Abstract
The influence of corticothalamic projections on the thalamus during different stages of reorganization was determined in anesthetized raccoons that had undergone previous removal of a single forepaw digit. Single-unit recordings were made from 522 sites in the somatosensory nucleus of the thalamus (ventroposterior lateral nucleus) before and after lesioning parts of primary somatosensory cortex. In those parts of ventroposterior lateral nucleus that had intact input from the periphery, the cortical lesion resulted in an immediate 85% increase in receptive field (RF) size. In animals studied 2–6 weeks after digit amputation, peripherally denervated thalamic neurons had unique RFs that were larger than normal, and these were not further enlarged by cortical lesion. However, at longer periods of reorganization (>4 mo), when the new RFs of denervated neurons had decreased in size, cortical lesion again produced expansion of RF size. These data demonstrate that corticothalamic fibers modulate the spatial extent of thalamic RFs in intact animals, probably by controlling intrathalamic inhibition. This corticothalamic modulation is ineffective during the early stages of injury-induced reorganization when new RFs are being formed, but is reinstated after the new RFs have become stabilized. The fact that neurons in the denervated thalamic region retained their unique RFs after cortical lesion indicates that their new inputs are not being relayed from a reorganized cortex and support the view that some plasticity occurs in or below the thalamus.
The processing of sensory information by the CNS is complicated by the complex interconnections between subcortical and cortical areas. Connections between the thalamus and cortex are largely reciprocal (1) with information being processed in both feed-forward (periphery to thalamus to cortex) and feedback (corticothalamic) directions. Although the majority of studies have concentrated on the ascending or feed-forward system, the feedback connections are actually much more numerous than the thalamocortical axons (1) and may contribute to focusing of information processing in the visual (2), auditory (3, 4), and somatosensory thalamus (5). Recently it has been proposed that this feedback from the cortex may contribute to plasticity in the ventroposterior lateral (VPL) nucleus, the main thalamic relay in the somatosensory system (6–8).
The corticothalamic axons are excitatory, glutamatergic, and reciprocally focused on the thalamic neurons that project to the same cortical region (1). In addition to exciting thalamocortical relay neurons directly, they also produce inhibition by means of local γ-aminobutyric acid (GABA)ergic interneurons, which are present in most species but not rats (9), and GABAergic neurons of the reticular thalamic nucleus, which project back into VPL (10). Electrophysiological studies have confirmed the monosynaptic excitatory and disynaptic inhibitory responses in VPL relay cells when cortical output cells are stimulated (11). Studies on the function of this feedback indicate that it facilitates on-line projections that are responsible for the center of receptive fields (RFs) and blocks projections that are slightly off-line (the RF surround) (12).
Recently, interest has grown on the reorganization that occurs in the somatosensory system after peripheral nerve injury. Similar to the case in somatosensory cortex, VPL thalamus also shows massive reorganizational plasticity (13–16). Rapid (minutes to hours) reorganization in VPL thalamus can be blocked if somatosensory cortex is inactivated in the rat (6). The question is raised as to whether new thalamic responses result from synaptic changes within the thalamus, or are simply relayed back from a reorganized cortex. To clarify this issue, we examined the thalamus of raccoon at different stages of thalamic reorganization. The raccoon has proven useful in somatosensory research because of its large and highly differentiated representations of the forepaw digits in the cuneate and VPL nuclei and primary somatosensory (S1) cortex. The present study investigated the role of S1 cortex in thalamic plasticity by examining RF properties of VPL neurons before and immediately after lesioning selective parts of somatosensory cortex in normal adult raccoons and in raccoons that had undergone amputation of the fourth forepaw digit. Differences are reported that depend on the length of time that had elapsed since the peripheral injury, and thus on the stage of reorganization.
