Abstract
Gene therapy of the lung has the potential to treat life-threatening diseases such as cystic fibrosis and α1-antitrypsin or surfactant deficiencies. A major hurdle for successful gene therapy is the development of an immune response against the transgene and/or viral vector. We hypothesized that by targeting the airways in the perinatal period, induction of an immune response against the vector particle could be prevented because of immaturity of the immune system, in turn allowing repeated gene transfer later in adult life to ensure long-term gene expression. Therefore, we readministered recombinant adeno-associated viral vector serotype 5 (rAAV2/5) to mouse airways 3 and 6 months after initial perinatal gene transfer. Our findings demonstrate that perinatal rAAV2/5-mediated gene transfer to the airways avoids a strong immune response. This immunological ignorance allows the readministration of an autologous vector later in adult life, resulting in efficient and stable gene transfer up to 7 months, without evidence of a decrease in transgene expression. Together, these data provide a basis to further explore perinatal gene therapy for pulmonary conditions with adequate gene expression up to 7 months.
Introduction
Breakthroughs in gene therapy for congenital blindness and adrenoleukodystrophy demonstrate the feasibility of successful and safe gene therapy (reviewed in Sheridan, 2011). Although gene therapy for pulmonary disorders (i.e., cystic fibrosis [CF]) has been at the forefront of the gene therapy field, efficient gene transfer to the airways remains more challenging than anticipated at first. Initial viral vector-mediated gene therapy trials for inherited disorders of the airways were based on adenoviral vectors (AdVs) (Zabner et al., 1993; Crystal et al., 1994) and later recombinant adeno-associated viral (rAAV) vectors (Wagner et al., 1999; Flotte et al., 2003). In contrast to promising results in preclinical animal models, in which long-term gene expression was demonstrated (Limberis and Wilson, 2006; Sumner-Jones et al., 2006; Liqun Wang et al., 2009), clinical trials have shown only limited success so far, due to transient and/or inefficient gene expression in the human airways (Harvey et al., 1999; Wagner et al., 2002; Moss et al., 2007). This could be attributed to the nonintegrating nature of the vector, the lack of appropriate receptors at the apical side of the respiratory epithelium, induction of an immune response against the transgene or vector particle, and the presence of preexisting immunity to the vector (reviewed in Mingozzi and High, 2011). Further preclinical research is therefore a prerequisite to investigate how to overcome the obstacles encountered in clinical trials.
rAAV vectors are promising viral vectors for gene therapy because many different AAV serotypes with a specific tissue tropism exist (Gao et al., 2002, 2004). Several of these serotypes (i.e., AAV5, AAV6, AAV6.2, and AAV9) have been shown to target the airway epithelium (Zabner et al., 2000; Auricchio et al., 2002; Limberis and Wilson, 2006; Limberis et al., 2009; Carlon et al., 2010).
rAAV vectors are regarded as safe gene delivery vehicles because they are devoid of all viral genes and the wild-type AAV they are derived from has, to date, not been associated with any known human pathology (Dismuke et al., 2013).
A major hurdle for gene therapy is the development of an immune response against the transgenic protein and/or the viral vector itself. This was illustrated by a cellular immune response raised against the vector capsid reducing factor IX expression in patients with hemophilia B after rAAV2/2-based gene therapy (Manno et al., 2006). Also, in clinical gene therapy studies conducted in adults, an adaptive immune response against the rAAV capsid, induced after the first vector dose, hampered repeated administrations (Moss et al., 2004; Mueller and Flotte, 2008). Expression of foreign proteins in the fetus, before the immune system is fully developed, may avoid immune activation. Proof-of-principle fetal gene therapy studies have shown long-term expression both of reporter and therapeutic proteins and induction of immune tolerance against the transgene in small and large animals (Tran et al., 2001; Waddington et al., 2003; Sabatino et al., 2007). Other theoretical advantages of fetal gene transfer include the administration before the onset of pathological changes that hamper gene delivery (e.g., mucus in CF), a longer contact time of the vector, a higher vector-to-target-cell ratio, and the presence of expanding stem and progenitor populations. As long as a phenotypic correction is not required before birth, these concepts also stand for gene transfer applications at the early neonatal stage. Although most theoretical advantages relate to a fetal approach, the benefits of the intervention must be weighed against the inherent risk for complications of the procedure (Deprest et al., 2009), rendering a neonatal approach favorable and more easily accepted from an ethical point of view.
In previous work, we demonstrated efficient reporter gene expression in the lungs up to 4 weeks after rAAV delivery to fetal airways (Carlon et al., 2010). In the current study, we validated the efficiency of rAAV2/5-mediated perinatal (fetal and neonatal) gene delivery to the murine airways and monitored long-term reporter gene expression. Transduction of actively dividing tissue, such as the airway epithelium, with a nonintegrating rAAV vector requires repeated vector administration(s) to achieve long-term gene correction. We hypothesized that, because of the immaturity of the immune system during perinatal gene transfer, an immune response against the vector particle could be prevented, allowing repeated administration(s) in adult life. Our results indeed demonstrate long-term gene expression after perinatal gene transfer and subsequent readministration. Analysis of capsid-specific neutralizing antibody (nAb) indicated low immunoreactivity against the rAAV vector after perinatal gene transfer. This allowed effective vector readministration in adult life, resulting in efficient reporter gene expression in the murine airways up to 7 months. Because multiple readministrations were not feasible, we conclude that immunological ignorance rather than tolerance underlies successful readministration of an autologous vector.
Materials and Methods
rAAV2/5 production
rAAV2/5-CBA-eGFP-P2A-fLUC (CBA, chicken β-actin promoter; eGFP, enhanced green fluorescent protein; fLUC, firefly luciferase) and rAAV2/5-CBA-β-Gal (β-Gal, β-galactosidase) were produced and purified as previously described (Van der Perren et al., 2011). Vector titers were determined by real-time PCR, using the primer probe set for the poly(A) sequence (Limberis et al., 2009). rAAV titers are presented as DNase-resistant genome copies (GC) per milliliter (Gao et al., 2002).
Vector delivery to perinatal or adult murine airways
For all experiments, FVB/N mice were used (Janvier, Le Genest-Saint-Isle, France). Fetal vector delivery by intraamniotic injection (60 μl of vector suspension) was performed as previously described (Carlon et al., 2010, 2012). Neonatal vector administration to the airways was performed by sniffing on day 3 postpartum. Pups were immobilized in an upright position and vector (60-μl vector suspension) was administered drop by drop into one nostril over a time period of 2 hr; pups were kept warm by placing them on a heating pad in between intranasal vector administrations. Intratracheal vector instillation was performed in adult mice 8–12 weeks of age. After exposure of the trachea through a midline incision, 160 μl of vector diluted in phosphate-buffered saline (PBS) was instilled into the trachea with a 26-gauge polytetrafluoroethylene (PTFE) catheter (BD Vasculon Plus; VWR, Vienna, Austria), using a stereoscopic zoom microscope (magnification, ×3). All animal procedures were carried out under Biosafety Level 2 conditions and were approved by the KU Leuven Biosafety Committee (laboratory accreditation number LA1210579; ethics committee approval number P065/2009).
