Abstract
Objective
A descriptive study was carried out to determine the significance of IgG-affinity in the serological diagnosis of rubella infections in pregnancy.
Materials and methods
A total of 92 pregnant women who had never received antirubella vaccines were recruited by simple random selection and did not exceed 24 weeks of gestation were recruited from the antenatal clinics of the University of Ilorin Teaching Hospital. Rubella virus-specific IgG, IgG-affinity and IgM were tested, using the Indirect ELISA methods.
Results
IgG-Affinity tests showed that 2 (2.2%) out of the 92 pregnant women, who were in their first and second trimester pregnancies respectively, had primary Rubella infections, while 1 (1.1%) primigravidae had a re-infection with rubella virus. It was also discovered that out of the 13 multigravid subjects that reported to have lost previous pregnancies, 2 (15.4%) cases may have been due to rubella infections that occurred during organogenesis.
Conclusion
Although the isolation of the whole virus or the viral nucleic material is the best basis for diagnosis, IgG-affinity is a proven supplementary serological diagnosis, to distinguish reinfection or viral persistence from primary exposure for prompt and accurate diagnosis. This is necessary for proper counselling of pregnant women especially in low economies where molecular diagnosis may not be affordable.
Keywords: IgG-Avidity, Rubella, Antenatal, Diagnosis, Antibodies
Introduction
Rubella virus is the monotypic agent of rubella (1). Viral particles are spread through airborne droplets, contact with oral-pharyngeal secretion of infected persons and vertically from infected mother to foetus during pregnancy (2, 3, 1). Rubella remains the mildest of viral exanthems in adults and may remain sub-clinical in 25% to 50% of cases (4, 5). Acute infections that occur in early pregnancy may induce foetal death or congenital malformations in the foetus, mostly affecting the brain, heart, eyes and ears (2).
Rubella has an average incubation period of 12 – 18 days but can extend to 23 days (3, 5). The infectious period of the virus is from 7 days before to 5 – 7 days after the onset of rash (2). Virions invoke a serological response that is detectable at the onset of its characteristic rash and evolves over the next few weeks (6). Viral-specific IgM antibodies are first detected 10 days post infection, and peaks at about 4 weeks post infection and may persist for more than 7 months after acute infections (6). Three weeks after infection, anti-rubella virus antibodies are present in all immunoglobulin classes, including IgG, IgA, IgD, and IgE (6). At early stages of primary infection, anti-rubella IgG antibodies have a low antigenic affinity; this however matures progressively over the next 3 months (6).
Differential diagnosis of rubella infections remains unreliable (7). Currently, laboratory confirmation of suspected cases is based on the detection of Rubella virus-specific IgM in a single serum sample collected soon after the onset of symptoms (8, 6). However, a positive IgM result may not always the onset of a primary infection. Re-infections are generally asymptomatic and are detected by serological investigations (7, 6). Although reasons for re-infections have not been clearly defined, cases associated with viraemia do not seem to be due to a defective immunologic response. However, viral strains from cases of reinfection do not seem to differ from other strains (8).
Studies carried out amongst pregnant women in Nigeria have revealed prevalence rates of 68.5% and 76% in two south-western (9, 10), 16.3% in a North-central region (2), and 54.1% in a North-western region (11). Despite the development of successful vaccines over four decades ago (7, 8), the global burden of disease as a result of Congenital rubella syndrome remains a major concern (3, 12). However this could, be significantly reduced by immunization and diagnostically-informed antenatal counselling (2).
This study was designed to determine the significance of IgG-avidity testing in the serologic diagnosis of antenatal rubella.
Materials and methods
A descriptive study was carried out on a cohort group of 92 pregnant women attending the Antenatal clinics at the University of Ilorin Teaching Hospital (UITH), Ilorin, Nigeria. Ethical approval was obtained from the Ethical Committee of the University of Ilorin Teaching Hospital. An informed consent form was signed by each participant. Selection criteria included pregnant women in their first and second trimesters of pregnancy, and had never received antirubella vaccines. Pregnant women above 24 weeks of gestation, including other pregnant women who were not registered at, nor attended the antenatal clinics of UITH were excluded from this study.
A structured questionnaire was designed and standardised. Data was collected by 3 trained research assistants through structured interviews. Three millilitres of blood were collected from the subjects by Venepuncture into labelled sterile sample tubes and allowed to clot undisturbed at room temperature. Sera were separated by centrifugation at 3,000 revolutions per minute (rpm) for 5 minutes and stored in 3 serum vial aliquots at -20°C until analyses.
