Abstract
The aim of cancer chemoprevention is disruption or delay of the molecular pathways that lead to carcinogenesis. Chemopreventive blocking and/or suppressing agents disrupt the molecular mechanisms that drive carcinogenesis such as DNA damage by reactive oxygen species, increased signal transduction to NF-κB, epigenomic deregulation, and the epithelial mesenchymal transition that leads to metastatic progression. Numerous dietary phytochemicals have been observed to inhibit the initiation phase of carcinogenesis, and therefore are useful in primary chemoprevention. Moreover, phytochemicals are capable of interfering with the molecular mechanisms of metastasis. Likewise, numerous synthetic compounds are relevant and clinically viable as chemopreventive agents during the fundamental stages of carcinogenesis. While molecularly targeted anti-cancer therapies are in constant stages of development, superior patient outcomes are observed if carcinogenic processes are prevented altogether. This article reviews the role of chemopreventive compounds in inhibition of cancer initiation and their ability to reduce cancer progression.
Keywords: blocking agents, chemoprevention, phytochemicals, suppressing agents
Introduction
Cancer is a public health burden that is predicted to be diagnosed in excess of 1.6 million new cases and results in more than 585,000 deaths in the United States in 2014.1 An ever-increasing understanding of cancer cell pathobiology has led to a molecular approach in treating neoplasms and reducing cancer mortality rates. These advances have also been instrumental in developing chemopreventive strategies that could reduce cancer incidence or oppose progression of disease. In light of the increasing financial strain of treating cancer and the favorable outcomes observed in investigational cancer prevention strategies, the Food and Drug Administration (FDA) has approved agents for the prevention of breast (tamoxifen and raloxifene) and cervical (human papillomavirus (HPV) vaccination) cancers and several other intervention agents to reduce cancer risk in precancerous conditions.2
Clinically, chemoprevention is categorized as primary, secondary, or tertiary. Primary chemoprevention is suited for the general population and for those who may be at increased risk for disease. Secondary chemoprevention is intended for patients with premalignant lesions that may progress to an invasive disease. Generally, both primary and secondary chemoprevention is now grouped under the category of “primary chemoprevention.” Examples of primary chemopreventive agents include dietary phytochemicals and non-steroidal anti-inflammatory drugs (NSAIDs). On the other hand, tertiary chemoprevention is targeted to prevent disease recurrence or additional (second) primary disease in those individuals who have already endured potentially curative therapy, such as treatment with aromatase inhibitors.
At the molecular level, cancer chemoprevention is characterized by the disruption of, or at least the delay of, multiple pathways and processes among the three stages of carcinogenesis: initiation, promotion, and progression.3,4 Chemicals or biomolecules that inhibit the initiation stage are important for the preservation of DNA in its native state and are referred to as “blocking agents” since they “block” mutagenic interactions with DNA.4 Blocking agents may circumvent the permanent, irreparable DNA damage that occurs during initiation by inactivating or metabolizing carcinogens directly, acting as free-radical scavengers, or physiologically inducing anti-oxidative enzyme activity and triggering mechanisms of DNA repair.5 Furthermore, blocking agents can also modify the epigenomic landscape.6
In contrast to blocking agents, compounds that affect later stages of carcinogenesis (promotion and progression) are termed “suppressing agents” for their ability to decrease the proliferative capacity of initiated cells.4 They interfere with cancer cell proliferation by down-regulating signal transduction pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 3 (STAT3), and many others,7–10 and by inhibiting cytochrome P450 enzymes that modulate signal transduction to hormone responsive elements.11 Additionally, suppressing agents are likely to reduce or delay the ability of cancer cells to evolve metastatic properties by promoting pathways leading to apoptosis10,12 and inhibiting pathways leading to angiogenesis, epithelial mesenchymal transition (EMT), invasion, and dissemination.5,13 Since metastasis is the most significant challenge related to cancer treatment and is the major cause of cancer-related death,14 this article will discuss not only the role of chemopreventive compounds in cancer formation but also their ability to reduce cancer progression.
Primary Chemopreventive Phytochemicals as Blocking Agents
Redox signaling
Dietary- and non-dietary-derived phytochemicals (molecules found in plants) have been directly observed to inhibit all stages of carcinogenesis in in vitro and in vivo models.5,10,15,16 Importantly, phytochemicals appear to prevent the initiation phase of carcinogenesis (protecting DNA from mutation) by modulation of cytoprotective enzyme activation. In fact, transcription factors responsible for the expression of these cytoprotective enzymes, including the well-documented NF-E2-related factor 2 (Nrf2), are induced not only in the presence of oxidative stress but also in the presence of various phytochemicals.
