Abstract
In the last 20 years, atrial fibrillation (AF) has become one of the most important public health problems and a significant cause of increasing health care costs in western countries. The prevalence of AF is increasing due to our greater ability to treat chronic cardiac and noncardiac diseases, and the improved ability to suspect and diagnose AF. At the present time, the prevalence of AF (2%) is double that reported in the last decade. The prevalence of AF varies with age and sex. AF is present in 0.12%–0.16% of those younger than 49 years, in 3.7%–4.2% of those aged 60–70 years, and in 10%–17% of those aged 80 years or older. In addition, it occurs more frequently in males, with a male to female ratio of 1.2:1. The incidence of AF ranges between 0.21 and 0.41 per 1,000 person/years. Permanent AF occurs in approximately 50% of patients, and paroxysmal and persistent AF in 25% each. AF is frequently associated with cardiac disease and comorbidities. The most common concomitant diseases are coronary artery disease, valvular heart disease, and cardiomyopathy. The most common comorbidities are hypertension, diabetes, heart failure, chronic obstructive pulmonary disease, renal failure, stroke, and cognitive disturbance. Paroxysmal AF occurs in younger patients and with a reduced burden of both cardiac disease and comorbidities. Generally, the history of AF is long, burdened by frequent recurrences, and associated with symptoms (in two thirds of patients). Patients with AF have a five-fold and two-fold higher risk of stroke and death, respectively. We estimate that the number of patients with AF in 2030 in Europe will be 14–17 million and the number of new cases of AF per year at 120,000–215,000. Given that AF is associated with significant morbidity and mortality, this increasing number of individuals with AF will have major public health implications.
Keywords: atrial fibrillation, epidemiology, risk factors, mortality, stroke
Introduction
In the last two decades, atrial fibrillation (AF) has become one of the most important public health issues and an important cause of health care expenditure in western countries. Even if AF is not a life-threatening arrhythmia, it influences quality of life significantly as a result of its anatomic, hemodynamic, and hemocoagulative consequences. In addition, AF is frequently associated with disturbing symptoms and very important socioeconomic problems, such as permanent disability, cognitive disturbance, hospitalization, and absence from work.1 Given that the prevalence of AF continues to increase, it is crucial to have an updated picture of the epidemiological, clinical, and social impact of AF to plan appropriate interventions and adequately allocate human and economic resources.
Search strategy
A systematic review of the studies reported on the epidemiology of AF in Europe was performed using the electronic MEDLINE and PubMed databases. The search terms included: “atrial fibrillation”, “atrial tachyarrhythmias”, “epidemiology”, “burden”, “rhythm control”, “rate control”, “stroke”, “heart failure”, “outcome”, “anticoagulant therapy”, and “antiplatelet therapy”. Studies published from 2005 to 2014 with a prespecified protocol (how AF was ascertained, diligence with which medical records were sought, modes of clinical assessment, and follow-up of patient subgroups) analyzing the epidemiology of AF exclusively in the general population were considered. When necessary (ie, absence in the literature of data on temporal trends regarding the incidence/prevalence of AF and risk of stroke), investigations performed before 2005 were used. The projected burden of AF in the European population in 2030 is based on estimates of demographic progression in Europe according to Eurostat.2
Prevalence and incidence of AF
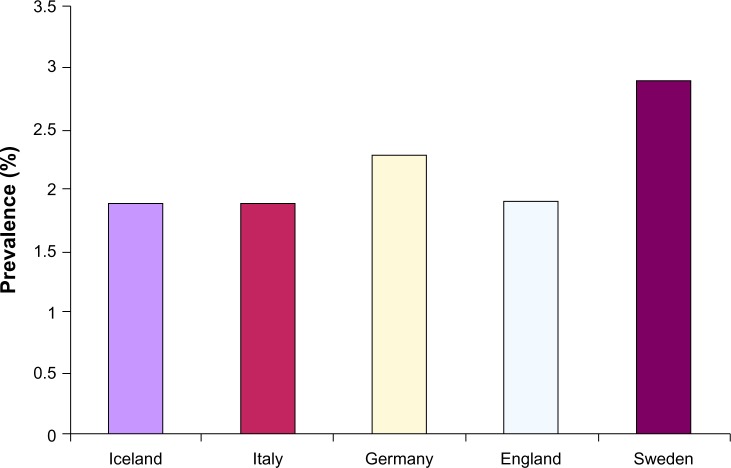
The most important studies on the epidemiology of AF carried out in developed countries and published between the end of the 20th century and the first years of the 21st century estimated that the prevalence of AF ranged between 0.5% and 1% in the general population.3,4 However, in the last decade, the common opinion was that the prevalence of AF, as perceived by number of hospitalizations, emergency room visits, and burden of outpatient visits for AF, had to be markedly higher.4–6 The most recent studies have confirmed this perception and shown that the prevalence of AF in the general adult population of Europe is more than double that reported just one decade earlier, ranging from 1.9% in Italy, Iceland, and England to 2.3% in Germany and 2.9% in Sweden (Figure 1). In particular, for Iceland, the mean increase of AF prevalence in the global population is 0.04% per year (data for the period 1998–2008), with a mean increase of 0.12% and 0.07% per year, respectively, in men and women aged 65–74 years, 0.27% and 0.23% in those aged 75–84 years, and 2.8% and 0.27% in those older than 84 years.
