (Male,  ), UnT2 (Male,
), UnT2 (Male,  ), UnT3 (Male,
), UnT3 (Male,  ), UnT4 (Male,
), UnT4 (Male,  ), UnT5 (Female,
), UnT5 (Female,  ), and UnT6 (Female,
), and UnT6 (Female,  ). The HIV-1SDmEF1αmurSigHutMCM-treated mice are M1 (Male,
). The HIV-1SDmEF1αmurSigHutMCM-treated mice are M1 (Male,  ), M2 (Female,
), M2 (Female,  ), M3 (Female,
), M3 (Female,  ), M4 (Male,
), M4 (Male,  ), and M5 (Male,
), and M5 (Male,  ). The averaged plasma concentration of wild-type mice (normal control) is shown in (
). The averaged plasma concentration of wild-type mice (normal control) is shown in ( ). (b) The averaged plasma MMA concentration of treatment groups over time. The data points of the mice in different treatment groups over the course of treatment were averaged. Data for normal mice (n=6,
). (b) The averaged plasma MMA concentration of treatment groups over time. The data points of the mice in different treatment groups over the course of treatment were averaged. Data for normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and treated Mut−/−MUTh2 mice (n=5,
), and treated Mut−/−MUTh2 mice (n=5,  ) are shown. The result shows the continuous accumulation of plasma MMA in Mut−/−MUTh2 untreated mice, with a mean±SEM plasma MMA concentration of 1.15±0.16 mM at 6 months, which is significantly different from normal mice (p<0.05). LV-treated mice had a mean±SEM plasma MMA concentration of 1.07±0.4 mM before treatment, and the level was greatly reduced to 0.38±0.074 mM 6 months after treatment, which is significantly different from normal and untreated groups. The error bars represent SEM; α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated), and (untreated vs. treated, respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. (c) The comparison of plasma MMA levels between treatment groups. The data points of individual mice were integrated by calculating the AUC using the trapezoidal rule as stated. Data for normal mice (n=6,
) are shown. The result shows the continuous accumulation of plasma MMA in Mut−/−MUTh2 untreated mice, with a mean±SEM plasma MMA concentration of 1.15±0.16 mM at 6 months, which is significantly different from normal mice (p<0.05). LV-treated mice had a mean±SEM plasma MMA concentration of 1.07±0.4 mM before treatment, and the level was greatly reduced to 0.38±0.074 mM 6 months after treatment, which is significantly different from normal and untreated groups. The error bars represent SEM; α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated), and (untreated vs. treated, respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. (c) The comparison of plasma MMA levels between treatment groups. The data points of individual mice were integrated by calculating the AUC using the trapezoidal rule as stated. Data for normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and treated Mut−/−MUTh2 mice (n=5,
), and treated Mut−/−MUTh2 mice (n=5,  ) are shown. The Mut−/−MUTh2 untreated mice had a mean±SEM of 17±3, which is significantly different from normal mice (p<0.05). The LV-treated mice had a mean±SEM of 9±1, which is significantly different from normal and untreated groups. The error bars represent SEM. α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated) and (untreated vs. treated), respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. MMA, methylmalonic acid; AUC, area-under-the-curve; LV, lentiviral vector.
) are shown. The Mut−/−MUTh2 untreated mice had a mean±SEM of 17±3, which is significantly different from normal mice (p<0.05). The LV-treated mice had a mean±SEM of 9±1, which is significantly different from normal and untreated groups. The error bars represent SEM. α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated) and (untreated vs. treated), respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. MMA, methylmalonic acid; AUC, area-under-the-curve; LV, lentiviral vector.
It should be noted that two untreated affected control mice died during the course of the experiment (at 17 and 16 weeks of age, respectively). Both of these mice presented a high level of MMA in plasma before death, with values of 993 and 954 μM, respectively, at 12 weeks of age (Fig. 4a). Plasma MMA at the time of death in one of these mice was 1024 μM (Fig. 4a). A sample was unable to be obtained from the other.
