Abstract
Donor race matching (both recipients and donors belonging to the same race) might be a factor in outcomes of donor allogeneic hematopoietic cell transplantation (alloHCT). A total of 858 patients who underwent umbilical cord blood (UCB) (475 patients: 202 double UCB and 273 single UCB) or unrelated donor (URD) (383 patients) alloHCT between January 1995 to December 2010 were studied. Most patients were Caucasian (87%), followed by Asians (4%), African Americans (3%), Hispanics (3%), mixed race (3%), and American Indians (<1%). Caucasians comprised 88% of the donor grafts; Caucasians are the most common race of the donor grafts for all races except for Asians. As a result, donor race matching was significantly higher in Caucasian recipients than ethnic minorities (95% vs. 47%, p<0.01). Donor race matching did not affect non-relapse mortality, relapse, acute or chronic graft-versus-host disease or overall survival. Acknowledging the limitations of this study (mainly, self-reported race information and small number of ethnic minorities), at present, there is no data supporting that donor race should be considered a factor in donor selection.
Keywords: Race, donor, minority, ethnicity, Caucasians, allogeneic hematopoietic cell transplantation
Introduction
The effect of ethnicity/race of recipients on the outcomes of allogeneic hematopoietic cell transplantation (alloHCT) has been evaluated since the 1980s [1-7]. Most studies showed that Caucasians had favorable survival over African American (AA) patients and Hispanics [1-3,5,6]. In addition to socio-economic status, an increased rate of graft-versus-host disease (GVHD) and treatment-related mortality were suggested causes of the worse outcome for these minorities [2,3]. Some risks might be explained by polymorphisms in cytokine genes, including tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-6 and -10, and interferon-gamma [8]. In addition, minor histocompatibility antigen (mHA) mismatch may induce GVHD [9], modulate the graft-versus-tumor effect [10-12], and lower overall survival (OS).[13] mHAs differences have been noted among various ethnic groups [14]. These antigenic targets are not routinely tested and may be responsible for differences in observed outcomes for different races. Therefore, we hypothesized that race and ethnic match status between recipient and donor may impact outcomes of alloHCT, even in patients with comparable Human Leukocyte Antigen (HLA) match status. In most alloHCT studies, donor race and donor–recipient race match have not been evaluated. Only one study evaluated the effect of donor race match status on various outcomes after unrelated donor (URD) transplantation [15].
In this study, we evaluated donor race/ethnic match and its impact on the major clinical outcomes of alloHCT from URD and umbilical cord blood (UCB) donors.
Material and Methods
A total of 858 patients who underwent UCB (475 patients: 202 double UCB and 273 single UCB) or URD (383 patients) HCT at the University of Minnesota between January 1995 to December 2010 were studied in our primary analysis. Race/ ethnicity information on recipients and donors was obtained from their self-reported data at the time of transplantation. Donor race was obtained from the National Marrow Donor Program (NMDP) and other reporting UCB banks. Only double UCB transplantations in which race information was known for both units were included. The primary study cohort does not include double UCB transplants with one donor unit matched and one mismatched with recipient race. However, in a supplemental analysis, if the race of the dominant unit was known in double UCB, this double UCB was included in the supplemental analysis even the race of other unit was unknown and analyzed based upon matching of the dominant unit.
Additionally, there were 470 alloHCT (34% of all URD or UCB HCT) in which the donor race was not reported and these were excluded from the analysis. A sensitivity analysis comparing this cohort to the primary study cohort was performed.
Patients received conditioning regimens, GVHD prophylaxis and supportive care per specific HCT protocols which varied over the period of study and have been described previously.[16-23] Reduced intensity conditioning (RIC) consisted of fludarabine (150 mg/m2), low dose total body irradiation (200 cGy) (TBI), and cyclophosphamide (50 mg/kg). Myeloablative conditioning regimens consisted of TBI (13.2 Gy) and cyclophosphamide (120 mg/kg) with fludarabine (75 mg/m2) in the case of UCB grafts.
UCB and URDs are considered when there is no HLA-matched sibling available and depending on urgency of transplant (e.g., concern for early relapse) or available study protocols, UCB was prioritized over URD. Using UCB selection criteria that we have previously published [20,24], UCB grafts were matched at 4–6 of 6 HLA-A, -B (Ag level) and -DRB1 (allele level) to the recipient, and in patients receiving two UCB units, to each other. In addition to the HLA matching, cell dose in the cryopreserved UCB unit was important in donor selection.
