Abstract
Introduction
Uptake of glutamate in the hippocampus by specialized transporters appears to be important for the prevention of glutamate-induced neurotoxicity. However, the role of these transporters in synaptic plasticity and learning is still unclear. We examined the expression pattern of glutamate transporters at different stages of spatial learning using a one-day (three blocks) version of the Morris Water Maze.
Methods
Male rats (Sprague Dawley, 3 months old) were divided into three groups (learner, swim control, or naïve control) and animals were sacrificed after the first, second, or third block of training. The hippocampi were immediately extracted and flash frozen for RNA analysis. Real time polymerase chain reaction was employed to examine the expression of glutamate transporter 1 (Glt-1), Glt1b, glutamate-aspartate transporter (GLAST) and excitatory amino acid carrier-1 (EAAC1) in whole hippocampi.
Results
EAAC1 and GLAST RNA were down-regulated in the learner and swimmer groups (compared to naïve) after the first two blocks of training during the one-day protocol but EAAC1 returned to control levels by the end of the third block. GLAST levels were upregulated by the third block of training. Glt-1b expression was downregulated during the second block of training but returned to control by the third block.
Conclusions
The observed decreases in glutamate transporter expression may be important during the early stages of spatial learning as a possible mechanism to enhance glutamatergic availability during critical stages of learning. However, similar decreases in glutamate transporter expression in both the learner and swimmer groups indicate that the observed differences may be task-induced. Additional experiments are currently underway to examine this possibility.
Keywords: Hippocampus, Glutamate Transporters, GLAST, EAAC1, Glt-1
INTRODUCTION
Glutamate is the main excitatory neurotransmitter in the hippocampus. The main mechanism for termination of glutamatergic transmission is uptake of glutamate by specialized transporters localized mainly to adjacent astrocytes. Within the hippocampus, at least three of the five glutamate transporters appear to be localized within the synapses and immediate peri-synaptic areas: glutamate transporter 1 (Glt-1); excitatory amino acid carrier-1 (EAAC1); and glutamate-aspartate transporter (GLAST).1 The Glt-1 is the main transporter for glutamate clearance in the brain. It has two identified splice variants: Glt-1, expressed by both neurons and astrocytes; and Glt-1b, expressed by astrocytes.2,3 The EAAC1 is mainly localized to neurons,4,5 whereas GLAST is found mainly in astrocytes.6,7 All three, GLAST, EAAC1 and Glt-1, are conserved among several species,8 and are important for glutamate homeostasis.
Glutamate uptake by its transporters appears to be important for the prevention of glutamate-induced neurotoxicity.9 More recently, a possible role in synaptic plasticity has been suggested. Changes in glutamate uptake and differential translocation of EAAC1 to the plasma membrane have occurred in the hippocampus after long-term potentiation (LTP) induction and contextual fear conditioning.10 In addition, previous studies showed an increase in Glt1 expression after in vivo induction of LTP at the mossy fiber-cornus ammonius 3 (MF/CA3) area of the hippocampus in rats,11 suggesting a possible role of this transporter in MF/CA3 plasticity. Because plasticity is a necessary process for learning and memory, this study, using the Morris water maze task, investigates whether modulation of glutamate transporters in the hippocampus occurs during the different stages of spatial learning.
METHODS
Animals and Behavior
Male Sprague Dawley (approx. 3 mo. old, 350–400g) rats were obtained from the Ponce School of Medicine Animal Care Facility and housed in pairs on a 12 hour light-dark cycle. Food and water was provided ad libitum. To reduce stress-mediated effects on behavior, animals were handled for one week before training. Each rat was randomly assigned to a learner (swim with platform), swimmer (swim without platform) or naïve (don’t swim) group and trained in the water maze using a one-day protocol.12 On training day, learner rats had to find a hidden transparent platform submerged 3 cm from the surface of the water of a 6 ft diameter pool. The rats underwent one, two or three blocks of training with one hour rest between blocks. In each block of training the learner rats underwent four 60 second trials (or until platform reached) with the platform located at the fourth quadrant. If after 60 sec the rat did not find the platform, it was gently directed towards it and allowed to sit for 15 sec on the platform before being removed from the pool, towel-dried and allowed to rest for 60 sec. After the last training trial, an additional trial (probe trial) was performed in which the rats were placed in the pool and allowed to swim for 60 sec without the platform. Swimmer rats swam without the platform for the duration of each trial (60 sec). Rats in the naïve group were not exposed to the water maze and remained in their home cages for the duration of experiment. Each trial was recorded using a ceiling-mounted video camera connected to a computerized tracking/imaging analyzer system (HVS Image, Hampton, UK; Watermaze Software, Edinburgh, UK).