Methods
Data were obtained from 12 adult raccoons (Procyon lotor) of either sex weighing between 5.2 and 7.8 kg. Two raccoons were intact controls. The other 10 raccoons underwent amputation of the fourth digit (D4) in right hand at the metacarpo-phalangeal joint, as described (14). In five animals (short-term group), the interval between amputation and recording was between 2 and 6 weeks. In five additional animals (long-term group), the interval was between 4 and 7 mo. During recording sessions, the raccoons were initially anesthetized with ketamine hydrochloride (100–200 mg, i.m.; Bimeda-MTC, Cambridge, ON, Canada) followed by isoflurane (2–4%). A catheter was inserted into the right radial vein for subsequent administration of α-chloralose (5% in propylene glycol; Sigma) as necessary to maintain a state of areflexia. A glucocorticoid (50 mg, Solu-Delta-Cortef; Upjohn) was injected i.v. immediately and 1–2 h later to reduce brain edema. Body temperature was regulated near 37°C, and end tidal CO2 was monitored continuously throughout the experiment (CWE, Ardmore, PA).
The animal was placed in a stereotaxic frame and a large craniotomy (≈25-mm diameter) was made over the left somatosensory cortex. Recordings were made in VPL thalamus by using tungsten microelectrodes [0.005 in, (1 in = 2.54 cm) 2–5 MΩ impedance; A-M Systems, Everett, WA] advanced with a piezo-electric microdrive (Burleigh Instruments, Fishers, NY). Penetrations were made at a slight angle to match the orientation of the digit regions more closely (Fig. 1B). Action potentials were amplified (CWE), band pass filtered (200–5,000 Hz), passed through a Humbug noise eliminator (Quest Scientific, North Vancouver, BC, Canada), and monitored on a digital storage oscilloscope and audio speaker. The entry of the microelectrode into the thalamus was indicated by a dramatic increase in background firing, often in bursts. The surface of VPL was located between 14 and 18.2 mm below the cortical surface.
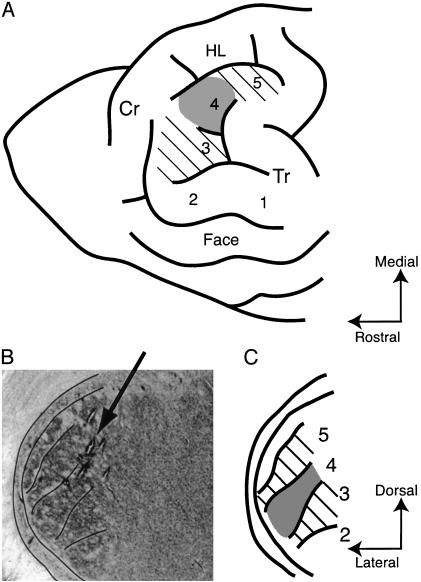
Fig. 1.
(A) Diagram of the forepaw representation in raccoon S1 cortex. The cortical lesion included the representational areas for D3 and D5 (hatched). D4 area (shaded) was lesioned and peripherally denervated in the amputation groups. Cr, cruciate sulcus; HL, hindlimb representation; Tr, triradiate sulcus. (B) Photomicrograph showing the VPL thalamic region and several penetrations passing through at the angle indicated by the arrow. (C) Diagram corresponding to B, showing digit regions in VPL. Hatched areas had their corticothalamic input removed. Shaded area 4 was also peripherally denervated.
Adjacent penetrations through VPL were separated by 200 μm, except when necessary to avoid blood vessels. Although recordings were performed in all digit areas in the VPL, penetrations were concentrated in the lateral part of VPL where D3–5 are represented. The activity of neurons was recorded at 200-μm steps within VPL. At each recording site, the RF was first localized with a glass probe or a large von Frey hair monofilament (Touch Test Sensory Evaluator, North Coast Medical, San Jose, CA). Von Frey hairs of decreasing size were tested to determine the smallest hair that produced an evoked response. This threshold von Frey hair was then used to determine the RF. The edges of the RF were drawn on the hand and the dimensions measured by using calipers. The RF area was calculated from these dimensions by using geometrical formulas for circles, ellipses, or rectangles. In the case of odd shapes, the RF was broken into simple shapes from which the total area could be calculated. In some cases, larger RFs were noted with higher thresholds, but only the RF area with the minimal threshold was used for calculation of RF size.