Figure 1.
Prevalence of atrial fibrillation in European countries.
In the USA, although updated information on the prevalence of AF in the global population is not available, it appears that the prevalence of AF has increased by 0.3% per year in Medicare beneficiaries older than 65 years, with an absolute growth of 4.5% (from 4.1% to 8.6%) in the period 1993–2007.7–12 The most likely explanation for such a significant increase is the greater ability to treat chronic cardiac and noncardiac diseases, the aging population, and the improved ability to suspect and diagnose AF.13 However, despite this increase, the real prevalence of AF is probably still underestimated because it is well known that AF, in a discrete proportion (10%–25%) of cases, occurs in the absence of symptoms.13,14 Very likely, if AF was detected appropriately by active screening, its prevalence would be higher and closer to 3%, as estimated for 2015 in the USA.3,14 In developing countries, AF occurs in approximately 0.6% of males and 0.4% of females. Although these rates are markedly lower than in developed nations, it appears that the burden of AF in these countries is enough to be a potential problem for health care systems.15 The prevalence of AF varies with age and sex. AF is present in 0.12%–0.16% of subjects younger than 49 years, 3.7%–4.2% of those aged 60–70 years, and 10%–17% of those aged 80 years or older. In addition, it occurs more frequently in males, with a male to female ratio of approximately 1.2:1. Despite the greater prevalence in men, women represent the bulk of patients with AF due to their longer survival.7–12,15,16
Less information is available on the incidence of AF. One of the first investigations of this problem was the Framingham study that in 1982 reported the overall incidence of chronic AF at two per 1,000 on biannual electrocardiography in patients aged 32–65 years.17 Subsequently, only few studies have analyzed this issue, with results that are not easily comparable because of a lack of homogeneity with regard to the characteristics of enrolled populations, ie, different age limits, different types of AF analyzed (all together, only permanent AF), different reservoirs of recruitment (members of health insurance funds or national health care systems), and different ways of diagnosing AF (classification based on administrative data, identification based on judgment of general practitioners). However, from these studies, it appears that among US Medicare beneficiaries aged 65 years or older, the incidence of AF has not changed substantially from 1993 (27.3 per 1,000 person/years) to 2007 (28.3 per 1,000 person/years), has always remained higher among men (34 per 1,000 person/years in men versus 25 per 1,000 person/years in women), and has continued to be much higher in the elderly population (18.8 per 1,000 person/years in those aged 70–74 years versus 53.9 per 1,000 person/years in those aged 84–89 years).10 Similar data were reported for another study performed in the USA, ie, the Olmsted County population in subjects aged 18 years and over. The incidence of AF has increased only slightly in this region between 1980 (3.04 per 1,000 person/years) and 2000 (3.68 per 1,000 person/years).18 In Europe, the most recent studies performed in the global population over the last decade report an incidence of AF ranging from 0.23 per 1,000 person/years in Iceland to 0.41 in Germany and 0.9 in Scotland, respectively.4,8,11 Also, in Europe, the incidence of AF increases with age and differs with sex, even if the reported rates are lower than those of the USA population. In Scotland, Germany, and the USA, subjects aged 65 years or older show an AF incidence of 4.7, 4.1, and 28.3 per 1,000 person/years, respectively; those aged 65–74 years show an incidence of 3.2, 10.8, and 15.5 (mean value between those aged 65–69 years and 70–74 years) per 1,000 person/years; and those aged 75–84 years show an incidence of 6.2, 16.8, and 33.5 (mean value between those aged 75–79 years and 80–84 years) per 1,000 person/years. A possible explanation for such marked differences lies in the method used to define the first episode of AF, ie, on the basis of administrative data (uncertainty in classifying the types of AF by the International Classification of Diseases diagnosis codes, the definition of prevalent AF generally made in inpatients, difficulties in assessing patients with a first episode of AF) or a documented history of AF as in Germany and Scotland.4,8,10
