Abstract
The basis of constitutive activation of NF-κB, essential for survival and resistance to apoptosis in many tumors, is not well understood. We find that transforming growth factor β2 (TGFβ2), predominantly in its latent form, is secreted by several different types of tumor cell lines that exhibit constitutively active NF-κB and that TGFβ2 potently stimulates the activation of NF-κB in reporter cells. Suppression of TGFβ2 expression by small interfering RNA kills prostate cancer PC3 cells, indicating that the TGFβ2–NF-κB pathway is important for their viability. These findings identify TGFβ2 as a potential target for therapeutic strategies to inhibit the growth of tumor cells that depend on constitutively active NF-κB, or to sensitize them to treatment with cytotoxic drugs.
Keywords: prostate cancer, small interfering RNA, ELISA, Smad
During progression, tumor cells become increasingly independent of negative regulatory controls, including resistance to apoptotic stimuli, one of the most important aspects of how the interaction of normal cells with their tissue-specific environment is regulated. Resistance to apoptosis can be achieved by means of different genetic alterations, including the loss of proapoptotic mechanisms (i.e., loss of p53-dependent signaling in response to stress), and induction or up-regulation of antiapoptotic mechanisms (i.e., expression of Bcl-2) (1). Consistently, many tumors acquire constitutive activation of the normally inducible antiapoptotic transcription factor nuclear factor κB (NF-κB) (2, 3), a central element in many physiological processes, including responses to cytokines and various stresses (4). Constitutively active NF-κB provides tumor cells with important selective advantages because it helps to determine their resistance to both natural [i.e., tumor necrosis factor (TNF), Fas, or TRAIL] and pharmacological (chemotherapeutic drugs) death stimuli. Factors mediating the constitutive activation of NF-κB are likely to be targets for anticancer treatments, making their identification an important clinical issue.
Transforming growth factor β2 (TGFβ2), identified here as a determinant of the constitutive activation of NF-κB in PC3 tumor cells, belongs to a superfamily of more than 40 factors, found in vertebrates, insects, and nematodes (5). Three isoforms of TGFβ are known in mammals (6). The TGFβs and their receptors are expressed ubiquitously in normal tissues and in most cell lines (7). All TGFβs are secreted as latent precursors containing active TGFβ and latency-associated peptide. In most cells, latency-associated peptide is linked to an additional protein, latent TGFβ binding protein, forming the large latent complex (8, 9). Latent TGFβs must be activated to the mature forms to activate the receptors that mediate Smad-dependent signaling (10). The activation of TGFβ is a complex process involving conformational changes of latent TGFβ, induced either by the cleavage of latency-associated peptide by proteases through the actions of endoglycosylases, or by the binding of latency-associated peptide to proteins such as integrin αvβ5 or thrombospondin-1 (11). Members of the TGFβ superfamily play essential roles in early embryonic development, cell mobility, growth, differentiation, apoptosis, and tumorigenesis (10). Despite the name “transforming growth factor,” the role of TGFβ family members in tumorigenesis is complex. Depending on the cell type, these factors can promote either tumor suppression or oncogenesis (7). In general, the TGFβs are potent inhibitors of the growth of various cell types, including epithelial, endothelial, and hematopoietic, but act as mitogens for fibroblasts.
Here, we have analyzed the mechanism of constitutive NF-κB activation in the prostate cancer cell line PC3, which is known to be resistant to TNF-induced apoptosis due to NF-κB activation (12), and in several other tumor cell lines. A potent NF-κB-activating factor has been newly identified as TGFβ2. The viability of PC3 cells depends on the production of TGFβ2, presumably because of their addiction to the constitutive activation of NF-κB.
Materials and Methods
Cell Culture and Preparation of Conditioned Media. Human prostate cancer PC3, DU145, and LNCaP, breast cancer MCF7, 293C6, and the derived mutant line C6P1Z12 (13), normal fibroblast WI38, fibrosarcoma HT1080, glioma T98G, mink lung epithelial CCL64, mouse fibroblast BALB/c-3T3 (American Type Culture Collection), and human melanoma Mel-29 cells (14) were cultured in DMEM with 10% FBS. Media conditioned for 24 h were collected from cells at 90% confluency, filtered, and stored at -70°C.