Sera were tested for Rubella virus-specific IgM, IgG and IgG-avidity antibodies by indirect quantitative Enzyme-Linked Immunosorbent Assay (ELISA) techniques. This was carried out, using the SERION ELISA classic Rubella Virus IgG/IgM kit, manufactured by SERION® Immunologics, Friedrich-Bergius-Ring 19, 97076, Würzburg, Germany. IgG test results were interpreted as a ratio of the sample optical density (OD) and the sample rate/cut-off. OD was read within 60 minutes at 405 nm against the substrate blank, while reference wave length between 620 nm and 690 nm. Cut-off values included, <0.30 U/ml = negative; 0.30–0.55 U/ml = Borderline; and >0.55 U/ml = positive. IgM test results were interpreted similarly as follows: <0.135U/ml = negative; 0.135–0.230 U/ml = Borderline; and >0.230 U/ml = positive. The controls and the calibrators passed the validation check recommended by the manufacturers of both the IgG and the IgM kits.
The avidity of IgG for rubella virus was also by indirect quantitative ELISA, but measured as a percentage ratio of the OD values of two IgG set-ups (i.e. with and without 35mM diethylamine). This was also carried out according to the manufacturer's validity ranges as displayed on the batch-specific quality control certificate, and interpreted as Low (<45%), Borderline (≥45% < 50%) and High (≥50%), representing recent infections (less than 12 weeks), Ongoing/ progressive infection, and Long-past infection (over 12 weeks) or a re-infection respectively.
Data entry was with the SPSS 11 software. Data was statistically tested at a critical level for statistical significance of 95% (p = 0.05) using the Chi-square and 95% Confidence Interval.
Results
Of the 92 pregnant women that participated in the study, 50 (54.4%) were multigravid, 13 (14.1%) had history of previous pregnancy losses (all of which occurred in the first and second trimesters of pregnancy), while 39 (42.4%) had living children (Figure 1). Results further revealed a directly proprtional relationship between age and gestational period amongst the participants.
Figure 1.
Pregnancy History
This figure shows the pregnancy history of the study subjects, including previous pregnancy losses – most of which occurred around the 10th week of gestation.
A total of 15 pregnant women had detectable levels of Rubella virus-specific IgG and IgM antibodies in their sera. Table 1 shows the clinical history and diagnostic results of the 15 (16.3%) participants who tested positive to the presence of antirubella IgG and IgM antibodies in their sera. Of the 13 pregnant women who had lost previous pregnancies, 2 tested positive for the presence of antirubella IgG antibodies in their sera, while 1 had a borderline antibody test result.
Table 1.
Patient's Diagnosis Result and Test History
| Patient | Details | Age (Years) | Gestation (Weeks) | Rubella Serology | ||
|---|---|---|---|---|---|---|
| IgM | IgG | Avidity | ||||
| 1. | Primigravid. No history of rubella vaccination or contact | 30 | 24 | + | ++ | High |
| 2. | Primigravid. Developed rash two weeks earlier. History of pregnancy loss at 6 weeks gestation. | 26 | 20 | − | ++ | High |
| 3. | Primigravid. No rash or known contact. No history of rubella vaccination. | 25 | 16 | − | ++ | Low |
| 4. | Primigravid. No history of rubella vaccination. History of pregnancy loss | 28 | 23 | − | ++ | High |
| 5. | Primigravid. No rash or known contact. No history of rubella vaccination | 29 | 16 | − | ++ | Nil |
| 6. | Primigravid. No rash or known contact. No history of rubella vaccination. Complained of joint pains in legs | 23 | 16 | − | + | High |
| 7. | Primigravid. Rashes, 6-week old fever with joint pains. No history of rubella vaccination. | 27 | 8 | − | + | High |