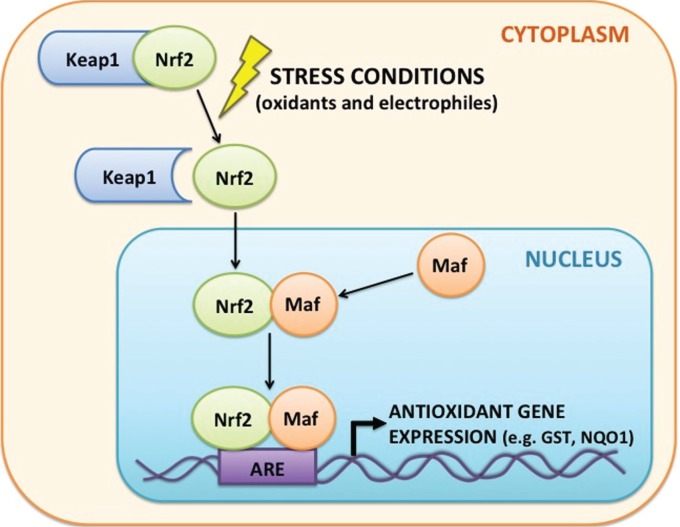
Nrf2, the “master regulator of antioxidant defenses,”17 binds to cis-enhancer sequences called antioxidant responsive elements (ARE) that are 5′-flanking of the promoter regions for genes encoding detoxifying and antioxidant enzymes18,19 such as glutathione S-transferases (GST) and NADPH:quinone oxidoreductase (NQO1) (Fig. 1). Under normal, homeostatic conditions, Nrf2 is regulated by cytosolic sequestration and interaction with Kelch-like ECH-associating protein 1 (Keap1). Under stress conditions (eg oxidants and electrophiles), Nrf2 is released from Keap1 and translocates to the nucleus, where it forms a heterodimer with Mafs before activating ARE-regulated genes. Expression of Nrf2 target genes is therefore advantageous to maintaining genomic integrity as these cytoprotective enzymes reduce the effects of electro-philes, chemical challenges, and oxidative stress on DNA.20
Figure 1.
Mechanism of Nrf2 signaling and activation of antioxidant gene expression. Nrf2 is sequestered in the cytoplasm by Keap1. In response to external stressors (eg oxidants and electrophiles), Nrf2 is released from Keap1 and translocates to the nucleus where it forms a heterodimer with Maf that binds to antioxidant-responsive elements (ARE). The Nrf2–Maf heterodimer increases transcription of genes downstream from the ARE that encode detoxifying and antioxidant enzymes such as GST and NQO1. These cytoprotective enzymes limit the effects of electrophiles and oxidants on DNA and help preserve genomic integrity.
Abbreviations: Nrf2, NF-E2-related factor 2; Keap1, Kelch-like ECH-associating protein 1; GST, glutathione S-transferase; NQO1, NADPH: quinone oxidoreductase 1.
Dietary phytochemicals such as isothiocyanate and sulphoraphane (Table 1) found in cruciferous vegetables and the phytochemicals found in the dietary supplement Protandim (Bacopa monniera, Camellia sinensis [green tea], Curcuma longa [turmeric], Silybum marianum [milk thistle], and Withania somnifera [Ashwagandha]) not only appear to act as potent activators of Nrf2 in both cell culture and animal models,5,20,21 but many are also known to inhibit the conversion of procarcinogens to their electrophilic (DNA damaging) species.22 Therefore, in reducing the potential for carcinogenic initiation, phytochemicals behave as blocking agents that prevent DNA mutations.
Table 1.