At the end of the last trial each rat was deeply anesthetized with sodium Nembutal solution (100 mg/kg; ip) and decapitated. The brain was removed and the hippocampi dissected out whole and flash frozen for further processing.
Tissue Processing and Real Time Polymerase Chain Reaction
RNA was isolated with Trizol Reagent as described by the manufacturer (Invitrogen, Carlsbad, CA). Then 1 μg of RNA from each sample was reversed transcribed using iScript cDNA Synthesis Kit (Bio-Rad Labs, Hercules, CA). A total of 3.75 ng of cDNA and 200 nM of primer were used for the real time (RT) polymerase chain reaction (PCR) using iQ SYBR Green Supermix (Bio-Rad Labs, Hercules, CA). The PCR and detection were accomplished in a iCycler iQ Multicolor Real-Time PCR Detection System (Bio-Rad Labs, Hercules, CA). A dissociation curve protocol was performed at the end of the amplification following typical procedures. All amplifications were run in triplicate with a no-template control for each primer pair. The target gene expression levels was computed by comparative CT method (against hipoxantine-guanine phosphoribosyl transferase[HPRT]) and the data transformed by the formula 2−ΔCt.
Primer Design
The sequence used for the HPRT gene primers were those described by Pennequin, et al.13 Each of the reminding primer was designed after the known mRNA sequences published at the Nucleotide tool of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Primers for Glt1-a, Glt1-b, GLAST and EAAC1 were designed using BioEdit software (Ibis Bioscience, Carlsbad, CA) and care was used to prevent homology areas between the different glutamate transporter genes. The sequences and expected product sizes were as follows: (1) Glt1-a forward (F) 5′-TGTTGAGGAAGAACCTTGGAAACGTG-3′, reverse (R) 5′-AGCATCTTTTACCATAAGATACGCTGG-3′; 96 bps (2) GLT1-b F 5′-CAATTTTCTTCACTCT TTGGGCCAAG-3′, R 5′-TTGGTCTACATATTTTTCTGACCCTTCC-3′; 105 bps (3) GLAST F 5′-GACATTTCTCTCTAGGGGCAGGCTG-3′, R 5′-GGTTTGGGAAGAGTACCCAAGAAGG-3′; 187 bps (4) EAAC1 F 5′-AACTGTTAGGATTAGGTCATGGGTC TGG-3′, R 5′-AATTGGGCACTAGTTGACTGGGTGG-3; 197 bps. (Annealing temperature = 61°C). Each primer was used for RT-PCR of hippocampal tissue to confirm the size of the products by running the resulting reaction mix on an agarose gel.
Statistics
Statistical analysis was performed using SigmaStat Software (Point Richmond, CA). One-Way ANOVA, One-Way Repeated measures ANOVA or t-tests were performed on particular data as indicated in the figure legends. Holm-Sidak test was performed as post-hoc analysis for each significantly different ANOVA.
RESULTS
Rats Learned the Location of the Platform During Training in the Morris Water Maze
With each block of training, rats in the learner group took progressively less time to reach the target platform (fig. 1A). In addition, by the end of block 2, learner rats preferred the target quadrant significantly more than swim control rats, whereas swim control rats maintained the same preference across training.
Fig 1.
