Abstract
Objectives: This study seeks to determine if variation in the dopamine transporter gene (SLC6A3/DAT1) moderates the dose-response effects of long-acting dexmethylphenidate (D-MPH) and mixed amphetamine salts (MAS) in children with attention-deficit/hyperactivity disorder (ADHD).
Methods: Fifty-six children and adolescents (mean age=11.7±2.2) participated in a double-blind, two period crossover, dose-response study with a randomized placebo week in each 4 week drug period. Each period consisted of sequential week-long exposures to three dose levels (10, 20, 25–30 mg, depending upon weight) of D-MPH or MAS.
Results: Doses of 10–20 mg of either D-MPH or MAS had little to no effect on hyperactivity-impulsivity and total ADHD symptom scores in subjects with the 9/9 genotype; this was in contrast to the dose-response curves of subjects with either the 10/10 or 10/9 genotype.
Conclusions: ADHD youth with the 9/9 genotype may require higher stimulant doses to achieve adequate symptom control.
Introduction
Stimulant treatment for attention-deficit/hyperactivity disorder (ADHD) typically employs a “trial and error” approach, using one or another formulation from the methylphenidate (MPH) or amphetamine (AMP) class, and gradually increasing the dose until clinically significant improvement is observed. Both MPH and AMP formulations have demonstrated robust acute efficacy, as indicated by large effect sizes on ADHD symptom measures (Conners 2002; Pliszka et al. 2006). In general, higher doses are needed to treat more severe symptoms, but may also be associated with higher levels of side effects, such as appetite loss and insomnia (Douglas et al. 1986; Stein et al. 2003; Newcorn et al. 2010). When AMP and MPH formulations are dosed comparably, few differences in response or tolerability are reported at the group level (Faraone 2009). However, there have been few systematic comparator trials. Moreover, despite overall comparability in response at the group level, findings to date suggest that many individuals have a superior response to one stimulant class over the other (Elia et al. 1991; Arnold 2000). In recognition of this point, current treatment algorithms recommend prescribing a different stimulant if there is lack of efficacy or poor tolerability to the first agent or formulation selected (Dulcan 1997; Pliszka et al. 2006). However, there are not yet established predictors of which medication class is likely to be more effective and/or better tolerated by individual children, as well as predictors of optimal dose (Denney and Rapport 1999; Elia et al. 1999).
Polymorphisms in a variable number tandem repeat (VNTR) in the 3′ untranslated region of the dopamine transporter (SLC6A3/DAT1) gene located on chromosome 5p15.3 have been studied with regard to both susceptibility for ADHD (Cook et al. 1995), and prediction of stimulant response (Winsberg and Comings 1999; Froehlich and Stein 2010). This variant has been highly investigated because of its direct relevance to the mechanism of action of stimulant medications, which characteristically enhance synaptic dopamine through blockade of the presynaptic dopamine transporter (Volkow and Swanson 2003). The VNTR varies from 3 to 11, with the 10/10 and 10/9 repeat being the most common.
In the first ADHD pharmacogenetic study examining DAT1 (Winsberg & Comings, 1999), the homozygous 10 repeat allele of DAT1 was associated with a diminished response to MPH relative to heterozygotes in a sample of 30 stimulant-naive children. However, other studies have shown no relationship with the 9 repeat allele (McGough et al. 2009; Froehlich et al. 2010), and a recent meta-analysis of 16 studies concluded that DAT1 is not a reliable predictor of treatment success based upon comparisons of response in individuals who were homozygous for the 10 repeat versus non-10/10 carriers (Rambeitz et al. 2014). Although this genotype grouping has been inconsistent in relationships with response across studies, there appears to be growing evidence that examining DAT1 genotypes in an additive fashion across three genotype groups may be informative.
Stein et al. (2005) reported that children with the less common 9/9 genotype of DAT1 displayed a dose-response curve distinct from that of those with more common genotype groups containing a 10 repeat allele, in a placebo-controlled trial of osmotic controlled-release oral delivery system (OROS) MPH in 46 youth (Stein et al. 2005). Utilizing a dose response methodology (as opposed to a flexible or optimal dose design), ADHD symptoms of children with the 9/9 were less responsive to increasing stimulant doses. Subsequently, two other studies reported that ADHD youth with the 9/9 genotype displayed a lower response rate, based upon parent ratings of ADHD symptoms (McGough et al. 2006; Joober et al. 2007). However, Froehlich et al. recently examined dose-response effects of MPH in 89 children with ADHD, including 5 with the 9/9 genotype, and found that those with the 9/9 genotype displayed a more robust response than other genotype groups, especially at the higher dose level (Froehlich et al. 2011). It is of note that in the latter study, hyperactive-impulsive symptom scores were more severe for children with the 9/9 genotype relative to children with genotypes containing the 10 repeat until relatively high doses (i.e., >1.5 mg/kg) were attained. Therefore, emerging data from several studies indicate that youth who have the 9/9 genotype of DAT1 display a different dose-response curve than more common genotype groups when treated with MPH.