Selected dietary and synthetic chemopreventive compounds and their chemopreventive effects represented herein.
| DIETARY | CHEMOPREVENTIVE EFFECTS |
|---|---|
| 2-methoxystypandrone (Polygonum cuspidatum) | Inhibits JAK and IKKβ kinase signaling to NF-κB |
| Apigenin (numerous plant sources) | Inhibits NF-κB DNA binding, IκB-α phosphorylation, and IKKβ activity |
| Curcumin (curcuma longa; turmeric) | Up regulates Nrf2 signaling; Induces apoptosis; Inhibits NF-κB signaling; decreases cell invasion and motility |
| EGCG (camillia sinensis; green tea) | Inhibits proteasome activity for IκBα stabilization and NFkB inhibiton; Increases expression of E-cadherin; Inhibitis signal transduction through ERK |
| [6[-gingerol (Zingiber officinale; ginger root) | Increases expression of E-cadherin; Inhibits signal transduction through ERK; Inhibits MMP-9 expression |
| Isothiocyanate/Sulphoraphane (cruciferous vegetables) | Increases Nrf2 expression |
| Luteolin (Terminalia chebula) | Inhibits MMP-9 expression |
| Resveratrol (skin of red grapes) | Increases expression of E-cadherin; Inhibits signal transduction through PI3K/Akt |
| SYNTHETIC | |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Inhibits inflammation and NF-κB signaling |
| Aromatase inhibitors | Inhibits CYP19 activity and estrogen activation |
| Tamoxifen | Inhibits signal transduction through estrogen receptor |
Epigenetics
While 90% of all cancers are presumably caused by somatic mutations, in recent years, there has been considerable interest in the contribution of epimutations (epigenetics) to the development of cancer. Epigenetics comprise alterations to gene expression without changes to the genome (DNA) sequence and include cytosine methylation within CpG islands, histone tail modifications (acetylation, methylation, and ubiquitination), and the effects of non-coding RNA.6,23
DNA methylation is the most commonly described epigenetic alteration with more than 600 cancer-related genes known to carry hypermethylated promoter regions.6 DNA methyl-transferases (DNMT3a and DNMT3b) preferentially target CpG regions for de novo methylation, and their activity is responsible for the hypermethylation and silencing of tumor suppressor genes. In contrast, decreased activity of DNMT1, the enzyme responsible for the maintenance methylation following DNA replication, can lead to a state of hypomethylation, chromosomal instability, and activation or excessive expression of oncogenes. In contrast to normal cells, cancer cells display profiles of “global hypomethylation” that are complemented with promoter-associated CpG island hypermethylation.23 While the presence or absence of DNA methylation contributes directly to gene expression, methylated DNA binding proteins (MBDs) can also influence gene expression indirectly by binding to methylated cytosine residues. The MBDs can then complex with histone deacetylase (HDAC), an event that leads to gene silencing through chromatin compaction.24
An emerging field of epigenetic study involves non- coding RNAs and their roles in modifying genes post- transcriptionally.25 These non-coding RNAs (18–25 nucleotides long) are commonly referred to as microRNA (miRNA) and function to down-regulate the expression of genes. This is accomplished through base pairing between the miRNA and an mRNA and subsequent mRNA degradation or translational repression. It is estimated that nearly 30% of all human genes are regulated by miRNAs.26 Although non-coding RNA is essential to normal cell processes (eg introns and splicing), aberrant miRNA expression patterns are linked to chromosomal instability25 by the silencing of epigenetic regulators such as DNMTs and HDACs.6
In the context of primary chemoprevention, phytochemicals have been shown to alter the epigenome and reverse abnormal gene expression through modulation of DNMTs, MBDs, HDACs, miRNAs (Table 2), and several other epigenetic mechanisms. In doing so, phytochemicals promote genomic integrity and cellular homeostasis in both in vitro and in vivo models.6 Importantly, numerous synthetic compounds are in clinical trials for the treatment of various cancers, and four have garnered FDA approval as epigenetic drugs against cutaneous T-cell lymphoma and myelodysplastic syndromes (Table 2).27
Table 2.
Natural and synthetic epigenetic modulators.