| 8. | Multigravid. No rash or known contact. No history of rubella vaccination | 23 | 18 | − | + | High |
| 9. | Multigravid. No rash or known contact. No history of rubella vaccination | 30 | 24 | − | + | Low |
| 10. | Multigravid. History of pregnancy at 20 weeks of gestation. No history of rubella vaccination. | 29 | 26 | − | + | Low |
| 11. | Multigravid. No rash or known contact. No history of rubella vaccination. | 35 | 12 | ++ | ++ | Low |
| 12. | Primigravid. No rash or known contact. No history of rubella vaccination | 20 | 20 | − | + | Moderate |
| 13. | Multigravid. Complained of fever with joint pains. No rash or known contact. No history of rubella vaccination | 38 | 12 | − | + | High |
| 14. | Primigravid. No rash or known contact. No history of rubella vaccination | 28 | 24 | − | + | Low |
| 15. | No history of rubella vaccination. | 34 | 16 | + | − | Nil |
−Negative, + Borderline, ++ Positive
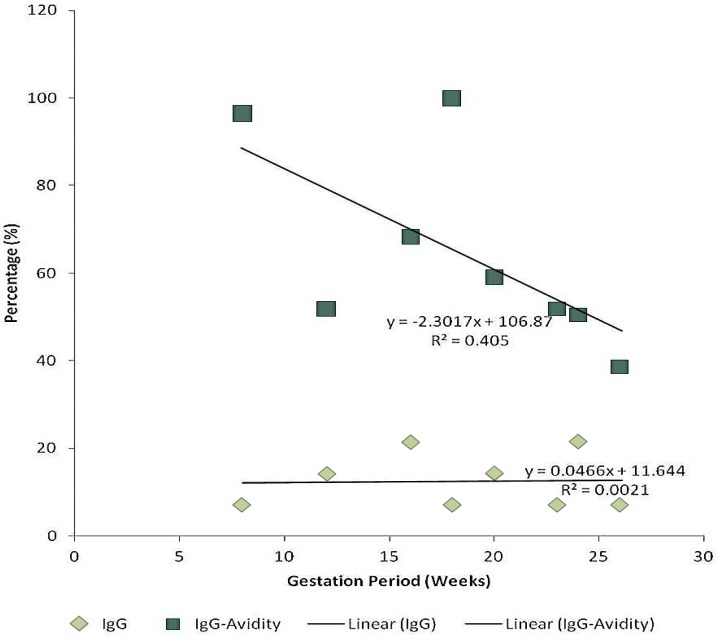
Among the 15 pregnant women whose sera tested positive for the for antirubella IgM and IgG antibodies, 7 (46.7%) had high IgG-Avidity levels, 1 (6.7%) had a moderate IgG-Avidity level, 5 (33.3%) had low IgG-Avidity levels, while 2 (13.3%) had no IgG-avidity. Furthermore, there was an inversely proportional relationship between age bracket and antirubella IgG antibodies (y = -0.667x + 22.001; r2 = 0.0029; p = 0.47) and also between gestational period and the IgG-Avidity levels (y = -2.3017x + 106.87; r2 = 0.0021; p = 0.46). Multiple symptoms were however reported.
Figures 2 and 3 show the relationship between clinical Symptoms and Rubella Virus-specific IgG and IgM Antibodies respectively.
Figure 2.
Correlation between Age Bracket, IgG and IgG-Avidity
Age Bracket and IgG: Linear Regression (y = -0.667x + 22.001) (r2 = 0.0029; p = 0.047)
Age Bracket and IgG Avidity: Linear Regression (y = 0.549x + 56.357) (r2 = 0.0015; p = 0.48)
Figure 3.
Correlation between Gestational Period, IgG and IgG-Avidity
Gestation and IgG: Linear Regression (y = 0.0466x + 11.644) (r2 = 0.0405; p = 0.044)
Gestation and IgG Avidity: Linear Regression (y = -2.3017x + 106.87) (r2 = 0.0021; p = 0.46)
Discussion
The detection of Rubella virus-specific IgG antibody in the sera of 14 (15.22%) of the pregnant women (p < 0.05) confirmed previous exposure to the virus (13), since, none of the pregnant women that participated in this study had ever received antirubella vaccines. The detection of rubella virus-specific IgM antibodies in the sera of 3 (3.26%) of the 92 pregnant women confirms that rubella infection was ongoing in those pregnant women (2). Such infections possibly occurred at least 10 days earlier (6). Detection of IgM antibodies was reported as positive and borderline – which highlights the course of the ongoing infection. The two borderline IgM test results indicate the possibility of an ongoing infection that was captured after about four months and at least 10 days since the infection occurred respectively (6).
Although most of the pregnant women that participated in this study had living children (Figure 1), 13 (14.1%) reported pregnancy losses that could have occurred during organogenesis. Out of the 13 who had lost previous pregnancies, 2 had positive IgG antibody tests, while 1 had a borderline antibody test. This result shows that the pregnancy losses could have been a result of Rubella infections in the 2 women with high IgG avidity levels. However, the pregnant woman with borderline IgG and low avidity must have lost her previous pregnancy to other teratogens (biological or chemical), however, previous studies carried out have shown that the development of IgG often coincides with diminished levels of serum IgM (6); although, this may be confirmed by further laboratory investigations (3).