An important point to consider with regard to stimulant treatment is that there is considerable variability across individuals in tolerability, which presumably contributes to the well-acknowledged poor long-term adherence to treatment (Charach et al. 2004). Therefore, in addition to predicting efficacy, it is also important to consider possible differences in tolerability as a function of genotype. For example, Gruber et al. (2009) systematically examined the relationship between DAT1 polymorphisms and three parent-reported stimulant side effects factors (i.e., emotionality, somatic complaints, being overfocused) derived from the Side Effects Rating Scale in children treated with MPH. Children with the 9/9 genotype displayed higher scores on the emotionality factor (i.e., irritability, sadness, anxiety, being prone to crying) during placebo and MPH treatment relative to the other genotype groups (Gruber et al. 2009), whereas children with the 10/10 genotype displayed a significant, dose-dependent increase in somatic complaints (e.g. decreased appetite, insomnia). Taken together, these and other findings (Leddy et al. 2009), suggest that when analyzed separately, youth with the 9/9 DAT1 genotype display a response to MPH that differs from that of those with the more common genotypes containing a 10 repeat.
Therefore, the goals of the present study are to: 1) test for differences in dose-response curves of MPH in children and adolescents with the 9/9 genotype relative to the 10/10 and 10/9 groups; 2) examine the association of DAT1 with stimulant side effect factors; and 3) examine whether DAT1 genotype moderates dose-response effects for mixed amphetamine salts (MAS) and dexmethylphenidate (D-MPH). As yet, the effects of DAT1 have not been studied for the AMP class of stimulants in youth with ADHD, and no studies have considered possible differential effects of DAT1 genotype on response to MPH and AMP.
Methods and Materials
Participants
Sixty-five children and adolescents 9–17 years of age (mean=11.7, SD=2.24) met the study inclusion criteria (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [DSM-IV] criteria for ADHD based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version) (American Psychiatric Association 1994; Kaufman et al. 1997), and 56 participated in the clinical trial; 7 decided not to participate or were lost to follow-up, and 2 did not comply with the protocol. The sample was predominantly male (47 boys [73%] and 17 girls), with a higher prevalence of the combined subtype (CT) (67%). The sample was ethnically diverse (per self-report) with equivalent percentages of subjects from African-American and Caucasian, or European-American, ethnic backgrounds (41% each); in addition, 7% were Latino, 7% were biracial, 2% were Asian, and 2% were Native American. Written consent was obtained from parents and participants prior to study participation. This study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Measures
During the initial assessment and at each weekly visit, participants met with clinical staff to discuss medication effects and to complete the ADHD Rating Scale (ADHD RS) –IV: Home version (DuPaul et al. 1998), and a structured side effect scale that included items from the Barkley Side Effects Rating Scale (Barkley et al. 1990). Three side effect factor scores (emotionality, somatic complaints, overfocused) based on Gruber et al (2009) were calculated for each subject.
Procedure
The details of the recruitment, study design, demographics, and comparative efficacy have been published preciously (Stein et al. 2011). In brief, participants were treated with extended release (ER) MAS (Adderall XR), dexmethylphenidate (D-MPH; Focalin XR), or placebo in an 8 week, randomized, double-blind, placebo-controlled, two-period crossover dose-response study with weekly dose escalations. Three dose conditions of ER D-MPH and ER MAS (10, 20, and 25–30 mg) were administered sequentially from lowest dose to highest dose, with a randomized week of placebo included in each 4 week drug period. The maximum dose was 25 mg in children <35 kg, to minimize potential side effects. The research pharmacist developed a randomization schedule for order of study drug and randomization of the placebo weeks, and prepared weekly blister packs for each subject containing capsules of study drug, which were indistinguishable from each other.
Blood was collected from all participants and genomic DNA extracted using a PureGene kit from 10 mL whole blood. The genotyping procedures for this assay have been described previously (Bedard et al. 2010). Briefly, 50 ng of DNA was used for polymerase chain reaction (PCR) amplification and fragment detection was performed on a 3730XL capillary instrument to identify the number of VNTRs with the most commonly observed 9 and 10 repeat alleles utilized for the analyses herein.
Dose-response effects were examined via hierarchical linear models using the linear mixed models module in SPSS version 20 (Bryk and Raudednbush 1992). Specifically, for each of the outcome variables we included the following main effect and interaction terms in the linear mixed modeling equation: sex, age, weight, ADHD subtype, prior stimulant exposure, ethnicity (self-report of African American, European, or Hispanic ancestry), DAT1 genotype, and dose, and then the interaction of dose with DAT1 polymorphism, drug with DAT1, and dose by drug by DAT1. In addition, a second set of analyses was conducted with baseline ADHD symptom severity added as a covariate to control for baseline differences in symptom severity. We tested for both linear and curvilinear components of dose-response in these models. Variables other than dose, DAT1, and drug type were entered as main effects to serve as covariates, to control for any possible contribution to variance in the outcome measures and to prevent any potential bias in estimates of the effects of the focal explanatory variables dose, drug type, and DAT1 genotype. With the exception of tests of self-identified ethnicity, which has two degrees of freedom, all tests had one degree of freedom.
Departure from Hardy–Weinberg equilibrium (HWE) was used to evaluate genotyping reliability. The DAT1 genotype frequencies for our sample were as follows: 9/9, 12%; 9/10, 36%; and 10/10, 52%, consistent with HWE (p=0.388).