| DIETARY | EPIGENETIC MODIFICATION(S) | ROLE(S) IN CHEMOPREVENTION |
|---|---|---|
| Curcumin | Inhibits DNA methylation (DAMT) Inhibits histone acetylation (HAT) and deacetylation (HDAC) Modulates miRNA expression |
Prevents DNA damage and inhibits NF-κB signalling59 |
| EGCG | Inhibits DNA methylation (DNMT) Inhibits histone acetylation (HAT) Modulates miRNA expression |
Triggers apoptosis and cell cycle arrest in tumor cells60 |
| Isothiocyanate/Sulphoraphane | Inhibits DNA methylation (DNMT) Inhibits histone deacetylation (HDAC) |
Inhibits tumor cell growth and triggers cell cycle arrest and apoptosis61–63 |
| Resveratrol | Inhibits DNA methylation (DNMT) Inhibits histone deacetylation (HDAC) |
Inhibits NF-κB signalling64 |
| Romidepsin (derived from the bacteria Chromobacterium violaceum) | Inhibits histone deacetylation (HDAC) | Reverses abnormal silencing of tumor suppressor genes, leading to growth arrest, differentiation, and/or apoptosis of tumor cells27,65 |
| SYNTHETIC | EPIGENETIC MODIFICATION(S) | EPIGENETIC OUTCOME(S) |
| Vorinostat and Hydroxamic acid (LAQ824) | Inhibits histone deacetylation (HDAC) Modulate miRNA expression |
Reverses abnormal gene silencing and alters miRNA expression in tumor cells, resulting in cell cycle arrest, differentiation, and/or apoptosis25,27 |
| 5-azacytidine (vidaza) and 2′-deoxycytidine (decitabine) | Inhibits DNA methylation (DNMT) | induces re-expression of silenced tumor suppressor genes and stimulates cell responses to dna damages |
Tertiary Chemoprevention by Suppressing Agents
NF-κB
The role of the NF-κB family of transcription factors is well established in immunity and inflammation. Inflammation is now recognized as one of the “hallmarks of cancer,” since it has been shown to contribute to both initiation and progression of tumor cells.28 While uncontrolled and persistent inflammation in the tumor microenvironment is driven by the actions of NF-κB target genes, NF-κB is also oncogenic.29 More than 150 genes are regulated by NF-κB and these genes function in mechanisms of cell survival including apoptosis inhibition,30 increased proliferative capacity of cells,31 and promotion of tumor cell progression.32
In unstimulated cells, the five NF-κB family members (p65 [RelA], RelB, c-Rel, p50/p105 [NF-κB1], and p52/p100 [NF-κB2]) are found as hetero- or homodimers and remain bound to IκB family proteins (IκBα, IκBβ, IκBγ, IκBε, and the non-IκB, Bcl-3). The IκB proteins sequester the NF-κB proteins in the cytoplasm. Upon stimulation by pro-inflammatory cytokines (eg TNF-α), IκB is phosphorylated by an IκB kinase (IKK) complex (IKKα, IKKβ, and IKKγ) and subsequently ubiquitinated and degraded by the proteasome. IκB degradation exposes a nuclear localization signal in NF-κB, enabling its translocation to the nucleus and activation of its target genes.33
Inflammation that is supported in the presence of aberrant NF-κB signaling likely promotes the development of tumors after the initiation of carcinogenesis.34 Consequently, there exists a conceivable strategy for the use of anti-inflammatory compounds as suppressing chemopreventive agents. Numerous dietary phytochemicals have been cited as inhibitors of NF-κB signaling or having anti-inflammatory properties.13 For example, through proteasomal inhibition, both the green tea polyphenol (−)-Epigallocatechin-3-gallate (EGCG) and curcumin stabilize IκBα in cell-based and animal models,12,35 thereby preventing nuclear translocation NF-κB and transcription of its target genes. Additionally, the compound 2-methoxystypandrone derived from the Chinese medicinal herb Polygonum cuspidatum and the phytochemical apigenin have been identified as IKK inhibitors that block NF-κB sig-naling.36,37 Although the use of anti-inflammatory compounds is interesting as a potential chemopreventive approach, no synthetic IKK inhibitors or NF-κB inhibitors have yet been clinically approved despite showing anti-tumor effects in numerous cancer models.29,38–40 Finally, the use of NSAIDs such as aspirin (used at a minimum dose of 75 mg/day for 5 years or longer) is an effective approach to chemoprevention for individuals at risk for various cancers, especially colorectal cancer.41
Cytochrome P450s
Cytochrome P450s (CYPs) are a superfamily of proteins involved in metabolism of environmental and dietary chemicals for elimination from the body and activation of procarcinogenic exogenous compounds and endogenous molecules (hormones) to their carcinogenic forms. The CYPs therefore represent a unique paradigm for chemoprevention and carcinogenesis.