The average of total AUC shows that the untreated control mice had a mean±SEM of 17±3, significantly higher (p<0.05, Tukey's post hoc test) than the normal control (with a mean±SEM of 0.004±0.003). The HIV-1SDmEF1αmurSigHutMCM-treated group displayed a mean±SEM of 9±1, which was significantly lower (p<0.05, Tukey's post hoc test) than that displayed by the untreated Mut−/−MUTh2 mice, but still significantly higher than that showed by the normal control group (Fig. 4c).
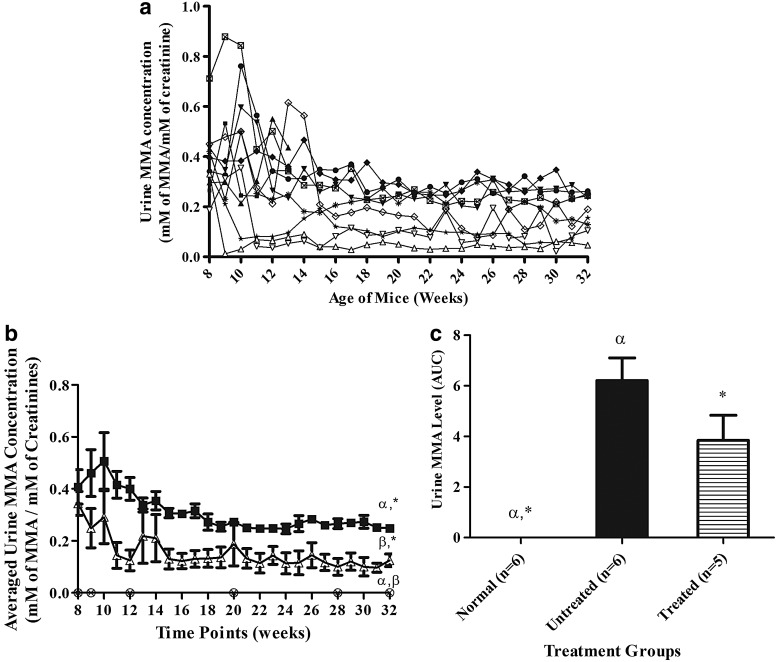
Urine MMA analysis
The level of urinary MMA for each mouse over time (Fig. 5a) showed a similar pattern to the results of the plasma analysis, with the MMA level rapidly decreasing 1 week after treatment in all the surviving HIV-1SDmEF1αmurSigHutMCM-treated mice and then being maintained at a lower level, with a mean±SEM urinary MMA level at 0.13±0.02 mM of MMA/mM of creatinine, compared with the untreated Mut−/−MUTh2 mice (0.25±0.01 mM of MMA/mM of creatinine) at 6 months posttreatment (Fig. 5b).
FIG. 5.
(a) The urinary MMA concentrations of individual mice over time. Mice were treated at 8 weeks of age on graph. The Mut−/−MUTh2 mice from untreated group are UnT1 (Male,  ), UnT2 (Male,
), UnT2 (Male,  ), UnT3 (Male,
), UnT3 (Male,  ), UnT4 (Male,
), UnT4 (Male,  ), UnT5 (Female,
), UnT5 (Female,  ), and UnT6 (Female,
), and UnT6 (Female,  ). The HIV-1SDmEF1αmurSigHutMCM-treated mice are M1 (Male,
). The HIV-1SDmEF1αmurSigHutMCM-treated mice are M1 (Male,  ), M2 (Female,
), M2 (Female,  ), M3 (Female,
), M3 (Female,  ), M4 (Male,
), M4 (Male,  ), and M5 (Male,
), and M5 (Male,  ). The urine MMA level of normal mice was too low to be detected by the GC/MS methodology used. (b) The averaged urinary MMA concentration of treatment groups over time. The data points of the mice in different treatment groups over the course of treatment were averaged. Data for normal mice (n=6,
). The urine MMA level of normal mice was too low to be detected by the GC/MS methodology used. (b) The averaged urinary MMA concentration of treatment groups over time. The data points of the mice in different treatment groups over the course of treatment were averaged. Data for normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and treated Mut−/−MUTh2 mice (n=5,