Bioluminescence imaging
Bioluminescence imaging (BLI) was performed as previously described (Carlon et al., 2010). Measurements are reported as the total photon flux from a 4.3-cm2 rectangular region of interest (ROI) for the lung and a 2.0-cm2 circular ROI for the nose.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside staining for β-Gal expression in tissue sections
Processing of lung samples and quantification of transduction efficiency in the conducting airways was determined as previously described (Carlon et al., 2010). Briefly, transduction efficiency of the conducting airways was determined with a computerized image analysis system (StereoInvestigator; MicroBrightField, Magdeburg, Germany). Cell type status was assigned on the basis of morphology of β-Gal-positive cells counterstained with Mayer's paracarmine. Three sections, spaced approximately 200 μm apart, were analyzed per animal and the data are presented as the number of β-Gal-positive cells relative to the total number of cells in the conducting airways. To determine the percentage of positive cells in the conducting airways, the trachea, bronchi, or bronchioles were delineated and all β-Gal-positive cells were counted, followed by quantification of all the epithelial cells.
Noses were processed and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) solution (Sigma, Diegem, Belgium) as described (Limberis et al., 2007), followed by Mayer's paracarmine counterstaining. The number of transduced cells was counted in three standard cross-sections covering the proximal, central, and distal nose region as described previously (Parsons et al., 1998). Cross-sections were analyzed by counting the number of β-Gal-positive cells in the epithelium around the entire perimeter of the nasal airway in the three sections relative to the total amount of epithelial cells. Only respiratory epithelial cells (from all three section levels) were included in the cell counts because the respiratory epithelium possesses similarities to lung conducting airway respiratory epithelium (Grubb and Boucher, 1999). β-Gal-positive cells were visualized with a Leica light microscope equipped with a BioPoint 2 motorized stage (Ludl Electronic Products, Hawthorne, NY). Brightness and contrast were optimized with Adobe Photoshop CS3 (Adobe Systems, Mountain View, CA).
Immunohistochemistry and hematoxylin–eosin staining on lung sections
Frozen sections (6 μm) were fixed in cold (−20°C) acetone for 15 min. Sections were stained with a rat monoclonal antibody (mAb) specific for CD4 (GK1.5; in-house supernatant) and a rat mAb specific for CD8 (clone 2.43; in-house supernatant). For immunofluorescence, the following detection antibodies were used: Alexa Fluor 488–donkey anti-rat (A21208; Molecular Probes, Gent, Belgium) and 4′,6-diamidino-2-phenylindole (DAPI) (D1306; Molecular Probes). Images were acquired with an LSM 510 Meta confocal microscope (Zeiss). Adjacent lung sections of 6 μm were stained with hematoxylin and eosin (H&E) to visualize immune cell infiltration, using a Leica BioPoint 2 light microscope.
rAAV transduction inhibition assay
Individual blood samples were collected at various time points from animals by retro-orbital bleeding. Bronchoalveolar lavage fluid (BALF) samples were collected at the time point of harvest (1 month after the first or second readministration) by flushing 2 ml of PBS through the trachea. Serum and BALF samples were heat-inactivated for 45 min at 56°C. HEK293T cells were seeded at 5.0×104 cells per well into 96-well plates on day 1. The next day, 4-fold dilutions of serum or BALF samples in Dulbecco's modified Eagle's medium (DMEM) without fetal calf serum (FCS) were prepared. Wells with the same volume of DMEM served as no-serum or no-BALF controls. Approximately 1.0×109 GC of rAAV2/5-CBA-eGFP-P2A-fLUC in DMEM was added, per well, to serum or BALF sample or control wells, incubated at 37°C for 60 min, and added to the cells (100-μl final volume). One hour later, 100 μl of DMEM–20% FCS was added to each well. Twenty-four hours later, the transduction efficiency was analyzed by quantifying fLUC activity. The percentage of inhibition was calculated relative to positive (i.e., no-serum or no-BALF) controls.
Luciferase assay
Cells were lysed in 70 μl of lysis buffer (50 mM Tris [pH 7.5], 200 mM NaCl, 0.2% Nonidet P-40 [NP-40], 10% glycerol) and the lysate was assayed according to the manufacturer's protocol (ONE-Glo luciferase assay system; Promega, Madison, WI). Luciferase activity was normalized to total protein determined by bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL).
Fluorescence-activated cell-sorting analysis of BALF samples
After BALF collection, samples were centrifuged at 400×g for 5 min to collect cell pellets. Lymphocytes were surface stained for 30 min at 4°C with phycoerythrin-conjugated anti-CD45R (B220; RA3-6B2; eBioscience, Vienna, Austria) and APC-H7-conjugated anti-CD4 (GK1.5; BD Biosciences, Erembodegem, Belgium), before fixation and permeabilization with an eBioscience Foxp3 fixation/permeabilization kit (cat. no. 00-5523-00; eBioscience). Cells were then stained for 30 min at 4°C with anti-Foxp3-APC (FJK-16s; eBioscience). Analysis was performed with a BD FACSCanto II flow cytometer (BD Biosciences) with FlowJo version 10.0.6 analysis software (Tree Star, Ashland, OR).
Statistical analysis
General changes in BLI signal over time were analyzed by repeated-measures analysis of variance (ANOVA). Comparisons between groups at specific time points were performed by one-way ANOVA followed by a Tukey HSD (honest significant difference) post-hoc test. Changes in β-Gal signal in each group separately over time were analyzed with a mixed-model ANOVA, including animal identification as a random effect, group as a fixed effect, and time as a covariate. For fluorescence-activated cell-sorting (FACS) analysis and rAAV transduction inhibition data, statistical significance was evaluated by ANOVA to compare different treatment groups. A Student t test was subsequently used for pair-wise comparisons. A p value less than 0.05 was considered statistically significant. Data are presented as means±SEM, unless stated otherwise. STATISTICA (version 8.0) software (StatSoft, Tulsa, OK) was used for statistical analysis.
Results
Efficient but transient gene expression in murine airways after perinatal rAAV2/5-based gene transfer
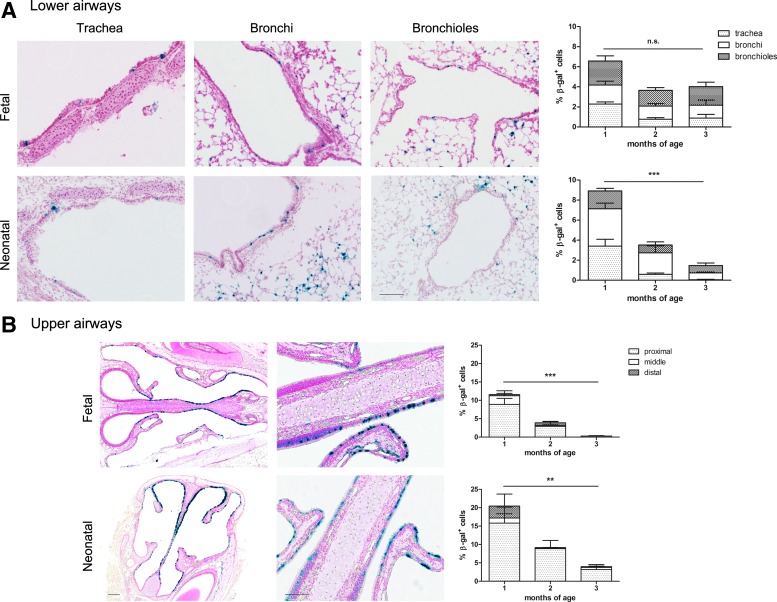
To study stability of gene expression after perinatal rAAV2/5 delivery in the upper (nose) and lower (lungs) murine airways, we compared gene expression over time after fetal and neonatal rAAV2/5 delivery of fLUC (1.5×1010 GC/animal) and β-Gal (1×1010 GC/animal) (n=3 or 4 per time point per group). Analysis of lung sections showed average transduction efficiencies (percent β-Gal-positive cells) of 6.6±1.1% in the fetal group and 8.9±1.5% in the neonatal group at 1 month postinjection (Fig. 1A). A 6-fold decrease was noted between 1 and 3 months in the neonatal group (p<0.001), in contrast to the fetal group where the transduction efficiency did not change significantly (1.7-fold decrease; p=0.18). Similar data were obtained for fLUC until 3 months postinjection (Supplementary Fig. S1; supplementary data are available online at www.liebertpub.com/hum). In parallel, we quantified the efficiency of nasal transduction (Fig. 1B). Starting at 11.6±1.5% and 20.5±3.6% β-Gal-positive cells 1 month after gene delivery for fetal and neonatal treatment, respectively, the number of transduced cells decreased significantly over the next 2 months for both groups (p<0.001 for fetal and p<0.01 for neonatal), resulting in a residual transduction level at 3 months of 0.3±0.1% in the fetal group compared with 4.0±1.1% in the neonatal group. Although the airways were efficiently transduced, gene expression markedly decreased by month 3, providing grounds for vector readministration to establish long-term expression.