Since increased CYP expression is observed in human tumors,11 it is important to understand the relationship of the CYPs to tumor development and malignant transformation. The standard pharmacological treatment for hormone-dependent breast cancer has traditionally been to block estrogen from binding to its receptor. This has been achieved through administration of the drug tamoxifen. While tamoxifen was the first FDA-approved chemopreventive agent for individuals at high risk for developing breast cancer, there are some considerable caveats for its use; tamoxifen is linked to an increased incidence of endometrial cancer42 and when used as a cancer therapy, resistance to its use is inevitable.43 Aromatase inhibitors (anastrozole, letrozole, and exemestane) are an alternative approach to tamoxifen treatment and have demonstrated superior treatment effectiveness in postmenopausal women.44 Aromatase inhibitors bind to and block the activity of CYP19 (aromatase), the enzyme that converts androgens to estrogens.45 As such, aromatase inhibitors behave as suppressing agents that down-regulate the survival signal mediated by estrogen to hyperproliferative estrogen responsive cells. They therefore limit the capacity of cells to progress to metastasis.
Phytochemicals Suppress Metastasis
In addition to the chemopreventive effects of synthetic aromatase inhibitors, dietary phytochemicals have also shown great promise in reducing cancer cell progression to metastasis. Metastasis is a complex process that involves cancer cell migration, invasion, dissemination through the lymphatics or vasculature, and, ultimately, colonization. Since the vast majority of cancer-related deaths are fundamentally linked to the onset of metastasis, essentially all physical manipulations and radio-, chemo-, and biological therapies seek to prohibit dissemination of cancer cells to distant sites. While molecularly targeted anti-metastasis drugs are in development,46 superior patient outcomes are achieved if the metastatic process is prevented altogether.
Dietary phytochemicals are well documented to block the molecular pathways that lead to metastatic events. During the EMT, cancer cells acquire properties of motility and invasiveness by loss of the epithelial phenotype. Cancer cells become capable of metastasis during EMT by reduced expression of the epithelial-specific proteins (eg E-cadherin)47 and gain of mesenchymal properties through increased expression of mesenchymal-specific proteins (eg N-cadherin).48 In vitro exposure of cancer cells to phytochemicals such as silibinin,49 EGCG,50 curcumin,51,52 [6]-gingerol,53 resveratrol,54 and numerous others has been shown to induce increased expression of E-cadherin and therefore decreases the mesenchymal phenotype. These phytochemicals appear to inhibit several EMT pathways, but commonly function through inhibition of receptor and non-receptor tyrosine kinases (ERK, Src, PI3K, etc.).
As tumor cells acquire the phenotypic EMT, the ability of those cells to become invasive is determined in large part by remodeling of the extracellular matrix. This is accomplished by increased expression and activation of matrix metalloproteinase (MMPs) in the tumor microenvironment that function to degrade the extracellular matrix and basement membrane. MMPs therefore eliminate the barrier that should restrict cancer cell dissemination to distant sites. Numerous phytochemicals inhibit MMP expression and function as suppressing agents in tertiary chemoprevention. For instance, recent evidence suggests that curcumin inhibits extracellular signaling to and decreased expression of MMP-9 by tumor cells in thyroid, colorectal, pancreatic, ovarian, and numerous other cancer cells, in vitro and in animal models.51,52,55 Furthermore, [6]-gingerol and luteolin, among many other phytochemicals, appear to reduce MMP-9 expression in pancreatic and colon cancer cells, respectively.53,56 Thus, chemoprevention may be viable not only in blocking the initiation of cancer but also in averting cancer progression.
Conclusions
The “state of the art” in chemoprevention has revealed that numerous natural and synthetic compounds are able to inhibit carcinogenesis through novel molecular mechanisms, with epigenetic alterations at the forefront of current research. The molecules discussed in this article were selected because of their historical medicinal use, consumption among certain populations, and their roles in various biological systems.
Although there is an exciting amount of new empirical information in support of chemopreventive strategies, there is also “art” in the realm of chemoprevention. Chemoprevention studies are generally well received by the nutraceutical research community, but the long-term safety and tolerability of any chemopreventive compound (natural or synthetic) for human consumption must be considered, since it is likely that the agent will have to be consumed/administered for a long period of time. Therefore, a major hurdle for potential clinical trials in chemoprevention is defining a minimal dose that remains clinically beneficial, while also avoiding any adverse effects.57 Furthermore, biotransformation reactions limit the potential for disseminating bioactive ingested dietary phytochemicals to the sites of carcinogenesis.58 Since the goal of primary chemoprevention is to reduce the incidence of cancer in the general population and those at high risk of developing the disease, chemopreventive agents will vary in their effectiveness depending on the genotype of the individual exposed to them. Hence, there is great promise in the “state of the art” in cancer chemoprevention, yet there still remains a considerable amount of “art” in such endeavors.