Plasmids, Transfections, and Luciferase Assays. To construct the small interfering RNA (siRNA)-TGFβ2 vector, a DNA fragment containing an inverted repeat of the target sequence GAAATGTGCAGGATAATTG, homologous to the 932–950 region of human TGFβ2 mRNA, spaced by the 9-nt sequence TTCAAGAGA and a poly(T) stretch as a stop codon for RNA polymerase III, was synthesized and cloned under control of the H4 promoter (15) into the 3′LTR of the retroviral vector pLPCX (16). Colonies of transfected cells were counted 10 days after puromycin selection. Mixed cell populations were propagated and tested for TGFβ2 secretion. The κB-luciferase construct p5XIP10 κB (contains five tandem copies of the NF-κB site from the IP10 gene) or the Smad binding element-luciferase construct was transfected transiently into the indicated cells. All transfections were carried out by using the Lipofectamine Plus reagent (Invitrogen Life Technologies, Carlsbad, CA). Efficiencies of transfections were normalized to β-galactosidase activity, expressed from a pCMVLacZ β-galactosidase reporter plasmid added to each DNA sample. Luciferase assay was performed 24 h after the cells were treated with TGFβ2 (R & D Systems) or conditioned medium, following the protocol provided by Promega. Relative luminescence was normalized to total protein, assayed with the Bio-Rad Protein Assay reagent.
Cytotoxicity Assays. Mouse fibroblast BALB/c-3T3 cells were treated with conditioned medium or with TGFβ2. To determine TGFβ activity, conditioned medium from PC3 cells was pretreated with polyclonal anti-TGFβ2 or anti-TGFβ1 (R & D Systems) for 1 h at room temperature before being added to BALB/c-3T3 cells. After incubation, TNFα (0.2 ng/ml, PeproTech, Rocky Hill, NJ) and cycloheximide (CHX; 0.4 μg/ml, Sigma) were added. Control cells were treated with CHX alone. After incubation overnight, the cells were washed with 1× PBS, and the number of cells was estimated by using a methylene blue assay.
Electrophoretic Mobility Gel Shift Assay (EMSA). The oligomer used for an NF-κB binding site (Santa Cruz Biotechnology), was 5′-AGTTGAGGGGACTTTCCCAGGC-3′, labeled with [γ-32P]ATP by the polynucleotide kinase method, following the protocol provided by Promega. Treated cells were washed, collected in 1× PBS and pelleted at 3,000 × g at 4°C for 4 min. Cytoplasmic extracts were prepared in binding buffer (13). The binding reaction was carried out at room temperature for 20 min in a total volume of 20 μl. Samples were loaded into 6% polyacrylamide gels in 0.25× Tris borate buffer, pH 8.0. After electrophoresis, the gels were dried and analyzed by autoradiography at -80°C.
Northern Analysis. A human IL-8 cDNA fragment was labeled with [α-32P]dCTP by using the Megaprime DNA labeling system, following the protocol provided by Amersham Biosciences. 293C6 cells were treated with TGFβ2 (4 nM) for 4, 10, or 24 h, then washed with cold 1× PBS. Total RNA was extracted with the TRIzol reagent at room temperature, following the protocol provided by Invitrogen Life Technologies. After 15 μg of total RNA was loaded, each lane was electrophoresed in an agarose/formaldehyde gel and transferred to a Hybond-N+ membrane (Amersham Biosciences). After UV crosslinking, the transfers were hybridized with [α-32P]dCTP-labeled probes and analyzed by autoradiography at -80°C.
Western Analysis. Cells treated with TGFβ2 for 30 or 60 min were washed with 1× PBS and pelleted at 3,000 × g at 4°C for 4 min. Cell pellets were lysed with radioimmunoprecipitation assay buffer (13). Cellular debris was removed by centrifugation at 16,000 × g for 10 min. The amount of protein in the supernatant solution was determined, and samples were heat-treated in 2× SDS sample loading buffer (13) at 100°C for 5 min. Equal amounts of samples were fractioned by SDS/PAGE and transferred to nitrocellulose membranes. Western analysis was performed with primary antibodies, which were visualized with horseradish peroxidase-coupled secondary antibodies by using the ECL Western blotting detection system (PerkinElmer Life and Analytical Sciences).
elisa. ELISA (Quantikine-Human TGFβ2 or β1 Immunoassay) was carried out according to the protocol from R & D Systems. The amount of TGFβ2 or β1 in conditioned medium was normalized to cell numbers. Neutralizing anti-TGFβ2 or anti-TGFβ1 (R & D Systems) was used to test the specificity of the ELISA.