), and treated Mut−/−MUTh2 mice (n=5,  ) are shown. The result shows that the LV-treated mice had a mean±SEM urinary MMA concentration of 0.3±0.04 mM before treatment, and the level was reduced to 0.13±0.02 mM of MMA/mM of creatinine 6 months after treatment, which is significantly different from normal and untreated groups (0.25±0.01 mM of MMA/mM of creatinine). The error bars represent SEM. α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated), and (untreated vs. treated), respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. (c) The comparison of urinary MMA levels between treatment groups. The data for each individual mouse were quantified by integrating the AUC as described. The averaged AUC values for each group are shown. The Mut−/−MUTh2 untreated mice (n=6,
) are shown. The result shows that the LV-treated mice had a mean±SEM urinary MMA concentration of 0.3±0.04 mM before treatment, and the level was reduced to 0.13±0.02 mM of MMA/mM of creatinine 6 months after treatment, which is significantly different from normal and untreated groups (0.25±0.01 mM of MMA/mM of creatinine). The error bars represent SEM. α, β, and * indicate a significant difference between treatment groups, (normal vs. untreated), (normal vs. treated), and (untreated vs. treated), respectively. The p-values were calculated using one-way ANOVA with Tukey's post hoc test. (c) The comparison of urinary MMA levels between treatment groups. The data for each individual mouse were quantified by integrating the AUC as described. The averaged AUC values for each group are shown. The Mut−/−MUTh2 untreated mice (n=6,  ) had a mean±SEM of 6±1, which is significantly different from normal mice (n=6,
) had a mean±SEM of 6±1, which is significantly different from normal mice (n=6,  ) (p<0.05). However, there is no significant difference between the untreated and treated group (n=5,
) (p<0.05). However, there is no significant difference between the untreated and treated group (n=5,  ). The error bars represent SEM. α and * indicate that a significant difference between treatment groups, (normal vs. untreated) and (normal vs. treated), respectively. The p-value among the groups was calculated using one-way ANOVA with Tukey's post hoc test.
). The error bars represent SEM. α and * indicate that a significant difference between treatment groups, (normal vs. untreated) and (normal vs. treated), respectively. The p-value among the groups was calculated using one-way ANOVA with Tukey's post hoc test.
The averaged total AUC was determined for each treatment group (Fig. 5c). The untreated Mut−/−MUTh2 mice show a mean±SEM of 6±1, which is significantly higher (p<0.05, Tukey's post hoc test) than that of normal mice, which had an average value below the limit of detection. A mild reduction, approximately 38%, was observed in the treated group, with a mean±SEM of 4±1; however, this did not reach statistical significance when compared with the untreated Mut−/−MUTh2 mice.