Types of AF, characteristics of AF patients, and impact of AF on health care systems
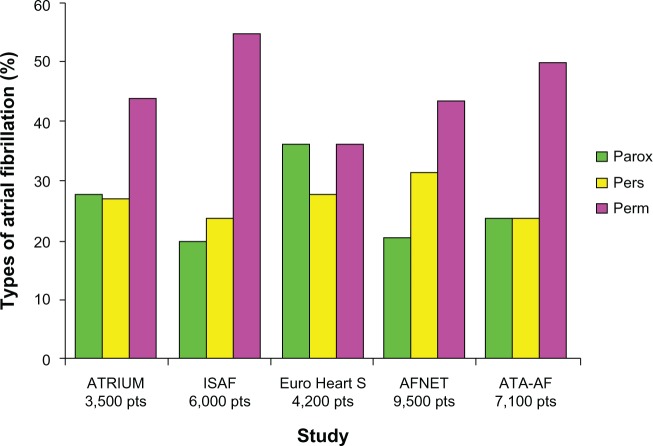
Observational studies performed in the general population or in patients hospitalized for AF show that permanent AF is the most frequent form of diagnosed AF; it occurs in 40%–50% of patients, followed by the paroxysmal and persistent forms that each occur in 20%–30% of cases (Figure 2).9,19–22 Generally, the history of AF is long and burdened by frequent recurrences. In France and Germany, the ALFA (Etude en Activité Libérale de la Fibrillation Auriculaire)23 and ATRIUM (OutpAtienT RegIstry Upon Morbidity of atrial fibrillation)19 studies showed that the mean time elapsed between the first episode of AF and the date of patient assessment was 47±63 months and 61±66 months, respectively; in Italy, the ISAF (Italian Survey of Atrial Fibrillation management) study showed that the history of AF was less than one year in only 13% of patients in whom AF was detected, 5–10 years in 30%, and >10 years in 18%. Approximately one fifth of AF patients are found to have suffered at least two recurrences during the previous year of observation and three quarters during the previous 5 years.9,19,22,23 AF is often associated with symptoms, sometimes disabling, that are resistant to drug therapy and necessitate transcatheter ablation. More specifically, despite adequate treatment, palpitations occur in 42%–55% of patients, asthenia in 15%–49%, dyspnea in 24%–49%, and angina in 10%–20%. Arrhythmia occurs without symptoms in only 12%–25% of patients with AF.9,19,21,23 The vast majority of patients have a European Heart Rhythm Association score of II to IV; those with permanent AF complain more frequently of dyspnea or asthenia and fatigue, and those with the paroxysmal form complain more of palpitations.19,23,24 The high rates of recurrence, disturbing symptoms, and clinical sequelae (stroke, heart failure, initiation of new antiarrhythmic drugs, drug-related complications, interventional therapy) contribute strongly to the use of health care resources. In Italy, AF is the cause of 1.5% of all emergency room visits; in Germany, the average number of hospitalizations with an AF diagnosis is 0.24 per year and the average number of outpatient consultations is 5.62 per year; in Scotland, the one-year out-of-hospital contact rate for AF is eight per 1,000 person/years, ranging from 0.2 in subjects aged <45 years to 62 per 1,000 person/years in those aged 74–85 years.4,6,8,9 In the USA, the bed utilization for AF as a primary diagnosis was approximately one million days in 1991 and 1.38 million days in 1999, representing a bed utilization that is similar to that needed for all the other cardiac arrhythmias combined. RealiseAF (REAl-LIfe global Survey Evaluating patients with Atrial Fibrillation) showed that one third of patients with AF had been hospitalized in the previous 12 months.24 Acute decompensation of heart failure is the leading cause of hospitalization in the persistent and permanent forms of AF (12.9% and 13.6%, respectively), followed by an acute coronary event (7.7% and 7.3%, respectively) and stroke (5% and 7.4%, respectively). The need for new use of antiarrhythmic drugs and proarrhythmic events from antiarrhythmic drugs are the most frequent causes of hospitalization among patients with paroxysmal AF (12.3%).24,25
Figure 2.