Results
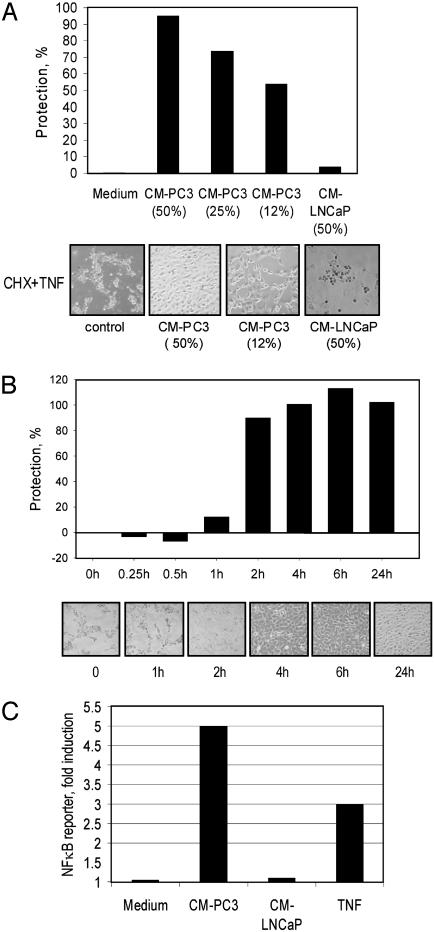
Conditioned Media from TNF-Resistant Prostate Cancer Cells Protect TNF-Sensitive Cells from Apoptosis by Inducing NF-κB. In the prostate tumor cell lines PC3 and DU145, but not LNCaP, the DNA-binding activity of NF-κB is constitutively high (12, 17). Consistently, PC3 cells are resistant to treatment with TNF, whereas LNCaP cells are sensitive. To investigate whether resistance to TNF is an intrinsic or transmissible trait, cell-free media conditioned by PC3 or LNCaP cells were transferred to BALB/c-3T3 indicator cells. These cells are highly sensitive to treatment with TNF in the presence of CHX, which prevents the blockade of apoptosis caused by the TNF-mediated activation of NF-κB. Treatment with cell-free media conditioned by PC3 but not LNCaP cells protected BALB/c-3T3 cells from the apoptosis mediated by TNF in a dose- and time-dependent manner (Fig. 1 A and B). The anti-TNF effect did not require the continued presence of conditioned medium, suggesting that the mechanism is unlikely to involve the direct inactivation of TNF, but rather to require the induction of TNF resistance in the indicator cells, manifest ≈2 h after pretreatment (Fig. 1B).
Fig. 1.
Conditioned medium from PC3 cells protects normal BALB/c-3T3 fibroblasts from TNF-induced cell death through NF-κB activation. (A) Pretreatment with medium conditioned by PC3 cells (CM-PC3) protects BALB/c-3T3 cells from TNF-induced death in a dose-dependent manner. CM-PC3 collected after 24 h was used at different concentrations to treat BALB/c-3T3 cells overnight before adding TNF and CHX. Photographs were taken 24 h later. Cell protection was assayed as described in Materials and Methods. (B) Time course of the protection mediated by CM-PC3. BALB/c-3T3 cells were treated with 50% CM-PC3. Photographs of the cells and the percentages of BALB/c-3T3 cells protected are shown. (C) Activation of NF-κB in BALB/c-3T3 fibroblast by CM-PC3. The cells were transfected with a κB-luciferase construct for 24 h and then treated with TNF (control), CM-PC3, or CM-LNCaP. Luciferase was assayed 24 h later.
Resistance of PC3 and many other types of cells to TNF is known to be determined by the activity of NF-κB (18). Therefore, we tested whether culture media conditioned by PC3 cells that are capable of inhibiting TNF-mediated apoptosis could induce NF-κB. Addition of medium conditioned by PC3 or LNCaP cells to BALB/c-3T3 cells, after transient transfection with an NF-κB reporter construct, showed that the NF-κB activating capacity was characteristic of PC3 but not LNCaP cells (Fig. 1C). Hence, constitutively active NF-κB in PC3 cells is accompanied by the secretion of factor(s) capable of inhibiting apoptosis and activating NF-κB in reporter cells.