For cortical lesions, the boundaries of digit areas in the cortex were determined on the basis of the sulcus pattern (17) and confirmed by electrophysiological recordings (Fig. 1 A). The cortical areas representing D3–5 (Fig. 1 A, hatched and shaded areas) were lesioned by careful suction after cutting between adjacent areas with a scalpel blade. The entire gray matter of those areas was removed to ensure removal of corticothalamic neurons in layers V and VI. The remaining regions of the forepaw representation (palm and D1 and D2 areas) were left intact. After the cortical ablation, recordings were again made in VPL at approximately the same locations as before the lesion.
Small electrolytic lesions were made at the end of selected penetrations in the thalamus. At the end of the recording session, the animal was killed by an overdose of pentobarbital (130 mg i.v.), and the brain was removed. Blocks containing the thalamus were postfixed for several days and then placed in 15% sucrose for at least 24 h. Frozen sections, 60 μm thick, were cut in the frontal plane and stained with thionin to match the penetrations with the digit regions within VPL.
Statistical analysis was carried out by using the statview 5.0 program (SAS Institute, Cary, NC), and a 5% type 1 error rate was used to assess statistical significance.
Results
Data were collected from a total of 522 VPL neurons. Each digit representation in VPL is a discrete subnucleus with the D5 area most lateral (Fig. 1 B and C). As one progresses from dorsal to ventral in VPL, the neuronal RFs progress from the palm distally down a digit. An example of the progression in an intact animal is shown in Fig. 2A Left. The first neuron encountered had a RF on pad C of the palm, and at successive depths the RFs progressed distally down the fourth digit. A penetration lateral to this showed a progression down the fifth digit and a more medial penetration down the third digit (data not shown). The sizes of the RFs illustrated are similar in all of the digit regions of the intact raccoon.
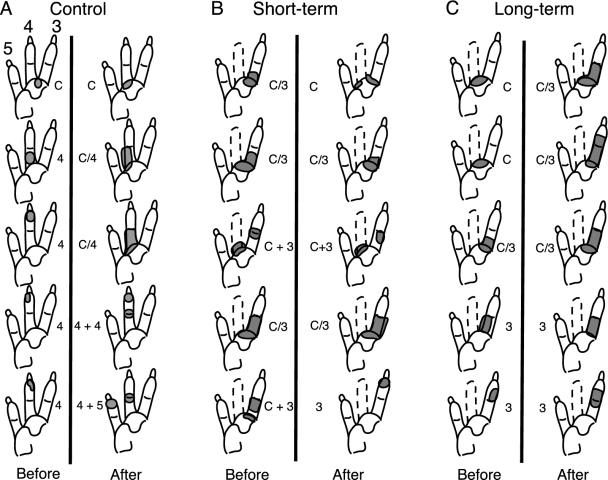
Fig. 2.
Diagram of the lateral half of the raccoon paw showing RFs (shaded) in representative penetrations passing through the D4 representation of VPL. Examples are from three animals in the control (A), short-term (B), and long-term (C) groups. Each column is from a single penetration from dorsal to ventral through VPL with 200 μm between recording sites. The locations of penetrations and relative depths before (Left) and after (Right) the cortical lesion are only approximately the same.
The denervated D4 region of VPL was identified on the basis of the normal D3 and D5 RFs in surrounding penetrations and on the abnormal RFs within a penetration thought to be in the D4 region, as described (14). Examples are shown in Fig. 2 B Left and C Left. The results from the deafferented, D4 representation studied before cortical lesion confirmed previous results indicating that peripherally denervated thalamus does undergo reorganization (14). The neurons in this area (n = 135) had RFs that were located on the adjacent D3, D5, and/or the underlying palm area. The occurrence of RFs on the palm at the bottom of the thalamus, as seen in Fig. 2B, is also characteristic of the reorganized VPL. In addition, many of these RFs were abnormally large and included skin from more than one peripheral region (digits and/or palm). These “multiregional” fields were defined as continuous (e.g., the first, second, and fourth RFs in Fig. 2B) or split (the third and fifth RFs in Fig. 2B). The number of multiregional fields in the denervated D4 region was 24 of 29 in the short-term group (83%) and 13 of 35 in the long-term group (37%). The remaining RFs in the denervated zone were restricted to a single region of the hand, usually D3 or D5. The proportions of multiregional fields in the short- and long-term groups were significantly different (χ2 = 13.5, P < 0.001), confirming our previous observation that the incidence of multiregional fields decreased with time after the amputation.
