Abstract
Background
Detection of clinically relevant pain relief in cats with degenerative joint disease (DJD) is complicated by a lack of validated outcome measures and a placebo effect.
Hypothesis/Objectives
To evaluate a novel approach for detection of pain relief in cats with DJD.
Animals
Fifty‐eight client‐owned cats.
Methods
Prospective, double‐masked, placebo‐controlled, stratified, randomized, clinical study. Enrolled cats were 6–21 years of age, with owner‐observed mobility impairment, evidence of pain in at least 2 joints during orthopedic examination, and overlapping radiographic evidence of DJD, and underwent a 2‐week baseline period, 3‐week treatment period with placebo or meloxicam, and 3‐week masked washout period. Outcome measures were evaluated at days 0, 15, 36, and 57.
Results
Both groups significantly improved after the treatment period (day 36) on client‐specific outcome measures (CSOM) and feline musculoskeletal pain index (FMPI) (P < .0001 for both); there was no difference between the groups on CSOM or FMPI score improvement. After the masked washout period, more cats that received meloxicam during the treatment period had a clinically relevant decrease in CSOM score (P = .048) and FMPI score (P = .021) than cats that received placebo.
Conclusions and Clinical Importance
Using both a client‐specific and a general clinical metrology instrument, owners of cats with DJD were able to detect evident recurrence of clinical signs after withdrawal of active medication than after withdrawal of placebo, and that this study design might be a novel and useful way to circumvent the placebo effect and detect the efficacy of pain‐relieving medications.
Keywords: Client‐specific outcome measures, Degenerative joint disease, Osteoarthritis
Abbreviations
- CSOM
client‐specific outcome measures
- DJD
degenerative joint disease
- FMPI
feline musculoskeletal pain index
- OA
osteoarthritis
Clinical responsiveness to an analgesic is typically measured by quantifying improvement during a treatment period, whether by self/proxy report or objective measurement. In noninferiority trials, efficacy of an analgesic is measured against a known effect whereas in placebo‐controlled trials, typically considered the reference standard, efficacy above any placebo effect is required. In evaluating the efficacy of a pain‐relieving medication in humans, questionnaire‐based results are frequently used as people can simply be asked to rate their response to a given treatment. Despite the direct questioning of an individual's experience, a large placebo effect exists and is seen repeatedly in clinical trials.1 In veterinary medicine, questionnaire‐based studies involve owners as proxies, with owners and caregivers being asked to rate a response in their pet. The questionnaires employed may be general, as in the feline musculoskeletal pain index (FMPI),2 inquiring about activities common to most cats, or they may be individualized, such as with the client‐specific outcome measures (CSOM)2 assessment. With both general and specific assessment techniques, large placebo effects occur in dogs3 and cats with DJD‐associated pain.2, 4
Degenerative joint disease (DJD) is a frequent cause of pain in older cats. The prevalence of radiographic DJD in older cats is high, ranging from 22 to 90% of cats.5 Long‐term treatment with nonsteroidal anti‐inflammatory medications, useful in treating osteoarthritis (OA) in humans and dogs, has been suggested to be effective in cats in studies without a placebo control.5 However, when evaluated against a placebo in client‐owned animals, the strong placebo effect obscures the presumed treatment‐related effect.2 This placebo response makes it difficult to demonstrate the efficacy of treatments to relieve pain in companion animal species.
In a novel clinical study design measuring responsiveness to a nonsteroidal anti‐inflammatory medication, meloxicam,1 we have used change in pain status after cessation of treatment as a proxy measure of efficacy. We hypothesize that though efficacy over a placebo may be difficult to detect during a treatment period because of the placebo effect, owners of cats with DJD will notice recurrence of clinical signs after withdrawal of active medication compared to placebo, and that this may be a useful way to detect the utility of pain‐relieving medications.
