Abstract
Purpose
Cancer survivors frequently receive care from a large number of physicians, creating challenges for coordination. We sought to explore whether cancer survivors whose providers have more patients in common (e.g. shared-patients) tend to have higher quality and lower cost care.
Methods
We performed a retrospective cohort study of 8661 patients diagnosed with loco-regional breast, prostate, or colorectal cancer. We examined survivorship care from Days 366 to 1095 following their cancer diagnosis. Our primary independent variable was “care density”, a novel metric of the extent to which a patient’s providers share patients with one another. Our outcome measures were health care utilization, quality metrics, and costs.
Results
In adjusted analyses, we found that patients with high care density—indicating high levels of patient-sharing among their providers—had significantly lower rates of hospitalization (OR 0.87, 95% CI 0.75–1.00) and higher odds of an eye examination for diabetes (OR 1.31, 95%CI 1.03–1.66) compared to patients with low care density. High care density was not associated with emergency department visits, avoidable outcomes, lipid profile following an angina diagnosis, or odds of glycosylated hemoglobin testing for diabetes. Patients with high care density had significantly lower total costs of care over 24 months (Beta coefficient −$2,116, 95%CI −$3107 to −$1125) along with lower inpatient and outpatient costs.
Conclusion
Cancer survivors treated by physicians who share more patients with one another tend to have some higher aspects of quality and lower cost care.
Implications of cancer survivors
If validated, care density may be a useful indicator for monitoring care coordination among cancer survivors and potentially targeting interventions that seek to improve care delivery.
Keywords: Care coordination, care density, cancer survivorship, social networking, survivors, referral and consultation
BACKGROUND
The 2005 Institute of Medicine report ‘From Cancer Patient to Cancer Survivor: Lost in Transition’ highlights the importance of care coordination for cancer survivors.[1] Patients with cancer frequently see a large number of health care providers both during their cancer treatment and into survivorship,[2–6] creating challenges for achieving coordinated care and increasing its likely value. Poor coordination among providers has been shown to be widespread, with adverse impacts on health care costs, patient outcomes, and experiences with care.[7, 8] However, the impact of care coordination among cancer survivors remains largely unknown.
Care coordination has received increasing attention in recent years. First, multiple health care reforms—including primary care medical homes and Accountable Care Organizations—are intended to improve quality through enhanced coordination.[9–13] Second, with improvements in diagnosis and treatment, life expectancy among cancer survivors has grown.[14] The five-year relative survival for breast cancer is 90%, for prostate cancer is 100%, and for colorectal cancer is 65%.[15] Third, the extent of comorbidity among cancer survivors is high.[16] The result is that cancer survivors are living longer and are more likely to have chronic disease(s) for which coordinated care may be important.
Most research in care coordination for cancer survivors has focused on the type of providers that a cancer survivor sees, generally finding that patients who visit both a primary care provider and oncology specialist are most likely to receive appropriate care.[4–6, 17–20] The current research assesses whether the potential relationships between a patient’s providers are associated with health care utilization, quality, and cost.
In particular, we examine a novel metric termed “care density” that may reflect aspects of care coordination. Prior work has validated that doctors who share more patients with one another are more likely to know one another and communicate about patient care.[21] Care density extends this concept by measuring—at the patient-level—how frequently his or her doctors share patients with one another.[22, 23] Prior work has found that, among patients with diabetes and congestive heart failure, patients whose doctors more frequently share patients with one another (i.e., have higher levels of care density) had significantly lower costs of care and fewer hospitalizations.[22] We hypothesized that cancer survivors with higher care density would have the potential for more highly coordinated care, potentially resulting in higher quality and lower costs.
METHODS
Research Design
This is a retrospective cohort study of patients in the time period after they have completed active cancer treatment and are transitioning to survivorship.[24] Assuming that cancer treatment would occur in the first year following diagnosis, we examined the association between care density and outcomes starting on Day 366 post-diagnosis and continuing through Day 1095. The Johns Hopkins School of Medicine Institutional Review Board deemed this project exempt.
Data Source
We used the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database, which combines the clinical information from the SEER registries with Medicare claims.
Study Subjects
We identified patients diagnosed with loco-regional breast, prostate, or colorectal cancer in the year 2004 who survived for at least three years. Patients who were not continuously enrolled in fee-for-service Medicare from one year prior to diagnosis through three years post diagnosis were excluded. Because we were interested in patients who had completed acute treatment and had no evidence of active malignancy, we excluded patients with a subsequent cancer diagnosis, or if they received chemotherapy, radiation, or hospice care during Days 366–1095.
Calculating Care Density
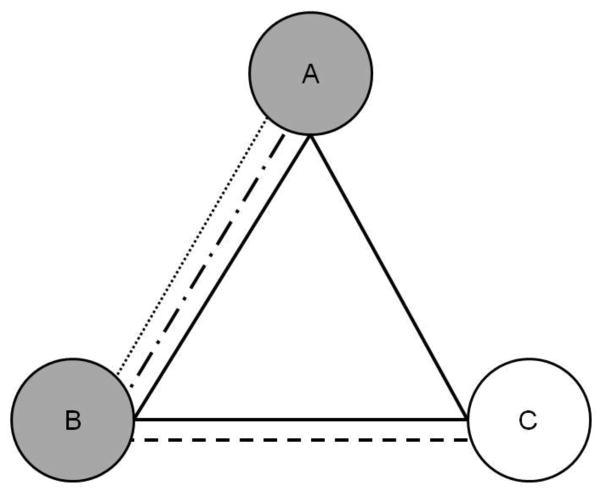
We determined the extent of patient-sharing using a measure called ‘care density’. Care density is a patient-level measure that describes the amount of patient-sharing among his or her outpatient providers. The numerator in this measure is the total number of patients shared by his/her providers. The denominator is the total number of provider pairs seen by that patient. Figure 1 provides an example.