All transplant protocols were approved by the University of Minnesota Institutional Review Board. All patients or their legal guardians provided written informed consent for the transplantation procedure including collection of long-term prospective outcome data. The Institutional Review Board also reviewed and approved this specific study.
Definitions
Malignant diseases were stratified as high risk and standard risk: standard risk includes acute leukemia in complete remission (CR) 1 or 2 and chronic myelogenous leukemia (CML) in chronic phase (CML-cp). High risk included all the remaining malignant diseases. Non-Malignant diseases include bone marrow failure, including aplastic anemia, Fanconi anemia and immune deficiency syndromes. HLA match status for URD was defined by refined HLA matching criteria (well-matched cases had either no identified HLA mismatch and informative data at 4 loci or allele matching at HLA-A, -B, and -DRB1, partially matched pairs had a defined, single-locus mismatch and/or missing HLA data, and mismatched cases had ≥ 2 allele or antigen mismatches) [25]. In vitro T-cell depletion (e.g., CD34+ positive selection), or in vivo T-cell depletion, including alemtuzumab or antithymocyte globulin use in conditioning regimen was considered as T-cell depletion. Primary graft failure is defined as when a patient did not achieve absolute neutrophil count (ANC) > 0.5 × 109/L for three consecutive days and <5% cellularity in bone marrow by day 42 or achieve ANC engraftment with < 10% donor chimerism in bone marrow by day 42.
The dominant double UCB unit was defined as the unit with >70% bone marrow chimerism at day 100. If the patient died prior to day 100, but had a unit with >70% bone marrow chimerism at day 21 or any later measure, that unit was considered the dominant unit.
Statistical analysis
The effect of recipient-donor race matching for alloHCT was evaluated for the following outcomes: overall survival (OS), disease free survival (DFS), relapse, non-relapse mortality (NRM), acute GVHD (aGVHD), chronic GVHD (cGVHD), and graft failure. Multivariable regression models were used to analyze the effect of recipient-donor race matching on outcomes. Unless otherwise stated in results, all models included factors for race match status, recipient race (Caucasians vs. all ethnic minorities), donor type and HLA match (URD well matched, URD partially matched, URD mismatched, UCB 5/6 and 6/6, or UCB 4/6), disease category (malignant high risk, malignant standard risk, or non-malignant), recipient cytomegalovirus (CMV)serostatus, and conditioning regimen intensity (myeloablative or RIC). Cox regression [26] was used for analysis of OS and DFS. Fine and Gray regression [27] was used for NRM (competing risk = relapse), and relapse, graft failure, aGVHD, and cGVHD (competing risk = death). OS and DFS were censored at 5 years after HCT. Relapse, non-relapse mortality (NRM), and cGVHD were censored at 3 years after HCT, aGVHD was censored at 100 days after HCT, and graft failure was censored at 42 days after HCT. The DFS, relapse and NRM models excluded non-malignant patients. Proportionality of hazards was checked using a log time-dependent effect; no departures were detected for the primary race match variable. Among other covariates, only the URD HLA mismatched group exhibited non-proportionality with a greater mortality hazard ratio in the first six months relative to later times.
Logistic regression was used to estimate the proportion of dominant units that came from a matched race donor after double UCB transplantation, controlling for differences in HLA and total nucleated cell count between the two units. The Kaplan-Meier method was used to estimate OS and the log-rank test was used to compare groups.
Results
Ethnicity and Race Match Status
The majority of study patients were Caucasians (87%) (Table 1). The remaining patients (13%) were most commonly Asians (4%), followed by African Americans (AA) (3%), Hispanics (3%), Mixed race patients (3%), and American Indians (<1%). Caucasians were much more likely to have a race-matched donor than ethnic minorities (91% vs. 33%, p<0.01).
Table 1.