Frequency of the different types of atrial fibrillation.
Abbreviations: ATRIUM, OutpAtienT RegIstry Upon Morbidity of atrial fibrillation; ISAF, Italian Survey of Atrial Fibrillation management; Euro Heart S, Euro Heart Survey; AFNET, Central Registry of the German Competence Network on Atrial Fibrillation; ATA-AF, AntiThrombotic Agents in Atrial Fibrillation; pts, patients; Parox, paroxysmal; Pers, persistent; Perm, permanent.
AF is frequently associated with cardiac disease and with cardiac/noncardiac comorbidity. Hypertensive heart disease (22%–36%), coronary heart disease (14%–32%), valvular heart disease (12%–26%), and cardiomyopathy (6%–10%) are the most common illnesses associated with AF, while the most frequent comorbidities are hypertension (67%–76%), heart failure (22%–42%), diabetes (20%–24%), obesity (20%–35%), chronic pulmonary disease (10%–18%), thyroid dysfunction (8%–11%), renal failure (11%–22%) stroke/transient ischemic attack (9%–16%), and neuropsychiatric disturbances (19%). In particular, one third of AF patients have at least three associated comorbidities; no comorbidity or cardiac disease is present in one fifth and one quarter of patients, respectively. Lone AF (defined on the basis of the strict criteria of the European Society of Cardiology) is present in a low proportion of cases, ranging between 2% and 12%.4,9,16,20–23 Patients with paroxysmal AF are younger and have a lower frequency of heart disease and comorbidity than those with permanent AF.16,19–21
Progression to permanent AF
In the past, the majority of AF studies have been carried out by considering AF as a whole, without a clear distinction of the subtypes. As shown previously, the different types of AF are associated not only with different clinical patient profiles, but also with different long-term outcomes. Epidemiological studies have shown also that the clinical setting of AF is an evolving picture that generally begins with the paroxysmal form and ends with the permanent form, covers different clinical pathways and treatment strategies, and results in different long-term outcomes.26–30 From these studies, it appears that the progression from paroxysmal to permanent AF is characterized by a trend that shows an initial peak rate at the first year after AF diagnosis followed by a continuous “crescendo” that depends on the clinical profile of the patient and the site of the patient’s clinical management. As reported by De Sisti et al in France and by the Canadian Registry of Atrial Fibrillation investigators in Canada, during the first year, AF evolves to the permanent form in approximately 4% of patients managed in tertiary arrhythmia centers and in 9% of those managed in the real-world setting. Over time, the progression becomes less evident, with a slow but steady trend that is strictly related to poorer clinical conditions and reaches rates of 18% and 25%, respectively, at 5 years of follow-up. In particular, progression is slower for the paroxysmal form and faster for the persistent form.27,28 In Europe, the Euro Heart Survey investigators reported a progression to permanent AF in 15% of patients with paroxysmal AF and 30% of those with persistent AF.29 These rates are significantly higher than those found in Canada, but in the European investigation patients showed either less severe cardiac disease or comorbidities. This consideration is consistent with the finding that the variables independently related to progression to permanent AF are age, an enlarged left atrium, lack of antiarrhythmic drugs, VVI pacing, and the presence of valvular heart disease, heart failure, hypertension, or chronic obstructive pulmonary disease.9,26–29 In this regard, the HATCH score (an acronym for “heart failure, age, previous transient ischemic attack, chronic pulmonary disease, hypertension”) can predict the risk with satisfactory power (area under the curve 0.68).31
Prognosis