FIG. 1.
Perinatal rAAV2/5-mediated gene transfer to murine airways. (A and B) β-Galactosidase (β-Gal) expression in the airways after fetal and neonatal rAAV2/5 administration (1.0×1010 genome copies [GC]/animal) at 1 month postinjection. (A) Lower airways (lung), showing representative images of the conducting airways (trachea, bronchi, and bronchioles). Original magnification, ×200. (B) Upper airways (nose), showing representative images of the proximal nose region (left) and a portion of the nasal epithelium (right) (original magnification: left, ×50; right, ×200). The graphs visualize the average transduction efficiency in the conducting airways and nasal epithelium. For both the lower and upper airways, the relative contribution of the different regions to the total transduction efficiency is visualized by the various shadings described in the legends. Mixed-model ANOVA, mean±SEM, **p<0.01, ***p<0.001. Scale bars: 100 μm for lung sections; 200 and 100 μm, respectively, for low- and high-magnification nose sections. Color images available online at www.liebertpub.com/hum
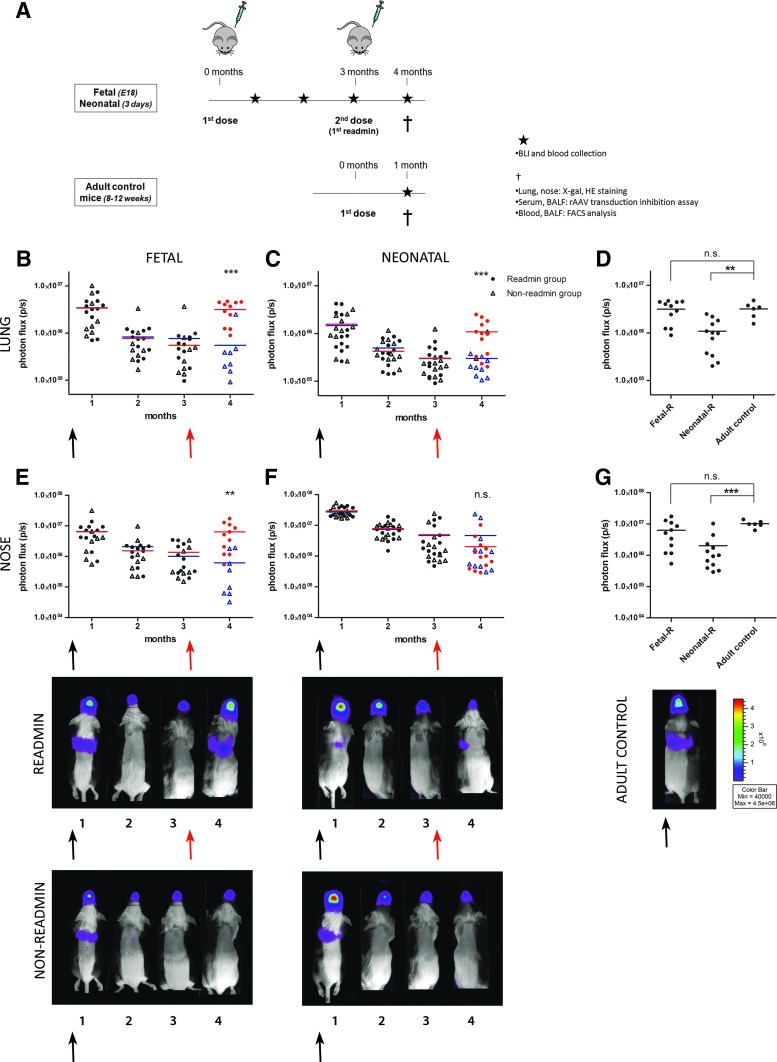
Successful readministration of rAAV2/5 after initial perinatal gene transfer
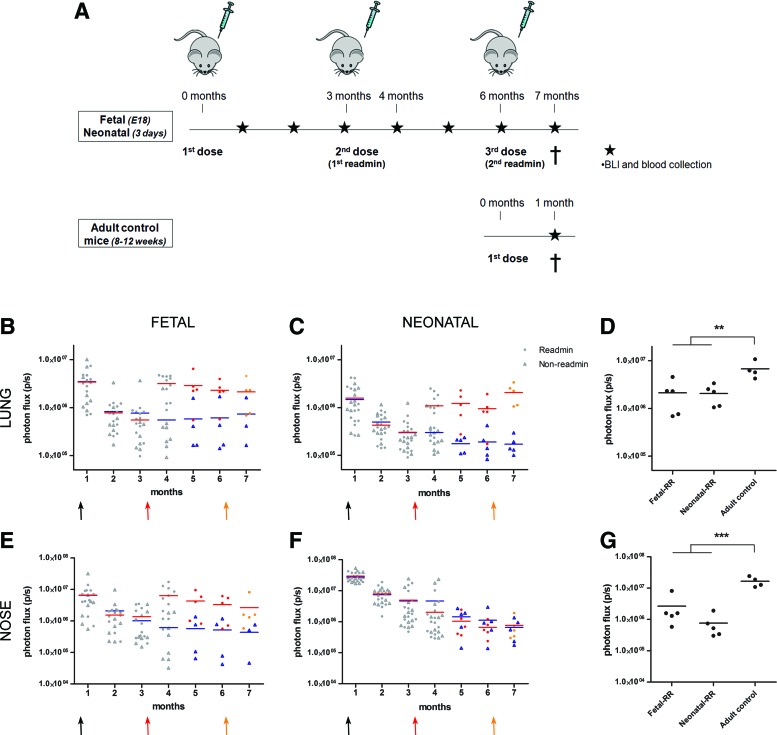
Next, we wanted to evaluate whether readministration of an autologous vector was successful after initial perinatal gene transfer. In Fig. 2A, a schematic overview is given of the experiment designed to study the feasibility of rAAV2/5 readministration to the murine upper and lower airways. rAAV2/5 encoding fLUC (1.5×1010 GC/animal) was delivered by intraamniotic injection to fetal FVB/N mice on embryonic day 18 (E18) (term, E19.5; n=19) and by sniffing to neonatal pups on day 3 (n=22). At 3 months postinjection, a second dose of the same vector was given by intratracheal instillation to approximately half of the animals (readministration group, n=11 for the fetal group, n=12 for the neonatal group), whereas the other half of the group was kept as non-readministration controls (n=8 for the fetal group, n=10 for the neonatal group). Gene expression levels were monitored by BLI at monthly intervals until 4 months after primary administration (Fig. 2B and C and Fig. 2E and F for lung and nose, respectively). An adult control group (2–3 months of age, n=6) was included to control for the maximum level of gene expression expected in the upper and lower airways after a single intratracheal instillation of rAAV2/5-fLUC (Fig. 2D and G). At 1 month postinjection, a comparable BLI signal was detected in the lungs between the groups (Fig. 2B and C). The photon flux, however, declined significantly in both groups at 3 months postinjection (5.3-fold, p<0.001 for the fetal group and 5.8-fold, p<0.001 for the neonatal group). Also for the nose, a significant decrease was observed between 1 and 3 months, resulting in a 5.4-fold decrease for the fetal group (p<0.001) and a 7.3-fold decrease for neonatal animals (p<0.001).