Patient and transplant characteristics of race matched and mismatched donors
| Donor race/ethnicity matched (N=713) N (%) |
Donor race/ethnicity mismatched (N=145) N (%) |
P | |
|---|---|---|---|
| Recipient race | < .01 | ||
| Caucasian | 675 (95) | 68 (47) | |
| Race/Ethnic minority | 38 (5) | 77 (53) | |
| African American | 9 (1) | 19 (13) | |
| Hispanic | 9 (1) | 16 (11) | |
| American Indian | 0 (0) | 5 (3) | |
| Asian | 17 (2) | 18 (12) | |
| Mixed | 3 (0) | 19 (13) | |
| Sex | .37 | ||
| Male | 427 (60) | 81 (56) | |
| Female | 286 (40) | 64 (44) | |
| Age | < .01 | ||
| < 18 years | 381 (53) | 95 (65) | |
| ≥ 18 | 332 (47) | 50 (34) | |
| Karnofsky/Lansky Score | .99 | ||
| < 80 | 41 (6) | 7 (5) | |
| 80 | 89 (12) | 19 (13) | |
| 90 | 258 (36) | 51 (35) | |
| 100 | 256 (36) | 53 (37) | |
| Unknown | 69 (10) | 15 (10) | |
| Diagnosis | .02 | ||
| Malignant | 464 (65) | 79 (54) | |
| Non-malignant | 249 (35) | 66 (45) | |
| Malignant Disease Risk | .79 | ||
| High Risk | 210 (45) | 37 (47) | |
| Standard Risk | 254 (55) | 42 (53) | |
| Malignant diagnosis category | .08 | ||
| AML, ALL, MDS | 302 (65) | 49 (62) | |
| Myeloproliferative, CML | 71 (15) | 16 (20) | |
| NHL, Hodgkins, CLL | 68 (15) | 6 (8) | |
| Other malignant disease | 23 (5) | 8 (10) | |
| Donor relation + HLA match2 | < .01 | ||
| URD well matched | 120 (17) | 19 (13) | |
| URD partially matched | 114 (16) | 15 (10) | |
| URD mismatched | 85 (12) | 29 (20) | |
| UCB 5/6 or 6/6 locus matched | 266 (37) | 39 (27) | |
| UCB 4/6 | 126 (18) | 43 (30) | |
| CMV serostatus | .02 | ||
| R+ | 321 (45) | 80 (55) | |
| R-/D+ | 67 (9) | 17 (12) | |
| R-/D- | 313 (44) | 45 (31) | |
| Unknown | 12 (2) | 3 (2) | |
| Transplant Year | .23 | ||
| 1995-1999 | 225 (32) | 46 (32) | |
| 2000-2004 | 225 (32) | 55 (38) | |
| 2005-2010 | 263 (37) | 44 (30) | |
| Conditioning Intensity | .02 | ||
| Myeloablative, TBI | 398 (56) | 86 (59) | |
| Myeloablative, no TBI | 119 (17) | 34 (23) | |
| RIC | 196 (27) | 25 (17) | |
| T-depletion/ATG/Campath | 290 (41) | 76 (52) | .01 |
Abbreviations: AML, acute myelogenous leukemia, ALL, acute lymphoblastic leukemia; CML, chronic myelogeneous leukemia; D, donor; NHL, non-Hodgkin’s lymphoma; R, recipient; TBI, total body irradiation; UCB, umbilical cord blood; URD, unrelated donor
Race-mismatched recipients were more likely to be an ethnic minority, HLA mismatched, under age 18, have a non-malignant disease, be CMV seropositive, and receive myeloablative conditioning and T-depletion (Table 1). No statistically significant associations were observed between race match status and recipient sex, malignant diagnosis category, Karnofsky/Lansky Score, or year of transplant.
Eighty-eight percent of all donors were Caucasians, followed by Hispanics (4%), Asians (3%), AAs (3%), and Mixed races (2%); American Indians were <1% (Table 2). Caucasians were the most frequent donors of single UCB (46%) and URD (44%) for ethnic minorities. Caucasians were the major donor group for every ethnic minority except Asians.
Table 2.