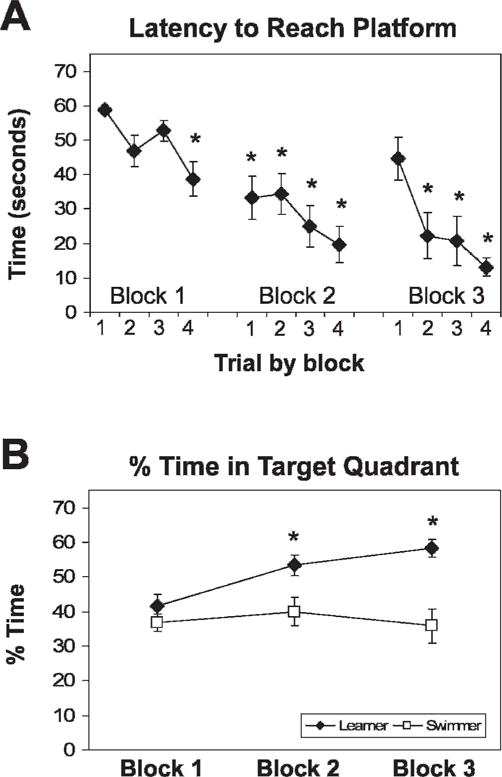
Rats learned the position of the platform. The latency to platform of learner rats progressively decreased within blocks and with each trial of training; *P>.001 vs B1T1 (A). By block 2, learner rats spent more time in the platform quadrant than the swimmer rats and than in other quadrants *P>.05 vs B1 and vs swimmer (B) during the probe trial (no platform trial)
EAAC1 and GLAST Expression Were Downregulated During Learning of the Morris Water Maze Task
Glutamate transporter expression of the learner group was compared to that of two controls, naïve and swim control. GLAST expression was decreased compared to naïve after the first and second block of training when memory acquisition is presumably taking place (Fig. 2A). A similar pattern was observed for EAAC1 (Fig. 2B), in which expression was decreased by the end of the first and second block. However, this down-regulation was also observed in the swim control group for both EAAC1 and GLAST, suggesting that the differences were task related. The levels of EAAC1 and GLAST RNA returned to control by the end of the third block.
Fig 2.
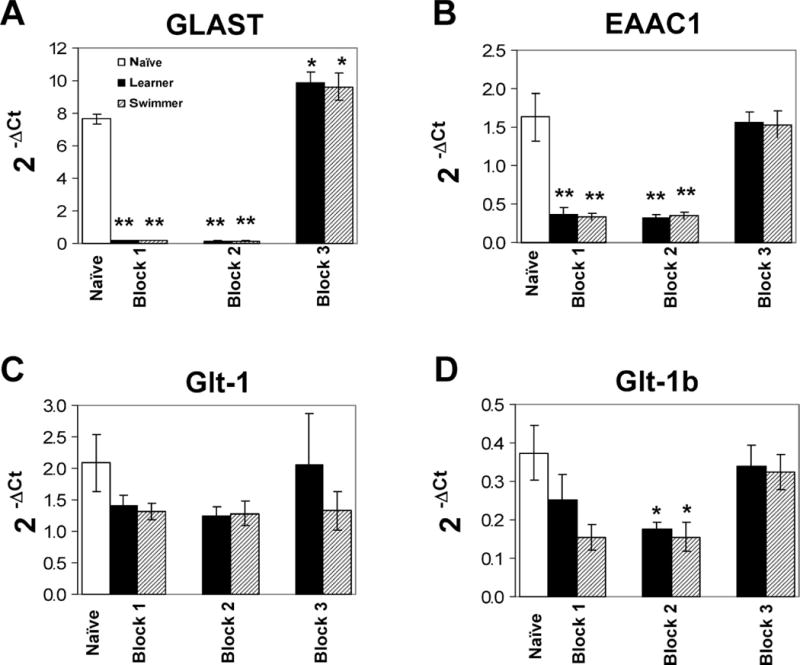
Glutamate transporter expression after water maze learning. RNA expression of GLAST (A) and EAAC1 (B) was down-regulated in learner and swimmer rats compared to naïve after the first and second block of training. GLAST expression was upregulated by the third and last block. Glt-1 expression did not differ between groups (C) whereas Glt-1b was down-regulated by the second block but returned to control levels by the third block (D). ΔCt = cycle threshold of transporter gene – cycle threshold of HPRT housekeeping gene. *P>.05 vs naïve **P>.001 vs naïve
Glt-1b Expression Was Downregulated During Learning
Glt-1b expression was also decreased compared to naïve control in both learner and swim control groups after the second block of training (Fig. 2D). Its expression returned to control levels by the third block of training. No differences were observed in Glt-1 expression levels (Fig. 2C).