FIG. 2.
Successful readministration of rAAV2/5 after initial perinatal gene transfer. (A) Overview of the experiment designed to study the feasibility of rAAV2/5 (1.5×1010 GC/animal) readministration to murine airways after perinatal gene delivery. Firefly luciferase (fLUC) expression was visualized (photos, bottom) and quantified (B–G) over time using bioluminescence imaging (BLI). The pseudocolor scale of bioluminescent images depicts the photon flux per second, per square centimeter per steradian (p/sec/cm2/sr). Total photon flux (p/sec) was quantified over time for nose and lung. Measurements of individual animals were plotted as single values and the average BLI signal per group per time point is depicted. The black arrow depicts the first vector dose received as fetus/neonate; the red arrow indicates readministration in adult life. Red circles, BLI signal after readministration; blue triangles, non-readministration controls. (D and G) The lung and nose signals of animals that received a second vector dose after perinatal gene delivery were compared with the signal measured in adult controls who received a single vector dose, 1 month postinjection. One-way ANOVA and Tukey HSD post-hoc test, **p<0.01, ***p<0.001. Abbreviations: BALF, bronchoalveolar lavage fluid; FACS, fluorescence-activated cell sorting; Fetal-R or Neonatal-R, fetal or neonatal vector delivery and readministration at 3 months; HE, hematoxylin and eosin; n.s., not significant.
At this point (3 months after primary administration), rAAV2/5-fLUC (1.5×1010 GC/animal) was readministered by intratracheal instillation (indicated by a red arrow; Fig. 2B, C, E, and F). fLUC activity showed a significant 5.8-fold increase in lung signal after readministration to the fetal group (3.2 [±0.5]×106 p/sec, n=11, compared with 5.5 [±2.8]×105 p/sec for non-readministered controls, n=8, p<0.001; Fig. 2B); likewise, the signal in the nose increased 10.3-fold in readministered fetal animals (6.3 [±1.7]×106 p/sec) compared with controls (6.1 [±2.8]×105 p/sec, p<0.01; Fig. 2E). For the neonatal group, a 5-fold increase was noted in the lung (1.1 [±0.2]×106 p/sec, n=12, compared with 2.2 [±0.3]×105 p/sec, n=10, p<0.001; Fig. 2C), whereas no increase was observed in the nose (2.0 [±0.8]×106 p/sec compared with 2.8 [±1.7]×106 p/sec, p=0.98; Fig. 2F). When comparing the BLI signal after readministration with that obtained in adult control mice receiving a single vector dose (Fig. 2D and G), animals in the fetal group reached comparable transduction efficiencies as adult controls for lung and nose, whereas the signal in neonatal animals was significantly lower (34%, p<0.01 and 20%, p<0.001 for lung and nose signal, respectively).
Comparable results were obtained in an independent experiment with a similar setup (Supplementary Fig. S2). Only here, rAAV2/5-β-Gal (1.0×1010 GC/animal) was coinjected to evaluate vector distribution in tissue sections. At 3 months, a subset of animals was readministered with rAAV2/5-β-Gal (2.0×1010 GC/animal) and rAAV2/5-fLUC (3.0×1010 GC/animal). fLUC activity in the lung increased 16.2-fold in the fetal group and the neonatal group (n=4 and 3, respectively) compared with non-readministered animals (n=4 and 6, p=0.08 and p=0.12, respectively) (Supplementary Fig. S2A and B). Whereas a 33.3-fold increase in nose signal was obtained for the fetal group (p=0.24), only a minimal increase of 4.4-fold was measured in the neonatal group (p=0.65) (Supplementary Fig. S2D and E). On X-Gal staining, an increase in β-Gal-positive cells was noted in the conducting airways 1 month after readministration both in the fetal group as well as in the neonatal group (Supplementary Fig. S3). In the nasal respiratory epithelium, however, an increase was noted only after readministration in the fetal group (Supplementary Fig. S4).
Together, our experiments, carried out twice, demonstrate that readministration of an autologous vector 3 months after fetal or neonatal rAAV2/5 delivery results in efficient retransduction of the lungs, but results only in an increase in nasal gene expression after initial fetal rAAV2/5 delivery.
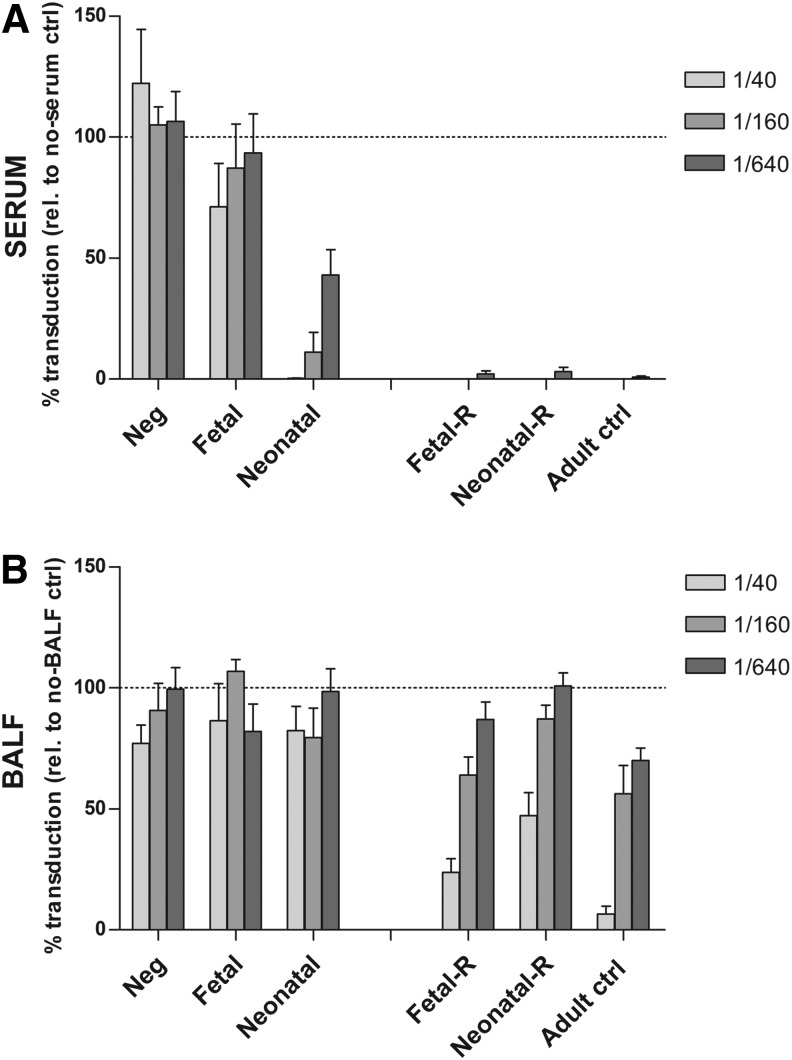
Analysis of neutralizing antibody against rAAV2/5 capsid after perinatal gene transfer and after readministration
To study the immunological mechanism underlying this successful readministration, we evaluated whether an adaptive immune response against the rAAV2/5 capsid was induced after perinatal gene transfer and after readministration by analyzing nAb against the capsid in serum and BALF (Fig. 3). As controls, we included nontreated animals (Neg, n=5) and adult control mice that received a single vector dose (Adult ctrl, n=6). Four months after perinatal gene transfer, only a weak capsid-specific humoral immune response was observed in serum of the fetal group (n=8; inhibition of 28.8, 12.8, and 6.7% at 1:40, 1:160, and 1:640 serum dilutions; Fig. 3A, left), whereas in the neonatal group (n=10) inhibition reached 99.8, 89.9, and 56.9%, respectively. For comparison, serum of adult controls inhibited transduction completely at a 1:640 dilution 1 month postinjection (99.1% inhibition).