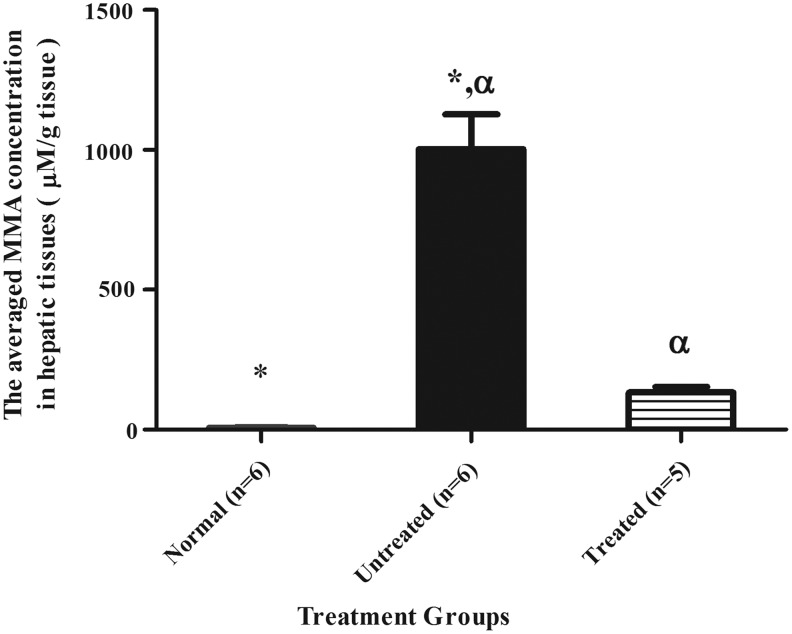
Assessment of MMA level in liver samples
The mean±SEM hepatic MMA level was significantly reduced to 133±20 μM/g tissue in the HIV-1SDmEF1αmurSigHutMCM-treated group (p<0.05, Tukey's post hoc test), compared with untreated Mut−/−MUTh2 control (1003±124 μM/g tissue), and this was not significantly different from the normal group (7±1 μM/g tissue) (Fig. 6). Individual hepatic MMA measurements indicated a range of results, from 68 to 194 μM/g tissue (Supplementary Table S1). MMA was undetectable in the brain, spleen, and kidneys of all mice, including untreated MMA mice, preventing evaluation of the effect of treatment on these tissues.
FIG. 6.
The comparison of hepatic MMA concentrations between treatment groups. Average hepatic MMA concentrations of normal mice (n=6,  ), Mut−/−MUTh2 untreated mice (n=6,
), Mut−/−MUTh2 untreated mice (n=6,  ), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,
), and HIV-1SDmEF1αmurSigHutMCM-treated mice (n=5,  ) are shown. The mean±SEM concentrations of hepatic MMA for normal mice, Mut−/−MUTh2 untreated group, and HIV-1SDmEF1αmurSigHutMCM-treated mice are 7±1 μM/g tissue, 1003±124 μM/g tissue, and 133±20 μM/g tissue, respectively. Statistical analysis demonstrated a significant difference between the untreated and normal group. Also, the analysis demonstrated a significant reduction (p<0.05) of hepatic MMA concentration in HIV-1SDmEF1αmurSigHutMCM-treated mice compared with the untreated group. However, the analysis showed no significant difference between the HIV-1SDmEF1αmurSigHutMCM-treated group and the normal group. The error bars represent SEM; * and α indicate a significant difference between treatment groups, (normal vs. untreated) and (untreated vs. treated), respectively. The p-value among the groups was calculated using one-way ANOVA with Tukey's post hoc test.
) are shown. The mean±SEM concentrations of hepatic MMA for normal mice, Mut−/−MUTh2 untreated group, and HIV-1SDmEF1αmurSigHutMCM-treated mice are 7±1 μM/g tissue, 1003±124 μM/g tissue, and 133±20 μM/g tissue, respectively. Statistical analysis demonstrated a significant difference between the untreated and normal group. Also, the analysis demonstrated a significant reduction (p<0.05) of hepatic MMA concentration in HIV-1SDmEF1αmurSigHutMCM-treated mice compared with the untreated group. However, the analysis showed no significant difference between the HIV-1SDmEF1αmurSigHutMCM-treated group and the normal group. The error bars represent SEM; * and α indicate a significant difference between treatment groups, (normal vs. untreated) and (untreated vs. treated), respectively. The p-value among the groups was calculated using one-way ANOVA with Tukey's post hoc test.
