Abstract
Adenosine triphosphate is a critical neurotransmitter in the gustatory response to the 5 primary tastes in mice. Genetic deletion of the purinergic P2X2/P2X3 receptor greatly reduces the neural and behavioral response to prototypical primary taste stimuli. In this study, we examined the behavioral response of P2X double knockout mice to maltodextrin and fat stimuli, which appear to activate additional taste channels. P2X double knockout and wild-type mice were given 24-h choice tests (vs. water) with ascending concentrations of Polycose and Intralipid. In Experiment 1, naive double knockout mice, unlike wild-type mice, were indifferent to dilute (0.5–4%) Polycose solutions but preferred concentrated (8–32%) Polycose to water. In a retest, the Polycose-experienced double knockout mice, like wild-type mice, preferred all Polycose concentrations. In Experiment 2, naive double knockout mice, unlike wild-type mice, were indifferent to dilute (0.313–2.5%) Intralipid emulsions but preferred concentrated (5–20%) Intralipid to water. In a retest, the fat-experienced double knockout mice, like wild-type mice, strongly preferred 0.313–5% Intralipid to water. These results indicate that the inherent preferences of mice for maltodextrin and fat are dependent upon adenosine triphosphate taste cell signaling. With experience, however, P2X double knockout mice develop strong preferences for the nontaste flavor qualities of maltodextrin and fat conditioned by the postoral actions of these nutrients.
Key words: experience, Intralipid, Polycose, postoral conditioning
Introduction
There is widespread agreement that humans and many other mammals have 5 basic tastes (bitter, sour, salty, umami, and sweet). However, there is evidence that other taste modalities exist in some mammalian species. In 1987, we proposed that rats and other rodents taste starch-derived maltodextrins (e.g., Polycose) as distinct from sugars and subsequently suggested maltodextrin as a “sixth” taste modality (Sclafani 1987, 2004). The existence of a distinctive maltodextrin taste was initially suggested by the findings that rats prefer Polycose to water at very low to high concentrations and do not cross-generalize conditioned taste aversions to Polycose and sucrose (Sclafani 1987). Subsequent electrophysiological findings also indicated that Polycose and sucrose activated separate taste channels in rats (Somenarain and Jakinovich 1990; Giza et al. 1991; Sako et al. 1994). Consistent with these early findings, recent mouse studies revealed that deletion of one or both components (T1R2 and T1R3) of the sweet taste receptor substantially impairs the attraction to sucrose but not to Polycose (Treesukosol et al. 2009, 2011; Zukerman, Glendinning, et al. 2009; Treesukosol and Spector 2012). The receptor responsible for Polycose taste has yet to be identified, but it appears to activate the same downstream taste signaling elements as sucrose. This is indicated by the preference deficits for sucrose and Polycose displayed by mice missing the G protein gustducin and the Ca2+-activated cation channel Trpm5 (Sclafani, Zukerman, et al. 2007). Sucrose and other primary tastants stimulate the release of adenosine triphosphate (ATP) as a neurotransmitter that activates purinergic receptors (P2X2/P2X3) on gustatory nerve fibers (Kinnamon and Finger 2013). Double knockout (P2X DoKO) mice missing the P2X2/P2X3 receptor do not prefer sucrose or other sweeteners in 24-h 2-bottle tests, but their response to Polycose was not measured (Finger et al. 2005). In Experiment 1 of this study, we determined if P2X2/P2X3 receptors also mediate Polycose taste by comparing Polycose preferences in P2X DoKO mice and wild-type (WT) mice. We also determined if prior Polycose experience influenced preferences in P2X DoKO mice as it does in other taste-impaired KO mice (gustducin KO, Trpm5 KO) (Sclafani, Zukerman, et al. 2007).