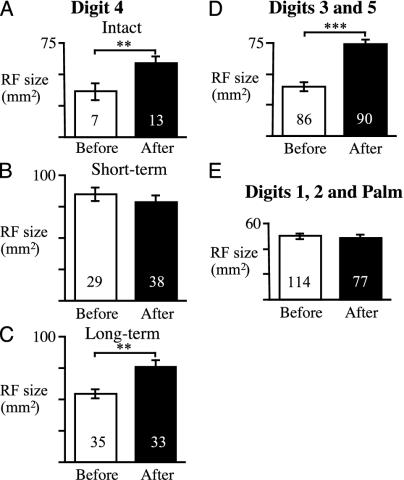
RF sizes were significantly larger in the deafferented D4 region than in nonamputated animals (Fig. 3 B and C, open bars, compared with Fig. 3A; F2,68 = 17.3, P < 0.001). The mean area was larger in the short-term group (85 ± 5 mm2) than in control animals (35 ± 6 mm2; t34 = 4.28, P < 0.001) and partially returned toward normal in the long-term group (55 ± 4 mm2). The long-term group had smaller RFs than the short-term group (t62 = 4.74, P < 0.001), but they were still larger than in the intact animals (t40 = 2.18, P < 0.05). The mean RF sizes in the reorganized D4 area were also significantly larger than in the surrounding regions with intact peripheral input (Fig. 3 D and E; t = 5.7 and 3.2 for short-term and long-term groups, respectively; P < 0.001 and P < 0.01). Note that any mistakes in assigning points to the deafferented region, by including normal D3 and D5 neurons in this sample, would decrease rather than increase the average RF size.
Fig. 3.
Summary of RF sizes before (open bars) and after (filled bars) cortical lesion. (A–C) The RF size in D4 area of intact (A), short-term (B), and long-term (C) animals. (D) RF size in D3 and D5 regions, which had intact peripheral input but loss of cortical input. (E) RF size in the regions that had intact peripheral and cortical input. Mean ± SEM. The number of recording sites in each group is shown at the bottom of each bar. **, P < 0.01; ***, P < 0.001.
The results obtained before cortical lesion confirm previous findings that the new RFs that appear after peripheral deafferentation are larger than normal in the short-term but gradually become smaller in parallel with an elimination of multiregional RFs.
Effect of S1 Ablation on VPL Regions with Intact Peripheral Input. Recordings were made from 176 neurons in D3 and D5 areas of VPL. These neurons had intact peripheral input and provided evidence about the effect of cortical lesions on control thalamus. The intact, short- and long-term groups did not differ in terms of RF size before the lesion (F2,83 = 0.38, P > 0.50). After the lesion, the RFs in D3 and D5 areas were 85% larger (74 ± 4 mm2) than before the lesion (40 ± 2mm2) (Fig. 3D; F1,170 = 43.9, P < 0.001). The expansion produced by cortical lesion was significant in each of the three groups (post hoc t = 3.5, 4.8, and 6.2 for intact, short-, and long-term groups, respectively; P < 0.001). The differences among these three groups were not statistically significant (F2,87 = 2.31, P > 0.10) even though expansion appeared to be greater in the short-term group (114%) compared with the intact (57%) and long-term groups (73%). The similar expansion for intact and amputation animals indicates that the amputation did not affect the adjacent, non-denervated regions of VPL thalamus.