Figure 1. Simplified calculation of care density.
Doctors are represented by circles (A to C) and patients by different lines styles (solid, dashed). The patient represented by the solid line saw all three doctors. The weight (strength) of connections between a pair of doctors is the number of shared patients. Doctor pair AB is given a weight of 3, pair BC a weight of 2, and pair AC a weight of 1. Care density for the solid line patient represents the sum of the weights of his/her doctor pairs (AB + BC + AC=3 + 2 + 1) divided by the number of doctor pairs he/she sees (3). This yields a care density of 2. In sensitivity analyses, we examine the care density among a patient’s primary care and oncology specialists. If the primary care and oncology specialists are shaded gray in the Figure, then the care density for the patient represented by the solid line would be the weight of AB (3) divided by 1 pair of doctors equaling a care density of 3.
To examine the amount of shared patients between physician pairs, we used data from the cancer cases and data from the 5% sample of patients enrolled in fee-for-service Medicare during 2004 and 2005. The larger population was used to provide more accurate estimates of the number of shared patients.
Two physicians shared in the care of a given patient if they both billed for outpatient evaluation and management visits based on CPT/HCPCS codes. In calculating care density, we trimmed the number of patients that a provider pair could share at the 99th percentile of the distribution (representing 6 shared patients).
We defined three groups of physicians based on specialties: primary care providers, oncology providers, and all other providers. Primary care providers included internal medicine doctors without subspecialty training, family practitioners, and general practitioners, geriatricians, and obstetricians and gynecologists. Oncology specialists included hematologists and oncologists, surgeons, urologists, and radiation oncologists. Specialty type was determined using American Medical Association physician specialty codes and, when unavailable, Centers for Medicare and Medicaid Services codes. For providers with more than one specialty, they were assigned to the specialty listed in the majority of their claims. Our primary measure of care density examined patient-sharing among all of a patient’s physicians seen during days 366 to 1,095 post-diagnosis.
With the skewed distribution of care density and hypothesized non-linear association with outcomes, care density was divided into tertiles. Care density cannot be calculated when a patient has only a single provider (the denominator is zero); however, these patients with less than 2 providers were retained in the analyses in a separate category.
Outcome Measures
Health care utilization
We determined whether patients had at least one hospitalization or emergency department visit during days 366 to 1095.
Costs
Costs of care during days 366 to 1095 were determined by summing the Medicare payment amounts from related claims. Costs were classified as total, outpatient, and inpatient costs.
Quality measures
We used published indicators for the quality of comorbid condition care.[25] These indicators included 7 different avoidable outcome indicators for chronic disease: ≥3 emergency department visits for cardiovascular-related diagnoses for patients with angina, perforated gallbladder for patients with cholelithiasis, admission for hyperosmolar or ketotic coma for diabetes, subsequent admission for a respiratory diagnosis for patients with chronic obstructive pulmonary disease or for patients with emphysema, diagnosis of lung abscess or empyema for patients with pneumonia, and non-elective admission for congestive heart failure for patients with cardiovascular disease (Appendix Table 1). These indicators were summarized as no avoidable outcome versus some avoidable outcome. We also examined process of care indicators for chronic disease management. For example, among patients who had a claim for diabetes from Days 1–365 from diagnosis (the denominator), we examined whether appropriate diabetes care was provided during the observation period (Days 366–1095) (the numerator). We examined receipt of yearly eye examinations and glycosylated hemoglobin or fructosamine testing every 6 months. We similarly examined the receipt of lipid testing for patients with a new angina diagnosis.
Appendix Table 1.
Definition of potentially avoidable complications
| Among patients with known angina, ≥3 emergency department visits for cardiovascular-related diagnoses in 1 year |
| Among patients with known cholelithiasis, diagnosis of perforated gallbladder |
| Among patients with known diabetes, admission for hyperosmolar or ketotic coma |
| Among patients with known chronic obstructive pulmonary disease, subsequent admission for a respiratory diagnosis |
| Among patients with known emphysema, subsequent admission for a respiratory diagnosis |
| Among patients with pneumonia, diagnosis of lung abscess or emphysema |
| Non-elective admission for congestive heart failure |
Covariates
Patient age, gender, and race were determined from beneficiary files. We included urban/rural status and SEER region. State buy-in was used as an indicator of low socioeconomic status. We determined comorbidity in the year prior to cancer diagnosis using the Charlson score as modified by Deyo and implemented by Klabunde.[26–28] Total number of physician outpatient visits was included as it may be associated with disease severity and complexity of care coordination. We assessed whether the patient had at least one visit to a PCP during the 2-year time period due to its known association with outcomes.[29]
Analyses
After presenting descriptive statistics, bivariate analyses were used to examine the association between covariates and care density (chi-square for categorical and 2-sample t-tests for continuous variables). Multivariable logistic regression models were used to examine the association of care density (independent variable) with outcomes (health care utilization and with quality measures), adjusting for all covariates. We used multivariable linear regression models to assess the relationship between care density and total and outpatient costs. Because a high proportion of patients did not have any inpatient costs, we used two-part models for this outcome. The first part was a logistic regression model predicting the incidence of inpatient hospitalization; the second part was a generalized linear regression model with gamma variance distribution and log-link function estimating inpatient costs for patients that had at least one inpatient admission. We used recycled predictions to generate the differences in cost associated with care density and 1,000 bootstrap samples with replacement to estimate the confidence intervals. The following sensitivity analyses were performed: (a) stratifying analyses by cancer type, (b) log transforming care density; (c) including only primary care providers and oncology specialists in constructing care density; (d) to address the issue of reverse causality, constructing care density only using data from days 366 to 730 and examining outcomes from days 731 to 1095, and (e) including a covariate for the total number of providers seen, recognizing that pairs of doctors are used to construct the denominator of care density.