DISCUSSION
Our results indicate a transient decrease in EAAC1 and GLAST glutamate transporter RNA expression during the early stages of a spatial learning task (20 min and 80 min after initiation of training). This decrease returned to baseline levels by the end of training (140 min). The transient decrease in expression suggests that regulation of transporter expression may be important during the acquisition phase of learning. By decreasing the expression of the glutamate transporters at early stages, more glutamate becomes available to activate receptors such as NMDA and mGluRs, known to be necessary for the early stages of hippocampal LTP.14–17 In fact, similar transient changes in expression of the NR1 subunit of the NMDA receptor were observed in the hippocampus 30 min but not 120 min after inhibitory-avoidance learning.18 Also, changes occurred in RNA expression of glutamate receptor subunits and of the Glt1 transporter following learning and LTP.19,20
One limitation of our study is that while we observed changes in RNA expression of the transporters, we do not know if these changes are paralleled by changes in transporter protein expression or activity. However, our results support recent in vitro studies indicating that increased glutamate concentrations induce a cell-death independent down-regulation of GLAST and Glt-1 protein.21 Additionally, increases in glutamate concentration in the ventral hippocampus occur during the acquisition phase of inhibitory avoidance learning,22 further validating the importance of glutamate modulation during early learning.
Our results indicate a decreased glutamate transporter expression after a spatial learning task, yet other studies have shown increases in glutamate transporter expression after hippocampal plasticity. For example, LTP induction in area CA1 of the hippocampus results in increased glutamate uptake and increased expression of EAAC1, and GLAST 30 min after induction.10 Moreover, during contextual fear conditioning, increases in uptake and surface expression of EAAC1 were observed 24 hours after conditioning. Although differences in protocol (learning paradigm, and assessment of protein vs. RNA) could account for the contradicting results, it is also possible that the difference in time-point assessment and of learning phase is responsible for the variations. In fact, other studies that observed changes in glutamate uptake and expression during contextual fear conditioning examined changes 24 hours after conditioning (presumably after consolidation) and no post-training (acute) data is available.10 Our study is the first to examine RNA levels immediately after training and during the acquisition phase of learning, thus contributing significantly to understanding the effects of acute modulation of glutamate transmission during early stages of learning. Currently, we are examining whether the transporter protein changes in parallel with RNA after Morris Water Maze learning.
Our work describes the time-dependent expression pattern of glutamate transporter RNA during early stages of spatial learning. Much evidence shows that a tight regulation of various molecular events and the timing of these events are important for the induction of synaptic plasticity, such as in the case of LTP. For example, inhibiting the NMDA receptor during LTP induction results in abolishment of LTP, but had no effect if the inhibitors were applied later (for review).23 In addition, both pre-synaptic changes such as increased probability of transmitter release and post-synaptic changes seem to occur during LTP.24 Glutamate transporters regulate clearance of glutamate at synapses, and are modified in the hippocampus during LTP.10 Our results indicate a clear alteration of glutamate transporter RNA expression during the early stages of a spatial learning task, thus supporting the idea that regulation of glutamate concentrations is essential at all stages of spatial learning.
Our results also show decreased EAAC1 and GLAST expression following swimming (no learning), which implies that these changes are not due to spatial learning but are task related instead. One possibility is that the changes observed are due to the physical activity inherent to the task. In fact, voluntary exercise enhances synaptic plasticity in the hippocampus, and improves performance in the water maze and consolidation of hippocampus-dependent memories.25,26 In addition, environmental enrichment has effects similar to those of exercise on learning processes.27,28 Due to the exposure to a novel task, handling, and visual cues in the room, exposure to the water maze may constitute environmental enrichment on its own. If a down-regulation of glutamate transporters is involved in improved learning and synaptic plasticity and exercise also results in such a reduction, it is possible that glutamate transporters may modulate the effects of exercise on learning. In that case, similar changes would be expected in glutamate transporter expression in both swimmer (no learner) and learner groups. Additional experiments in our lab are ongoing to determine whether the physical activity during the one-day water maze training protocol also results in enhancement of synaptic plasticity and learning.
Our findings provide important information regarding glutamate transporter modulation on hippocampal learning, which could be essential for the development of novel pharmacotherapies to treat hippocampal-related memory disorders, which are prevalent in Hispanic populations.