The D4 region of the nonamputated animals also showed a large (63%) increase in mean RF size as a result of cortical lesion (from 36 to 59 mm2, Fig. 3A). These data indicates that the D4 thalamic region was not inherently different from the surrounding areas. An example of the RFs seen in a penetration through the intact D4 region after cortical lesion is shown on the right of Fig. 2 A.
Lesioning the cortical representations of D3–5 did not produce any change in RF size within the palm or D1 or 2 representations (n = 191). The mean RF areas in these regions were approximately the same after the lesion (49 ± 2 mm2) as they were before (50 ± 2 mm2; t189 = 0.37, P = 0.35; Fig. 2E). These regions with intact peripheral and cortical inputs provide a control for any widespread suppressive effects of the cortical lesion and also confirm that the lesion was restricted to the intended cortical areas.
Effect of S1 Ablation on Peripherally Deafferented VPL. Examination of the reorganized D4 area revealed that the cortical lesion had different effects depending on the time that had elapsed after amputation (Fig. 3 B and C). Analysis of variance revealed a significant difference between the short- and long-term groups (F1,131 = 11.7, P < 0.001) and a significant interaction effect (F1,131 = 7.1, P < 0.01). In the short-term group (Fig. 3B), the cortical lesion did not affect RF size; the mean before the lesion (85 ± 5 mm2) was not significantly different after the cortical lesion (79 ± 5 mm2, t65 = 0.82, P = 0.21). An example of RFs from one penetration through the deafferented region is shown in the right column of Fig. 2B. However, in the long-term group the cortical lesion produced a 36% increase in RF size (from 55 ± 4 to 75 ± 6 mm2; Fig. 3C: t66 = 3.00, P < 0.01), similar to that seen in peripherally intact VPL. An example of a penetration in this group is shown in the right column of Fig. 2C. It is interesting that the RF sizes in the thalamic regions that lost their cortical input were of similar magnitude in all groups after the lesion, regardless of whether the periphery was intact or not (range of means was 59–83 mm2, Fig. 3 A–D).
Discussion
Although there is increasing evidence for somatosensory reorganization in both cortical and subcortical regions (13), the numerous reciprocal connections between levels make it difficult to identify where the connections are changing or the time at which changes occur at different levels. A “bottom-up” hypothesis of reorganization would maintain that plastic changes occurring at the early stations in the ascending pathway are relayed to the cortex. An alternative is that the changes occur only in the cortex, and the new RF information is fed back to subcortical regions through corticofugal fibers (18). To explore the role of the cortex in thalamic plasticity, we examined the RF properties of peripherally intact and peripherally deafferented thalamic neurons in the presence and absence of cortical inputs. We removed the corticothalamic inputs to VPL by lesioning the specific cortical digit areas for the amputated (D4) and surrounding digits (D3 and D5), which are clearly defined and easily recognized in raccoons. Anatomical studies show that each digit region of raccoon S1 projects to and receives input from the corresponding somatotopic region of VPL (19, 20) with few or no collaterals to adjacent digit regions. Pharmacological inactivation of the cortex would have the advantage of being reversible, but the extent of drug diffusion is difficult to determine in that case. In the present experiment, the absence of changes in neurons with RFs on D1 or 2 or the palm confirmed that the lesions were restricted to the intended cortical areas. They also indicate that the lesions did not have any widespread debilitating effect on the animal. Recording within a few hours after the cortical lesion also eliminates the possibility that any changes are caused by retrograde degeneration of the thalamocortical neurons.