RESULTS
The final sample included 8661 cancer survivors, of whom 4559 had prostate cancer (53%), 2231 had colorectal cancer (26%), and 1871 breast cancer (22%). The mean age was 75, and 65% were male (Table 1). On average, patients had 16 visits during the 2-year interval and 9% had 2 or more comorbidities. Care density was calculated for 7509 patients with at least 2 providers. Care density ranged from 1.0 to 6.0. Patients in the lowest care density tertile were more likely to be females, non-White, without a PCP, and live in an urban area compared to the other tertiles. There were 1152 patients who had zero or one providers and for whom care density could not be calculated. These patients were more likely to be male, non-white, have state buy-in, and lack a PCP compared to patients who had at least 2 providers.
Table 1.
Descriptive and bivariate analyses for cancer survivors. Care density is constructed using visits to all primary care providiers, oncology specialists, and other providers during Days 366 to 1,095 following diagnosis.
| Total | <2 docs | Low care density (1.0–1.2) | Medium care density (1.2–1.7) | High care density (1.7–6.0) | |
|---|---|---|---|---|---|
|
| |||||
| N (%) | 8661 (100) | 1152 (100) | 2577 (100) | 2399 (100) | 2533 (100) |
| Age (mean, SD) | 74.8 (7) | 74.9 (7) | 74.6 (7) | 74.7 (6) | 75.1 (7)* |
| Female | 3047 (35) | 334 (29) | 1026 (40) | 867 (36) | 820 (32)*** |
| Race | |||||
| White | 7330 (85) | 872 (76) | 2175 (84) | 2083 (87) | 2200 (87) |
| Black | 721 (8) | 188 (16) | 210 (8) | 163 (7) | 160 (6) |
| Other/Unknown | 610 (7) | 92 (8) | 192 (7) | 153 (6) | 173 (7)* |
| SEER region | |||||
| Connecticut | 621 (7) | 69 (6) | 175 (7) | 210 (9) | 167 (7) |
| Detroit | 586 (7) | 66 (6) | 248 (10) | 157 (7) | 115 (6) |
| Hawaii | 131 (2) | 27 (2) | 30 (1) | 35 (1) | 39 (2) |
| Iowa | 620 (7) | 61 (5) | 134 (5) | 156 (7) | 269 (11) |
| NewMexico | 215 (3) | 29 (3) | 56 (2) | 51 (2) | 79 (3) |
| Seattle | 633 (7) | 111 (10) | 184 (7) | 181 (8) | 157 (6) |
| Utah | 356 (4) | 42 (4) | 129 (5) | 95 (4) | 90 (4) |
| Atl/RuralGA | 233 (3) | 34 (3) | 90 (3) | 67 (3) | 42 (2) |
| California | 2905 (34) | 407 (35) | 811 (31) | 813 (34) | 874 (35) |
| Kentucky | 579 (7) | 76 (6) | 137 (5) | 140 (6) | 226 (9) |
| Louisiana | 572 (7) | 89 (8) | 141 (5) | 147 (6) | 195 (8) |
| NewJersey | 1210 (14) | 141 (12) | 442 (117) | 347 (14) | 280 (11)*** |
| State buy-in | 883 (10) | 169 (15) | 237 (9) | 202 (8) | 275 (11)** |
| Urban residence | 7824 (90) | 1028 (89) | 2397 (93) | 2222 (93) | 2177 (86)*** |
| Total number of visits (mean, SD) | 16.4 (12) | 5.25 (9) | 16 (11) | 20.6 (12) | 18 (12)*** |
| Charlson comorbidity index | |||||
| 0 | 6114 (71) | 920 (80) | 1850 (72) | 1618 (67) | 1726 (68) |
| 1 | 1764 (20) | 133 (12) | 530 (21) | 546 (23) | 555 (22) |
| 2+ | 783 (9) | 99 (9) | 197 (8) | 235 (10) | 252 (10)** |
| Had PCP visit | 7158 (83) | 443 (38) | 2263 (88) | 2179 (91) | 2273 (90)** |
p<0.05 for comparison is among care density levels,
p<0.01,
p<0.001
Table 2 presents the outcome measures. In terms of health care utilization, 22% had been hospitalized at least once and 29% had been to the emergency room. Avoidable outcomes were observed in nearly a fifth (19%) of our sample. Median total costs were $3552. Patients with low care density had the lowest percentage who met quality indicators for angina and diabetic eye examination. There was significant variation in total and outpatient costs across tertiles of care density. Patients with less than 2 providers were less likely to have had a hospitalization, emergency department visit, and to achieve appropriate quality indicators compared to patients with at least 2 providers. They were more likely to have had an avoidable outcome and tended to have lower total and outpatient costs of care.
Table 2.