Discussion
We used a lentivirus construct containing codon-optimized human MCM gene sequence under the control of a murine mitochondrial targeting sequence to restore MCM metabolism in vivo. Initial in vivo experiments with a vector utilizing the native human MCM coding sequence under the transcriptional control of the EF1-α gene promoter, HIV-1SDmEF1αhMCM, suggested that MCM expression was too low to significantly rescue enzyme activity in the liver or to significantly decrease the MMA levels in blood or urine, despite the vector being effective in biochemically correcting (propionate incorporation assay) MMA knockout cells in vitro (results not shown). Therefore, several strategies were adopted to enhance the expression and hence improve vector-mediated restoration of MCM metabolism in vivo. These strategies include optimizing codon usage in the human MCM gene sequence and the use of a murine mitochondrial targeting sequence. The use of the latter modification is supported by the study conducted by Ye et al. (2001) in which it was demonstrated that the use of murine mitochondrial targeting sequence improved expression of the human OTC cDNA in mouse hepatocytes. Our in vitro results demonstrated a considerable improvement in the ability of the vector to restore MCM expression in MCM knockout fibroblasts, with an average value of 179±18 nM succinyl coenzyme A/min/μg of total cell protein (p<0.05), approximately twice the activity in the (nonoptimized) HIV-1SDmEF1αhMCM-vector-treated MCM knockout fibroblasts (85±13 nM/min/μg of total cell protein). Importantly, this level was significantly higher, by 5-fold, compared with the level found in the HEK293T normal control cells (24±1 nM/min/μg of total cell proteins) (data not shown). However, it was not possible to assess which change (codon optimization or use of a murine signal) was more important in improving expression of MCM or whether both contributed to the result. In practice, the only issue of importance is that expression was significantly improved; hence, it was considered appropriate to assess the new vector in the Mut−/−MUTh2 mouse model.
Plasma MMA levels are considered by physicians who manage patients with MMAuria the best guide to validate the effect of treatment. We demonstrated that the plasma MMA levels of the HIV-1SDmEF1αmurSigHutMCM-treated mice were significantly reduced, to half the level of the untreated group. The decrease in plasma MMA can only be the result of substantial restoration of MCM enzyme activity. Moreover, the liver MMA concentration was significantly decreased, indicating the rapid metabolism and clearance of these MMA metabolites from the animals. The correction we obtained in these mice is comparable to the results described by Chandler and Venditti (2010), who reported a mean plasma MMA level of approximately 400 μM in their AAV8-vector-treated MMAuria mice. This vector utilized the cytomegalovirus immediate early promoter to drive MCM expression.
Unlike the plasma MMA, only a modest decrease in MMA levels was found in the urine. This is consistent with the outcomes reported in patients who received liver transplantation, but do not show normalization of urinary MMA levels (Lubrano et al., 2007). The role of the kidneys in the whole-body MCM activity is not known, although kidney contains only about 18% of the enzyme activity found in the liver (Andrews et al., 1993; Lubrano et al., 2007). Liver has been the main target for gene therapy in ameliorating the MMAuria disease, based on the success in liver transplantation (Meyburg and Hoffmann, 2005). However, kidney transplantation alone in a 26-year-old patient resulted in the stabilization of the patient's metabolic decompensation, raising the possibility of considering kidney as a substitute organ for the treatment of MMAuria (Lubrano et al., 2007). This is also suggested by Kaplan et al. (2006) and Kasahara et al. (2006), who both challenge the rationale of liver transplantation. In fact, a few reports have also suggested that the benefit of either renal or combined transplantation (van't Hoff et al., 1999; Chakrapani et al., 2002; Nyhan et al., 2002; Hsui et al., 2003; Kaplan et al., 2006; Kasahara et al., 2006) is greater than that of liver transplantation alone (Van Calcar et al., 1998; Lubrano et al., 2001; Coman et al., 2006).
Vector copy number studies of the liver, spleen, and kidney showed that the liver is the main target for gene delivery by intravenous injection, with an average of 3.2 copies per cell, followed by the spleen. It should also be noted that the biodistribution of LV vector was measured in other tissues, including gut, brain, and skeletal muscles; however, no vector was detected in tissues other than the liver, spleen, and kidneys (data not shown). This observation is in accordance with previous findings that liver appears to be the main target after IV gene delivery (Follenzi et al., 2002; Pan et al., 2002; McIntyre et al., 2010). A high level of vector copy was detected in one of the HIV-1SDmEF1αmurSigHutMCM-treated mice (M1), with 10 copies per cell, indicating that very high levels of transduction are possible. However, it is unclear why this mouse responded so well, as the other treated mice had an average of only approximately 1.4 vector copies per cell (range from 0.8 to 1.6 copies/cell).