Cortical Lesions Produce Expansion of RFs in Intact VPL. Recordings in the peripherally intact VPL (representations of D3 and D5) demonstrated that removal of their corticothalamic inputs produced a significant (85%) expansion of thalamic RFs. These results indicate that S1 cortex imposes an inhibitory spatial restriction on VPL neurons (Fig. 4A). In addition to the direct excitatory inputs on the distal dendrites of thalamocortical relay neurons (21–23), corticothalamic afferents synapse (21–23) on two types of GABAergic neurons: local interneurons (24) and thalamic reticular neurons (22, 25) that project back into the main thalamic nuclei. Physiological studies have demonstrated that cortical stimulation produces monosynaptic activation and disynaptic inhibition of thalamic relay cells (11, 26). More detailed analysis of the spatial constraints on this feedback suggests that corticothalamic fibers facilitate inputs from the center of a neuron's RF and suppress the RF surrounds, which results in “focusing” of spatial information processing (5, 12). Similarly in the visual (2, 4, 27) and auditory systems (3, 28), the corticofugal feedback reinforces the core of the subcortical activity by densely focusing on the relay neurons that are matched to the cortical neurons while inhibiting a fringe of surrounding poorly matched neurons. This kind of feedback improves the processing of sensory input by sharpening their tuning and augmenting the response magnitude, a process called “egocentric selection” by Suga and coworkers (29, 30). The increase of the RF size described here after cortical lesion could result from the loss of excitatory corticothalamic drive on the thalamic inhibitory neurons, with the result that inputs from the skin surrounding the original RF are unmasked (Fig. 4A Lower). Corticofugal output might also influence inhibition in the dorsal column nuclei, which could also contribute to smaller RFs in VPL (12, 31).
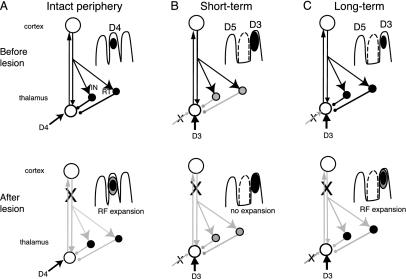
Fig. 4.
Possible circuitry to account for effects of S1 lesion on VPL RF size. (A) In the peripherally intact animal, corticothalamic input inhibits thalamic relay neurons by means of interneurons (IN) and reticular nucleus neurons (RT); cortical lesion produces RF expansion by removing this inhibition (Lower; gray lines indicate reduced activity). (B) At short intervals after amputation of D4, but after new inputs have started to appear from adjacent digits (D3), the thalamic GABAergic neurons are down-regulated (shaded circles) resulting in larger than normal RFs (Upper) and lack of an effect of cortical lesion (Lower). (C) At long intervals after amputation, GABA levels have recovered, leading to smaller, novel RFs (Upper) and expansion after cortical lesion (Lower).
Cortical Influence on Deafferented VPL. To examine the role of corticothalamic inputs in peripheral injury-induced reorganization of the thalamus, we studied the effect of specific cortical ablation on the D4 area in the VPL after amputation of D4. The first important finding was that the new RFs in deafferented VPL were maintained after the cortical lesion. If reorganization occurred only in the cortex, a cortical lesion should eliminate the responses of the peripherally denervated VPL neurons. The present results plus previous studies in the raccoon (14, 15, 32) clearly indicate that some changes in synaptic connections occur in and/or below VPL and that the subcortical responses are not due to cortical reorganization being relayed back to the thalamus. This conclusion is consistent with a recent study in the rat in which thalamic reorganization, induced by lesioning the gracile nucleus, was not eliminated by cortical lesion after reorganization, but was blocked if the lesion was made before reorganization (8). As they describe it, although the cortex may be involved in the induction of thalamic reorganization, it is certainly not necessary for its subsequent expression.
Although the reorganized cortex was not responsible for the responses in VPL, the results show that the cortex modulates VPL neurons differently at different times after amputation. In the early stage of reorganization (<6 weeks), when the new RFs in the deafferented VPL are larger than normal, removal of corticofugal influences did not affect RF size. However, after a long period of reorganization (>4 mo) when the new RFs of VPL neurons have shrunk to a size approaching the intact situation (14, 15), cortical lesion again produced expansion of RFs. This indicates that the cortex reestablishes its ability to restrict VPL RFs and to modulate some of the response properties of reorganized thalamic neurons. Studies in other species also support this role of corticothalamic input. When the cortex of rats was silenced pharmacologically, the RF size of many whisker-barrel neurons was altered, as was the amount of unmasking, or “rapid reorganization,” of novel inputs when the periphery was subsequently deafferented (6). In the monkey, both acute and prolonged chemical block of cortical activity induced a form of reorganization characterized by enlarged RFs (7).