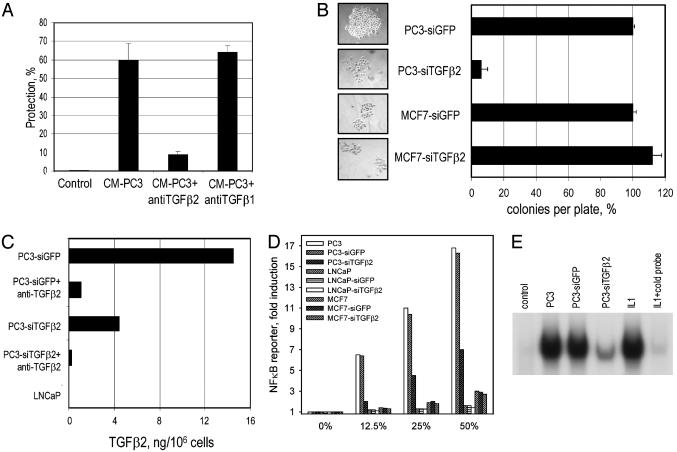
TGFβ2 Is the Major Protective Factor Secreted by PC3 Cells and Is Essential for Their Survival. The activation of NF-κB that results in blockade of apoptosis could be mediated by cytokines known to be produced by prostate cancer cells (19). By using polyclonal neutralizing antibodies against several NF-κB-inducing cytokines, we found that anti-TGFβ2 (but not anti-TGFβ1 or anticlusterin) almost completely blocked protection (Fig. 2A), indicating that TGFβ2 is likely to be primarily responsible for the antiapoptotic effect of media conditioned by PC3 cells. Consistently, purified recombinant TGFβ2 mediated a protective effect similar to that of conditioned media (data not shown).
Fig. 2.
TGFβ2 mediates the survival of PC3 cells by inducing NF-κB. (A) Protection of BALB/c-3T3 cells from TNF/CHX by CM-PC3 is prevented by anti-TGFβ2, but not by anti-TGFβ1. CM-PC3 was pretreated with polyclonal antibodies for 1 h at room temperature before being added to BALB/c-3T3 cells. (B) Reduction in colony number and colony size is caused by transduction of siTGFβ2 into PC3 cells. The number of colonies per well (average three) was determined 10 days after puromycin selection. The experiment was repeated three times with similar results. Colony numbers were normalized for transfection efficiencies, determined by using a β-galactosidase reporter assay. The CMV-LacZ plasmid was added to each transfection mixture. (C) PC3 cells stably transfected with a construct expressing siRNA against TGFβ2 express reduced amounts of TGFβ2. The amount of TGFβ2 in 5 ml of culture media conditioned for 24 h by 106 PC3 cells per ml was analyzed by ELISA after activation of TGFβ2 by treatment with 1 M HCl. Anti-TGFβ2 was used to show the specificity of the assay. (D) Conditioned media from PC3-siTGFβ2 cells have decreased NF-κB activation ability in 293C6κB indicator cells. Indicator cells were incubated with conditioned media for 24 h. The luciferase assay was done as described in Materials and Methods. si-GFP was used as the plasmid control, and LNCaP and MCF7 cells were used as cell controls. (E) Conditioned media from PC3-siTGFβ2 cells showed decreased ability to activate NF-κB in LNCaP cells, as assayed by EMSA. LNCaP cells were treated with different conditioned media. To show the specificity, IL-1 was used as a control. The binding of labeled probe was blocked by competition with a 100-fold excess of unlabeled probe.
The secretion of NF-κB-inducing factors by PC3 cells suggests that constitutively active NF-κB is maintained in these cells by autocrine regulation, and TGFβ2 seems to be the major factor. To test this possibility further, we analyzed the consequences of suppressing TGFβ2 production on the phenotype of PC3 cells by using siRNA. PC3 cells expressing GFP were infected with a retrovirus (pLPCX-siTGFβ2) encoding a hairpin loop siRNA representing a fragment of TGFβ2 mRNA, expressed from an H4 promoter (15). A construct expressing siRNA against GFP was used as a control. MCF7 and LNCaP cells, infected with the same virus, were used as examples of cells that do not produce TGFβ2. Interestingly, the number and sizes of colonies from pLPCX-siTGFβ2-infected PC3 cells were dramatically reduced in comparison to cells infected with the control virus, and the siTGFβ2 RNA had no effect on MCF7 (Fig. 2B) or LNCaP (data not shown) cells.