Results
As previously reported in Stein et al. (2011), there were significant and substantial dose-related decreases in total and hyperactive-impulsive symptom scores (p<0.001, R 2=0.59 and p<0.001, R2=0.46, respectively) that did not differ by type of stimulant, as well as significant dose-related decreases for inattention symptom scores (p<0.001, R2=0.11) that were more modest in magnitude, but which also did not differ by type of stimulant.
Does DAT1 genotype moderate dose response effects on total ADHD score and ADHD symptom dimensions?
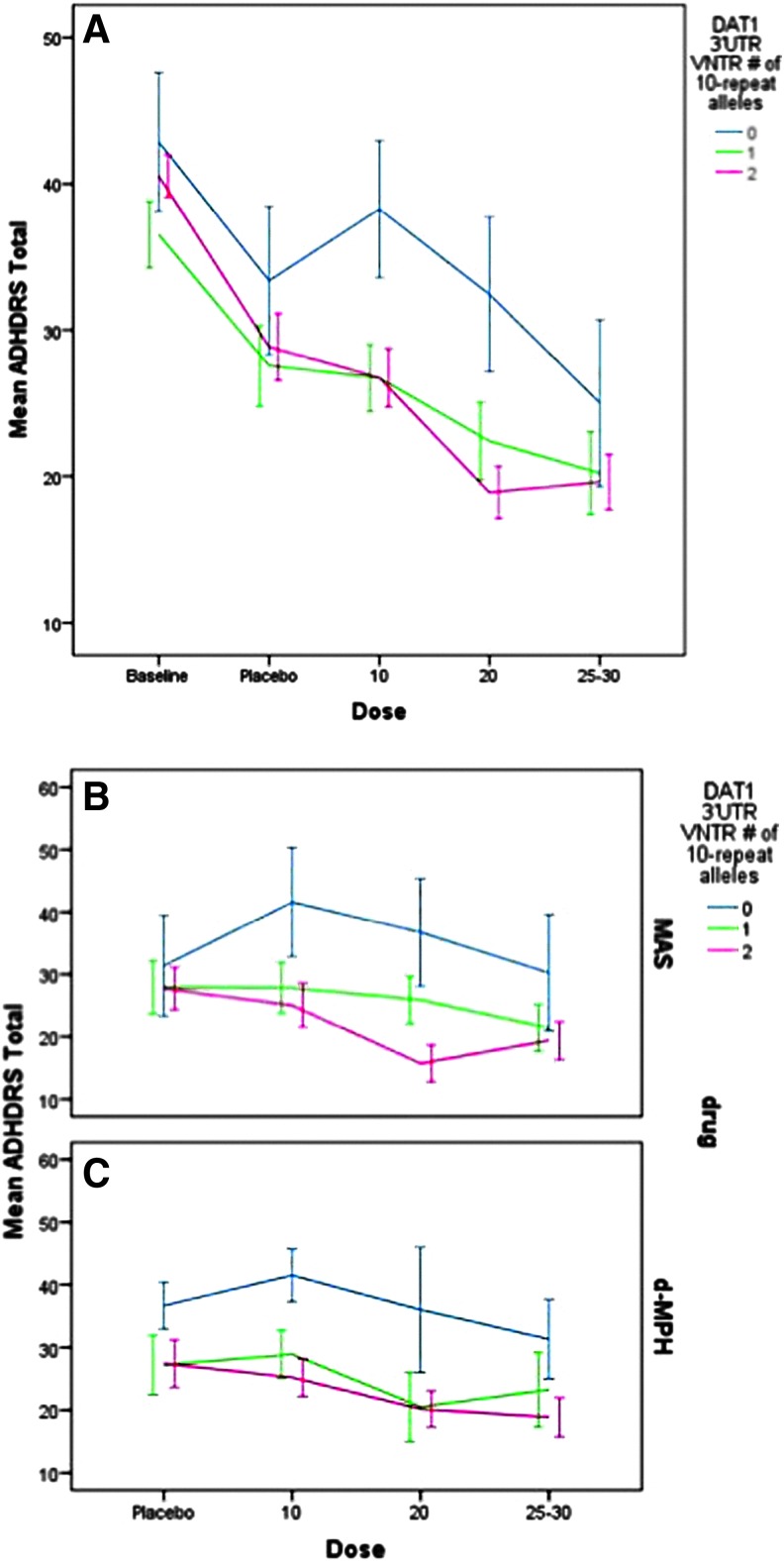
As seen in Figure 1A (data for both stimulants combined), there was a significant main effect for dose (F=17.08, p<0.001) and DAT1 (F=12.5, p<0.001) on total ADHD RS and a significant curvilinear dose-response curve that was different for subjects absent the 10 repeat allele (F=5.1, p=0.024). This dose response by DAT1 interaction remained after adjusting for baseline ADHD symptom severity, as subjects with the 9/9 genotype continued to display a different dose-response curve from those with genotypes containing the 10 repeat allele (F=3.7, p=0.05). Subjects with the 9/9 genotype displayed higher ADHD symptoms at all dose levels and displayed a less adequate response to increasing dose of either D-MPH or MAS (see Fig. 1B and C). DAT1 genotype did not interact with type of stimulant. ADHD symptom levels also varied by age (F=11.2, p<0.001), subtype (F=28.9. p<0.001), stimulant history (F=8.5, p<0.01), and weight (F=16.3, p<0.001), but not by ethnicity.