Another candidate “sixth” taste has been proposed, that is, the taste for fat (or fatty acids) (Mattes 2003; Passilly-Degrace et al. 2014). Although the preference for fatty foods has long been assumed to be due to their textural and olfactory properties, there is now substantial evidence for a gustatory component to fat detection (Montmayeur and le Coutre 2010). Furthermore, putative taste receptors have been identified including the fatty acid–binding protein CD36 and the G proteins GPR120 and GPR40 (Laugerette et al. 2005; Cartoni et al. 2010). In particular, CD36 KO, GPR120 KO, and GPR40 KO mice displayed reduced gustatory neural responses to fatty acids as well as attenuated preferences for fatty acid solutions (e.g., linoleic acid), but normal responses to stimuli representing sweet, bitter, salty, and sour tastes (Laugerette et al. 2005; Sclafani, Ackroff, et al. 2007; Gaillard et al. 2008; Cartoni et al. 2010). In addition, CD36 KO mice display reduced preferences for fat (soybean oil) emulsions (Sclafani, Ackroff, et al. 2007). Unlike sweet and Polycose preference, fat preference does not involve signaling by gustducin but does require Trpm5 signaling (Sclafani, Zukerman, et al. 2007; Cartoni et al. 2010; Liu et al. 2011). The role of ATP neurotransmission in fat taste signaling is not known (Abdoul-Azize et al. 2013), and in Experiment 2, we determined if deletion of the purinergic receptor P2X2/P2X3 impairs fat preference in mice. We also determined if prior experience with fat influenced preferences in P2X DoKO mice as it does in other taste-impaired KO mice (CD36 KO and Trpm5 KO) (Sclafani, Ackroff, et al. 2007; Sclafani, Zukerman, et al. 2007).
Experiment 1: Maltodextrin preference
Materials and methods
Animals
Naive adult male P2X2/P2X3 double knockout mice (P2X DoKO, n = 11, 11–14 weeks old) and WT mice (n = 11, 10–12 weeks old) were used. As previously described, these mice were generated on a mixed C57BL/6 and 129Ola background (Finger et al. 2005; Stratford and Finger 2011). The DoKO mice weighed more than WT mice (29.2 vs. 26.2g, P < 0.01). The animals were singly housed in plastic tub cages with ad libitum access to chow (LabDiet 5001; PMI Nutrition International) and water in a room maintained at 22 °C with a 12:12h light:dark cycle. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Test solutions
Polycose (Polycose Powder, 00746, Ross Laboratories) solutions were prepared at concentrations of 0.005–32%, and a saccharin (sodium saccharin, S1002, Sigma Chemical Co.) solution was prepared at a 0.2% concentration. The solutions were made using deionized water on a w/w basis because solution intakes were recorded by weight.
Procedure
The mice were given a 2-day choice test with 0.2% saccharin versus water to confirm their ageusic and normal phenotypes, respectively, as in prior studies (Zukerman, Glendinning, et al. 2009; Zukerman, Glendinning, et al. 2013). Three days later, they were given a series (Test 1) of 24-h 2-bottle Polycose versus water tests at Polycose concentrations of 0.5%, 1%, 2%, 4%, 8%, 16%, and 32%. The mice were then given water only for 4 days, followed by a second series (Test 2) of preference tests with Polycose concentrations of 0.005%, 0.05%, 0.5%, 1%, 2%, 4%, and 8% (w/w). Each concentration was presented for 2 days in an ascending order, and the left-side positions of the bottles were alternated daily.
Data analysis
Fluid intakes were averaged over the 2 days at each concentration. Polycose preferences were also expressed as percent intakes (Polycose solution intake/total intake × 100). Group differences in Polycose intakes and preferences were evaluated using separate mixed model analyses of variance (ANOVAs) with genotype and concentration as between-group and within-group factors, respectively. Significant differences between Polycose and water intake at each concentration were evaluated within each group using paired t-tests corrected for multiple comparisons using the Bonferroni procedure. Because the P2X DoKO mice weighed more than WT mice, Polycose intakes were also expressed as intake per 30g body weight (BW), as in prior studies (Bachmanov, Tordoff, et al. 2001; Sclafani, Zukerman, et al. 2007; Glendinning et al. 2008), and group differences were evaluated with ANOVA. The results of this ANOVA were only slightly different from the analysis of the absolute intake data (see below) and only the absolute data are presented in detail.
Results
In the pretest, the DoKO mice did not differ in their saccharin and water intakes (3.9 vs. 4.3g/day), whereas the WT mice consumed more (P < 0.01) saccharin than water (8.5 vs. 1.5g/day).