Rare, slowly growing colonies, formed after transduction with anti-TGFβ2 siRNA, were expanded and tested for TGFβ2 production in comparison with similar colonies generated after transfection with control siRNA. Conditioned media from PC3 cells transduced with siRNA against TGFβ2 contained about one-third as much total TGFβ2 as did media from PC3 cells transduced with siRNA against GFP, determined by ELISA (Fig. 2C). Anti-TGFβ2 was used to pretreat the media to show the specificity of the assay (Fig. 2C). The former media were proportionally less capable of inducing NF-κB-mediated transcription in 293C6 indicator cells (Fig. 2D) and specific DNA binding in LNCaP (Fig. 2E) or BALB/c 3T3 cells (data not shown). TGFβ2 production increased gradually during propagation of the siTGFβ2-PC3 cell population, up to the level of the original PC3 cells, suggesting that TGFβ2 provides a selective advantage (data not shown). We generated cells in which the superrepressor IκB (SR-IκB) suppressed the NF-κB response (20). These cells were sensitive to TNF even in the absence of CHX, and the sensitivity to TNF induced by SR-IκB could not be overcome by preincubating the cells with conditioned media from PC3 cells or by recombinant TGFβ2 (data not shown). Hence, we have shown that the TGFβ2 secreted by PC3 cells is the major factor that protects indicator cells from TNF-mediated apoptosis through the activation of NF-κB.
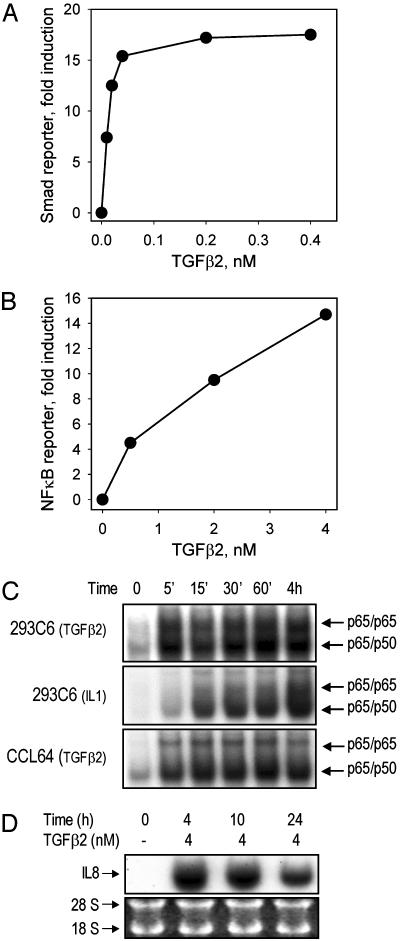
The Rapid and Direct Activation of NF-κB by TGFβ2 May Go Through a Smad-Independent Signaling Pathway. To test the effect of TGFβ2 on NF-κB activation in human cell lines, we established an indicator assay by stably transfecting 293C6 cells with a κB-luciferase construct. In contrast to the results of an activity assay that depends upon Smad activation, in which TGFβ2 reached its maximal effect at ≈0.4 nM (Fig. 3A), NF-κB was activated in a dose-dependent manner up to 4.0 nM (Fig. 3B). EMSA showed that the activation occurred within 5 min and persisted for at least 4 h (Fig. 3C). Consistently, activation of IL-8, a typical NF-κB target gene, was induced by treatment with TGFβ2 (Fig. 3D). Because the maximal activation of NF-κB or Smad occurs at concentrations of TGFβ2 that differ by about 10-fold, these two responses are likely to be due to the activation of distinct signaling pathways.
Fig. 3.
The activation of NF-κB by TGFβ2 is rapid and dose-dependent. (A) Dose–response of Smad promoter activation after TGFβ2 treatment. 293C6 cells were transfected transiently with a Smad binding element-luciferase construct for 24 h and then treated with TGFβ2 for another 24 h before luciferase assay. (B) Dose–response of NF-κB activation after TGFβ2 treatment. Stable 293C6κB indicator cells were treated with TGFβ2 for 24 h before luciferase assay. (C) Time course of activation of NF-κB by TGFβ2. Cells were treated with TGFβ2 (2 nM). IL-1-treated cells were used as controls. Note that the time of exposure of the gel in the experiment was much less than in the TGFβ2 experiment. EMSAs were done as described in Materials and Methods. (D) TGFβ2-induced IL-8 expression in 293C6 cells. Cells were treated with TGFβ2. Northern analysis was done as described in Materials and Methods.