Footnotes
Author Contributions
Contributed to the writing of the manuscript: KRLP and NRI. Agree with manuscript results and conclusions: KRLP and NRI. Jointly developed the structure and arguments for the paper: KRLP and NRI. Made critical revisions: KRLP and NRI. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Marc D. Basson, Editor in Chief
COMPETING INTERESTS: KRLP holds a patent for (−)-epigallocatchin gallate derivitatives for inhibiting proteasomes (8710248). NRI discloses no potential conflicts of interest.
FUNDING: Authors disclose no funding sources.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants. Provenance: the authors were invited to submit this paper.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Patterson S, Hawk E. Chemoprevention—history and general principles. Best Pract Res Clin Gastroenterol. 2011;25:445–459. doi: 10.1016/j.bpg.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 3.De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 6.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Gan FF, Ling H, Ang X, et al. A novel shogaol analog suppresses cancer cell invasion and inflammation, and displays cytoprotective effects through modulation of NF-κB and Nrf2-Keap1 signaling pathways. Toxicol Appl Pharmacol. 2013;272:852–862. doi: 10.1016/j.taap.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 9.Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Landis-Piwowar K, Chan TH, Dou QP. Green tea polyphenols as proteasome inhibitors: implication in chemoprevention. Curr Cancer Drug Targets. 2011;11:296–306. doi: 10.2174/156800911794519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg Med Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landis-Piwowar K, Chen D, Chan TH, Dou QP. Inhibition of catechol-Omicron-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (−)-EGCG. Oncol Rep. 2010;24:563–569. doi: 10.3892/or_00000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre-Ghiso JA, Bragado P, Sosa MS. Metastasis awakening: targeting dormant cancer. Nat Med. 2013;19:276–277. doi: 10.1038/nm.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slamenova D, Horvathova E. Cytotoxic, anti-carcinogenic and antioxidant properties of the most frequent plant volatiles. Neoplasma. 2013;60:343–354. doi: 10.4149/neo_2013_046. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Reuland DJ, Khademi S, Castle CJ, et al. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–111. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Das BN, Kim YW, Keum YS. Mechanisms of Nrf2/Keap1-dependent phase II cytoprotective and detoxifying gene expression and potential cellular targets of chemopreventive isothiocyanates. Oxid Med Cell Longev. 2013;2013(7):839409. doi: 10.1155/2013/839409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(1):3291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 23.Shankar S, Kumar D, Srivastava RK. Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther. 2013;138:1–17. doi: 10.1016/j.pharmthera.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duthie SJ. Epigenetic modifications and human pathologies: cancer and CVD. Proc Nutr Soc. 2011;70:47–56. doi: 10.1017/S0029665110003952. [DOI] [PubMed] [Google Scholar]
- 25.Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet, and cancer: new mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog. 2012;51:213–230. doi: 10.1002/mc.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Mair B, Kubicek S, Nijman SM. Exploiting epigenetic vulnerabilities for cancer therapeutics. Trends Pharmacol Sci. 2014;35:136–145. doi: 10.1016/j.tips.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF[alpha]-induced apoptosis by NF-[kappa]B. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 31.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 34.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci Signal. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 35.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuang S, Qi C, Liu J, et al. 2-Methoxystypandrone inhibits signal transducer and activator of transcription 3 and nuclear factor-κB signalling by inhibiting Janus kinase 2 and IκB kinase. Cancer Sci. 2014;105:473–480. doi: 10.1111/cas.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu DG, Yu P, Li JW, et al. Apigenin potentiates the growth inhibitory effects by IKK-β-mediated NF-κB activation in pancreatic cancer cells. Toxicol Lett. 2014;224:157–164. doi: 10.1016/j.toxlet.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Schön M, Wienrich BG, Kneitz S, et al. KINK-1, a novel small-molecule inhibitor of IKKbeta, and the susceptibility of melanoma cells to antitumoral treatment. J Natl Cancer Inst. 2008;100:862–875. doi: 10.1093/jnci/djn174. [DOI] [PubMed] [Google Scholar]