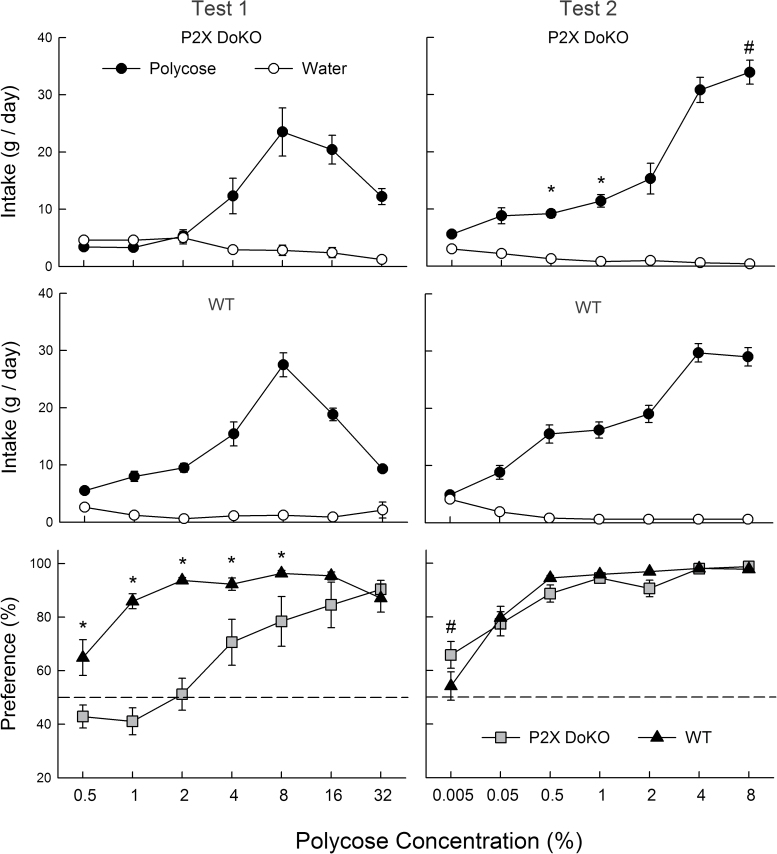
Overall, in Test 1, the P2X DoKO and WT mice did not differ in their Polycose solution intakes and they increased and then decreased their intakes as concentration increased [F(6,120) = 55.7, P < 0.001] (Figure 1). The DoKO mice tended to consume less of the dilute (0.5–8%) concentrations but more of the 32% concentration [Group × Concentration interaction, F(6,120) = 2.1, P < 0.05], but there were no significant differences at individual concentrations. With respect to Polycose preference (Figure 1), the percent intakes of the WT mice exceeded (P < 0.05) that of the DoKO mice at 0.5–8% concentrations [Group × Concentration interaction, F(6,120) = 14.8, P < 0.001]. Furthermore, whereas the WT mice drank significantly more Polycose than water at all concentrations, the P2X DoKO mice drank significantly more Polycose only at 8–32% concentrations. Analysis of the Polycose data expressed as intake per 30g BW (data not shown) revealed a marginal Group × Concentration interaction [F(6,120) = 2.15, P < 0.053]. In this analysis, the P2X DoKO mice consumed less (P < 0.05) Polycose than WT mice at 1–8% concentrations.
Figure 1.
Intake (means ± SE) of Polycose solutions and water for the P2X DoKO group (top) and WT group (middle) and percent preference for Polycose over water for both groups (bottom) during 2-bottle Polycose versus water choice tests. In Test 1 (left), naive mice were tested with 0.5–32% Polycose. In Test 2 (right), the same mice were tested with 0.005–8% Polycose. An asterisk (*) indicates significantly (P < 0.05) higher values in the WT than P2X DoKO group; a number sign (#) indicates a significantly higher value in the P2X DoKO than WT group.
When retested with Polycose at a lower concentration range (Test 2), there were no overall group differences in Polycose intake or preference, but there were some concentration-specific differences (Figure 1). In particular, the P2X DoKO mice consumed less (P < 0.05) 0.5–1% Polycose than WT mice but more 8% Polycose [Group × Concentration interaction, F(6,120) = 8.0, P < 0.01]. When expressed as intake per 30g BW (data not shown), the P2X DoKO mice consumed less 0.05–2% Polycose than did WT mice, but 8% Polycose intakes did not differ. The DoKO group had a higher (P < 0.01) percent intake of 0.005% Polycose than WT mice, but otherwise the groups displayed very similar preference for 0.05–8% Polycose [Group × Concentration interaction, F(6,120) = 2.4, P < 0.05] and both groups consumed significantly more Polycose than water at 0.5–8% concentrations (Figure 1).