The hepatic MCM enzyme activity, measured by HPLC, showed a statistically significant increase between the treated and untreated animals. Unexpectedly, despite detecting a low level of protein expression in the Mut−/−MUTh2 untreated mice, no hepatic enzyme activity could be detected using our specific HPLC enzyme assay, in contrast to the findings as previously reported (Peters et al., 2012) that this Mut−/−MUTh2 mouse model possesses approximately 20% of normal control MCM enzyme activity level. The most likely explanation for this is the threshold of detection for our HPLC-based enzyme assay, estimated to be about 25% of normal (data not shown).
The high level of gene transduction in the HIV-1SDmEF1αmurSigHutMCM-treated mice (M1) resulted in a small but noticeable improvement across the whole range of results, including Western blot analysis, and plasma, urinary, and liver MMA levels (Supplementary Table S1). However, the improvement was small compared with the difference in copy number, suggesting that expression of MCM enzyme activity in the liver may be limited by other factors.
This idea is supported by the in vitro study of Wilkemeyer et al. (1992), who investigated the restoration of propionate metabolism in cultured cells after transfection with retroviral-based vector that expressed murine MCM (Wilkemeyer et al., 1992), demonstrating that MCM is not a rate-limiting enzyme in propionate metabolism and that overexpression of MCM did not increase propionate metabolism above the normal level in cultured fibroblasts and hepatoma cells.
These studies, in combination with the reported somewhat limited efficacy of liver transplantation, support the need for future studies directed toward additional gene delivery into the kidneys, for example, through parenchymal or ureteral administration (Gusella et al., 2002).
Conclusion
Administration of the HIV-1SDmEF1αmurSigHutMCM vector can provide significant, although incomplete, biochemical correction of MMAuria in a Mut−/−MUTh2 mouse model, providing additional support for gene therapy as a potential treatment for MMAuria. Further studies should investigate the potential to target other tissues in addition to the liver.
Supplementary Material
Acknowledgments
This work was supported by funding from the Women's and Children's Hospital Foundation and the Australian National Health and Medical Research Council. The authors would like to thank Dr. Nicole Buck for providing assistance with the mouse model; Dr. David Johnson, Rosemarie Gerace, and Minh-Uyen Trinh for technical assistance with biochemical analyses; Dr. Peter Clements for technical support with the HPLC experiments; and Lynn Marsden for her assistance with animal sampling procedures.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Andrews E., et al. (1993). Expression of recombinant human methylmalonyl-CoA mutase: in primary mut fibroblasts and Saccharomyces cerevisiae. Biochem. Med. Metab. Biol. 50, 135–144 [DOI] [PubMed] [Google Scholar]
- Anson D.S., and Fletcher J.M. (2007). Gene therapy for disorders affecting children, progress and potential. J. Paediatr. Child Health 43, 323–330 [DOI] [PubMed] [Google Scholar]
- Baumgarter E.R., and Viardot C. (1995). Long-term follow-up of 77 patients with isolated methylmalonic acidaemia. J. Inherit. Metab. Dis. 18, 138–142 [DOI] [PubMed] [Google Scholar]
- Chace D.H., et al. (2001). Rapid diagnosis of methylmalonic and propionic acidemias: quantitative tandem mass spectrometric analysis of propionylcarnitine in filter-paper blood specimens obtained from newborns. Clin. Chem. 47, 2040–2044 [PubMed] [Google Scholar]
- Chakrapani A., et al. (2002). Metabolic stroke in methylmalonic acidemia five years after liver transplantation. J. Pediatr. 140, 261–263 [DOI] [PubMed] [Google Scholar]