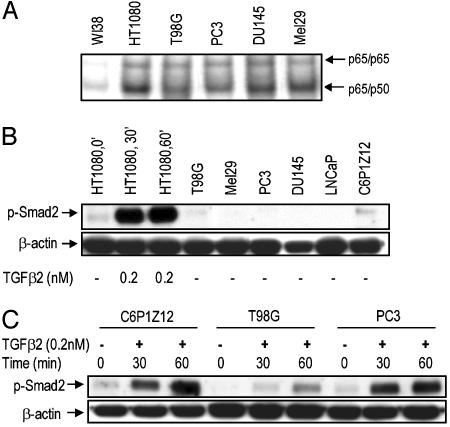
Some Tumor Cell Lines with Constitutive NF-κB Secrete TGFβ2. To estimate how general the phenomenon of production of TGFβ2 by tumor cells with constitutively active NF-κB might be, we tested its presence in media from PC3 and eight other cell lines by using a quantitative ELISA. Low or insignificant levels of active TGFβ2 were found in the media conditioned by all cells except PC3. However, much higher levels of TGFβ2 were found in media conditioned by several cell lines after treatment of the media with HCl, which converts latent TGFβ to the active form (Table 1). Therefore, several of the cell lines tested produce TGFβ2, predominantly in its latent form. Interestingly, all of these cell lines display constitutive activation of NF-κB, assayed by ELISA (Table 1) or EMSA (Fig. 4A), including C6P1Z12 (Table 1), a mutant line derived from 293C6 cells after selection for constitutive activation of NF-κB (13). Consistent with the predominant presence of latent TGFβ2, none of the cells examined showed appreciable constitutive activation of Smad2 (Fig. 4B), although the Smad pathway was capable of responding to active TGFβ2 in these cells (Fig. 4 B and C). These observations suggest that latent TGFβ2 production is a property of many tumor cell lines with constitutively active NF-κB. Although TGFβ1 is also capable of activating NF-κB (data not shown), the NF-κB activating and antiapoptotic effect of media conditioned by PC3 cells is conferred not by TGFβ1 but by TGFβ2, as shown by the results with neutralizing antibodies (Fig. 2A). We do not see effects of TGFβ1, probably because low levels of this cytokine are secreted by these cells as compared with TGFβ2 (data not shown).
Table 1. ELISA of TGFβ2 in conditioned media.
| Active TGFβ2, pg/106 cells
|
Total TGFβ2, pg/106 cells
|
||||
|---|---|---|---|---|---|
| Conditioned media | Constitutively active NF-κB | Without antibody | With antibody | Without antibody | With antibody |
| No cells | — | 10 | 0 | 29 | 3 |
| 293C6 | — | 0 | 0 | 68 | 0 |
| WI38 | — | 0 | 0 | 85 | 9 |
| LNCaP | — | 0 | 0 | 100 | 16 |
| HT1080 | + | 0 | 0 | 370 | 0 |
| T98G | + | 48 | 16 | 1,700 | 76 |
| Mel29 | + | 0 | 0 | 510 | 0 |
| PC3 | + | 450 | 100 | 10,000 | 530 |
| DU145 | + | 27 | 0 | 920 | 0 |
| C6P1Z12 | + | 200 | 70 | 8,500 | 320 |
Media collected after 24 h were assayed with and without treatment with polyclonal anti-TGFβ2. Total TGFβ2 was assayed after exposure to 1 M HCl, to release latency-associated peptide. Active TGFβ2 was assayed without exposure to HCl.
Fig. 4.
Smad2 is not constitutively active in tumor cells with constitutive NF-κB activity and TGFβ2 overexpression. (A) Constitutive NF-κB activation in several tumor cell lines. Cells were cultured, samples were collected, and EMSAs were performed. (B) Smad2 is not constitutively phosphorylated in the tumor cells. Cells were collected, and Western assays were performed. (C) Phosphorylation of Smad2 on Ser-465/467 upon TGFβ2 treatment of the tumor cells. Cells with high TGFβ2 expression (according to the ELISA) were treated with TGFβ2 for 30 or 60 min, and Western assays were performed.