Descriptive and bivariate analyses of outcome measures. Care density is constructed using all primary care, oncology specialists, and other providers during Days 366 to 1095.
| Total | <2 docs | Low care density | Medium care density | High care density | |
|---|---|---|---|---|---|
|
| |||||
| Hospitalization, N (%) | 1936/8661 (22.4) | 167/1152 (14.5) | 578/2577 (22.4) | 596/2399 (24.8) | 595/2533 (23.5) |
| ED visit | 2548/8661 (29.4) | 235/1152 (20.4) | 745/2577 (28.9) | 780/2399 (32.5) | 788/2533 (31.1)* |
| Avoidable outcome | 744/3740 (19.9) | 98/383 (25.6) | 196/1050 (18.7) | 212/1115 (19.0) | 238/1192 (20.0) |
| Lipid profile, angina | 308/449 (68.6) | 16/41 (39.0) | 74/121 (61.2) | 94/116 (81.0) | 124/171 (72.5)** |
| Eye examination, diabetes | 884/1984 (44.6) | 36/200 (18.0) | 237/577 (41.1) | 312/588 (53.1) | 299/619 (48.3)*** |
| Glycosylated hemoglobin or fructosamine testing, diabetes | 536/1984 (27.0) | 21/200 (10.5) | 148/577 (25.6) | 177/588 (30.1) | 190/619 (30.7) |
| Total costs, median $ (IQR) | 3552 (1544–8608) | 344 (0–2543) | 3483 (1625–8544) | 4749 (2526–10982) | 3767 (1876–9054)** |
| Inpatient costs, median $ (IQR)^ | 12674 (5936–27279) | 16253 (5764–35215) | 13957 (6336–30134) | 12606 (5847–25064) | 11516 (5745–24028) |
| Outpatient costs, median $ (IQR) | 2929 (1362–5336) | 253 (0–1512) | 2935 (1504–5163) | 4013 (2330–6695) | 3058 (1659–5340)*** |
Among patients who had >$0 inpatient costs
p<0.05 for comparison is among care density levels,
p<0.01,
p<0.001
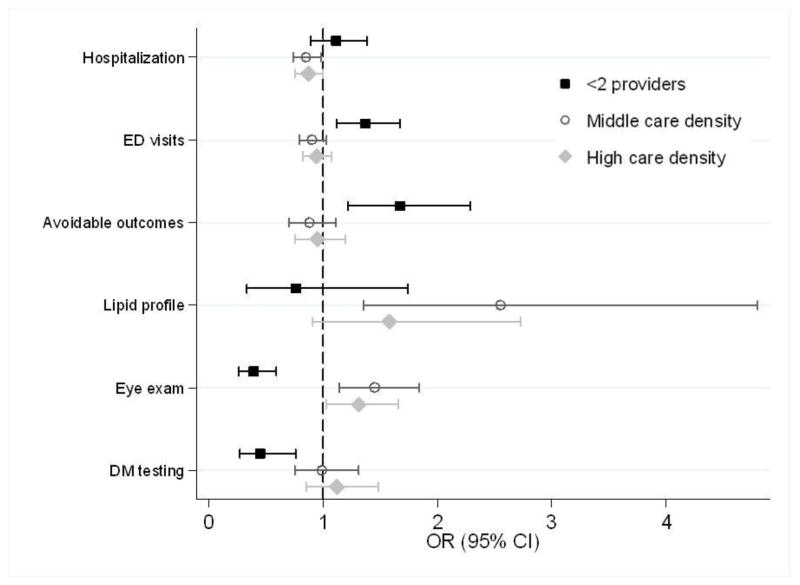
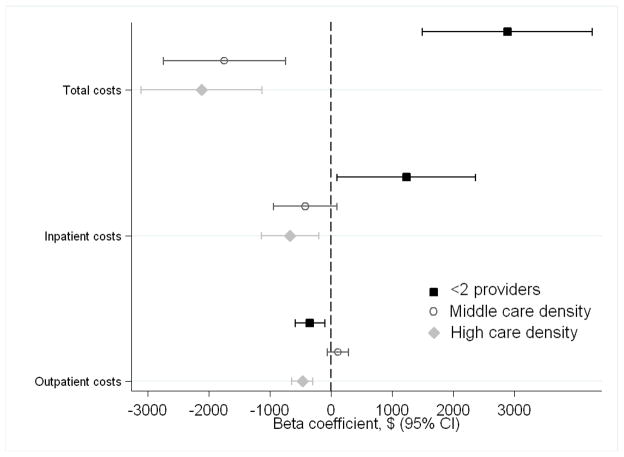
In adjusted analyses (Figure 2), our comparison group was patients in the lowest tertile of care density. Patients in the highest tertile had significantly lower odds of hospitalizatioin (OR 0.87, 95%CI 0.75–1.00, p=0.05) and higher odds of a diabetic eye examination (OR 1.31, 95%CI 1.03–1.66) relative to those with the lowest care density. The total, inpatient, and outpatient costs of care were significantly lower for patients in the highest tertile (Figure 3). Similarly, compared to the lowest tertile, we found that patients in the middle tertile had significantly lower rates of hospitalization (OR 0.85, 95%CI 0.74–0.98), higher odds of having a lipid profile following an angina diagnosis (OR 2.55, 95%CI 1.35–4.80) and eye examination for diabetes (OR 1.45, 95%CI 1.14–1.84). Patients in the middle tertile had significantly lower total costs of care. We did not consistently observe a stepwise gradient in which patients in the highest care density had lower utilization/costs and better quality metrics than those in the medium group. Patients with less than two providers had a higher odds of having an emergency department visit and an avoidable outcome and lower odds of meeting diabetic quality indicators compared to patients in the lowest care density tertile. Total costs were significantly higher—representing a change from the unadjusted results—as were inpatient costs; outpatient costs were lower.
Figure 2.
Multivariable logistic regression analyses showing the association between care density and outcomes. The comparison group contains patients in the lowest tertile of care density. Analyses are adjusted for age, gender, race, SEER site, cancer type, state buy-in, urban/rural residence, number of visits, PCP visit, and comorbidity.
Figure 3.