Donor and Recipient Race Pairs
| RECIPIENT RACE (N=858 patients) | Caucasian donor (units) | AA donor (units) | Hispanic donor (units) | AI donor (units) | Asian donor (units) | Mixed Race donor (units) |
|---|---|---|---|---|---|---|
| Caucasians (N=743, 87%) | 855 | 16 | 21 | 6 | 7 | 18 |
| AA (N=28, 3%) | 18 | 10 | 3 | 0 | 1 | 1 |
| Hispanic (N=25, 3%) | 16 | 1 | 9 | 1 | 2 | 0 |
| AI (N=5, <1%) | 4 | 0 | 1 | 0 | 0 | 0 |
| Asian (N=35, 4%) | 13 | 0 | 5 | 0 | 20 | 3 |
| Mixed race (N=22, 3%) | 22 | 2 | 1 | 0 | 1 | 3 |
| Total Donor Units (N=1060 units) | 928 (88%) | 29 (3%) | 40 (4%) | 7 (<1%) | 31 (3%) | 25 (2%) |
Total donor unit numbers are higher than total recipient numbers because of inclusion of 202 transplants using double UCB.
Abbreviations: AA, African Americans; AI, American Indians.
Effects of Race Matching on Overall Survival
The median follow-up interval among survivors was 5 years; minimum was 1 year. We were primarily interested in the association of a binary factor, donor: recipient race match or mismatch, on HCT outcomes including OS. Race mismatched recipients had a hazard ratio (HR) of 1.0 (95% confidence interval (CI): 0.7-1.3, p=0.86) relative to race matched recipients for overall mortality in a multivariable analysis (MVA) controlled for ethnicity group, donor type and HLA match status, disease risk, recipient CMV serostatus, and conditioning intensity (Table 3). The observed absence of any effect of race matching was apparent in the nearly identical 5-year OS estimates of 45% and 46% for matched and mismatched recipients, respectively (Figure 1).Survival was similar for matched and mismatched recipients in all combinations of donor and HLA categories (Table 4). We formally checked for an interaction between HLA and race matching in MVA, but the interaction effect was not significant for any outcome. We also examined any possible race match effect in a more homogeneous, but smaller patient cohort (n=243) of Caucasian recipients with a malignant diagnosis who were URD HLA well-matched or UCB 5/6 or 6/6 HLA matched. Adjusting for donor type (UCB or URD), disease risk (high or standard), and age (<18 or ≥ 18), race mismatched recipients had a hazard ratio of 0.8 (95% CI: 0.3-1.8, p=0.56) for overall mortality.
Table 3.
Multivariable models for four major outcomes
| Overall Survival | Disease Free Survival | Relapse | Non-relapse mortality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Factor | Hazard Ratio |
Lower 95% CL |
Upper 95% CL |
P | Hazard Ratio |
Lower 95% CL |
Upper 95% CL |
P | Hazard Ratio |
Lower 95% CL |
Upper 95% CL |
P | Hazard Ratio |
Lower 95% CL |
Upper 95% CL |
P |
| Race match | ||||||||||||||||
| Matched | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| Mismatched | 1.0 | 0.7 | 1.3 | .86 | 0.9 | 0.6 | 1.4 | .77 | 0.8 | 0.4 | 1.5 | .48 | 1.1 | 0.7 | 1.8 | .68 |
|
| ||||||||||||||||
| Recipient race | ||||||||||||||||
| Caucasian | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| Ethnic minority | 1.0 | 0.7 | 1.5 | .84 | 1.1 | 0.7 | 1.7 | .74 | 1.6 | 0.8 | 3.3 | .23 | 0.8 | 0.4 | 1.5 | .51 |
|
| ||||||||||||||||
| Donor & HLA match | ||||||||||||||||
| URD well matched | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
| URD partially matched | 1.5 | 1.0 | 2.1 | .03 | 1.4 | 0.9 | 2.1 | .14 | 1.0 | 0.5 | 2.0 | .98 | 1.3 | 0.8 | 2.2 | .27 |
| URD mismatched | 1.7 | 1.2 | 2.4 | <.01 | 1.5 | 1.0 | 2.2 | .07 | 0.6 | 0.3 | 1.3 | .19 | 1.9 | 1.1 | 3.1 | .01 |
| UCB 5/6 or 6/6 | 0.9 | 0.7 | 1.2 | .50 | 1.0 | 0.7 | 1.4 | .82 | 1.6 | 0.9 | 2.6 | .12 | 0.6 | 0.3 | 1.0 | .03 |
| UCB 4/6 | 1.1 | 0.8 | 1.6 | .45 | 1.1 | 0.8 | 1.6 | .63 | 1.6 | 0.9 | 2.8 | .10 | 0.7 | 0.4 | 1.2 | .18 |
|
| ||||||||||||||||
| Disease Category | ||||||||||||||||
| Malignant, high risk | 2.4 | 1.9 | 3.2 | <.01 | 1.7 | 1.4 | 2.2 | <.01 | 2.0 | 1.4 | 2.7 | <.01 | 1.1 | 0.8 | 1.5 | .67 |
| Malignant, standard risk | 1.6 | 1.3 | 2.1 | <.01 | 1.0 | 1.0 | 1.0 | |||||||||
| Non-malignant | 1.0 | excluded | ||||||||||||||
|
| ||||||||||||||||
| CMV | ||||||||||||||||
| R+ | 1.1 | 0.9 | 1.4 | .21 | 1.1 | 0.9 | 1.3 | .50 | 0.9 | 0.7 | 1.2 | .49 | 1.1 | 0.8 | 1.6 | .41 |
| R- | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
|
| ||||||||||||||||
| Conditioning | ||||||||||||||||
| Myeloablative | 1.0 | 0.8 | 1.2 | .90 | 0.8 | 0.6 | 1.0 | .02 | 0.6 | 0.5 | 0.9 | <.01 | 1.1 | 0.7 | 1.6 | .75 |
| RIC | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||||||
Abbreviations: URD, unrelated donor; UCB, umbilical cord blood; RIC, reduced intensity conditioning
Figure 1.