- Chandler R.J., and Venditti C.P. (2008). Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut(0) methylmalonic acidemia. Hum. Gene Ther. 19, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J., and Venditti C.P. (2010). Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol. Ther. 18, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman D., et al. (2006). Renal transplantation in a 14-year-old girl with vitamin B12-responsive cblA-type methylmalonic acidaemia. Pediatr. Nephrol. 21, 270–273 [DOI] [PubMed] [Google Scholar]
- Coulombe J.T., et al. (1981). Massachusetts Metabolic Disorders Screening Program. II. Methylmalonic aciduria. Pediatrics 67, 26–31 [PubMed] [Google Scholar]
- Cox E.V., and White A.M. (1962). Methylmalonic acid excretion: an index of vitamin-B12 deficiency. Lancet 280, 853–856 [DOI] [PubMed] [Google Scholar]
- Crane A.M., et al. (1992). Cloning and expression of a mutant methylmalonyl coenzyme A mutase with altered cobalamin affinity that causes mut- methylmalonic aciduria. J. Clin. Invest. 89, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deodato F., et al. (2006). Methylmalonic and propionic aciduria. Am. J. Med. Genet. C. Semin. Med. Genet. 142C, 104–112 [DOI] [PubMed] [Google Scholar]
- Follenzi A., et al. (2002). Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum. Gene Ther. 13, 243–260 [DOI] [PubMed] [Google Scholar]
- Gusella G.L., et al. (2002). Lentiviral gene transduction of kidney. Hum. Gene Ther. 13, 407–414 [DOI] [PubMed] [Google Scholar]
- Horster F., and Hoffmann G.F. (2004). Pathophysiology, diagnosis, and treatment of methylmalonic aciduria-recent advances and new challenges. Pediatr. Nephrol. 19, 1071–1074 [DOI] [PubMed] [Google Scholar]
- Hsui J.Y., et al. (2003). Living-related liver transplantation for methylmalonic acidemia: report of one case. Acta Paediatr. Taiwan 44, 171–173 [PubMed] [Google Scholar]
- Kahler S.G., et al. (1994). Pancreatitis in patients with organic acidemias. J. Pediatr. 124, 239–243 [DOI] [PubMed] [Google Scholar]
- Kaplan P., et al. (2006). Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol. Genet. Metab. 88, 322–326 [DOI] [PubMed] [Google Scholar]
- Kasahara M., et al. (2006). Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr. Transplant. 10, 943–947 [DOI] [PubMed] [Google Scholar]
- Kikuchi M., et al. (1989). Assay of methylmalonyl CoA mutase with high-performance liquid chromatography. Clin. Chim. Acta. 184, 307–313 [DOI] [PubMed] [Google Scholar]
- Koldej R., et al. (2005). Optimisation of a multipartite human immunodeficiency virus based vector system; control of virus infectivity and large-scale production. J. Gene Med. 7, 1390–1399 [DOI] [PubMed] [Google Scholar]
- La Marca G., et al. (2007). Rapid 2nd-tier test for measurement of 3-OH-propionic and methylmalonic acids on dried blood spots: reducing the false-positive rate for propionylcarnitine during expanded newborn screening by liquid chromatography-tandem mass spectrometry. Clin. Chem. 53, 1364–1369 [DOI] [PubMed] [Google Scholar]
- Ledley F.D., et al. (1990). Heterogeneous alleles and expression of methylmalonyl CoA mutase in mut methylmalonic acidemia. Am. J. Hum. Genet. 46, 539–547 [PMC free article] [PubMed] [Google Scholar]
- Lubrano R., et al. (2001). Kidney transplantation in a girl with methylmalonic acidemia and end stage renal failure. Pediatr. Nephrol. 16, 848–851 [DOI] [PubMed] [Google Scholar]
- Lubrano R., et al. (2007). Renal transplant in methylmalonic acidemia: could it be the best option? Report on a case at 10 years and review of the literature. Pediatr. Nephrol. 22, 1209–1214 [DOI] [PubMed] [Google Scholar]
- Manoli I., and Venditti C.P. (1993). Methylmalonic acidemia. In GeneReviews™ [Internet]. Pagon R.A., Dolan C.R., Stephens K., et al., eds. (University of Washington, Seattle, 1993–2013) [PubMed] [Google Scholar]