As presented in Table 1, clinical symptoms reported by the pregnant women are descriptive of Rubella virus infections at various stages of pathogenesis (5, 3) Although lymphadenopathy and maculopapula rash were the least observed symptoms amongst the subjects, the predominance of fever cases observed (Table 1) conforms to previous studies that showed that the most common symptoms of rubella (lymphadenopathy, erythematous rash and low-grade fever) can be readily confused with similar illnesses associated with maculopapula rash caused by other common viral and non-viral pathogens or even some drugs (6). Invariably, clinical diagnosis has been proven unreliable (7) as observed in the discordant laboratory diagnostic results (Table 1). This irrefutably confirms the evidence of subclinical Rubella virus infections (3, 5) as observed in the pregnant women whose sera tested positive for rubella virus-specific IgM antibodies but showed no clinical symptoms.
A positive IgM result may not necessarily mean a primary infection in every case; therefore, anti-rubella IgG avidity assay was used in differentiating between primary and re-infection (14, 3). Seven of the pregnant women that participated in the study were diagnosed to have past infections, 1 (1.1%) of them had infections that occurred over two weeks ago and were ongoing, 3 (3.3%) had primary infections that occurred at least two weeks prior to the laboratory test while 2 (2.2.%) had recent infections occurring in less than two weeks.
A confirmed case of rubella infection in the screened pregnant women is the laboratory diagnosis of the presence of IgM in the sera of the screened pregnant women. However, immunological responses to rubella infections have various implications such that a Borderline IgM serological test result implies that:
A recent rubella infection occurred because antirubella IgM is detected first, 10 days post infection during which antirubella IgG immunoglobulin have not been produced and therefore are not detected in the patient serum (6);
An acute rubella infection began less than two weeks ago, and the host (IgM) immune response is still being developed because; antirubella IgM peaks at about 4 weeks post infection. This can be best confirmed if the host IgG test is positive and IgG avidity index is very low because; by three weeks post infection, anti-rubella virus IgG antibodies are present (6);
An acute rubella infection occurred within the last seven months during which the IgM may be thinning out and IgG avidity maturing. A confirmatory test however is the antirubella IgG positive test with a moderate avidity index (6);
A secondary rubella infection (i.e. rubella reinfection) has occurred (excluding the possibility of residual IgM months or years after the primary infection) if antirubella IgG test is positive with a High avidity index (13). This is because a natural rubella infection normally confers lifelong immunity. Therefore, supplementary measurement of IgM together with the measurement of antirubella specific IgM is combined to distinguish reinfection or viral persistence from primary exposure (6).
Primary Rubella Virus Infection
It was observed that the sera of a 34-year old subject, who was in her second trimester of pregnancy, had borderline antirubella IgM and was negative for antirubella IgG. This indicates the possibility of a recent rubella infection, which may have occurred about ten days prior to the laboratory serological test (6). Only one subject however tested positive for antirubella IgM with a positive antirubella IgG as well, and a very low IgG avidity index; thus, implying that the infection was acute and began less than two weeks from date of sample collection. The subject's histories further revealed that she is a 35-year old married multigravidae in her first trimester of pregnancy. She has never been immunized against Rubella virus, without a living child. She never had an abortion (induced or spontaneous).
Secondary Rubella Virus Infection (i.e. reinfection)
It was observed that two of the screened pregnant women had borderline Rubella virus-specific IgM antibodies in their sera. However, one of these subjects also tested positive for Rubella virus-specific IgG antibodies with a High IgG-Avidity Index; thus indicating the possibility of a reinfection in that patient. The medical history of this patient further revealed that, she was a 30-year old primigravida with no history of immunization against rubella virus. However, she did not present with any clinical symptom of rubella virus infection, this is in accordance with previous studies that showed that reinfections are generally asymptomatic and are recognised by serological investigations (7). Further still, such occurrences pose an extremely small risk of foetal damage as a result of the high avidity antirubella IgG.
Acute rubella virus infections that occur in early pregnancy may induce foetal death or congenital rubella syndrome (1, 15, 16). Unfortunately, at early stages of primary Rubella virus infections, IgG is of low avidity, maturing gradually over three months (6), thus making IgG and IgM detection inaccurate for complete diagnosis. Detection of this low IgG avidity together with antiviral IgA has been promoted as an alternative or supplement to the measurement of rubella specific IgM, to distinguish reinfection or viral persistence from primary exposure (6). This has further been established through the cases presented in this study to aid prompt and accurate diagnosis, which is necessary for proper counselling of pregnant women.