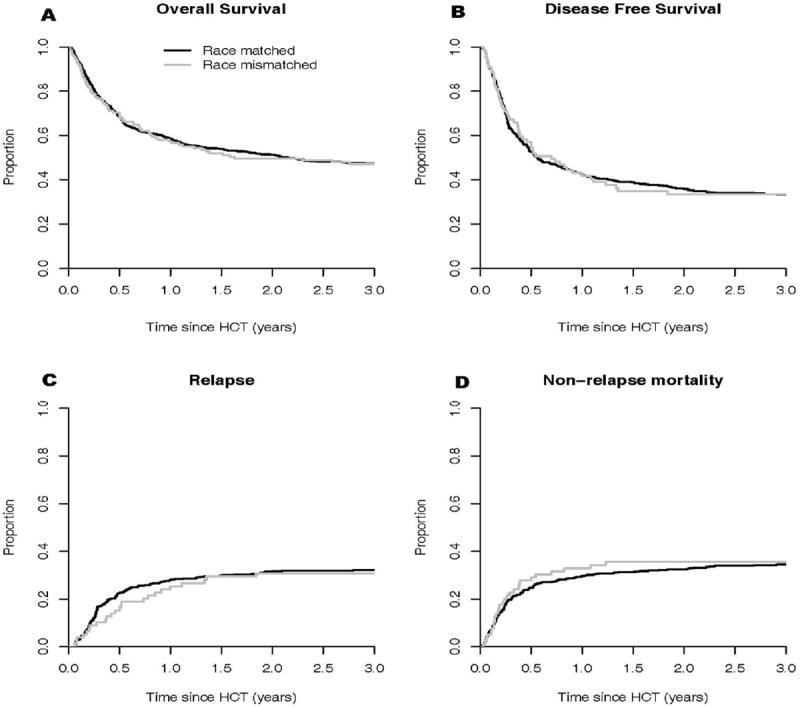
(A) Overall survival, (B) disease-free survival, (C) relapse and (D) non-relapse mortality. There was no difference between race matched and mismatched patients regarding OS, DFS, relapse and NRM.
Table 4.
5-year overall survival estimates by race and HLA match
| Donor Type and HLA match (N) | Donor race matched 5-year OS (95% CI) | Donor race mismatched 5-year OS (95% CI) | P |
|---|---|---|---|
| URD well matched (139) | 52% (43-61) | 68% (43-84) | .30 |
| URD partially matched (129) | 40% (31-49) | 27% (8-50) | .18 |
| URD mismatched (114) | 33% (23-43) | 34% (18-51) | .81 |
| UCB 5/6 or 6/6 (305) | 53% (47-59) | 54% (35-70) | .86 |
| UCB 4/6 (169) | 37% (28-45) | 43% (28-57) | .42 |
| Total (858) | 45% (41-49) | 46% (38-54) | .94 |
Survival estimates are not adjusted for any other factors.