Acknowledgments
We would like to thank Esther Jiménez, Eliezer Ruíz, and Ruth Quintana for their technical help. This work was supported by APA’s Diversity Program in Neuroscience Fellowship to Ada I. Fraticelli-Torres (ST32MH018882-21,22) by the RCMI Molecular Core Facility (12RR003050), the Grass Foundation, MBRS-SCORE (S06 GM008239-20S1) and MBRS-RISE (GM082406).
References
- 1.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 2.Berger UV, Desilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492(1):78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Mahadomrongkul V, Berger UV, et al. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24(5):1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coco S, Verderio C, Trotti D, Rothstein JD, Volterra A, Matteoli M. Non-synaptic localization of the glutamate transporter EAAC1 in cultured hippocampal neurons. Eur J Neurosci. 1997;9(9):1902–1910. doi: 10.1111/j.1460-9568.1997.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 5.Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8(2):108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- 6.Berger UV, Hediger MA. Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol (Berl) 1998;198(1):13–30. doi: 10.1007/s004290050161. [DOI] [PubMed] [Google Scholar]
- 7.Velaz-Faircloth M, McGraw TS, alandro MS, Fremeau RT, Jr, Kilberg MS, Anderson KJ. Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Physiol. 1996;270(1 Pt 1):C67–C75. doi: 10.1152/ajpcell.1996.270.1.C67. [DOI] [PubMed] [Google Scholar]
- 8.Levenson JM, Weeber EJ, Sweatt JD, Eskin A. Glutamate uptake in synaptic plasticity: from mollusc to mammal. Curr Mol Med. 2002;2(7):593–603. doi: 10.2174/1566524023362069. [DOI] [PubMed] [Google Scholar]
- 9.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 10.Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KJ, Orfila JE, Achanta P, Martinez JL., Jr Gene expression associated with in vivo induction of early phase-long-term potentiation (LTP) in the hippocampal mossy fiber-Cornus Ammonis (CA)3 pathway. Cell Mol Biol (Noisy-le-grand) 2003;49(8):1281–1287. [PubMed] [Google Scholar]
- 12.Kraemer PJ, Brown RW, Baldwin SA, Scheff SW. Validation of a single-day Morris Water Maze procedure used to assess cognitive deficits associated with brain damage. Brain Res Bull. 1996;39(1):17–22. doi: 10.1016/0361-9230(95)02028-4. [DOI] [PubMed] [Google Scholar]
- 13.Peinnequin A, Mouret C, Birot O, et al. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004;5:3. doi: 10.1186/1471-2172-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 15.Omrani A, Melone M, Bellesi M, et al. Upregulation of GLT-1 severely impairs LTD at mossy fibres-CA3 synapses. J Physiol. 2009;587(Pt 19):4575–4588. doi: 10.1113/jphysiol.2009.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riedel G. Function of metabotropic glutamate receptors in learning and memory. Trends Neurosci. 1996;19(6):219–24. doi: 10.1016/0166-2236(96)20012-8. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi M, Kobayashi K, Manabe T, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273(5275):645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 18.Cammarota M, de Stein ML, Paratcha G, Bevilaqua LR, Izquierdo I, Medina JH. Rapid and transient learning-associated increase in NMDA NR1 subunit in the rat hippocampus. Neurochem Res. 2000;25(5):567–572. doi: 10.1023/a:1007590415556. [DOI] [PubMed] [Google Scholar]
- 19.Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci. 2004;20(5):1355–1362. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee EH, Hsu WL, Ma YL, Lee PJ, Chao CC. Enrichment enhances the expression of sgk, a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur J Neurosci. 2003;18(10):2842–2852. doi: 10.1111/j.1460-9568.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann C, Bette S, Engele J. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 2009;1297:1–8. doi: 10.1016/j.brainres.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 22.Ballini C, Corte LD, Pazzagli M, et al. Extracellular levels of brain aspartate, glutamate and GABA during an inhibitory avoidance response in the rat. J Neurochem. 2008;106(3):1035–1043. doi: 10.1111/j.1471-4159.2008.05452.x. [DOI] [PubMed] [Google Scholar]
- 23.Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52(1):24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Isaac JT, Oliet SH, Hjelmstad GO, Nicoll RA, Malenka RC. Expression mechanisms of long-term potentiation in the hippocampus. J Physiol Paris. 1996;90(5–6):299–303. doi: 10.1016/s0928-4257(97)87901-6. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2008;19(10):988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26(23):6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39(4):569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]