Materials and Methods
Cats were screened and enrolled similarly to previous studies conducted by the authors to assess joint pain in cats.2 Sixty‐six client‐owned cats were identified by their caregivers or participating primary care veterinarians as study candidates based on owner‐reported mobility impairment of at least 3 months duration and a history of veterinarian diagnosis or suspicion of DJD. Cats had to live indoors only, not be receiving any anti‐inflammatory medication, and considered generally healthy before screening. At screening (day 0), cats received a physical, orthopedic, and neurologic exam, and blood and urine samples were taken for a hematology profile, serum biochemistry panel, urinalysis with sediment evaluation, and serum T4 analysis. Orthogonal radiographs of the complete axial and appendicular skeleton were completed under sedation and were reviewed by a board‐certified veterinary radiologist. Also on day 0, owners completed a battery of subjective evaluations, including a CSOM questionnaire2 (Fig 1) where owners selected 3 activities in which their cat was impaired and rated how much difficulty their cat had with each activity, and the FMPI.2 Inclusion criteria included owner‐perceived mobility impairment, evidence of pain in at least 2 joints during orthopedic examination and overlapping radiographic evidence of DJD, and absence of systemic illness. These criteria were more stringent (ie, resulted in recruitment of more highly impaired cats) than in previous studies.2, 4 Cats with stable chronic kidney disease (up to IRIS stage 2) were eligible for participation. Qualifying cats were then enrolled into the study, and randomized (stratified by impairment) to receive meloxicam at 0.035 mg/kg/d or volume‐matched placebo (identical to the vehicle for the active medication) during the treatment period. Meloxicam is approved in Europe and other countries for use in cats as a single subcutaneous injection and for repeated oral administration; however, in the United States it is only licensed for single subcutaneous injection. Stratification was done by owner‐perceived impairment level and based on day 0 CSOM score to ensure that the cats with the highest degree of owner‐perceived impairment were balanced in both treatment groups. No analysis based on level of impairment was performed.
Figure 1.

Client‐specific outcome measure form completed by owners of enrolled cats on days 15, 36, and 57.
After enrollment, cats began a 2‐week baseline period during which they received volume‐matched placebo (known to be placebo by both owners and investigators) in order to acclimate to the daily medication regimen, and to verify correct owner administration and record keeping. After this baseline, cats began one 3‐week double‐masked treatment period during which they received either meloxicam or placebo, and then a 3‐week masked washout period (all cats received placebo, unbeknownst to the owners). The CSOM and FMPI were completed after baseline (day 15) and after each 3‐week treatment period (on day 36 and 57) (Fig 2).
Figure 2.
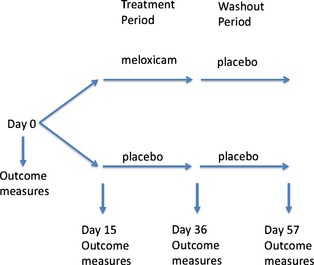
Study timeline. Outcome measures collected included client‐specific outcome measures and feline musculoskeletal pain index.
Differences between the treatment groups were analyzed using t‐tests for age and weight and chi‐square analysis for sex. CSOM scores were calculated by assigning a score from 0 to 4 (0 = impossible, 4 = no problem) for owners’ ranking of each of 3 activities to produce a score that could range from 0 (all activities were impossible for the cat) to 12 (all activities were no problem for the cat). A change in CSOM score greater than or equal to 2 (representing a 16.7% change in total score), as has been used in dogs,6 was considered relevant. To investigate improvement from day 15 to day 36, the number of cats in each group (meloxicam versus placebo) with CSOM changes greater than or equal to +2 were compared using one‐tailed Fisher's exact test. Similarly for deterioration from day 37 through day 57, the number of cats in each group with CSOM changes less than or equal to −2 were compared using one‐tailed Fisher's exact test. [Correction made after online publication February 10, 2014: “Fisher's exact test” has been updated to “one‐tailed Fisher's exact test”]
Feline musculoskeletal pain index scores were analyzed in a similar manner. Scores from 1 to 5 were assigned for the owners' response to each questionnaire item (1 = not at all, 5 = normal). The total score for the 17 items could range from 17 (cats were unable to perform each item) to 85 (cats were normal for each item). A change in FMPI score of greater than or equal to +8 for improvement, or less than or equal to −8 for deterioration (11.8% of total score range), was considered relevant. The number of cats with changes in FMPI score that were equal or greater in magnitude +8 for improvement or −8 for deterioration was compared using Fisher's exact tests. Changes from baseline (day 15) to day 36 for each group (CSOM and FMPI) were evaluated using t‐tests. A P‐value of >.05 was chosen for statistical significance. Data were analyzed using a statistical software package.2
All procedures were approved by the North Carolina State University Animal Care and Use Committee before study initiation.