FIG. 3.
Analysis of neutralizing antibody (nAb) against the rAAV2/5 capsid after perinatal gene transfer and after readministration. An rAAV transduction inhibition assay was performed to analyze nAb against the rAAV2/5 capsid in serum and BALF. Samples were analyzed 4 months after fetal or neonatal rAAV2/5 delivery, or 1 month after readministration. Serial dilutions of serum (A) or BALF samples (B) were incubated with rAAV2/5-fLUC and tested for inhibition of transduction in HEK293T cells. Transduction was quantified by measuring relative light units (RLU) per microgram of protein and is expressed as mean percent transduction relative to no-serum or no-BALF control±SEM. The dashed line represents 100% transduction as measured by no-serum or no-BALF control. Fetal or neonatal, non-readministration controls 4 months after fetal or neonatal rAAV2/5 delivery; Fetal-R or Neonatal-R, fetal or neonatal vector delivery and readministration at 3 months, serum/BALF collection 1 month later; Adult ctrl, adult controls that received a single vector dose, serum/BALF collection 1 month postinjection.
One month after vector readministration, however (Fig. 3A, right), a complete inhibition of transduction (≥97% inhibition at 1:640 serum dilution) was measured for both the fetal group (fetal-R; n=11) and neonatal group (neonatal-R; n=12), in line with adult control mice. Of note, further serum dilutions allowed rAAV2/5-fLUC transduction to the same extent in all three treatment groups, indicating comparable serum nAb concentrations (Supplementary Fig. S5). These results indicate that after fetal administration only a weak, capsid-specific humoral immune response is induced, whereas after neonatal gene transfer a more pronounced immune response is observed. However, after readministration, nAb levels increased in both groups to comparable levels as in the adult mice that received a single vector dose.
In addition, we explored the local immune response in the lungs by sampling the BALF. Four months after fetal (n=4) or neonatal (n=5) vector delivery, capsid-specific nAb were undetectable in BALF samples (Fig. 3B, left). However, 1 month after readministration, nAb increased in both treatment groups (fetal-R, n=6; neonatal-R, n=7), comparable to that of adult controls (n=6) (Fig. 3B, right).
Together, these results indicate that although only low immunoreactivity against the rAAV2/5 capsid was present after perinatal rAAV2/5 vector delivery either in serum or BALF, a strong capsid-specific humoral immune response was induced on readministration to the same extent as a single administration in adult animals (compare fetal-R and neonatal-R with Adult ctrl; Fig. 3A and B), arguing against the establishment of tolerance to the viral vector.
Increased immune cell infiltration in retransduced lung after rAAV2/5 readministration
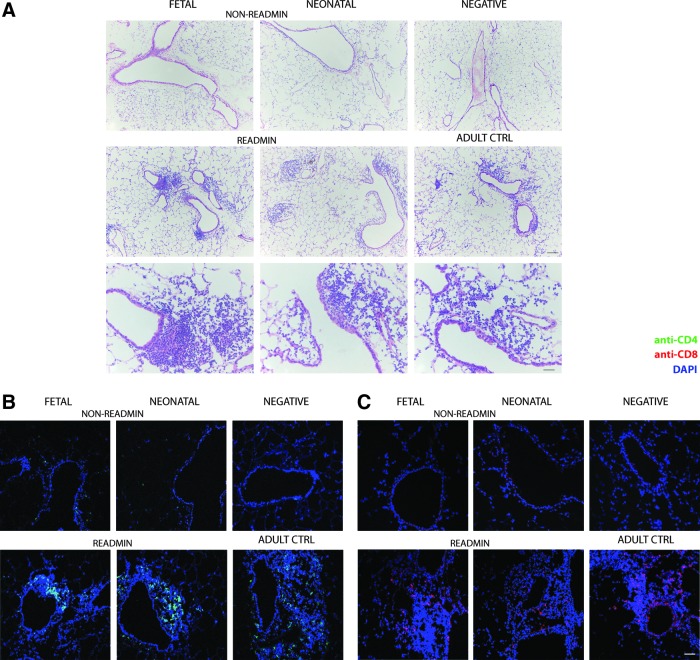
We then studied the local immune response in the retransduced lung in more detail. First, we analyzed relative differences in B cell and regulatory T cell (Treg) populations in BALF (Supplementary Fig. S6). As controls, we included BALF of nontreated animals (n=5) and adult controls (n=6). Four months after perinatal treatment, B cell numbers (B220+) in the fetal group (n=4) and neonatal group (n=5) were not different compared with negative controls (Supplementary Fig. S6A, left). Likewise, the increase in B cells in readministration animals (fetal-R, n=6; neonatal-R, n=7) was comparable to adult controls (Supplementary Fig. S6A, right). Treg (CD4+Foxp3+) levels increased in the perinatal treatment groups after readministration (Supplementary Fig. S6B, right); they were significantly higher than in negative controls (p<0.01 for both groups), but not different compared with adult controls. This response can be attributed to the fact that Tregs accumulate in regions undergoing tissue inflammation (Kamikozuru et al., 2009). H&E stainings of lung sections (Fig. 4A) demonstrated infiltration of mononuclear cells around conducting airways and blood vessels after readministration (Fig. 4A, middle and bottom rows), a signal that was absent in non-readministered animals (Fig. 4A, top row). Scoring of lung sections for inflammation after perinatal rAAV2/5 administration (Table 1) showed minimal infiltration, comparable to negative animals. Readministration resulted in increased lymphocytic infiltration around the conducting airways and blood vessels, comparable to adult controls. More detailed analysis detected CD4+ as well as CD8+ T cells in the lymphocytic infiltrates (Fig. 4B and C, bottom row).
FIG. 4.