Multivariable regression analyses showing the association between care density and costs. The comparison group contains patients in the lowest tertile of care density. Analyses are adjusted for age, gender, race, SEER site, cancer type, state buy-in, urban/rural residence, number of visits, PCP visit, and comorbidity. Inpatient costs are based on 2-part models.
Sensitivity analyses are presented in Appendix Tables 2 through 6. In analyses stratified by cancer type, we observed significantly lower total costs (prostate and colorectal cancer), inpatient (colorectal cancer), and outpatient costs (across all cancer types) for patients in the highest care density tertile compared to those in the lowest tertile (Appendix Tables 2 and 3). In analyses in which care density was log transformed, there was a significant association between care density and lower total, outpatient, and inpatient costs of care (Appendix Table 4). When reclassifying care density to include only PCPs and oncology specialists, we found a significant, inverse association between being in the highest care density tertile and total and outpatient costs of care (Appendix Tables 2 and 3). Care density was not significantly associated with avoidable outcomes or other measures of quality. In models that examined whether care density calculated in Year 2 predicted outcomes in Year 3 after diagnosis, we found that high care density continued to be associated with receipt of a diabetic eye exam, total costs, and outpatient costs (Appendix Tables 2 and 3). Including a covariate for the number of doctors did not significantly alter our findings (Appendix Tables 5 and 6).
Appendix Table 2.
Mutlivariable logistic regression analyses* showing the association between care density and outcomes, stratified by cancer type and with care density defined using varying specifications of providers and timeframes.
| Care density created using visits to PCPs, oncology specialists, and all other providers during days 366–1095 | Care density created using visits to PCPs and oncology specialists only during days 366–1095 | Care density created using visits to PCPs, oncology specialists, and all other providers during days 366–730; Outcomes calculated during days 731–1095 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| BREAST | PROSTATE | COLORECTAL | OVERALL SAMPLE | OVERALL SAMPLE | ||
|
| ||||||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
|
| ||||||
| Hospitalization | ||||||
| <2 providers | 1.48 (0.92,2.39) | 1.07 (0.74,1.54) | 0.97 (0.69,1.38) | 1.38 (1.18,1.63) | 1.49 (1.20,1.85) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Middle care density | 0.94 (0.71,1.26) | 0.78 (0.63,0.96) | 0.90 (0.69,1.17) | 0.73 (0.62,0.86) | 0.84 (0.70,1.01) | |
| High care density | 1.02 (0.74,1.39) | 0.81 (0.66,1.01) | 0.83 (0.64,1.07) | 0.91 (0.79,1.06) | 0.96 (0.80,1.16) | |
| ED visit | ||||||
| <2 providers | 1.40 (0.90,2.17) | 1.34 (0.97,1.85) | 1.35 (0.97,1.87) | 1.54 (1.33,1.79) | 1.29 (1.06,1.58) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Middle care density | 0.83 (0.64,1.08) | 0.98 (0.81,1.19) | 0.83 (0.64,1.07) | 0.89 (0.77,1.04) | 0.84 (0.71,0.99) | |
| High care density | 1.07 (0.81,1.42) | 0.88 (0.72,1.06) | 0.93 (0.73,1.19) | 1.00 (0.88,1.15) | 0.97 (0.83,1.13) | |
| Avoidable outcome | ||||||
| <2 providers | 1.36 (0.67,2.80) | 2.50 (1.35,4.64) | 1.71 (1.10,2.65) | 1.65 (1.30,2.08) | 1.72 (1.12,2.63) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Middle care density | 0.76 (0.46,1.24) | 0.63 (0.42,0.96) | 1.25 (0.88,1.78) | 0.85 (0.65,1.12) | 0.97 (0.66,1.42) | |
| High care density | 1.30 (0.79,2.16) | 0.66 (0.44,0.99) | 1.11 (0.79,1.55) | 1.02 (0.80,1.29) | 1.01 (0.69,1.48) | |
| Lipid profile, angina | ||||||
| <2 providers | 2.06 (0.26,16.31) | 0.23 (0.04,1.53) | 1.53 (0.39,5.96) | 0.55 (0.29,1.02) | 0.51 (0.24,1.06) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Middle care density | 42.07 (3.75,472.21) | 0.79 (0.26,2.45) | 4.41 (1.40,13.87) | 1.20 (0.60,2.41) | 1.86 (1.02,3.38) | |
| High care density | 3.27 (0.71,15.13) | 0.74 (0.26,2.13) | 3.03 1.13 | 0.90 (0.48,1.68) | 1.47 (0.83,2.58) | |
| Eye examination, diabetes | ||||||
| <2 providers | 0.58 (0.24,1.41) | 0.17 (0.07,0.42) | 0.56 (0.30,1.03) | 0.64 (0.49,0.84) | 0.47 (0.33,0.65) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Middle care density | 1.35 (0.80,2.29) | 1.53 (1.08,2.19) | 1.52 (0.97,2.38) | 1.13 (0.86,1.49) | 1.52 (1.19,1.96) | |
| High care density | 1.22 (0.68,2.20) | 1.32 (0.94,1.85) | 1.30 (0.83,2.03) | 1.07 (0.84,1.37) | 1.48 (1.15,1.90) | |
| Glycosylated hemoglobin or fructosamine testing, diabetes | ||||||
| <2 providers | 1.49 (0.56,3.95) | 0.13 (0.03,0.55) | 0.40 (0.18,0.90) | 0.69 (0.50,0.95) | 0.60 (0.42,0.86) | |
| Low care density | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Middle care density | 0.99 (0.54,1.83) | 1.06 (0.70,1.59) | 0.95 (0.57,1.61) | 1.30 (0.95,1.76) | 1.29 (0.99,1.68) | |
| High care density | 0.91 (0.45,1.81) | 1.24 (0.84,1.83) | 1.24 (0.74,2.08) | 1.12 (0.85,1.47) | 1.25 (0.96,1.62) | |
Analyses are adjusted for age, gender (except breast/prostate models), race, SEER site, cancer type, state buy-in, urban/rural residence, total number of visits, visit with PCP, and Charlson comorbidity index.