Results
Subjects
At enrollment, cats ranged in age from 6 to 21 years of age. There were no differences in age (P = .69), weight (P = .67), or sex (P = .79) between the placebo and meloxicam groups. A minority of cats in each group were rated as higher impairment (within our range of impairment) by their owners (based on day 0 CSOM scores) and were distributed evenly across both groups (n = 7 in the meloxicam group, n = 5 in the placebo group). Of the 66 cats enrolled in the study, 8 cats were excluded because of adverse events precluding the completion of day 57 (n = 5) and client noncompliance with the protocol (n = 3). A total of 58 cats (29 in each group) were included in the analysis of CSOM and FMPI improvement and FMPI deterioration, and 57 were included in the analysis of CSOM deterioration as one owner had failed to fully complete the CSOM questionnaire on day 57.
Improvement at Day 36 (from Day 15)
No significant difference was found between the placebo and meloxicam group in the number of cats with a change in CSOM score greater than or equal to +2 (P = .39) or FMPI score greater than or equal to +8 (P = .61) (Tables 1, 2). Both groups improved significantly compared to baseline (day 15) on CSOM (P < .0001) and FMPI (P < .0001).
Table 1.
Client‐specific outcome measures (CSOM) improvement: number of cats with at least each amount of change in CSOM scores at day 36 (from day 15). Bold numbers indicate number of cats in each group at the chosen cut‐off value.
| ≥0 | ≥1 | ≥2 | ≥3 | ≥4 | ≥5 | |
|---|---|---|---|---|---|---|
| Placebo (n = 29) | 27 | 24 | 19 | 15 | 9 | 5 |
| Meloxicam (n = 29) | 26 | 25 | 21 | 17 | 9 | 6 |
Table 2.
Feline musculoskeletal pain index (FMPI) improvement: number of cats with at least each amount of change in FMPI scores at day 36 (from day 15). Bold numbers indicate number of cats in each group at the chosen cut‐off value.
| ≥0 | ≥1 | ≥2 | ≥3 | ≥4 | ≥5 | ≥6 | ≥7 | ≥8 | ≥9 | ≥10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 29) | 25 | 25 | 22 | 21 | 17 | 13 | 12 | 12 | 11 | 10 | 9 |
| Meloxicam (n = 29) | 24 | 23 | 22 | 19 | 18 | 17 | 16 | 15 | 11 | 9 | 8 |
Deterioration at Day 57 (from Day 36)
A significant difference was found between the placebo and meloxicam group in the number of cats with a change in CSOM score greater than or equal to −2 (P = .048) and FMPI score greater than or equal to −8 (P = .021) (Tables 3 and 4).
Table 3.
Client‐specific outcome measures (CSOM) deterioration: number of cats with at least each amount of change in CSOM scores at day 57 (from day 36). Bold numbers indicate number of cats in each group at the chosen cut‐off value.
| ≤−5 | ≤−4 | ≤−3 | ≤−2 | ≤−1 | ≤0 | |
|---|---|---|---|---|---|---|
| Placebo (n = 28) | 0 | 4 | 4 | 5 | 9 | 17 |
| Meloxicam (n = 29) | 1 | 4 | 9 | 12 | 17 | 21 |
Table 4.