The largest epidemiological studies have clearly shown that AF is associated with an increased risk of stroke (both ischemic and hemorrhagic), hemorrhagic consequences (due to anticoagulation therapy), and death. Cognitive dysfunction, heart failure, and socioeconomic implications are further important consequences of AF. It is estimated that 20% of all strokes occur in the setting of AF; this rate increases to 25% in patients aged >80 years. Patients with AF have an age-adjusted risk of stroke that is five-fold higher than in the normal population regardless of the type of AF.32–34 Since the introduction of systematic use of anticoagulants, the absolute number of ischemic strokes has decreased dramatically. In USA, the rate of ischemic stroke among Medicare beneficiaries with AF aged 65 years or older was 48 per 1,000 person/years in 1992 and 17 per 1,000 person/years in 2007; this means a 65% decrease in the ischemic stroke rate over 15 years.35 In Sweden, the annual rate of ischemic stroke in the global population of AF patients is 25 per 1,000 person/years among those treated with anticoagulants and 45 per 1,000 person/years in those who are not treated.36 In France, the cumulative incidence of ischemic stroke is ten per 1,000 person/years in patients with a CHADS2 score ≥1.37 In such countries, during the same periods of observation and despite the wider use of anticoagulants, the rate of intracranial hemorrhage has remained unchanged at a rate of two per 1,000 person/years.35,36 Stroke secondary to AF is associated with a 50% increased risk of serious disability. Cognitive dysfunction, including vascular dementia, is present in 10%–15% of patients with AF, which is twice the rate in patients without AF. Cognitive disturbances are also frequently found in AF patients, even in the absence of overt stroke, as a consequence of multiple asymptomatic cerebral emboli that can be found on cerebral imaging in a discrete percentage of cases.9,38,39
AF and heart failure coexist in a large percentage of patients (22%–42%) and share risk factors. In addition, each of these conditions strongly predisposes to the other.40 Women and men with AF have an eleven-fold and three-fold higher risk, respectively, of developing heart failure in comparison with those without AF. Both these conditions are associated with an adverse prognosis “quod vitam”, and the occurrence of new AF in patients with heart failure is associated with a two-fold higher risk of death in comparison with those without AF.41–43
AF is also associated with increased mortality. In the USA, among Medicare beneficiaries aged 65 years or older followed from 1993 to 2007, the global mortality rate after incident AF was 10.8% in the short-term (30 days), 24.7% in the medium term (one year), and 42% in the long term (3 years). These rates have changed only slightly and not significantly over time.10 In Sweden, during the period 1995–2008, patients enrolled in the Swedish National Patient Registry showed a global mortality rate of 40% after diagnosis of incident AF and 60% at 5 and 10 years, respectively, versus a rate of 20% and 40% in those without AF. The relative risk of dying was higher in women than in men and decreased with age. Women and men aged <65 years had an adjusted relative risk of 4.88 and 3.07, respectively, of dying at one year, those aged 65–74 years had a risk of 2.88 and 2.07, and those aged 75–85 years had a risk of 2.09 and 1.72. AF was found to be a significant risk factor for all-cause mortality together with the presence of chronic renal failure, cancer, and chronic obstructive pulmonary disease.44 Also, in Olmsted County, during a period of observation of 24 years, new-onset AF was found to be associated with a significantly increased risk (67%) of all-cause mortality that was highest (hazard ratio 9.62) within the first 4 months of diagnosis and decreased significantly thereafter (hazard ratio 1.66).45
The relationship between mortality and type of AF is not clear. In the Women’s Health Study, persistent and permanent AF (but not paroxysmal AF) have been found to be significantly associated with mortality,46 and in the Loire Valley AF Project, only permanent AF was found to be significantly associated with mortality.47 Thus, even though there is not yet clear evidence that the increased mortality observed in patients with AF is directly due to arrhythmia or to the numerous serious associated conditions, AF remains a marker of a poor prognosis.