- 39.Amschler K, Schön MP, Pletz N, Wallbrecht K, Erpenbeck L, Schön M. NF-kappaB inhibition through proteasome inhibition or IKKbeta blockade increases the susceptibility of melanoma cells to cytostatic treatment through distinct pathways. J Invest Dermatol. 2010;130:1073–1086. doi: 10.1038/jid.2009.365. [DOI] [PubMed] [Google Scholar]
- 40.Mbalaviele G, Sommers CD, Bonar SL, et al. A novel, highly selective, tight binding IkappaB kinase-2 (IKK-2) inhibitor: a tool to correlate IKK-2 activity to the fate and functions of the components of the nuclear factor-kappaB pathway in arthritis-relevant cells and animal models. J Pharmacol Exp Ther. 2009;329:14–25. doi: 10.1124/jpet.108.143800. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow AJ, Jones ME, British Tamoxifen Second Cancer Study Group Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–384. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 43.Oida K, Matsuda A, Jung K, et al. A nuclear factor-κB plays a critical role in both intrinsic and acquired resistance against endocrine therapy in human breast cancer cells. Sci Rep. 2014;4:4057. doi: 10.1038/srep04057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams N, Harris LN. The renaissance of endocrine therapy in breast cancer. Curr Opin Obstet Gynecol. 2014;26:41–47. doi: 10.1097/GCO.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 45.Ponzone R, Mininanni P, Cassina E, Pastorino F, Sismondi P. Aromatase inhibitors for breast cancer: different structures, same effects? Endocr Relat Cancer. 2008;15:27–36. doi: 10.1677/ERC-07-0249. [DOI] [PubMed] [Google Scholar]
- 46.Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013;328(2):207–211. doi: 10.1016/j.canlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Guarino M, Rubino B, Ballabio G. The role of epithelial–mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima S, Doi R, Toyoda E, et al. N-cadherin expression and epithelial- mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 49.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res. 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Amicis F, Perri A, Vizza D, et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J Cell Physiol. 2013;228:2054–2062. doi: 10.1002/jcp.24372. [DOI] [PubMed] [Google Scholar]
- 51.Chen CC, Sureshbabul M, Chen HW, et al. Curcumin suppresses metastasis via Sp-1, FAK inhibition, and E-cadherin upregulation in colorectal cancer. Evid Based Complement Alternat Med. 2013;2013:541695. doi: 10.1155/2013/541695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen F, Cai WS, Li JL, et al. Synergism from the combination of ulinastatin and curcumin offers greater inhibition against colorectal cancer liver metastases via modulating matrix metalloproteinase-9 and E-cadherin expression. OncoTargets Ther. 2014;7:305–314. doi: 10.2147/OTT.S57126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SO, Kim MR. [6]-Gingerol prevents disassembly of cell junctions and activities of MMPs in invasive human pancreas cancer cells through ERK/NF-κB/snail signal transduction pathway. Evid Based Complement Alternat Med. 2013;2013:761852. doi: 10.1155/2013/761852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Ma J, Ma Q, et al. Resveratrol inhibits the epithelial–mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem. 2013;20(33):4185–4194. doi: 10.2174/09298673113209990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Pandurangan A, Dharmalingam P, Sadagopan S, Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol. 2014 doi: 10.1177/0960327114522502. [DOI] [PubMed] [Google Scholar]
- 57.Scott E, Gescher A, Steward W, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res. 2009;2:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- 58.Landis-Piwowar KR, Dou QP. Polyphenols: biological activities, molecular targets, and the effect of methylation. Curr Mol Pharmacol. 2008;1:233–243. doi: 10.2174/1874467210801030233. [DOI] [PubMed] [Google Scholar]
- 59.Mudduluru G, George-William JN, Muppala S, Asangani IA. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 60.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang LG, Beklemisheva A, Liu XM, Ferrari AC. Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer. Mol Carcinog. 2007;46:24–31. doi: 10.1002/mc.20258. [DOI] [PubMed] [Google Scholar]
- 63.Izzotti A, Calin GA, Steele VE, Cartiglia C. Chemoprevention of cigarette smoke-induced alterations of microRNA expression in rat lungs. Cancer Prev Res. 2010;3:62–72. doi: 10.1158/1940-6207.CAPR-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tili E, Michaille JJ, Alder H, Volinia S. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem Pharmacol. 2010;80:2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGraw AL. Romidepsin for the treatment of T-cell lymphomas. Am J Health Syst Pharm. 2013;70:1115–1122. doi: 10.2146/ajhp120163. [DOI] [PubMed] [Google Scholar]