Feline musculoskeletal pain index (FMPI) deterioration: number of cats with at least each amount of change in FMPI scores at day 57 (from day 36). Bold numbers indicate number of cats in each group at the chosen cut‐off value.
| ≤−10 | ≤−9 | ≤−8 | ≤−7 | ≤−6 | ≤−5 | ≤−4 | ≤−3 | ≤−2 | ≤−1 | ≤0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 29) | 2 | 2 | 2 | 5 | 7 | 8 | 10 | 15 | 16 | 17 | 19 |
| Meloxicam (n = 29) | 5 | 7 | 9 | 10 | 10 | 10 | 12 | 13 | 15 | 17 | 19 |
Discussion
In this study, we used deterioration after withdrawal of active medication to show efficacy, above placebo, of a nonsteroidal anti‐inflammatory medication for the treatment of DJD‐associated pain in cats. The use of the masked washout period allowed us to detect a difference in behavioral ratings from owners on individualized (CSOM) and general (FMPI) subjective outcome measures. This allowed us to use a clinical phenomenon, the return of clinical signs after withdrawal of active medication, to circumvent the placebo effect that often complicates clinical trials of medications for pain relief, where improvement over placebo is the most common endpoint. As this placebo effect is seen in human as well as veterinary clinical trials, our finding might have translational relevance.
It is well established that DJD in cats leads to owner‐perceived changes in behavior. Among the most commonly cited impairments include changes in a cat's ability to jump up or down, move smoothly up or down stairs, and ambulate normally. Changes are also seen in cats' activity level and mood. Several of these items have been shown to be responsive to treatment with analgesics,7 though the overwhelming placebo effect that may be seen makes interpretation of results difficult when evaluating analgesics in a clinical trial with client‐owned cats.
We used an individualized measure to evaluate changes in cats' performance of activities in which they were impaired. These client‐specific approaches have been found to be useful for veterinary and human pain research.8, 9 We also employed a previously developed and evaluated questionnaire (FMPI) that queries owners on multiple items thought to be common among cats with DJD or mobility impairment.2 In this study, responsiveness measured using the CSOM and FMPI at the end of the active treatment period was subject to the same placebo effect commonly seen in efficacy trials for nonsteroidal anti‐inflammatories2 and other analgesic medications, supplements, and diets.4 This placebo effect can mask findings of efficacy during a treatment period, as detecting improvement over this effect might be difficult.10 In addition, any natural waxing and waning of clinical signs, a feature of OA related pain, and the phenomenon of “regression to the mean,” might complicate the interpretation of repeated outcome measures. However, by comparing the ratings of owners during the masked washout period after a treatment period, we were able to show a greater change in ratings of the cats that had just received active medication versus those that received placebo. Our threshold for relevance of the change in CSOM scores was based on previous work in dogs6 where a change in 2 points (with 3 owner‐identified activities) was considered clinically relevant. In cats, the CSOM has been used previously with a change in 4 points being considered clinically significant,8 however that study identified 5 activities with a total possible CSOM score of 20, whereas this study used only 3 activities with a total possible CSOM score of 12. Therefore, a change in magnitude of greater than or equal to 2 was considered a relevant change for this study, essentially converting the scores into a threshold for success versus failure of the treatment. For the FMPI, we selected a change in over 10% of the total possible score for relevance, but future studies will be needed to establish the best threshold for clinical relevance.
This study stratified cats by owner‐perceived impairment based on day 0 CSOM scores. This was done to ensure that cats rated as highest impairment by their owners were equally distributed across the treatment groups. However, because of more stringent inclusion criteria based around disease burden and degree of mobility impairment, the cats in the present study were all more impaired than in previous studies we have conducted.2, 4 Cats began the study at different points along the assessment scales, and these thresholds for improvement and deterioration were chosen for applicability across impairment levels. Other ways to stratify cats for impairment could include radiographic scoring of severity or baseline activity measured by actimetry, however it is still unknown how well these signs correlate with pain or with treatment response.