FIG. 1.
Baseline and dose response for Attention-Deficit/Hyperactivity Disorder Rating Scale (ADHD-RS) total by DAT1 3′UTR VNTR genotype for mixed amphetamine salts (MAS) and dexmethylphenidate (D-MPH). (A) represents both stimulants combined and includes baseline characteristics. (B) and (C) represent MAS and D-MPH dose-response curves separately (excludes baseline). A color figure is available in the online version of this article at www.liebertonline.com/jcap
Examination of ADHD symptom dimensions revealed a main effect of DAT1 genotype (F=16.5, p=0.001) and an interaction with dose-response (F=8.8, p=0.003) for hyperactive-impulsive symptoms. Subjects with the 9/9 genotype displayed higher hyperactive-impulsive scores at all dose levels, and a distinct dose-response curve, with little benefit noted for either drug. The dose response by DAT1 interaction on hyperactive-impulsive symptoms remained after controlling for baseline ADHD symptom severity, as subjects with the 9/9 genotype continued to show a different dose-response curve from those with genotypes containing the 10-repeat allele (F=6.37, p=0.012). Significant covariates were age (F=32.1, p<0.001), ADHD subtype (F=33.5, p<0.001), and weight (F=24.2, p<0.001).
For inattentive symptoms, there was a main effect of DAT1 genotype (F=5.3, p=0.022); genotype did not interact with dose-response (F=1.8, p=0.185), with results remaining similar after controlling for baseline inattentive symptom levels (F=1.91, p=0.169). Subjects with the 9/9 genotype displayed higher inattention scores overall, but did not differ in their dose-response compared with other DAT1 genotype groups. Weight (F=6.9, p=0.009) and ADHD subtype (F=16.3, p<0.001) were significant covariates.
Does DAT1 genotype moderate dose-response effects of ER-MAS and D-MPH on side effect factors?
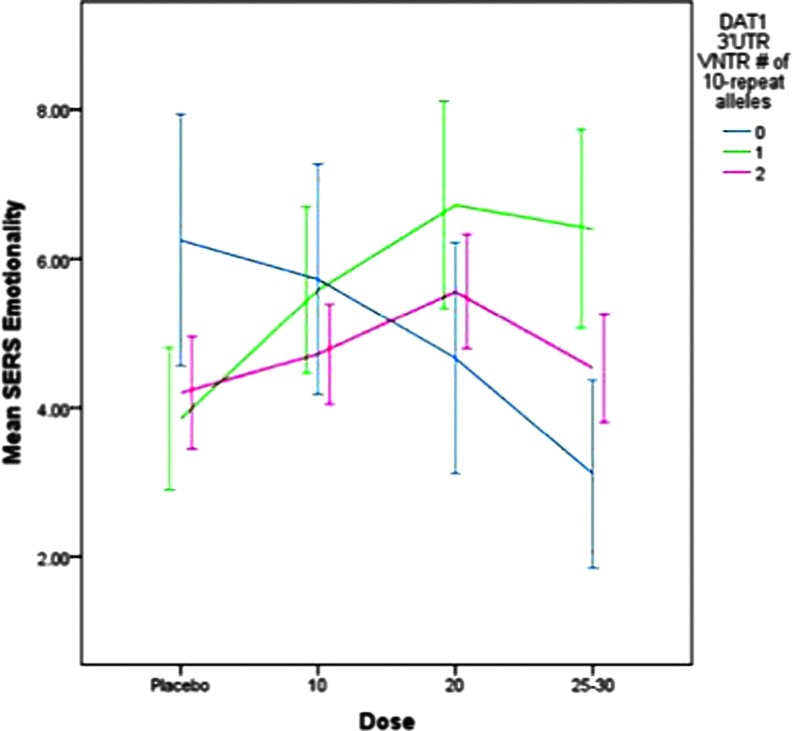
The relationships between DAT1 genotype, ER D-MPH and MAS dose and side effect factors were evaluated using empirically derived stimulant effect factor scores (Gruber et al. 2009). Dose-response effects on the emotionality factor (i.e., irritability, sadness, tendency to cry, and anxiety) are portrayed in Figure 2. There were no significant main effects of DAT1, no interaction with dose, and no differences between ER MAS and D-MPH. However, ADHD subtype was a significant covariate (F=16.1, p<0.001); subjects with CT displayed higher emotionality scores (mean=6.2±0.58) than did those with the predominantly inattentive subtype (mean=3.4±0.66).
FIG. 2.