Bold=significant at p<0.05
Appendix Table 6.
Multivariable regression analyses* showing the association between care density and outcomes; models do not and do include the number of doctors visits as a covariate. Care density is constructed using all primary care, oncology specialists, and other providers during Days 366 to 1095.
| Base model | Including total number of doctors as covariate | |
|---|---|---|
|
| ||
| beta-coefficient (95% CI) | beta-coefficient (95% CI) | |
|
| ||
| Total costs | ||
| <2 providers | 2888 (1499 – 4277) | 1938 (498 – 3379) |
| Low care density | 0 | 0 |
| Middle care density | −1742 (−2742 – −741) | −1292 (−2308 – −277) |
| High care density | −2116 (−3107 – −1125) | −2006 (−2997 – −1015) |
| Inpatient costs^ | ||
| <2 providers | 1236 (101 – 2371) | 773 (−300 – 1847) |
| Low care density | 0 | 0 |
| Middle care density | −419 (−936 – 99) | −277 (−813 – 258) |
| High care density | −666 (−1138 – −1957) | −609 (−1079 – −139) |
| Outpatient costs | ||
| <2 providers | −339 (−579 – −99) | 139 (−108 – 385) |
| Low care density | 0 | 0 |
| Middle care density | 119 (−54 – 291) | −108 (−281 – 66) |
| High care density | −465 (−636 – −294) | −520 (−690 – −351) |
Analyses are adjusted for age, gender (except breast/prostate models), race, SEER site, cancer type, state buy-in, urban/rural residence, total number of visits, visit with PCP, and Charlson comorbidity index
Two part models with recycled predictions and bootstrapping to estimate confidence intervals
bold = significant at p<0.05
Appendix Table 3.
Mutlivariable regression analyses* showing the association between care density and outcomes, stratified by cancer type and with care density defined using varying specifications of providers and timeframes.
| Care density created using visits to PCPs, oncology specialists, and all other providers during days 366–1095 | Care density created using visits to PCPs and oncology specialists only during days 366–1095 | Care density created using visits to PCPs, oncology specialists, and all other providers during days 366- 730; Outcomes calculated during days 731–1095 | |||
|---|---|---|---|---|---|
|
| |||||
| BREAST | PROSTATE | COLORECTAL | OVERALL SAMPLE | OVERALL SAMPLE | |
|
| |||||
| Beta-coeff. (95% CI) | Beta-coeff. (95% CI) | Beta-coeff. (95% CI) | Beta-coeff. (95% CI) | Beta-coeff. (95% CI) | |
|
| |||||
| Total costs | |||||
| <2 providers | 5270 (1971,8569) | 2889 (1220,4558) | 1083 (−2002,4168) | 3327 (2208,4446) | 2443 (1690,3195) |
| Low care density | 0 | 0 | 0 | 0 | 0 |
| Middle care density | −1966 (−4033,103) | −1434 (−2602,−266) | −2045 (−4590,501) | −3160 (−4299,−2021) | −1098 (−1743,−452) |
| High care density | −1786 (−4054,483) | −1831 (−2966,−696) | −2923 (−5351,−496) | −1139 (−2165,−112) | −640 (−1277,−2) |
| Inpatient costs^ | |||||
| <2 providers | 2163 (−144, 4470) | 1208 (−472, 2888) | 765 (−1167,2698) | 1234 (552, 1917) | 2004 (1053,−2953) |
| Low care density | 0 | 0 | 0 | 0 | 0 |
| Middle care density | 7 (−981, 996) | −158 (−935, 620) | −754 (−1745, 236) | −803 (−1286, −321) | −569 (−1055,−82) |
| High care density | −427 (−1450, 597) | −334 (−1044, 376) | −1149 (−2026, −271) | −4 (−486, 477) | −430 (−894,34) |
| Outpatient costs | |||||
| <2 providers | −75 (−622,473) | 40 (−308,388) | −876 (−1300,−451) | −69 (−263,125) | 113 (−17,244) |
| Low care density | 0 | 0 | 0 | 0 | |
| Middle care density | −56 (−399,287) | 71 (−172,315) | 422 (72,772) | −99 (−297,99) | −132 (−244,−20) |
| High care density | −412 (−789,−36) | −468 (−705,−232) | −471 (−805,−137) | −274 (−452,−96) | −177 (−287,−66) |
Analyses are adjusted for age, gender (except breast/prostate models), race, SEER site, cancer type, state buy-in, urban/rural residence, total number of visits, visit with PCP, and Charlson comorbidity index
Two part models with recycled predictions and bootstrapping to estimate confidence intervals
bold = significant at p<0.05
Appendix Table 4.