Six additional subgroup multivariate analyses were performed and in none did race match have a statistically significant influence on survival. These included: all Caucasian recipients (n=743, p=0.64), minority recipients (n=115, p=0.49), myeloablative recipients (n=637, p=0.75), RIC recipients (n=221, p=0.18), URD (n=383, p=0.83), or UCB (n=475, p=0.89).
Effects of Race Matching on other outcomes
We also examined the impact of race matching on other transplant outcomes and similarly showed no significant effects: HR for cGVHD in race-mismatched recipients was 1.2 (95% CI: 0.7-2.0, p=0.54); acute GVHD HR 1.1 (95% CI: 0.8-1.5, p=0.71); and graft failure HR 0.7 (95% CI: 0.3-1.5, p=0.35). In MVA for DFS, relapse, and NRM models including only recipients with a malignant disease (n=543), the HR for DFS in race mismatched recipients was 0.9 (95% CI: 0.6-1.4, p=0.77); for relapse HR 0.8 (95% CI: 0.4-1.5, p=0.48); and for NRM HR 1.1 (95% CI: 0.7-1.8, p=0.68).
Effect of Race Matching for the Dominant Unit in Double UCB
We investigated whether race matching might influence which unit in double UCB grafting would become the dominant hematopoietic unit over time. Out of 37 double UCB transplants in which exactly one unit was from the same race as the recipient, 22 matched race units (59%) became the dominant unit after transplantation. The predicted value of this proportion when the two units are balanced in terms of HLA match degree and total nucleated cell count is 58% (95% CI: 41-73%, p=0.42), suggesting that race matching had no favorable influence on this setting of competitive double unit UCB engraftment.
Supplemental Analysis including race category only for the dominant UCB unit
We added to our study an additional subset of double UCB transplantations (n=92) in whom the race of dominant unit was known. In this larger study group (n=950) the results were very similar to the primary analysis; for all seven outcomes (OS, DFS, relapse, NRM, aGVHD, cGVHD, graft failure), HRs were all near 1.0 (0.9, 0.8, 0.8, 0.9, 1.0, 1.3, 0.6, respectively) and the p-values for all analyses were >0.2.
Unknown Donor Races
There were 470 alloHCTs in which donor race data was not reported. Characteristics of patients with known and unknown donor races were compared and considering thatdouble UCB recipients had a greater likelihood of having at least one unknown donor race. Comparing HCTs with donor race known vs. unknown, there was no statistically significant differences in recipient sex, age, malignant or benign disease, disease risk, Karnofsky/Lansky performance score, CMV serostatus, 5-year OS, or 5-year DFS. For URD and single UCB transplantations, unknown donor race was more frequent over the last 6 years (2005-2010) compared to earlier era (1995-2004) (35% vs. 23%, p<0.01). For double UCB, unknown donor race was more frequent in ethnic minority recipients compared to Caucasian recipients (71% vs. 51%, p<0.01), and in patients with at least one 4/6 HLA match (62% vs. 46%, p<0.01). Although the high degree of unreported donor races is certainly relevant to this study, the clinical characteristics of this cohort were mostly similar to those with known donor race.
Discussion
In this large analysis of URD and UCB HCTs, we found that race match status did not have a significant effect on outcomes of either URD or UCB transplantations. Donor race match status was previously evaluated for its impact on outcome in only one NMDP study [15]. That study similarly identified no effect of donor race match status on clinical outcomes, but did not report the number of donor mismatch HCTs performed in either Caucasians or ethnic minorities and included no UCB HCTs. Although it might be expected that minor histocompatibility antigens (as potential tumor-associated target antigens) are associated with a potent graft-versus-tumor effect and mHAs difference might be more apparent between different racial or minority groups, we found no differences between race-matched and race-mismatched HCT in risks of relapse or DFS [10-12,14,28]. Importantly, after adjusting for cell dose and HLA match, donor race match did not influence the predominance of UCB units in double UCB transplantation [29].
This analysis showed that a donor selection strategy designed to choose the best HLA matched URD and optimally sized UCB units resulted in more frequent race/ethnic mismatch between donor and recipient in racial minorities in both URD and UCB transplantations, at least in this predominantly Caucasian donor and cord bank pool.