Experiment 2: Fat preference
Materials and methods
Animals
Naive adult P2X2/P2X3 DoKO (6 males and 7 females, 20–23 weeks old) and WT controls (8 males and 8 females, 16–22 weeks old) were used. The DoKO and WT mice did not significantly differ in body weights (27.7±1.1 vs. 28.5±1.2g), but males weighed more than females (31.4±0.4 vs. 25.0±1.0g, P < 0.01). The animals were housed as in the first experiment.
Test solutions
Intralipid emulsions were prepared using 20% Intralipid (2B6023, Baxter), a stable fat emulsion containing 20% soybean oil, 2.25% glycerol, and 1.2% egg yolk phospholipids. The 20% Intralipid was diluted with deionized water to produce emulsions that contained 0.003–10% soybean oil.
Procedure
The DoKO and WT mice were given a 2-day choice test with 0.2% saccharin versus water. Five days later, they were given a series (Test 1) of 2-bottle Intralipid versus water tests at Intralipid concentrations of 0.313%, 0.625%, 1.25%, 5%, 10%, and 20%. The mice were then given water only for 4 days, followed by a second test series (Test 2) at Intralipid concentrations of 0.003%, 0.03%, 0.313%, 0.625%, 1.25%, and 5%. Each concentration was presented for 2 days in an ascending order and the left-side positions of the bottles were alternated daily. The data were analyzed as in the first experiment.
Results
In the pretest, the DoKO mice did not differ in their saccharin and water intakes (3.2 vs. 3.2g/day), whereas the WT mice consumed significantly (P < 0.01) more saccharin than water (8.5 vs. 0.7g/day).
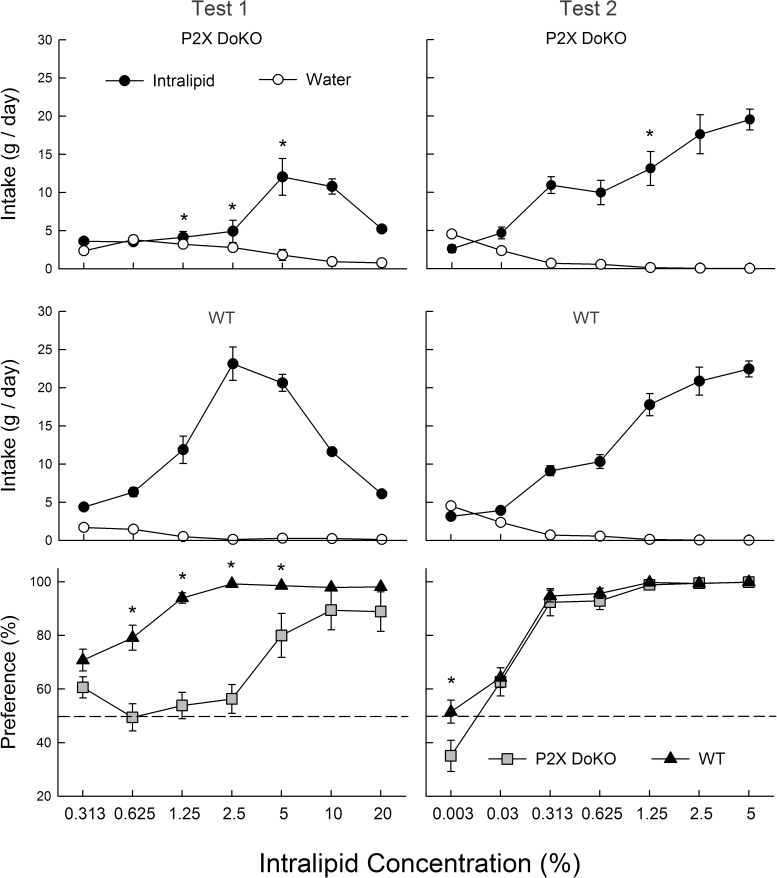
In Test 1, overall, the P2X DoKO mice consumed less Intralipid than WT mice [6.2 vs. 12.0g/day, F(1,27) = 22.6, P < 0.001] (Figure 2). Both groups increased and then decreased their Intalipid intake as concentrations increased [F(6,162) = 52.2, P < 0.001], but the DoKO mice consumed less (P < 0.001) than WT mice at 1.25–5% concentrations [Group × Concentration interaction, F(6,162) = 23.6, P < 0.001]. With respect to fat preference, the WT mice consumed significantly more Intralipid than water at all concentrations, whereas the DoKO mice preferred Intralipid to water only at 5–20% concentrations (Figure 2). Their percent Intralipid preferences were lower than those of the WT mice at 0.625–5% concentrations [Group × Concentration interaction, F(6,162) = 8.4, P < 0.001]. One unusual male DoKO mouse strongly avoided Intralipid at 0.625% and 5–20% concentrations, but there were no overall sex differences in Intralipid intake or preference. There was a Sex × Concentration interaction [F(6,150) = 2.6, P < 0.05] and males consumed more 1.25% and 2.5% Intralipid than females.