Mutlivariable regression analyses* showing the association between care density and outcomes. Care density is constructed using all primary care, oncology specialists, and other providers during Days 366 to 1095 and log transformed. Patients for whom care density could not be calculated (<2 providers) are excluded.
| OR (95% CI) | ||
|---|---|---|
| Hospitalization | ||
| care density | 0.89 (0.76, 1.05) | |
| ED visit | ||
| care density | 0.99 (0.85, 1.14) | |
| Avoidable outcome | ||
| care density | 0.98 (0.76, 1.25) | |
| Lipid profile, angina | ||
| care density | 1.20 (0.65, 2.22) | |
| Eye examination, diabetes | ||
| care density | 1.19 (0.92, 1.55) | |
| Glycosylated hemoglobin or fructosamine testing, diabetes | ||
| care density | 1.01 (0.75, 1.36) | |
|
| ||
| Beta coeff.** (95% CI) | ||
|
| ||
| Total costs | ||
| care density | −371 (−614, −128) | |
| Inpatient costs^ | ||
| care density | −255 (−400, −110) | |
| Outpatient costs | ||
| care density | −98 (−141, −56) | |
Analyses are adjusted for age, gender, race, SEER site, cancer type, state buy-in, urban/rural residence, total number of visits, visit with PCP, and Charlson comorbidity index
indicates predicted costs in dollars from moving from the 40th percential of care density to the 60th percentile
Two part models with recycled predictions and bootstrapping to estimate confidence intervals
bold = significant at p<0.05
Appendix Table 5.
Multivariable regression analyses* showing the association between care density and outcomes; models do not and do include the number of doctors visits as a covariate. Care density is constructed using all primary care, oncology specialists, and other providers during Days 366 to 1095.
| Base Model | Including total number of doctors as covariate | |
|---|---|---|
|
| ||
| OR (95% CI) | OR (95% CI) | |
|
| ||
| Hospitalization | ||
| <2 providers | 1.11 (0.89 – 1.38) | 1.02 (0.81 – 1.29) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 0.85 (0.74 – 0.98) | 0.88 (0.76 – 1.02) |
| High care density | 0.87 (0.75 – 1.00) | 0.87 (0.76 – 1.01) |
| ED visit | ||
| <2 providers | 1.37 (1.12 – 1.67) | 1.24 (1.00 – 1.53) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 0.90 (0.79 – 1.03) | 0.94 (0.82 – 1.07) |
| High care density | 0.94 (0.82 – 1.07) | 0.94 (0.83 – 1.08) |
| Avoidable outcome | ||
| <2 providers | 1.67 (1.22 – 2.29) | 1.62 (1.17 – 2.26) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 0.88 (0.70 – 1.11) | 0.89 (0.70 – 1.13) |
| High care density | 0.95 (0.75 – 1.19) | 0.95 (0.76 – 1.20) |
| Lipid profile, angina | ||
| <2 providers | 0.76 (0.33 – 1.74) | 1.05 (0.43 – 2.54) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 2.55 (1.35 – 4.80) | 2.24 (1.17 – 4.27) |
| High care density | 1.58 (0.91 – 2.73) | 1.49 (0.86 – 2.61) |
| Eye examination, diabetes | ||
| <2 providers | 0.39 (0.26 – 0.59) | 0.50 (0.32 – 0.77) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 1.45 (1.14 – 1.84) | 1.33 (1.04 – 1.69) |
| High care density | 1.31 (1.03 – 1.66) | 1.28 (1.01 – 1.63) |
| Glycosylated hemoglobin or fructosamine testing, diabetes | ||
| <2 providers | 0.45 (0.27 – 0.76) | 0.39 (0.23 – 0.68) |
| Low care density | 1.00 | 1.00 |
| Middle care density | 0.99 (0.75 – 1.31) | 1.04 (0.78 – 1.38) |
| High care density | 1.12 (0.85 – 1.48) | 1.14 (0.86 – 1.50) |
Analyses are adjusted for age, gender (except breast/prostate models), race, SEER site, cancer type, state buy-in, urban/rural residence, total number of visits, visit with PCP, and Charlson comorbidity index
DISCUSSION
Previous studies have found that the type of provider a patient sees is an important determinant of high quality survivorship care.[4–6, 17–20] The current findings suggest that the relationships between providers may also matter. Cancer survivors whose outpatient doctors tended to share more patients with one another—i.e., had higher care densities—had significantly lower odds of hospitalization, higher odds of a diabetic eye exam, and lower costs of care compared to survivors whose doctors shared fewer patients with one another.
Though exploratory in nature, the current findings are supported by previous research demonstrating that doctors who share more patients with one another are more likely to know, seek advice, and receive referrals from one another.[21] This may reflect increased ability of these doctors to coordinate care due to formal institutional arrangements such as working in the same practice or sharing an electronic medical record, or informal mechanisms such as the ability to call one another. At the patient level, this may indicate that patients whose doctors share more patients with one another may have an easier time coordinating care. Importantly, care density does not capture whether a pair of doctors actually communicate about a specific patient and care density has not been validated. In particular, care density should be examined with respect to patient- and doctor-reported experiences of care.
We found the most consistent results when including all outpatient providers in our care density calculation. When we limited the doctors to primary care providers and oncologists, care density remained associated with costs but was not associated with quality outcomes. Given their importance in cancer survivorship care, we had anticipated stronger findings with respect to quality when care density was limited to primary care providers and oncologists. Further research is necessary to determine among which providers patient-sharing is potentially important and for what specific quality outcomes (e.g. including endocrinologists when examining diabetes testing).
Patients with low care density tended to have higher costs and some lower quality metrics compared to patients with medium or high care density. However, contrary to our expectations, patients in the highest care density did not necessarily have better outcomes than those in the medium care density group. Given the lack of a stepwise gradient, our findings should be interpreted cautiously. Because care density was categorized based on the sample distribution, it is likely that the categories may reflect overlapping levels of physician patient-sharing and/or patient experiences of care. As noted above, comparing care density against patient report may provide insight into this finding.