Dose-response for emotionality by DAT1 3′UTR VNTR genotype for mixed amphetamine salts and dexmethylphenidate. SERS, Stimulant Side Effect Scale. A color figure is available in the online version of this article at www.liebertonline.com/jcap
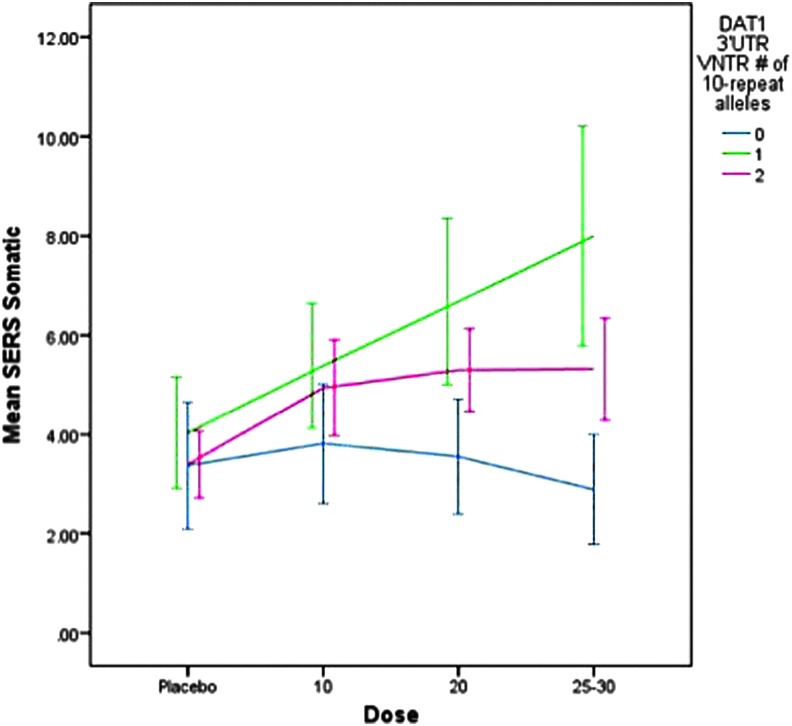
There was a trend for differences across DAT1 genotypes on the somatic complaints factor (e.g., decreased appetite, insomnia) (F=3.1, p=0.079). As portrayed in Figure 3, subjects who had at least one 10 repeat allele displayed higher scores on the somatic factor as did subjects with the 9/9 genotype, at all dose levels. Significant covariates were ADHD subtype (F=8.1, p=0.005), weight (F=5.8, p=0.016), and self-reported ethnicity (F=3.04, p=0.050). Subjects with ADHD CT, a previous history of stimulant treatment, and lower body weight were more likely to display somatic complaints, and subjects of Hispanic origin displayed slightly higher scores (mean=5.4±1.6) than did those European-American (mean=4.8±5.6) or African-American (mean=3.6±5.7) origin.
FIG. 3.
Dose effects on somatic complaints by DAT1 3′UTR VNTR genotype for mixed amphetamine salts and dexmethylphenidate. SERS, Stimulant Side Effect Scale. A color figure is available in the online version of this article at www.liebertonline.com/jcap
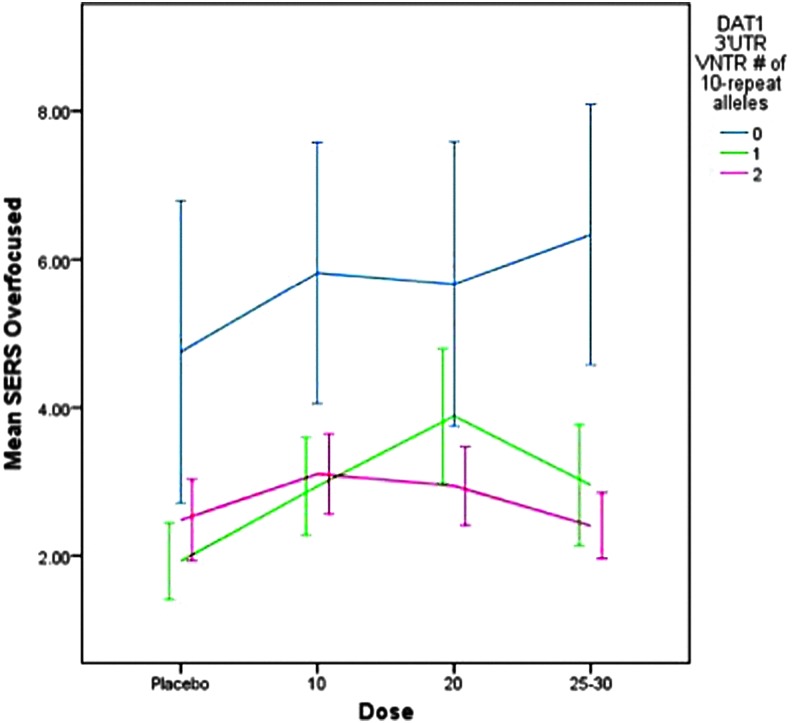
As portrayed in Fig. 4, subjects with the 9/9 genotype displayed significantly higher ratings on the overfocused factor (i.e., stares, bites nails) (F=19.6, p<0.001) at all dose levels, but there was no dose by genotype effect. Significant covariates included ADHD subtype (F=76.4, p<0. 001), weight (F=4.8, p=0.029), and stimulant history (F=7.3, p=0.007). Subjects with ADHD CT, lower body weight, and prior stimulant treatment displayed higher scores on the overfocused factor.
FIG. 4.