Discussion
A large body of literature suggests that, during the early phase of epithelial tumorigenesis, TGFβs, especially TGFβ1, inhibit primary tumor development and growth by inducing cell-cycle arrest and possibly apoptosis, serving as the proapoptotic factors through Smad-dependent signaling pathways (21, 22). However, in late stages of progression, as tumor cells evade the growth inhibition by TGFβs because of inactivation of their signaling pathways or aberrant regulation of cell-cycle machinery, the role of TGFβs is often switched from tumor suppression to promotion. For example, among TGFβ family members, TGFβ1 is regarded as an important regulator of normal and malignant prostate tissues. In the nonmalignant prostate, TGFβ1 stimulates cell differentiation, inhibits epithelial cell proliferation, and induces epithelial cell death (23). In contrast, prostate cancer cells frequently lose their TGFβ1 receptors and acquire resistance to the antiproliferative and proapoptotic effects of TGFβ1. Accordingly, high expression of TGFβ1 and loss of TGFβ receptor expression have been associated with a particularly bad prognosis in prostate cancer patients (23). We have found that TGFβ2 secreted by prostate cancer PC3 cells, and not TGFβ1, serves as the major protector against the TNF-mediated death of BALB/c-3T3 cells through NF-κB activation and is the key determinant of the survival of PC3 cells. Therefore, quite differently from the well accepted dominant role of TGFβ1 in prostate cancer development, our study reveals an important role of another TGFβ family member, TGFβ2, in this process.
Functional interactions between TGFβ and NF-κB in tumor cells have been addressed in previous publications, which, however, provide a confusing view, with TGFβ playing the role of either an inhibitor or activator of NF-κB-mediated signaling in different cell types (24–26). Assayed in stably transfected human 293 cells, we demonstrate that the activation of NF-κBbyTGFβ2 is rapid (Fig. 3C). However, because maximal activation of Smad (Fig. 3A) or NF-κB (Fig. 3B) occurs at concentrations of TGFβ2 that differ by 10-fold or more, these two responses to TGFβ2 are due to the activation of distinct pathways and might even use somewhat different receptors.
The growth-inhibiting effects of TGFβs on epithelial cells are mediated by Smad-dependent signaling, explaining why many tumor cells acquire defects in TGFβ receptors or Smad2 (27). However, this is not the case in PC3 cells, which still retain a normal response to TGFβ2 (Fig. 4C), indicating that the TGFβ receptors and Smads are still functional in these cells. Furthermore, Western analysis showed that Smad2 is not activated constitutively in PC3 cells or in several other cell lines (Fig. 4B). ELISA data (Table 1) further confirm that most cancer cell lines with constitutive NF-κB secrete different levels of TGFβ2, mainly in its latent form. These data strongly suggest that, although the Smad-dependent signaling pathway is still intact in PC3 cells, it is not activated in growing cells. Our findings, together with published information concerning functional interactions between TGFβ and NF-κB, allow us to build the following model: Secretion of TGFβ causes a dual effect on tumor cells of epithelial origin. On the one hand, it suppresses the growth of these cells through Smad-dependent signaling. On the other hand, TGFβ secretion can be beneficial for tumor cells by causing the constitutive activation of NF-κB. It is beneficial to tumors to keep the NF-κB-activating role of TGFβ but get rid of its growth-suppressive effect. To achieve this, tumor cells acquire mutations in either TGFβ receptors or Smads (27). Does latent TGFβ2 activate NF-κB directly to achieve a protective effect in PC3 cells? Further studies are needed to address this possibility.
Suppressing the production of TGFβ2 causes PC3 cells to die, and a similar effect is caused by inactivating NF-κB through the ectopic expression of the SR-IκB (12), suggesting that PC3 cells have become addicted to the constitutive autocrine activation of NF-κB mediated by TGFβ2. This observation identifies TGFβ2 as an important potential target for therapeutic suppression designed to inhibit the growth of tumor cells with constitutively active NF-κB and to sensitize them to treatment with cytotoxic drugs.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 CA62220 (to G.R.S.) and CA88071 (to A.V.G.).
Abbreviations: CHX, cycloheximide; EMSA, electrophoretic mobility gel shift assay; siRNA, small interfering RNA; SR-IκB, superrepressor IκB; TGFβ, transforming growth factor β; TNF, tumor necrosis factor.