Race information on patients and donors was obtained from self-reported data. It is obvious that self-reported race data may not reflect individuals’ actual genetic race. Therefore our study has this limitation, like other published studies evaluating the importance of race. Moreover, self-reported race/ethnicity allocation includes large genetic and ethnic variation within the same race/ethnicity and represents a crude surrogate for ancestry. Our study also had a relatively small number of ethnic minorities, and thus our results reflect a patient population that is mostly Caucasian. A small number of alloHCT in each ethnic minority prevents us from evaluating whether there are differences between each race (e.g., the effects of race mismatch in Asians are the same with that as in African Americans). Donor race information was missing in a large number of alloHCT, another consequence of using self-reported race data. However, adjusting as much as possible for the clinical factors which could influence outcomes, we did not find any effect of race matching on HCT outcomes. Given the limitations, our study starts to answer the important question of whether a race mismatch is detrimental in alloHCT. Clearly, evaluating the impact of donor race on alloHCT outcomes in a larger database by using genetic markers may offer further insights. On the other hand, this study is significant as being the first study looking at the impacts of donor race and race matching status on the outcomes of alloHCT. With our results, at present there is no data to show that using a donor from another race/ethnicity is deleterious, and thus ethnic group matching should not be contributory to donor selection for URD or UCB transplantation.
Acknowledgments
This work was supported in part by NIH P30 CA77598 utilizing the Biostatistics and Bioinformatics shared resource at the University of Minnesota Masonic Cancer Center.
Footnotes
Conflict of Interest: Authors have no conflict of interest to disclose related to this subject
References
- 1.Klingemann HG, Deeg HJ, Self S, Thomas ED, Storb R. Is race a risk factor for allogeneic marrow transplantation? Bone marrow transplantation. 1986;1:87–94. [PubMed] [Google Scholar]
- 2.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Easaw SJ, Lake DE, Beer M, Seiter K, Feldman EJ, Ahmed T. Graft-versus-host disease. Possible higher risk for African American patients. Cancer. 1996;78:1492–1497. [PubMed] [Google Scholar]
- 4.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3754–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Loberiza FR, Jr, Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–1416. doi: 10.1182/blood-2004-06-2385. [DOI] [PubMed] [Google Scholar]
- 6.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of Race/Ethnicity and Survival after Single Umbilical Cord Blood Transplantation for Adults and Children with Leukemia and Myelodysplastic Syndromes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;18:903–912. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone marrow transplantation. 2011 doi: 10.1038/bmt.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI Minority Workshops: Part IV. Human Immunology. 2004;65:1413–1419. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- 9.Toubai T, Tawara I, Sun Y, et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radio-sensitive host hematopoietic-derived antigen presenting cells. Blood. 2011;119:3844–3853. doi: 10.1182/blood-2011-10-384057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell SR, Murata M, Bryant S, Warren EH. Minor histocompatibility antigens - Targets of graft versus leukemia responses. International Journal of Hematology. 2002;76:155–161. doi: 10.1007/BF03165108. [DOI] [PubMed] [Google Scholar]
- 11.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunology and Cell Biology. 2011;89:396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen ME, Kornblit B, Larsen MV, et al. Degree of predicted minor histocompatibility antigen mismatch correlates with poorer clinical outcomes in nonmyeloablative allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:1370–1381. doi: 10.1016/j.bbmt.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Spierings E, Hendriks M, Absi L, et al. Phenotype frequencies of autosomal minor histocompatibility antigens display significant differences among populations. Plos Genetics. 2007;3:1108–1119. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 17.Oran B, Dolan M, Cao Q, Brunstein C, Warlick E, Weisdorf D. Monosomal karyotype provides better prognostic prediction after allogeneic stem cell transplantation in patients with acute myelogenous leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:356–364. doi: 10.1016/j.bbmt.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Warlick ED, Tomblyn M, Cao Q, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1025–1032. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachanova V, Brunstein CG, Burns LJ, et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone marrow transplantation. 2009;43:237–244. doi: 10.1038/bmt.2008.313. [DOI] [PubMed] [Google Scholar]
- 20.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1327–1334. doi: 10.1016/j.bbmt.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 25.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables. J Royal Stat Soc Bulletin. 1972;34:187–220. [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 28.Spierings E, Hendriks M, Absi L, et al. Phenotype frequencies of autosomal minor histocompatibility antigens display significant differences among ethnic populations. Tissue Antigens. 2007;69:374–375. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez P, Wagner JE, Defor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone marrow transplantation. 2011;47:799–803. doi: 10.1038/bmt.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]