Dose-response effects on overfocused factors by DAT1 3′UTR VNTR genotype for mixed amphetamine salts and dexmethylphenidate. SERS, Stimulant Side Effect Scale. A color figure is available in the online version of this article at www.liebertonline.com/jcap
Discussion
In this placebo-controlled, crossover study of two commonly utilized ER stimulants, ADHD youth with the 9/9 genotype displayed a dose-response curve distinct from that of carriers of the 10 repeat allele. Specifically, youth with the 9/9 genotype displayed little or no effect on total ADHD symptoms and hyperactive-impulsive symptoms at low and moderate dose levels of D-MPH or MAS. Consistent with findings from several previous dose- response studies of MPH (Stein et al. 2005; Joober et al. 2007; Froehlich et al. 2011) and AMP (Lott et al. 2005), children with the 9/9 phenotype displayed decreased sensitivity to the beneficial behavioral effects of stimulants at low and moderate dose levels. DAT1 genotype moderated dose-response to stimulant treatment even after controlling for baseline severity. Moreover, ADHD youth with the 9/9 genotype did not display dose-dependent increases in somatic complaint or other stimulant side effect factor scores.
The results of this study add to previous findings indicating that individuals homozygous for the 9 repeat allele of DAT1 represent a small but important biologically defined subgroup of ADHD children and adolescents, whose baseline characteristics and response to stimulant medication differs from those with the 9/10 genotype. Children and adolescents with the 9/9 genotype displayed higher base rates of ADHD symptoms, and more staring and nail biting, thus highlighting the importance of evaluating levels of these behaviors and purported side effects at baseline (Sonuga-Barke et al. 2009).
In contrast to our findings in which the 9/9 genotype was not associated with decreased appetite or other somatic complaints, Leddy et al. reported that children with 9/9 genotype consumed 28% and 49% less food at lunch than children with 10/10 or 10/9 genotypes, when receiving doses of 0.3 and 0.6 mg/kg of MPH, respectively (Leddy et al. 2009). It is unclear if the differences in findings across these two studies are related to subject differences, using an immediate release versus an extended release preparation, or different methodological factors of study design, dosing, or assessment method. Nevertheless, further study is warranted on the relationship between DAT1 genotype and stimulant and dose related effects on appetite and eating behaviors utilizing objective measures.
The neurobiological basis for our findings of altered dose-response characteristics in youth with the 9/9 genotype of DAT1 is interesting to consider. The 9 repeat allele has been associated with increased dopamine transporter binding in the striatum (Spencer et al. 2013). The issue of poor therapeutic response has recently been discussed by Volkow and colleagues (Volkow et al. 2009), who suggested that nonresponse to MPH may reflect very low dopamine activity while some adverse events may be the result of high neuronal activity. Based upon the current findings, one could speculate that the 9/9 genotype is associated with lower baseline striatal DAT activity, and that youth with the 9/9 genotype may require higher stimulant dosages to achieve symptom control. In contrast, individuals with DAT1 genotypes containing the 10 repeat allele appear to respond to lower stimulant dose levels. These findings are in agreement with the hypothesis that stimulant medications may be most effective in individuals with greatest transporter densities or activity (Bellgrove et al. 2005). In addition to data from the positron emission tomography (PET) radioligand studies cited, there is also some evidence from gene expression studies to support this model (Heinz et al. 2000; Mill et al. 2002).
Conclusions
The results of our study should be interpreted in the context of its limitations. As reported in other candidate gene studies examining the intermediate phenotype of drug response (Hart et al. 2013), there may be increased risk of type 1 error because of the modest sample size of the study. However, treatment intervention studies often allow for the detection of large effect sizes with smaller numbers of participants.
Additionally, we had a racially diverse study sample, which requires analyses to assure that population stratification is not influencing the results. Failure to adequately control for population stratification may lead to spurious results, especially in a sample with a high degree of admixture, such as samples comprising multiple ethnic groups from the United States (Baye and Wilke 2010). Our predefined analysis plan addressed potential biases caused by population stratification in two ways. First, we controlled for self-reported ethnicity (i.e., European or African ancestry, Hispanic) in all analyses presented herein. Second, given that a majority of the individuals with the 9/9 genotype were of African American self-reported ethnicity, we conducted a set of sensitivity analyses that also included ancestry informative markers (AIMS) that genetically characterize the degree of West African ethnicity, to examine whether our results did not adequately control for possible population stratification effects. Results from both analyses were consistent, indicating a primary effect of genotype that is unlikely to be confounded by population stratification. Adding strength to the findings of the present study is that similar results were obtained for both AMP and MPH stimulants used in the crossover design, and the findings here are generally consistent with previous placebo-controlled studies employing similar dose- response designs (Stein and McGough 2008; Froehlich et al. 2010).
However, because of the exploratory nature of the present study and small sample size, we did not control for multiple comparisons. We limited our planned analysis to effects on dimensional measures of ADHD symptoms and stimulant side effects. In order to reduce the number of comparisons, we utilized a data reduction technique and examined side effect factor scores rather than examining individual side effects. Furthermore, we compensated for the relatively small number of 9/9 subjects in our analytic plan by statistically controlling for a variety of extraneous factors that may influence drug response, such as age, weight, previous stimulant exposure, and ethnicity. Nonetheless, replication is needed with larger sample sizes, and as suggested by Hart et al. (2013), a prospective genotyping strategy to obtain more balanced genotyping groups may be helpful for evaluating uncommon alleles in future studies.