Evaluation of lung inflammation after perinatal rAAV2/5 administration or readministration in adult life. (A) Infiltration of immune cells in lung parenchyma was evaluated by hematoxylin–eosin (H&E) staining 4 months after fetal and neonatal rAAV2/5 administration (1.0×1010 GC of rAAV2/5-β-Gal per animal and 1.5×1010 GC of rAAV2/5-fLUC per animal) and 1 month after readministration at 3 months. Samples of negative and adult control mice that received a single vector dose were analyzed in parallel. Representative images at various time points depict peribronchial, peribronchiolar, and perivascular regions in which infiltration of immune cells was assessed. (B) Anti-CD4–Alexa 488 staining for identification of CD4+ T cells in lymphocytic infiltrates. (C) Anti-CD8–Alexa 555 staining for identification of CD8+ T cells in lymphocytic infiltrates. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Scale bars: 50 μm, except scale bar H&E bottom row: 200 μm. Abbreviations: readmin, readministration at 3 months and tissue collection at 4 months; non-readmin, non-readministered controls 4 months after perinatal gene transfer.
Table 1.
Scoring of Lung Inflammation After Perinatal rAAV2/5 Administration or Readministration in Adult Lifea
| Diffuse (sub-) mucosal infiltrate: central airways | Diffuse (sub-) mucosal infiltrate: peripheral airways | Alveolar/venous infiltrate | |
|---|---|---|---|
| Neg control | 0±0 | 0±0 | 0±0 |
| Non-readminb | |||
| Fetal | 0±0 | 0.4±0.8 | 0.5±0.7 |
| Neonatal | 0.5±1.0 | 0.3±0.5 | 0.4±0.5 |
| Readminc | |||
| Fetal | 1.3±1.2 | 1.7±1.5 | 1.3±1.2 |
| Neonatal | 1.7±0.6 | 1.8±1.3 | 2.0±1.0 |
| Adult control | 2.0±1.0 | 1.7±0.6 | 0.8±0.3 |
Neg, nontreated; Readmin, readministration at 3 months and tissue collection at 4 months; Non-readmin, non-readministered controls 4 months after perinatal gene transfer.
Lung sections were stained with hematoxylin and eosin and scored to evaluate the degree of lung inflammation after fetal and neonatal rAAV2/5 administration (1.0×1010 GC of rAAV2/5-β-Gal per animal; 1.5×1010 GC of rAAV2/5-fLUC per animal) and readministration at 3 months. Lung sections were scored according to the region as well as the degree of inflammation. Scores: 0, no immune cell infiltration; 0.5, few infiltrating cells with minimal significance; 1, mild lymphocytic infiltrate; 2, moderate lymphocytic infiltrate; 3, severe lymphocytic infiltrate. Data represent means±SD.
Non-readministration controls for the fetal and neonatal group 4 months after perinatal rAAV2/5 delivery.
Readministration animals for the fetal and neonatal group 1 month after readministration.
These data demonstrate that despite low immunoreactivity in the transduced lung after perinatal rAAV2/5 delivery, a strong local adaptive immune response is induced on retransduction of the lung, comparable to a single rAAV2/5 administration in adult animals. This indicates that immunological ignorance rather than tolerance is at play. The increase in Tregs after readministration is likely secondary to induced tissue inflammation and does not play a role in the induction and maintenance of immunological tolerance.
Second vector readministration does not increase gene expression after perinatal gene transfer
In a next step, we evaluated a second readministration of rAAV2/5-fLUC (1.5×1010 GC/animal) 6 months after perinatal treatment or 3 months after the first readministration (n=5 for the fetal and neonatal group) (Fig. 5A). After an increase in BLI signal measured 1 month after primary readministration (described previously in Fig. 2, but shown again in Fig. 5 as gray dots), only a minor decrease in BLI signal was noted for the fetal and neonatal groups between 4 and 6 months (1.4-fold, p=0.47 [fetal] and 1.2-fold, p=0.77 [neonatal] for lung [Fig. 5B and C]; 1.9-fold, p=0.80 [fetal] and 3.1-fold, p=0.50 [neonatal] for nose [Fig. 5E and F]). After the second readministration (orange arrow in Fig. 5B and C and Fig. 5E and F), lung and nose signals remained unchanged when analyzed 1 month later both in the fetal group (p=0.79 for lung; p=0.79 for nose) (Fig. 5B and E) and in the neonatal group (p=0.08 for lung; p=0.13 for nose) (Fig. 5C and F). Compared with adult controls receiving a single vector dose, the lung signal after the second readministration in the fetal group was only 32% (p<0.01; Fig. 5D) and the nose signal was only 16% (p<0.001; Fig. 5G). For the neonatal group, 31 and 5% of the lung and nose signals of adult control mice were obtained (p<0.01 and p<0.0001, respectively; Fig. 5D and G).
FIG. 5.
Evaluation of a second rAAV2/5 readministration after initial perinatal gene transfer. (A) Overview of the experiment designed to study the feasibility of repeated rAAV2/5-fLUC readministration (1.5×1010 GC/animal) to murine airways after perinatal gene delivery. (B–G) fLUC expression was quantified over time, using BLI. Total photon flux (p/sec) was quantified at various time points for nose and lung. Measurements of individual animals were plotted as single values and the average BLI signal per group per time point is depicted. Measurements in gray were taken from Fig. 2. Red and orange circles, BLI signal after the first and second readministration, respectively; blue triangles, non-readministration controls. The red and orange arrows indicate the first and second readministration respectively. (D and G) The lung and nose signals of animals that received a second readministration after perinatal gene delivery were compared with the signal measured in adult controls that received a single vector dose, 1 month postinjection. One-way ANOVA and Tukey HSD post-hoc test, **p<0.01, ***p<0.001. Fetal-RR or Neonatal-RR, fetal or neonatal vector delivery and readministration at 3 and 6 months. Color images available online at www.liebertpub.com/hum
The failure to retransduce the airways efficiently by a second readministration (Fig. 5) can be attributed to the presence of capsid-specific nAb, measured both 1 month (described previously in Fig. 3) and 3 months after primary readministration (i.e., before the second readministration; Supplementary Fig. S7), supporting the inhibitory role of nAb in preventing retransduction after the second readministration (Fig. 5).
In conclusion, our results demonstrate long-term gene expression up to 7 months after initial perinatal gene transfer followed by a single readministration in adult life. Analysis of capsid-specific nAb indicated low immunoreactivity against the rAAV vector after perinatal gene transfer, but a significant increase in nAb levels after readministration. In addition, multiple readministrations were not feasible, demonstrating that immunological ignorance rather than tolerance was induced against rAAV2/5 capsid after perinatal gene transfer.
Discussion
Transduction with a nonintegrating rAAV vector results in transient gene expression in actively dividing cells and remains a challenge in many gene therapy studies (Flageul et al., 2009; Wang et al., 2012). At present, many methods are being evaluated for rAAV readministration, for example, by serotype switching (Weinstein et al., 2010; Wang et al., 2012), capsid engineering (Vandenberghe et al., 2009), or immune modulation and tolerance induction to prevent immune responses to the vector and/or transgene (Goudy et al., 2011; Wang et al., 2011). Alternatively, the viral vector can be administered to an immature immune system by perinatal gene therapy (Sabatino et al., 2007; Sinn et al., 2008; Hu et al., 2011). We report here for the first time the successful readministration of rAAV2/5 to the upper and lower murine airways after initial perinatal gene transfer.