European perspective
Although the marked differences in population characteristics present in the majority of the published epidemiological studies (eg, different ages at enrolment, different types of AF considered), the available data indicate a similar prevalence and incidence of AF throughout the developed world. At the present time, among residents aged 15–20 years or over in Europe, the prevalence of AF can be estimated at 2%, together with an incidence ranging between 0.23 and 0.41 per 1,000 person/years. Among these patients, the annual rate of ischemic and hemorrhagic strokes is estimated to be 2% (1.3% in France, 1.7% in the USA, 4.5% in patients not anticoagulated and 2.5% in patients anticoagulated in Sweden) and 0.2%, respectively. The number of hospitalizations for AF is 0.25 per year and the number of outpatient contacts is approximately six per year. This means that at the present time in the European community (population 500 million people) there are approximately 10 million patients with AF and 100,000–200,000 with new-onset AF. If the estimated prevalence of AF increases by 0.04% per year (as observed in Iceland in the overall population between 1998 and 2008) or by 0.08% (as estimated in the USA on a population aged >20 years),3 and if the projected growth of the European population is 0.2%–0.3% (a combination of a decrease in the young population and an increase among people aged >65 years),2 in 2030 the prevalence of AF would be 2.7%–3.3% in a European population with 516–525 million inhabitants. Therefore, within 15 years, the number of the European citizens with AF will be 14–17 million and the number of new AF cases will be 120,000–215,000 per year (estimated incidence 0.23–0.41 per 1,000 person/years). In particular, 7–8.5 million people will be affected by permanent AF, 3.5–4.2 by persistent AF, and 3.5–4.2 by paroxysmal AF. These data are in agreement with those of Krijthe et al, who predict that there will be approximately 14 million AF patients among individuals aged >55 years in 2030.48 To these figures must be added a further 280,000–340,000 new ischemic strokes, 3.5–4 million hospitalizations for AF, and 100–120 million outpatient visits. The magnitude of these data seems to confer an endemic dimension to this health care problem, implying not only a greater engagement of physicians but also a significant effort of health care systems to improve AF prevention and treatment and to facilitate the organization of social interventions for the cure of its consequences. In the absence of proven effective therapies for the primary prevention of AF, the attention of physicians must be directed towards preventing the cardiac conditions and comorbidities that represent significant risk factors for AF. In particular, further efforts are needed to understand better the longitudinal course of patients at risk of developing AF in order to prevent complications.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 2.Eurostat Methodologies and Working papers Work Session on Demographic Progressions Lisbon, Portugal: April28–302010 [Google Scholar]
- 3.Go AS, Hylek EM, Philips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implication for rhythm management and stroke prevention. The AnTicoagulation and RIsk factors in Atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Murphy NF, Simpson CR, Jhund PS, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93:606–612. doi: 10.1136/hrt.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruskin JN, Singh JP. Atrial fibrillation endpoints: hospitalizations. Heart Rhythm. 2004 Jul;1(Suppl):B31–B35. doi: 10.1016/j.hrthm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Santini M, De Ferrari GM, Pandozi C, et al. FIRE Investigators Atrial fibrillation requiring urgent medical care. Approach and outcome in the various departments of admission. Data from the atrial Fibrillation/Flutter Italian Registry (FIRE) Ital Heart J. 2004;5:205–213. [PubMed] [Google Scholar]
- 7.Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461–468. doi: 10.1111/joim.12114. [DOI] [PubMed] [Google Scholar]
- 8.Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 9.Zoni-Berisso M, Filippi A, Landolina M, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation [From the Italian Survey of Atrial Fibrillation Management (ISAF) Study] Am J Cardiol. 2013;111:705–711. doi: 10.1016/j.amjcard.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Piccini JP, Hammil BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefansdottir H, Appelund T, Gudnason V, Adnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projection. Europace. 2011;13:1110–1117. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 12.Cowan C, Healicon R, Robson I, et al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart. 2013;99:1166–1172. doi: 10.1136/heartjnl-2012-303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzmaurice DA, Hobbs DR, Jowet S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:386–391. doi: 10.1136/bmj.39280.660567.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rho RW, Page RL. Asymptomatic atrial fibrillation. Progr Cardiovasc Dis. 2005;48:78–87. doi: 10.1016/j.pcad.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Doblas JJ, Muniz J, Alonso Martin JJ, et al. Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev Esp Cardiol. 