Clinical Significance
What is the clinical importance of identifying a small subgroup of youth based upon DAT1 genotype who require higher doses of stimulant medication to reduce ADHD symptoms? The findings from this and other studies (Froelich et al. 2010) raise the possibility that the 9/9 genotype of DAT1 could account for a disproportionate degree of poor response to stimulant medications in youth with ADHD because of early discontinuation and underdosing. Children and adolescents with the 9/9 genotype are likely to require dose levels ≥25 mg/day. In the community, clinicians are often reluctant to prescribe higher doses for fear of increased side effects (Vahue 2001). Knowledge that higher doses are likely to produce improvement without increasing side effects in this subgroup should positively affect treatment decisions. The importance of providing adequate treatment for this difficult to treat subgroup is noteworthy, as previous studies have demonstrated that the 9 repeat genotype is associated with persistence of ADHD (Barkley et al., 2006; Franke et al. 2010). Furthermore, children with the 9/9 genotype are at increased risk of future conduct problems (Lahey et al. 2011). If this finding is confirmed, it might justify the decision to incorporate genotype into the titration schedule and medication algorithm for ADHD. However, although this approach has considerable intuitive appeal, it is premature to recommend incorporating pharmacogenomic approaches into clinical care until larger scale replications and cost efficacy investigations are performed.
Acknowledgements
The authors would like to acknowledge the assistance of Michael Pacini, PharmD, Esperanza Salinas, MD, and Elizabeth Charney, MD. We also would like to thank the children and families who participated in this study.
Disclosures
Dr. Stein receives research support from Shire Pharmaceuticals and Pfizer. He is a consultant/advisor for Alcobra, Genco Sciences, and Novartis. Dr. Newcorn receives research support from Shire Pharmaceuticals. He is currently serving or has served as an advisor and/or consultant for Alcobra, BioBehavioral Diagnostics, Enzymotic, GencoSciences, Neos Therapeutics, Sunovion, and Shire Pharmaceuticals. Drs. Waldman, Bishop, and Kittles, and Mr. Cook have no conflicts of interest or financial ties to disclose.
An investigator-initiated study was sponsored by Novartis Pharmaceuticals, with additional support provided by the University of at Chicago (UIC) Center for Clinical and Translational Science Ilinois (CCTS) Award Number UL1RR029879 from the National Center for Research Resources and NIMH MH083888 (Bishop). The content is solely the responsibility of the authors and does not necessarily represent the offical views of the National Center for Research Resources or the National Institutes of Health.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Arnold L: Methylphenidate vs. amphetamine: Comparative review. J Atten Disord 3:200–211, 2000 [Google Scholar]
- Barkley R, McMurray M, Edelbrock CS, Robbins K: Side ffects of MPH in children with attention deficit hyperactivity disorder: A systematic placebo controlled evaluation. Pediatrics 86:184–192, 1990 [PubMed] [Google Scholar]
- Barkley RA, Smith KM, Fischer M, Navia B: An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. Am J Med Genet B Neuropsychiatr Genet 141B:487–498, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye TM, Wilke RA: Mapping genes that predict treatment outcome in admixed populations. Pharmacogenomics J 10:465–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Schulz KP, Cook EH, Jr, Fan J, Clerkin SM, Ivanov I, Halperin JM, Newcorn JH: Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. NeuroImage 53:935–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Fitzgerald M, Gill M, Robertson IH: Association between dopamine transporter (DAT1) genotype, left-sided inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology 30:2290–2297, 2005 [DOI] [PubMed] [Google Scholar]
- Bryk AT, Raudednbush S: Hierarchical Linear Models: Applications and Data Analysis Methods. Newbury Park,CA: Sage; 1992 [Google Scholar]
- Charach A, Ickowicz A, Schachar R: Stimulant treatment over five years: Adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry 43:559–567, 2004 [DOI] [PubMed] [Google Scholar]
- Conners CK: Forty years of methylphenidate treatment in attention-deficit/ hyperactivity disorder. J Atten Disord 6 (Suppl 1): S17–30, 2002 [DOI] [PubMed] [Google Scholar]
- Cook E, Stein M, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL: Association of attention deficit disorder and the dopamine transporter gene. Am J Hum Genet 56:993–998, 1995 [PMC free article] [PubMed] [Google Scholar]
- Denney C, Rapport M: Predicting methjylphenidate response in children with ADHD: Theoretical, empirical, and conceptual models. J Am Acad Child Adolesc Psychiatry 38:393–401, 1999 [DOI] [PubMed] [Google Scholar]
- Douglas V, Barr R, O'Neill ME, Britton BG: Short term effects of methylphenidate on the cognitive, learning, and academic performance of children with attention defict disorder in the laboratory and the classroom. J Child Psychol Psychiatry 27:191–211, 1986 [DOI] [PubMed] [Google Scholar]
- Dulcan M: Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 36(Suppl 10):85S–121S, 1997 [DOI] [PubMed] [Google Scholar]
- DuPaul G, et al. ADHD Rating Scale-IV:Checklists, Norms, and Clinical Interpretations. New York: Guilford Press; 1998 [Google Scholar]
- Elia J, Ambrosini PJ, Rapoport JL: Treatment of attention-deficit-hyperactivity disorder. N Engl J Med 340:780–788, 1999 [DOI] [PubMed] [Google Scholar]
- Elia J, Borcherding BG, Rapoport JL, Keysor CS: Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders? Psychiatry Res 36:141–155, 1991 [DOI] [PubMed] [Google Scholar]