In an effort to better understand the underlying immunological mechanism, we detected a significantly lower level of capsid-specific nAb after perinatal treatment compared with adult gene delivery (Fig. 3). These findings reflect the difference in immune system maturity at the moment of vector delivery, explaining successful rAAV2/5 readministration in adult life after initial perinatal gene transfer. However, 1 month after the first readministration, an increase in capsid-specific nAb, B cells, and Tregs was observed in serum and BALF for both the fetal and neonatal treatment groups (Fig. 3 and Supplementary Fig. S6), comparable to adult control mice that received a single vector dose. These data demonstrate that a primary immune response against the rAAV2/5 capsid was generated only after the first readministration, indicating that immunological ignorance rather than tolerance is induced against the vector particle. Similar to our observations, adenoviral fLUC-vector (AdV-fLUC) delivery to the preimmune fetus by intrahepatic injection in CD-1 mice did not induce immune tolerance to fLUC or AdV proteins (Lipshutz et al., 2000). These results demonstrate that fetal administration of a viral vector does not necessarily lead to induction of tolerance against foreign proteins, such as vector or transgenic proteins, despite the immaturity of the immune system.
We demonstrated that readministration to the mouse airways was feasible after both fetal and neonatal rAAV2/5 gene delivery. However, significantly higher expression levels were obtained after fetal compared with neonatal gene transfer. This might be explained by the degree of immune system maturity at the time of vector administration. Whereas the immune system of the mouse fetus is still immature on E18, 2- to 3-day-old neonatal pups are on the border of developing a relatively mature immune system (Darrasse-Jeze et al., 2005; Fontenot et al., 2005; Liston and Rudensky, 2007). This would suggest that full immune ignorance was induced after fetal gene delivery, in contrast to only partial ignorance after neonatal gene transfer on day 3. Although immune system immaturity of neonatal mice less than 3 days of age resembles that of fetal mice, the human immune system is relatively well developed in utero by gestational week 23 (West, 2002), supporting the importance to further study and optimize fetal gene therapy in a preclinical context.
Although this study did not focus on a potential immune response against the transgenes, CD4+ and CD8+ T cells were detected in peribronchial and perivascular infiltrates in the lung after retransduction after initial fetal and neonatal gene transfer, in line with the adult controls (Fig. 4B and C). This demonstrates that not only a humoral immune response was mounted against the foreign proteins (rAAV capsid and/or transgenes), but also a cellular immune response. However, we cannot discern whether CD4+ or CD8+ T cells infiltrated the lung as part of an immune reaction toward the transgene or the rAAV capsid. Of note, gene expression remained stable over the span of 7 months (the end point of our study) after initial perinatal gene transfer and subsequent readministration in the airways. Therefore, despite the presence of CD8+ T cells in the peribronchial and perivascular infiltrates, there was no apparent cytotoxic response giving rise to a loss of transduced cells. In clinical trials using rAAV for patients with hemophilia B, the stability of gene expression was hampered by a capsid-specific cytotoxic T cell response (Mingozzi et al., 2007; Nathwani et al., 2011), which was not predicted by preclinical animal studies (Li et al., 2007; Wang et al., 2007). This emphasizes the need to study the cellular immune response against the transgene and the rAAV capsid in more detail in different animal models.
The marked reduction in transduced cells in the upper and lower airways 3 months after fetal and neonatal gene transfer (Fig. 1) can be explained by profound proliferation of airway epithelium early in life. Although the turn-over of airway epithelium in adult mice is typically considered to be about 100 days (Rawlins and Hogan, 2006), the proliferation rate in the growing mouse lung is presumably considerably higher, although detailed information is lacking to date. Cell proliferation will lead to dilution of the episomal rAAV genomes. This is supported by the fact that contrary to the strong decrease in lung signal 3 months after perinatal treatment (Figs. 1 and 2), the same dose administered to adult mice resulted in stable gene expression (our unpublished observations), in line with data published by Wilson and colleagues (Wang et al., 2012).
Results on the feasibility of repeated rAAV delivery vary to a large extent and may depend on the host, delivery route, target organ, time point of administration, AAV serotype, or vector dose tested (Halbert et al., 1997, 2000; Beck et al., 1999). Although a single readministration was successful in our experimental set-up, a second readministration 3 months later did not lead to an increase in gene expression (Fig. 5). This failure correlated with an increase in capsid-specific nAb after the first readministration that persisted until the time point of the second readministration (Supplementary Fig. S7). Also, in a CF clinical trial, repeated administration of rAAV2/2 (three doses, 1 month apart) was unsuccessful (Moss et al., 2004). However, there is preclinical evidence that in certain settings, rAAV vectors can be readministered (Auricchio et al., 2002; Limberis and Wilson, 2006), for example, by delaying the time point of readministration (Limberis and Wilson, 2006).
In conclusion, perinatal gene transfer to the airways, using rAAV2/5, does not evoke a strong immune response. In our hands, this immunological ignorance allowed readministration of an autologous vector, resulting in efficient and stable gene transfer up to 7 months, without evidence for a decrease in gene expression (Fig. 5). For permanent gene correction, later time points should be monitored to evaluate the stability of gene expression. However, our data already provide a basis to study perinatal gene therapy for pulmonary conditions where temporary gene expression is sufficient, for example, bronchopulmonary dysplasia. Furthermore, the current preclinical gene therapy protocol can prove useful to delay pathological changes that already occur in utero until other therapeutic approaches can be applied after birth. As we did not observe a decrease in gene expression after 7 months, this gene therapy model also offers perspectives to study gene transfer for pulmonary diseases that require permanent genetic correction. Moreover, this mouse model allows basic immunological studies on immune tolerance and ignorance in the context of gene therapy.
Supplementary Material
Acknowledgments
The authors thank Caroline Van Heijningen, Joris Van Asselberghs (Neurobiology and Gene Therapy, KU Leuven, Flanders, Belgium), and Wim Pierson (Autoimmune Genetics Laboratory, VIB, Flanders, Belgium) for excellent technical assistance. M.S.C. is a postdoctoral fellow supported by funding from the Fund Alphonse and Jean Forton managed by the King Baudouin Foundation, Belgium, for CF research. D.V. is a doctoral fellow supported by a grant from KU Leuven, DBOF/10/062. M.M.d.C. is a postdoctoral fellow supported by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). M.M. is a postdoctoral fellow supported by funding from the Flemish Research Foundation (FWO Vlaanderen). J.T. holds a part-time Clinical Research Fellowship (KOOR) from UZ Leuven. Research was funded by IWT-Vlaanderen, by EC grant DIMI (LSHB-CT-2005-512146), and by the In Vivo Molecular Imaging Research (IMIR) program from KU Leuven. The authors acknowledge the Penn Vector Core, founded by James M. Wilson, for their kind gift of the AAV2/5 packaging plasmid for rAAV vector production.
Author Disclosure Statement
No competing financial interests exist.