Significance of IgG Avidity
Of the 14 pregnant women whose sera tested positive for IgG antibodies, 3 were observed to have clinical rubella symptoms. Although a correlation between serum IgG and clinical symptoms was statistically significant for all symptoms, further serology showed that these three women, who were between 8 – 16 weeks of gestation, had no laboratory diagnostic evidence of ongoing rubella infections because, their serum IgG-Avidity index levels were high. Furthermore, IgM antibodies tested negative; thus implying that the clinical symptoms observed were differential.
This result also shows that the pregnancy losses could have been a result of Rubella infections in the 2 women with high IgG avidity levels
The woman with borderline IgG and low avidity must have lost her previous pregnancy to other teratogens (biological or chemical), however, previous studies carried out have shown that the development of IgG often coincides with diminished levels of serum IgM (17)
References
- 1.Deka D, Rustgi R, Singh S, Roy KK, Malhotra N. Diagnosis of rubella infection during pregnancy. J. Obstet Gynecol India. 2006;56:44–66. [Google Scholar]
- 2.Agbede OO, Adeyemi OO, Olatinwo AWO, Salisu TJ, Kolawole OM. Sero-Prevalence of Antenatal Rubella in UITH. The Open Public Health Journal. 2011;4:10–6. [Google Scholar]
- 3.WHO EPI team. Manuals for the laboratory diagnosis of measles and rubella virus infection; Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 4.Brooks GF, Butel JS, Morse SA. Jawetz, Melnick and Adelberg's Medical Microbiology. USA: McGraw-Hill; Paramyxoviruses and rubella. [Google Scholar]
- 5.Dontigny L, Arsenault M, Martel M. Rubella in pregnancy. J Obstet Gynaecol Can. 2008;30:152–8. doi: 10.1016/S1701-2163(16)32740-2. [DOI] [PubMed] [Google Scholar]
- 6.Hobman T, Chantler J. Rubella virus. In: Knipe DM, Howley PM, Griffin DE, Martin, editors. Fields Virology. 5th ed. PA, USA: Lippincott Williams & Wilkins publishers; 2007. pp. 1069–1100. [Google Scholar]
- 7.Banatvala JE, Brown DWG. Rubella. Lancet. 2004;363:1127–37. doi: 10.1016/S0140-6736(04)15897-2. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Measles and rubella laboratory network: 2007 meeting on use of alternative sampling techniques for surveillance; Weekly Epidemiological Record; 2008. pp. 225–32. [PubMed] [Google Scholar]
- 9.Bamgboye AE, Afolabi KA, Esumeh FI, Enweani IB. Prevalence of rubella antibody in pregnant women in Ibadan, Nigeria. West Afr J Med. 2004;23:245–8. doi: 10.4314/wajm.v23i3.28131. [DOI] [PubMed] [Google Scholar]
- 10.Onyenekwe CC, Kehinde-Agbeyangi TA, Ofor US, Arinola OG. Prevalence of rubella-IgG antibody in women of childbearing age in Lagos, Nigeria. West Afr J Med. 2000;19:23–6. [PubMed] [Google Scholar]
- 11.Bukbuk DN, EL-Nafaty AU, Obed JY. Prevalence of rubellaspecific IgG antibody in non-immunized pregnant women in Maiduguri, North Eastern Nigeria. Cent Eur J Public Health. 2002;10:21–3. [PubMed] [Google Scholar]
- 12.Hamkar R, Jalilvand S, Abdolbaghi MH, Jelyani KN, Esteghamati A, Hagh-goo A, et al. Distinguishing between primary infection and reinfection with rubella vaccine virus by IgG avidity assay in pregnant women. Eastern Mediterranean Health Journal. 2009;15:94–103. [PubMed] [Google Scholar]
- 13.WHO. Weekly Epidemiological Record; 2000. pp. 161–72. [Google Scholar]
- 14.Wong DA, Lim WL. Diagnosis of Rubella infection in pregnancy. Hong Kong practitioner. 1994;16:179–85. [Google Scholar]
- 15.Katow S. Rubella virus gene diagnosis during pregnancy and mechanism of congenital rubella. Intervirology. 1998;41:163–9. doi: 10.1159/000024931. [DOI] [PubMed] [Google Scholar]
- 16.Rubin E, Farber JL, Pathology J.B. Pennysalvia: Lippincott Company; 1988. pp. 206–8. [Google Scholar]
- 17.Stokes A, Mims CA, Grahame R. Subclass distribution of IgG and IgA responses to rubella virus in man. J Med. Microbiol. 1986;21:283–85. doi: 10.1099/00222615-21-4-283. [DOI] [PubMed] [Google Scholar]