The absence of an effect of cortical lesions in the short-term animals might be explained by depression or decreased firing of corticothalamic neurons during this interval, so that they would not be able to evoke thalamic inhibition. In fact, the evidence suggests that the spontaneous activity of cortical neurons is higher than normal for several weeks after deafferentation (33). It has not been determined whether corticothalamic neurons might behave differently.
A more likely explanation for the decrease and subsequent recovery of corticothalamic modulation is that they result from changes in intrathalamic inhibition. GABAergic modification of cortical RFs has been shown to change during the course of reorganization in the raccoon (34, 35) and similar changes may occur in the GABAergic circuitry of the thalamus. Although many histo- and immunochemical studies have shown that GABAergic markers in the cortex are decreased after peripheral injury (36), few studies have examined the thalamus (37, 38). Both of these studies found a decrease in GABA markers similar to that seen in the cortex. A decrease in inhibition in VPL should permit weak excitatory lemniscal inputs to the thalamus, such as those from adjacent digits, greater opportunity to activate the thalamocortical relay cells (Fig. 4B), a condition thought to be necessary for the strengthening of weak connections (39). Strengthening of these weak inputs would account for the larger RFs in the short-term animals. A decrease in inhibition in the thalamus would also explain the lack of further expansion after cortical lesion (Fig. 4B Lower). When thalamic GABA levels recover, in the long-term animals, these inhibitory neurons could again contribute to shrinkage of the RFs (Fig. 4C). They would also permit recovery of corticothalamic modulation, so that cortical lesions at this later stage of reorganization would again produce RF expansion (Fig. 4C Lower).
Acknowledgments
This work was supported by Canadian Institutes of Health Research Grant MT-06673.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GABA, γ-amino butyric acid; RF, receptive field; VPL, ventroposterior lateral; D, digit.
References
- 1.Steriade, M., Jones, E. G. & McCormick, D. A. (1997) Thalamus (Elsevier, Amsterdam).
- 2.Tsumoto, T., Creutzfeldt, O. D. & Legendy, C. R. (1978) Exp. Brain Res. 32, 345-364. [DOI] [PubMed] [Google Scholar]
- 3.Zhang, Y. & Suga, N. (1997) J. Neurophysiol. 78, 3489-3492. [DOI] [PubMed] [Google Scholar]
- 4.Zhang, Y., Suga, N. & Yan, J. (1997) Nature 387, 900-903. [DOI] [PubMed] [Google Scholar]
- 5.Rapisarda, C., Palmeri, A. & Sapienza, S. (1992) Exp. Brain Res. 88, 140-150. [DOI] [PubMed] [Google Scholar]
- 6.Krupa, D. J., Ghazanfar, A. A. & Nicolelis, M. A. (1999) Proc. Natl. Acad. Sci. USA 96, 8200-8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ergenzinger, E. R., Glaxier, M. M., Hahm, J. O. & Pons, T. P. (1998) Nat. Neurosci. 1, 226-229. [DOI] [PubMed] [Google Scholar]
- 8.Parker, J. L. & Dostrovsky, J. O. (1999) J. Neurosci. 19, 8623-8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbaresi, P., Spreafico, R., Frassoni, C. & Rustioni, A. (1986) Brain Res. 382, 305-326. [DOI] [PubMed] [Google Scholar]