- Marcell P.D., et al. (1985). Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 150, 58–66 [DOI] [PubMed] [Google Scholar]
- Massoud A.F., and Leonard J.V. (1993). Cardiomyopathy in propionic acidaemia. Eur. J. Pediatr. 152, 441–445 [DOI] [PubMed] [Google Scholar]
- Matsui S.M., et al. (1983). The natural history of the inherited methylmalonic acidemias. N. Engl. J. Med. 308, 857–861 [DOI] [PubMed] [Google Scholar]
- Mc Guire P.J., et al. (2008). Combined liver-kidney transplant for the management of methylmalonic aciduria: a case report and review of the literature. Mol. Genet. Metab. 93, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C., et al. (2010). Correction of mucopolysaccharidosis type IIIA somatic and central nervous system pathology by lentiviral-mediated gene transfer. J. Gene Med. 12, 717–728 [DOI] [PubMed] [Google Scholar]
- Meyburg J., and Hoffmann G.F. (2005). Liver transplantation for inborn errors of metabolism. Transplantation 80, S135–S137 [DOI] [PubMed] [Google Scholar]
- Nagarajan S., et al. (2005). Management of methylmalonic acidaemia by combined liver-kidney transplantation. J. Inherit. Metab. Dis. 28, 517–524 [DOI] [PubMed] [Google Scholar]
- Nyhan W.L., et al. (2002). Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur. J. Pediatr. 161, 377–379 [DOI] [PubMed] [Google Scholar]
- Pan D., et al. (2002). Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol. Ther. 6, 19–29 [DOI] [PubMed] [Google Scholar]
- Peters H., et al. (2003). A knock-out mouse model for methylmalonic aciduria resulting in neonatal lethality. J. Biol. Chem. 278, 52909–52913 [DOI] [PubMed] [Google Scholar]
- Peters H.L., et al. (2012). Mouse models for methylmalonic aciduria. PLoS One 7, e40609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M.L., et al. (1991). Genetic characterization of a MUT locus mutation discriminating heterogeneity in mut0 and mut− methylmalonic aciduria by interallelic complementation. J. Clin. Invest. 87, 203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., and Ledley F.D. (1992). Correction of methylmalonyl-CoA mutase deficiency in Mut0 fibroblasts and constitution of gene expression in primary human hepatocytes by retroviral-mediated gene transfer. Somat. Cell Mol. Genet. 18, 507–516 [DOI] [PubMed] [Google Scholar]
- Sniderman L.C., et al. (1999). Outcome of individuals with low-moderate methylmalonic aciduria detected through a neonatal screening program. J. Pediatr. 134, 675–680 [DOI] [PubMed] [Google Scholar]
- Tanaka K., et al. (1980). Gas-chromatographic method of analysis for urinary organic acids. I. Retention indices of 155 metabolically important compounds. Clin. Chem. 26, 1839–1846 [PubMed] [Google Scholar]
- Van Calcar S.C., et al. (1998). Renal transplantation in a patient with methylmalonic acidaemia. J. Inherit. Metab. Dis. 21, 729–737 [DOI] [PubMed] [Google Scholar]
- Van't Hoff W., et al. (1999). Liver transplantation for methylmalonic acidaemia. Eur. J. Pediatr. 158Suppl 2, S70–S74 [DOI] [PubMed] [Google Scholar]
- White A.M., and Cox E.V. (1964). Methylmalonic acid excretion and vitamin B12 deficiency in the human. Ann. NY Acad. Sci. 112, 915–921 [DOI] [PubMed] [Google Scholar]
- Wilkemeyer M., et al. (1992). Propionate metabolism in cultured human cells after overexpression of recombinant methylmalonyl CoA mutase: implications for somatic gene therapy. Somat. Cell Mol. Genet. 18, 493–505 [DOI] [PubMed] [Google Scholar]
- Ye X., et al. (2001). Differences in the human and mouse amino-terminal leader peptides of ornithine transcarbamylase affect mitochondrial import and efficacy of adenoviral vectors. Hum. Gene Ther. 12, 1035–1046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.