The effect of worsening clinical signs after withdrawal of medication is often discussed as a clinical phenomenon. This might be especially true for drugs used to treat chronic or progressive diseases. As clinicians, we might try a period of medication withdrawal, even if just while switching from one drug to another, which might help both the owner and veterinarian to determine the efficacy of the medication. Despite its clinical use, this approach has not been used in drug trials in veterinary medicine (nor human medicine), though comments alluding to this phenomenon have been mentioned. In a nonplacebo‐controlled study on the efficacy of meloxicam treatment for cats with OA, the authors highlight one particular cat in the discussion, in which the veterinary rating of improvement was greater than the owners' rating on the questionnaire.5 They state that “when analgesic treatment was subsequently stopped, the owner soon became aware of a very obvious deterioration in the cat's condition, bringing their retrospective assessment of improvement in line with that of the veterinary surgeon.”
It has been suggested10 that using multiple outcome measures may be more beneficial than using a single measure. The same authors noted that the placebo effect seen in ratings of behaviors in companion animals closely mimics what is seen in human clinical trials, making clinical trials for pain relief in veterinary medicine of important comparative value as well as veterinary value. It is our belief that study designs that incorporate a masked washout period and measure deterioration after this washout period may prove to be a better design for the detection of treatment effects over placebo effects. In this study, owners were masked during the washout period, whereas the investigators were not, providing the potential for an investigator to bias the owner ratings. However, the interviews with owners were conducted by trained investigators, in the same manner at each visit, thus limiting the likelihood that owners would be influenced in their ratings. In addition, investigators were masked to the treatment the cat had received during the treatment period. In the future, a double‐masked washout period would increase the robustness of the findings by avoiding any potential for investigators to bias owner ratings. While this is a limited study, we believe that study designs that allow for analysis of this deterioration effect in addition to other efficacy measures may lead to breakthroughs in treatment options for chronic pain in our patients.
Acknowledgments
We thank all the participating veterinarians and their staff members as well as all the cats and cat owners that took part in this study. ME Gruen was supported by a Fellowship grant funded by Boehringer Ingelheim Vetmedica, Inc and currently receives support from the NIH Ruth L. Kirschstein National Research Service Award T32OD011130.
Conflict of Interest Declaration: Dr BDX Lascelles has received consulting and speaker fees from Boehringer Ingelheim Vetmedica, Inc.
Footnotes
Metacam® 0.5 mg/mL Oral Suspension; Boehringer Ingelheim Vetmedica, Inc, St. Joseph, MO
JMP Pro 9.0.0; SAS, Cary, NC
References
- 1. Rochon PA, Binns MA, Litner JA, et al. Are randomized control trial outcomes influenced by the inclusion of a placebo group? A systematic review of nonsteroidal anti‐inflammatory drug trials for arthritis treatment. J Clin Epidemiol 1999;52:113–122. [DOI] [PubMed] [Google Scholar]
- 2. Benito J, Hansen B, Depuy V, et al. Feline musculoskeletal pain index: Responsiveness and testing of criterion validity. J Vet Int Med 2013;27:474–482. [DOI] [PubMed] [Google Scholar]
- 3. Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc 2012;241:1314–1319. [DOI] [PubMed] [Google Scholar]
- 4. Lascelles BDX, DePuy V, Thomson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Int Med 2010;24:487–495. [DOI] [PubMed] [Google Scholar]
- 5. Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Fel Med Surg 2009;11:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cozzi EM, Spensley MS. Multicenter randomized prospective clinical evaluation of meloxicam administered via transmucosal oral spray in client‐owned dogs. J Vet Pharmacol Therap 2013;36:609–616. doi: 10.1111/jvp.12050. [DOI] [PubMed] [Google Scholar]
- 7. Klinck MP, Frank D, Guillot M, Troncy E. Owner‐perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can Vet J 2012;53:1181–1186. [PMC free article] [PubMed] [Google Scholar]
- 8. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client‐specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Int Med 2007;21:410–416. [DOI] [PubMed] [Google Scholar]
- 9. Mannion AF, Caporaso F, Pulkovski N, Sprott H. Goal attainment scaling as a measure of treatment success after physiotherapy for chronic low back pain. Rheumatology 2010;49:1734–1738. [DOI] [PubMed] [Google Scholar]
- 10. Malek S, Sample SJ, Schwartz Z, et al. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client‐owned dogs with hip osteoarthritis. BMC Vet Res 2012;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]