References
- 1.Gurova, K. V. & Gudkov, A. V. (2003) J. Cell. Biochem. 88, 128-137. [DOI] [PubMed] [Google Scholar]
- 2.Rayet, B. & Gelinas, C. (1999) Oncogene 18, 6938-6947. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A. S. (2001) J. Clin. Invest. 107, 241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karin, M., Cao, Y., Greten, F. R. & Li, Z. W. (2002) Nat. Rev. Cancer 2, 301-310. [DOI] [PubMed] [Google Scholar]
- 5.Massague, J. M. (1998) Annu. Rev. Biochem. 67, 753-791. [DOI] [PubMed] [Google Scholar]
- 6.Govinden, R. & Bhoola, K. D. (2003) Pharmacol. Ther. 98, 257-265. [DOI] [PubMed] [Google Scholar]
- 7.Massague, J. & Chen, Y. G. (2000) Genes Dev. 14, 627-644. [PubMed] [Google Scholar]
- 8.Gentry, L. E., Lioubin, M. N., Purchio, A. F. & Marquardt, H. (1988) Mol. Cell. Biol. 8, 4162-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray, A. M. & Mason, A. J. (1990) Science 247, 1328-1330. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence, D. A. (1996) Eur. Cytokine Network 7, 363-374. [PubMed] [Google Scholar]
- 11.Roberts, A. B. (1998) Miner. Electrolyte Metab. 24, 111-119. [DOI] [PubMed] [Google Scholar]
- 12.Gasparian, A. V., Yao, Y. J., Lu, J., Yemelyanov, A. Y., Lyakh, L. A., Slaga, T. J. & Budunova, I. V. (2002) Mol. Cancer Ther. 1, 1079-1087. [PubMed] [Google Scholar]
- 13.Sathe, S. S., Sizemore, N., Li, X., Vithalani, K., Commane, M., Swiatkowski, S. M. & Stark, G. R. (2004) Proc. Natl. Acad. Sci. USA 101, 192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kichina, J. V., Rauth, S., Das Gupta, T. K. & Gudkov, A. V. (2003) Oncogene 22, 4911-4917. [DOI] [PubMed] [Google Scholar]
- 15.Myslinski, E., Ame, J. C., Krol, A. & Carbon, P. (2001) Nucleic Acids Res. 29, 2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, A. D. & Rosman, G. J. (1989) BioTechniques 7, 980-990. [PMC free article] [PubMed] [Google Scholar]
- 17.Palayoor, S. T., Youmell, M. Y., Calderwood, S. K., Coleman, C. N. & Price, B. D. (1999) Oncogene 18, 7389-7394. [DOI] [PubMed] [Google Scholar]
- 18.Muenchen, H. J., Lin, D. L., Walsh, M. A., Keller, E. T. & Pienta, K. J. (2000) Clin. Cancer Res. 6, 1969-1977. [PubMed] [Google Scholar]
- 19.Teicher, B. A., Kakeji, Y., Ara, G., Herbst, R. S. & Northey, D. (1997) In Vivo 11, 453-461. [PubMed] [Google Scholar]
- 20.Miagkov, A. V., Kovalenko, D. V., Brown, C. E., Didsbury, J. R., Cogswell, J. P., Stimpson, S. A., Baldwin, A. S. & Makarov, S. S. (1998) Proc. Natl. Acad. Sci. USA 95, 13859-13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo, J., Ghiassi, M., Jirmanova, L., Balliet, A. G., Hoffman, B., Fornance, A. J., Liebermann, D. A., Bottinger, E. P. & Roberts, A. B. (2003) J. Biol. Chem. 278, 43001-43007. [DOI] [PubMed] [Google Scholar]
- 22.Valderrama-Carvajal, H., Cocolakis, E., Lacerte, A., Lee, E. H., Krystal, G., Ali, S. & Lebrun, J. J. (2002) Nat. Cell Biol. 4, 963-969. [DOI] [PubMed] [Google Scholar]
- 23.Wikstrom, P., Damber, J. & Bergh, A. (2001) Microsc. Res. Tech. 52, 411-419. [DOI] [PubMed] [Google Scholar]
- 24.Han, S. H., Yea, S. S., Jeon, Y. J., Yang, K. H. & Kaminski, N. E. (1998) J. Pharmacol. Exp. Ther. 287, 1105-1112. [PubMed] [Google Scholar]
- 25.Arsura, M., Panta, G. R., Bilyeu, J. D., Cavin, L. G., Sovak, M. A., Oliver, A. A., Factor, V., Heuchel, R., Mercurio, F., Thorgeirsson, S. S. & Sonenshein, G. E. (2003) Oncogene 22, 412-425. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai, H., Chiba, H., Sugita, T. & Toriumi, W. (1999) J. Biol. Chem. 274, 30353-30356. [DOI] [PubMed] [Google Scholar]
- 27.Schutte, M. (1999) Ann. Oncol. 10, Suppl. 4, 56-59. [PubMed] [Google Scholar]