Figure 2.
Intake (means ± SE) of Intralipid solutions and water for the P2X DoKO group (top) and WT group (middle) and percent preference for Intralipid over water for both groups (bottom) during 2-bottle Intralipid versus water choice tests. In Test 1 (left), naive mice were tested with 0.313–20% Intralipid. In Test 2 (right), the same mice were tested with 0.003–5% Intralipid. An asterisk (*) indicates significantly (P < 0.05) higher values in the WT than P2X DoKO group.
In Test 2, a lower range of Intralipid concentrations (0.003–5%) was presented and there were no overall group differences in intake or preference and only a few concentration-specific differences (Figure 2). Both the P2X DoKO and WT groups increased Intralipid intake as concentration increased and they differed only in that the WT mice consumed somewhat more (P < 0.05) 1.25% Intralipid than DoKO mice [Group × Concentration interaction, F(6,162) = 3.3, P < 0.001]. The WT mice also had a higher percent intake of 0.003% Intralipid than did the DoKO mice [Group × Concentration interaction, F(6,162) = 2.1, P < 0.05] (Figure 2). Otherwise, the 2 groups showed very similar Intralipid preferences at the 0.03–5% concentrations. However, whereas the WT mice consumed significantly more 0.03% Intralipid than water, this difference was not significant with the DoKO mice because of greater within-group variability. The DoKO mice significantly preferred Intralipid to water at 0.313% and higher concentrations. Note that the atypical male DoKO mouse that avoided Intralipid in Test 1 strongly preferred 1.25–5% Intralipid in Test 2.
General discussion
The present findings revealed significant preference deficits for maltodextrin (Polycose) and fat (Intralipid) in P2X DoKO mice. This extends the original findings of sucrose and monosodium glutamate preference deficits in P2X DoKO mice (Finger et al. 2005). Thus, it appears that the tastes of maltodextrin and fat, like that of other nutrients, require ATP signaling on P2X2/P2X3 receptors for their normal detection.
Maltodextrin taste
The WT mice were very attracted to the Polycose solutions as evidenced by their robust preference and their high intakes. Similar results were obtained with C57BL/6 (B6) and 129 mice, which show Polycose concentration–response functions similar to those of sucrose, the prototypical sweet taste stimulus (Bachmanov, Reed, et al. 2001; Sclafani 2006; Sclafani, Zukerman, et al. 2007). Yet, behavioral and electrophysiological findings demonstrate that Polycose and sucrose represent separate taste qualities (Sclafani 1987, 2004). In particular, mice missing the sweet taste receptor components T1R2 and/or T1R3 show greatly attenuated responses to sucrose and other sweeteners but normal or near-normal responses to Polycose (Treesukosol et al. 2009, 2011; Zukerman, Glendinning, et al. 2009; Treesukosol and Spector 2012). Deletion of other taste signaling components, however, impairs both sucrose and Polycose preferences in mice. In 24-h tests, gustducin KO and Trpm5 KO mice, unlike WT mice, failed to prefer 0.5–4% Polycose to water (Sclafani, Zukerman, et al. 2007). Experiment 1 revealed that naive P2X DoKO mice also did not significantly prefer 0.5–4% Polycose solutions but displayed significant preferences for 8–32% Polycose. The preferences for concentrated solutions are likely due to the potent postoral actions of glucose, the digestive product of Polycose. B6 mice acquire significant preferences (80–90%) for flavored solutions paired with intragastric (IG) infusions of 8% maltodextrin or 8–32% glucose (Sclafani and Glendinning 2005; Sclafani and Ackroff 2012; Zukerman, Ackroff, et al. 2013). Consistent with a postoral interpretation of Polycose preference, food-restricted P2X DoKO mice, unlike WT mice, did not prefer 8% Polycose to water in 1-min choice tests, which minimize postoral nutritive actions, but subsequently displayed a robust (95%) Polycose preference in 24-h tests (unpublished findings).