- 10.Cox, C. L., Huguenard, J. R. & Prince, D. A. (1997) Proc. Natl. Acad. Sci. USA 94, 8854-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsumoto, T., Nakamura, S. & Iwama, K. (1975) Exp. Brain Res. 22, 281-294. [DOI] [PubMed] [Google Scholar]
- 12.Canedo, A. & Aguilar, J. (2000) Eur. J. Neurosci. 12, 2515-2533. [DOI] [PubMed] [Google Scholar]
- 13.Jones, E. G. & Pons, T. P. (1998) Science 282, 1121-1125. [DOI] [PubMed] [Google Scholar]
- 14.Rasmusson, D. D. (1996) J. Comp. Neurol. 364, 92-103. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson, D. D. (1996) J. Neurophysiol. 75, 2441-2450. [DOI] [PubMed] [Google Scholar]
- 16.Florence, S. L., Hackett, T. A. & Strata, F. (2000) J. Neurophysiol. 83, 3154-3159. [DOI] [PubMed] [Google Scholar]
- 17.Welker, W. I. & Seidenstein, S. (1959) J. Comp. Neurol. 111, 469-501. [DOI] [PubMed] [Google Scholar]
- 18.Kaas, J. H. (1999) Proc. Natl. Acad. Sci. USA 96, 7622-7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doetsch, G. S., Standage, G. P., Johnston, K. W. & Lin, C.-S. (1988) J. Neurosci. 8, 1873-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmusson, D. D. & Nance, D. (1986) Brain Res. Bull. 16, 399-406. [DOI] [PubMed] [Google Scholar]
- 21.Jones, E. G. & Powell, T. P. S. (1969) Proc. R. Soc. London Ser. B 172, 173-185. [DOI] [PubMed] [Google Scholar]
- 22.Jones, E. G. (1975) J. Comp. Neurol. 162, 285-308. [DOI] [PubMed] [Google Scholar]
- 23.Liu, X.-B., Honda, C. N. & Jones, E. G. (1995) J. Comp. Neurol. 352, 69-91. [DOI] [PubMed] [Google Scholar]
- 24.Barbaresi, P. & Manzoni, T. (2003) Neurosci. Lett. 339, 211-214. [DOI] [PubMed] [Google Scholar]
- 25.Bourassa, J., Pinault, D. & Deschenes, M. (1995) Eur. J. Neurosci. 7, 19-30. [DOI] [PubMed] [Google Scholar]
- 26.Deschenes, M. & Hu, B. (1990) Eur. J. Neurosci. 2, 140-152. [DOI] [PubMed] [Google Scholar]
- 27.Sillito, A. M., Jones, H. E., Gerstein, G. L. & West, D. C. (1994) Nature 369, 479-482. [DOI] [PubMed] [Google Scholar]
- 28.Yan, J. & Suga, N. (1999) J. Neurophysiol. 81, 817-824. [DOI] [PubMed] [Google Scholar]
- 29.Suga, N., Yan, J. & Zhang, Y. (1997) Trends Cognit. Sci. 1, 13-20. [DOI] [PubMed] [Google Scholar]
- 30.Yan, J. & Suga, N. (1996) Science 273, 1100-1103. [DOI] [PubMed] [Google Scholar]
- 31.Malmierca, E. & Nuñez, A. (1998) Brain Res. 810, 172-180. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson, D. D. & Northgrave, S. A. (1997) J. Neurophysiol. 78, 2924-2936. [DOI] [PubMed] [Google Scholar]
- 33.Rasmusson, D. D., Webster, H. H. & Dykes, R. W. (1992) Somatosens. Mot. Res. 9, 279-289. [DOI] [PubMed] [Google Scholar]
- 34.Tremere, L., Hicks, T. P. & Rasmusson, D. D. (2001) J. Neurophysiol. 86, 94-103. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury, S. A. & Rasmusson, D. D. (2002) Exp. Brain Res. 145, 150-157. [DOI] [PubMed] [Google Scholar]
- 36.Jones, E. G. (1993) Cereb. Cortex 3, 361-372. [DOI] [PubMed] [Google Scholar]
- 37.Land, P. W. & Akhtar, N. D. (1987) Brain Res. 425, 178-181. [DOI] [PubMed] [Google Scholar]
- 38.Rausell, E., Cusick, C. G., Taub, E. & Jones, E. G. (1992) Proc. Natl. Acad. Sci. USA 89, 2571-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bienenstock, E. L., Cooper, L. N. & Munro, P. W. (1982) J. Neurosci. 2, 32-48. [DOI] [PMC free article] [PubMed] [Google Scholar]