References
- Auricchio A., O'Connor E., Weiner D., et al. (2002). Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 110, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S.E., Jones L.A., Chesnut K., et al. (1999). Repeated delivery of adeno-associated virus vectors to the rabbit airway. J. Virol. 73, 9446–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlon M., Toelen J., Van Der Perren A., et al. (2010). Efficient gene transfer into the mouse lung by fetal intratracheal injection of rAAV2/6.2. Mol. Ther. 18, 2130–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlon M.S., Toelen J., Da Cunha M.M., et al. (2012). A novel surgical approach for intratracheal administration of bioactive agents in a fetal mouse model. J. Vis. Exp. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R.G., McElvaney N.G., Rosenfeld M.A., et al. (1994). Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 8, 42–51 [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G., Marodon G., Salomon B.L., et al. (2005). Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood 105, 4715–4721 [DOI] [PubMed] [Google Scholar]
- Deprest J.A., Devlieger R., Srisupundit K., et al. (2009). Fetal surgery is a clinical reality. Semin. Fetal Neonatal Med. 15, 58–67 [DOI] [PubMed] [Google Scholar]
- Dismuke D.J., Tenenbaum L., and Samulski R.J. (2013). Biosafety of recombinant adeno-associated virus vectors. Curr. Gene Ther. 13, 434–452 [DOI] [PubMed] [Google Scholar]
- Flageul M., Aubert D., Pichard V., et al. (2009). Transient expression of genes delivered to newborn rat liver using recombinant adeno-associated virus 2/8 vectors. J. Gene Med. 11, 689–696 [DOI] [PubMed] [Google Scholar]
- Flotte T.R., Zeitlin P.L., Reynolds T.C., et al. (2003). Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum. Gene Ther. 14, 1079–1088 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Dooley J.L., Farr A.G., and Rudensky A.Y. (2005). Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202, 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Vandenberghe L.H., Alvira M.R., et al. (2004). Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P., Alvira M.R., Wang L., et al. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudy K.S., Annoni A., Naldini L., and Roncarolo M.G. (2011). Manipulating immune tolerance with micro-RNA regulated gene therapy. Front. Microbiol. 2, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb B.R., and Boucher R.C. (1999). Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 79, S193–S214 [DOI] [PubMed] [Google Scholar]
- Halbert C.L., Standaert T.A., Aitken M.L., et al. (1997). Transduction by adeno-associated virus vectors in the rabbit airway: Efficiency, persistence, and readministration. J. Virol. 71, 5932–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L., Rutledge E.A., Allen J.M., et al. (2000). Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74, 1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B.G., Leopold P.L., Hackett N.R., et al. (1999). Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin.Invest. 104, 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Cela R.G., Suzuki M., et al. (2011). Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc. Natl. Acad. Sci. U.S.A. 108, 2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikozuru K., Fukunaga K., Hirota S., et al. (2009). The expression profile of functional regulatory T cells, CD4+CD25high+/forkhead box protein P3+, in patients with ulcerative colitis during active and quiescent disease. Clin. Exp. Immunol. 156, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Murphy S.L., Giles-Davis W., et al. (2007). Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15, 792–800 [DOI] [PubMed] [Google Scholar]
- Limberis M., Bell P., and Wilson J.M. (2007). Detection of reporter gene expression in murine airways. Methods Mol. Biol. 411, 25–34 [DOI] [PubMed] [Google Scholar]
- Limberis M.P., and Wilson J.M. (2006). Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U.S.A. 103, 12993–12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis M.P., Vandenberghe L.H., Zhang L., et al. (2009). Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol. Ther. 17, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz G.S., Flebbe-Rehwaldt L., and Gaensler K.M. (2000). Reexpression following readministration of an adenoviral vector in adult mice after initial in utero adenoviral administration. Mol. Ther. 2, 374–380 [DOI] [PubMed] [Google Scholar]
- Liqun Wang R., McLaughlin T., Cossette T., et al. (2009). Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of α-1-antitrypsin using invasive and noninvasive delivery. Mol. Ther. 17, 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., and Rudensky A.Y. (2007). Thymic development and peripheral homeostasis of regulatory T cells. Curr. Opin. Immunol. 19, 176–185 [DOI] [PubMed] [Google Scholar]
- Manno C.S., Pierce G.F., Arruda V.R., et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and High K.A. (2011). Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 12, 341–355 [DOI] [PubMed] [Google Scholar]
- Mingozzi F., Maus M.V., Hui D.J., et al. (2007). CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13, 419–422 [DOI] [PubMed] [Google Scholar]
- Moss R.B., Rodman D., Spencer L.T., et al. (2004). Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: A multicenter, double-blind, placebo-controlled trial. Chest 125, 509–521 [DOI] [PubMed] [Google Scholar]
- Moss R.B., Milla C., Colombo J., et al. (2007). Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: A randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18, 726–732 [DOI] [PubMed] [Google Scholar]
- Mueller C., and Flotte T.R. (2008). Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 15, 858–863 [DOI] [PubMed] [Google Scholar]
- Nathwani A.C., Tuddenham E.G., Rangarajan S., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D.W., Grubb B.R., Johnson L.G., and Boucher R.C. (1998). Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum. Gene Ther. 9, 2661–2672 [DOI] [PubMed] [Google Scholar]
- Rawlins E.L., and Hogan B.L. (2006). Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133, 2455–2465 [DOI] [PubMed] [Google Scholar]
- Sabatino D.E., Mackenzie T.C., Peranteau W., et al. (2007). Persistent expression of hF.IX after tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol. Ther. 15, 1677–1685 [DOI] [PubMed] [Google Scholar]
- Sheridan C. (2011). Gene therapy finds its niche. Nat. Biotechnol. 29, 121–128 [DOI] [PubMed] [Google Scholar]
- Sinn P.L., Arias A.C., Brogden K.A., and McCray P.B., Jr (2008). Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J. Virol. 82, 10684–10692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Jones S.G., Davies L.A., Varathalingam A., et al. (2006). Long-term persistence of gene expression from adeno-associated virus serotype 5 in the mouse airways. Gene Ther. 13, 1703–1713 [DOI] [PubMed] [Google Scholar]
- Tran N.D., Porada C.D., Almeida-Porada G., et al. (2001). Induction of stable prenatal tolerance to β-galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood 97, 3417–3423 [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H., Wilson J.M., and Gao G. (2009). Tailoring the AAV vector capsid for gene therapy. Gene Ther. 16, 311–319 [DOI] [PubMed] [Google Scholar]
- Van der Perren A., Toelen J., Carlon M., et al. (2011). Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther. 18, 517–527 [DOI] [PubMed] [Google Scholar]
- Waddington S.N., Buckley S.M., Nivsarkar M., et al. (2003). In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood 101, 1359–1366 [DOI] [PubMed] [Google Scholar]
- Wagner J.A., Messner A.H., Moran M.L., et al. (1999). Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109, 266–274 [DOI] [PubMed] [Google Scholar]
- Wagner J.A., Nepomuceno I.B., Messner A.H., et al. (2002). A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 13, 1349–1359 [DOI] [PubMed] [Google Scholar]
- Wang L., Figueredo J., Calcedo R., et al. (2007). Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18, 185–194 [DOI] [PubMed] [Google Scholar]
- Wang L., Wang H., Bell P., et al. (2012). Hepatic gene transfer in neonatal mice by adeno-associated virus serotype 8 vector. Hum. Gene Ther. 23, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Tapscott S.J., Chamberlain J.S., and Storb R. (2011). Immunity and AAV-mediated gene therapy for muscular dystrophies in large animal models and human trials. Front. Microbiol. 2, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D.A., Correia C.E., Conlon T., et al. (2010). Adeno-associated virus-mediated correction of a canine model of glycogen storage disease type Ia. Hum. Gene Ther. 21, 903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West L.J. (2002). Defining critical windows in the development of the human immune system. Hum. Exp. Toxicol. 21, 499–505 [DOI] [PubMed] [Google Scholar]
- Zabner J., Couture L.A., Gregory R.J., et al. (1993). Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75, 207–216 [DOI] [PubMed] [Google Scholar]
- Zabner J., Seiler M., Walters R., et al. (2000). Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74, 3852–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.