- Faraone SV: Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T 34:678–694, 2009 [PMC free article] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti–Hummer A, Heine M, Jacob CP, Lesch KP, Casas M, Ribasés M, Bosch R, Sánchez-Mora C, Gómez-Barros N, Fernàndez-Castillo N, Bayés M, Halmøy A, Halleland H, Landaas ET, Fasmer OB, Knappskog PM, Heister AJ, Kiemeney LA, Kooij JJ, Boonstra AM, Kan CC, Asherson P, Faraone SV, Buitelaar JK, Haavik J, Cormand B, Ramos-Quiroga JA, Reif A: Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 35:656–664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich T E, Epstein JN, Nick TG, Melguizo Castro MS, Stein MA, Brinkman WB, Graham AJ, Langberg JM, Kahn RS: Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:1129–1139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, McGough JJ, Stein MA: Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS Drugs 24: 99–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Stein MA.Pharmacogenocis of attention deficit/hyperactivty disrder. In: Pharmacogenomics in Psychiatry. Edited by Schwab M. and Kaschka W. Basel: Karger; 2010; pp. 75–102, [Google Scholar]
- Gruber R, Joober R, Grizenko N, Leventhal BL, Cook EH, Jr, Stein MA: Dopamine transporter genotype and stimulant side effect factors in youth diagnosed with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:233–239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, deWit BH, Palmer AA: Candidate gene studies of a promising intermediate phenotype: Failure to replicate. Neuropsychopharamcology 38:802–816, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR: Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 22:133–139, 2000 [DOI] [PubMed] [Google Scholar]
- Joober R, Grizenko N, Sengupta S, Amor LB, Schmitz N, Schwartz G, Karama S, Lageix P, Fathalli F, Torkaman-Zehi A, Ter Stepanian M: Dopamine transporter 3'-UTR VNTR genotype and ADHD: A pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology 32:1370–1376, 2007 [DOI] [PubMed] [Google Scholar]
- Kambeitz J, Romanos M, Ettinger U: Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics J 14:77–84, 2014 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorder and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:989–988, 1997 [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Waxmonsky JG, Salis RJ, Paluch RA, Gnagy EM, Mahaney P, Erbe R, Pelham WE, Epstein LH: Dopamine-related genotypes and the dose-response effect of methylphenidate on eating in attention-deficit/hyperactivity disorder youths. J Child Adolesc Psychopharmacol 19:127–136, 2009 [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, de Wit H: Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology 30:602–609, 2005 [DOI] [PubMed] [Google Scholar]
- McGough JJ, McCracken JT, Loo SK, Manganiello M, Leung MC, Tietjens JR, Trinh T, Baweja S, Suddath R, Smalley SL, Hellemann G, Sugar CA: A candidate gene analysis of methylphenidate response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 48:1155–1164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough J, McCracken J, Swanson J, Riddle M, Kollins S, Greenhill L, Abikoff H, Davies M, Chuang S, Wigal T, Wigal S, Posner K, Skrobala A, Kastelic E, Ghuman J, Cunningham C, Shigawa S, Moyzis R, Vitiello B: Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 45:1314–1322, 2006 [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I: Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet 114:975–979, 2002 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Stein MA, Cooper KM: Dose-response characteristics in adolescents with attention-deficit/hyperactivity disorder treated with OROS methylphenidate in a 4-week, open-label, dose-titration study. J Child Adolesc Psychopharmacol 20:187–196, 2010 [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Crismon ML, Hughes CW, Corners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder: The Texas Children's Medication Algorithm Project: Revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45: 642–657, 2006 [DOI] [PubMed] [Google Scholar]
- Sonuga–Barke EJ, Coghill D, Wigal T, DeBacker M, Swanson J: Adverse reactions to methylphenidate treatment for attention-deficit/hyperactivity disorder: structure and associations with clinical characteristics and symptom control. J Child Adolesc Psychopharmacol 19:683–690, 2009 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, Batchelder H, Clarke A, Fischman AJ: Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biol Psychiatry 74:84–89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, McGough JJ: The pharmacogenomic era: Promise for personalizing attention deficit hyperactivity disorder therapy. Child Adolesc Psychiatr Clin N Am 17:475–490, xi-xii, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, Black DO, Seymour KE, Newcorn JH: A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 112: e404, 2003 [DOI] [PubMed] [Google Scholar]
- Stein MA, Waldman ID, Charney E, Aryal S, Sable C, Gruber R, Newcorn JH: Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol 21:581–588, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, Kim SJ, Cook EH: Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology 30:1374–1382, 2005 [DOI] [PubMed] [Google Scholar]
- Vahue HE: Methylphenidate dosage for children with ADHD over time under controlled conditions: Lessons from the MTA. J Am Acad Child Adolesc Psychiatry 40:188–196, 2001 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM: Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918, 2003 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM: Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA 302:1084–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsberg BG, Comings DE: Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry 38:1474–1477, 1999 [DOI] [PubMed] [Google Scholar]