It is possible that care density may be used by health care systems and insurers to help identify patients at risk for poor coordination. Interventions may facilitate communication between providers who share relatively few patients and/or direct patients towards providers who share a large number of patients. In addition, care density may be a tool to help identify how changes in health care delivery (e.g. through accountable care organizations) influence the connections among a patient’s providers.
Among patients who did saw less than 2 outpatient providers, we found comparatively lower odds of attaining quality metrics and higher costs after adjustment. On average, these patients appear to be falling through the cracks in the outpatient health care system as indicated by their lower outpatient care costs and lower rates of primary care provider visits. Given that they were more likely to be black and have state buy-in compared to patients with visits to 2 different providers, these findings may reflect and contribute to disparities in cancer survivorship care. Additional work is needed to explore why some patients have less than 2 providers and among those who saw one provider, whether the specialty of the provider is associated with costs and quality. Interventions that target this group of patients to ensure that they are receiving appropriate follow-up may be warranted.
This study has multiple limitations. First, care density is a novel measure of coordination that has not been validated. Second, we had a small sample size for some quality of care and avoidable outcome measures that may have limited our ability to see clinically meaningful associations. Third, we are unable to generalize to younger populations and those in managed care. Fourth, there is the potential for misclassification bias with claims data in the attribution of diseases and/or types of providers who are involved in care. Because we would not expect the misclassification to be differential between levels of care density, this is less likely to affect our main results.
In summary, patient-sharing may reflect important aspects of provider relationships that help enable coordinated care for cancer survivors. Correspondingly, we find that cancer survivors whose outpatient doctors share more patients with one another tend to have lower rates of hospitalization and lower costs of care. Further work is needed to explore the properties of care density as a potential metric of care coordination among cancer survivors and examine whether care density may be employed to target those at risk of poor coordination.
Acknowledgments
Financial Support: This was supported by the National Cancer Institute (R01CA149616 and K07 CA151910).
We would like to thank Eric Roberts and Klaus Lemke.
Footnotes
Conflicts of interest: None
References
- 1.Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Translation. Washington, DC: National Academy Press; 2006. [Google Scholar]
- 2.Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care Patterns in Medicare and Their Implications for Pay for Performance. N Engl J Med. 2007;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 3.Pollack LA, Adamache W, Ryerson AB, Eheman CR, Richardson LC. Care of long-term cancer survivors. Cancer. 2009;115(22):5284–5295. doi: 10.1002/cncr.24624. [DOI] [PubMed] [Google Scholar]
- 4.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073–9. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 5.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24:469–74. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder CF, Frick KD, Herbert RJ, et al. Preventive care in prostate cancer patients: following diagnosisna dn for five-year survivors. J Cancer Surviv. 2011:283–91. doi: 10.1007/s11764-011-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald K, Sundaram V, Bravata D, et al. Closing the quality gap: A critical analysis of quality improvement strategies. Volume 7 - Care Coordination. Rockville, MD: AHRQ; 2007. [PubMed] [Google Scholar]
- 8.Bodenheimer T. Coordinating care--a perilious journey through the health care system. N Engl J Med. 2008;358:1064–1071. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 9.National Priorities Partnership. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. National Quality Forum; Washington, DC: 2008. [Google Scholar]
- 10.Rittenhouse DR, Shortell SM, Fisher E. Primarry Care and Accountable Care--Two Essential Elements of Delivery-System Reform. N Engl J Med. 2009;361:2301–2303. doi: 10.1056/NEJMp0909327. [DOI] [PubMed] [Google Scholar]
- 11.Miller HD. From Volume to Value: Better Ways to Pay for Health Care. Health Aff. 2009;28:1418–1428. doi: 10.1377/hlthaff.28.5.1418. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ES, McClellan MB, Bertko J, et al. Fostering Accountable Health Care: Moving Forward In Medicare. Health Aff. 2009;28(2):w219–w231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal MB. Beyond Pay for Performance--Emerging Models of Provider-Payment Reform. N Engl J Med. 2008;359:1197–1200. doi: 10.1056/NEJMp0804658. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. Cancer Facts & Figures 2013. American Cancer Society; Atlanta, GA: 2013. [Google Scholar]
- 16.Rowland JH, Mariotto A, Alfano CM. Cancer survivors--United States, 2007. MMWR. 2011;60:269–72. [PubMed] [Google Scholar]
- 17.Earle CC, Burstein JJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 18.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712–9. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 19.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Trends in follow-up and preventive care for colorectal cancer survivors. J Gen Intern Med. 2008;23:254–9. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder CF, Frick KD, Kantsiper ME, et al. Prevention, screening, and surveillance care for breast cancer surivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27:1054–61. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping Physician Networks with Self-Reported and Administrative Data. Health Serv Res. 2011;46:1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: a netework analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–65. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bynum JP, Ross JS. A Measure of Care Coordination? J Gen Intern Med. 2013;28 (3):336–8. doi: 10.1007/s11606-012-2269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder CF, Frick KD, Herbert RJ, et al. Quality of Care for Comorbid Conditions During the Transition to Survivorship: Differences Between Cancer Survivors and Noncancer Controls. J Clin Oncol. 2013;31(9):1140–1148. doi: 10.1200/JCO.2012.43.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asch SM, Sloss EM, Hogan C, Brook RJ, Kravitz RL. Measuring underuse of necessary care among elderly Medicare beneficiaries using inpatient and outpatient claims. JAMA. 2000;284:2325–33. doi: 10.1001/jama.284.18.2325. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Alex KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Klabunde CN, Potosky AL, Legler JM, Warren JL. Deveopment of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 29.Starfield B, Shi L, Macinko J. Contribution of Primary Care to Health Systems and Health. Milbank Quarterly. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]