2014;67(4):259–269. doi: 10.1016/j.rec.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Abbot RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham Study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 18.Myasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and the implications on the future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 19.Meinertz T, Kirch, Rosin L, Pittrow D, Wilich SN, Kirchof P, ATRIUM Investigators Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100:897–905. doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabauer M, Gerth A, Limbourg T, et al. The Registry of German Competence Network on atrial fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieuwlaat R, Capucci A, Camm AJ, et al. European Heart Survey Investigators Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 22.Di Pasquale G, Mathieu G, Maggioni AP, et al. ATA-AF Investigators Current presentation and management of 7148 patients with atrial fibrillation in cardiology and internal medicine hospital centers: the ATA-AF study. Int J Cardiol. 2013;167:2895–2903. doi: 10.1016/j.ijcard.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Levy S, Maarek M, Coumel P, et al. College of French Cardiologists Characterization of different subsets of atrial fibrillation in general practice in France. The ALFA study. Circulation. 1999;99:3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CE, Naditch-Brulé L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice. insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 25.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–e146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year. Follow up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 27.De Sisti A, Leclercq JF, Halimi F, Fiorello P, Bertrand C, Attuel P. Evaluation of time course predicting factors of progression of paroxysmal or persistent atrial fibrillation to permanent atrial fibrillation. Pacing Clin Electrophysiol. 2014;37:345–355. doi: 10.1111/pace.12264. [DOI] [PubMed] [Google Scholar]
- 28.Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 29.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation, A 30-year follow up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 30.De Voos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation. Clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 31.Self WH, Wasserman BS, McNaughton CD, Darbar D. Evaluating the HATCH score for predicting progression to sustained atrial fibrillation in ED patients with new atrial fibrillation. Am J Emerg Med. 2013;31:792–797. doi: 10.1016/j.ajem.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Heart Association . Heart and Stroke Statistical Update. Dallas, TX, USA: American Heart Association; 1999. [Accessed May 29, 2014]. Available from: http://www.nanocorthx.com/Articles/HeartDiseaseStrokeStatistics.pdf. [Google Scholar]
- 33.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 34.Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockolm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–972. doi: 10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]
- 35.Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among Medicare patients with atrial fibrillation: a 15-year perspective (1992–2007) JAMA Intern Med. 2013;173:159–160. doi: 10.1001/jamainternmed.2013.1579. [DOI] [PubMed] [Google Scholar]
- 36.Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin use in patients with atrial fibrillation: a report of the Swedish Atrial Fibrillation Cohort Study. Circulation. 2012;125:2298–2307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- 37.Cotté FE, Chaize G, Kachaner I, Gaudin AF, Vainchtock A, Durand-Zaleski L. Incidence and cost of stroke and haemorrhage in patients diagnosed with atrial fibrillation. J Stroke Cerebrovasc Dis. 2014;23:73–83. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Ott AO, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hoffman A. Atrial fibrillation and dementia in a population-based study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 39.Cha MJ, Park H, Lee MH, Cho Y, Choi EK, Oh S. Prevalence and risk factors for silent ischemic stroke in patients with atrial fibrillation as determined by brain magnetic resonance imaging. Am J Cardiol. 2014;113:656–661. doi: 10.1016/j.amjcard.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 42.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get with the Guidelines – Heart Failure. Circ Heart Fail. 2012;5:191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4:740–746. doi: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson T, Magnuson A, Bryngelson IL, et al. All cause mortality in 272186 patients hospitalized with incident atrial fibrillation1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 46.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benerjee A, Taillandier S, Olesen JB, et al. Pattern of atrial fibrillation and risk of outcomes: the Loire Valley Atrial Fibrillation Project. Int J Cardiol. 2013;167:2682–2687. doi: 10.1016/j.ijcard.2012.06.118. [DOI] [PubMed] [Google Scholar]
- 48.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections of the number of individuals with atrial fibrillation in the European Union from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]