Although the P2X DoKO mice were indifferent to dilute Polycose solutions in Test 1, they were similar to WT mice in significantly preferring 0.05–8% Polycose in Test 2. Thus, prior experience with concentrated Polycose solutions induced preferences for dilute Polycose solutions in DoKO mice. This is similar to significant preferences displayed by gustducin KO, Trpm5 KO, and T1r3 KO mice for dilute Polycose, glucose, or sucrose solutions after they developed preferences for concentrated saccharide solutions (Zukerman, Glendinning, et al. 2009; Zukerman, Touzani, et al. 2009; Zukerman, Glendinning, et al. 2013). These findings demonstrate the potency of glucose-containing saccharides to condition flavor preferences and the ability of ageusic mice to use remaining taste, olfactory, and/or textual cues to discriminate saccharide solutions from water. A role for olfaction in saccharide preference is indicated by the finding that olfactory bulbectomy attenuated the experience-induced preference for dilute sucrose solutions in T1r3 KO mice (Zukerman, Touzani, et al. 2009). Even in the absence of unique flavor cues, mice and rats can utilize sipper spout cues or location cues to develop postoral conditioned saccharide preferences (Elizalde and Sclafani 1990; de Araujo et al. 2008).
Further evidence for a critical role of ATP signaling in Polycose taste detection is provided by the recent finding that mice missing the calcium homeostasis modulator 1 (Calhm1), which is thought to mediate ATP release from taste cells, do not show the normal stimulation of licking in brief access tests with 3.2–32% Polycose solutions (Taruno et al. 2013). Long-term (24-h) Polycose preference tests were not conducted by Taruno et al., but we observed that Calhm1 KO mice displayed a near-total preference for 8% Polycose over water after 1-bottle experience with the saccharide (unpublished data). This is consistent with our recent report that Calhm1 KO mice acquired a strong sucrose preference after 24-h experience with the sugar (Sclafani et al. 2014).
Although P2X DoKO mice showed a marked reduction in Polycose preference, they consumed nearly as much Polycose as did WT mice. This contrasts with gustducin KO and Trpm5 KO mice which, even after developing a preference for Polycose, underconsumed 2–8% Polycose solutions relative to WT controls. The reason why gustducin KO and Trpm5 KO mice but not P2X DoKO display persistent reductions in Polycose intake is not known. It may be related, however, to background strain differences; the gustducin KO and Trpm5 KO are expressed on a B6 background and the P2X DoKO is expressed on a mixed B6×129 background.
Fat taste
In Experiment 2, the naive P2X DoKO mice, unlike WT mice, were indifferent to dilute (0.313–2.5%) Intralipid emulsions in Test 1 and preferred only more concentrated Intralipid (5–20%) emulsions. However, in Test 2, the fat-experienced DoKO mice preferred low-to-high Intralipid concentrations relative to water and consumed amounts comparable to those of WT mice. In a preliminary experiment reported in summary form, we observed similar preference deficits in P2X DoKO mice tested with 0.313–20% soybean oil emulsions (Ackroff and Sclafani 2010). Together, these findings provide further evidence for an important role for gustation and specifically for ATP neurotransmission in fat preference in mice.
The fat preference deficits displayed by the naive P2X DoKO mice are similar to those previously observed in Trpm5 KO and CD36 KO mice (Sclafani, Ackroff, et al. 2007; Sclafani, Zukerman, et al. 2007). These mice, like P2X DoKO mice, displayed no or attenuated preferences for soybean oil/Intralipid emulsions at low concentrations but significant preferences at high concentrations. Furthermore, they displayed normal fat preferences when retested with low oil concentrations. The experience-induced fat preference displayed by all 3 KO genotypes can be attributed to an acquired attraction to the nongustatory flavor properties (texture and odor) of fat conditioned by the nutrient’s postoral reinforcing actions. In B6 mice, IG infusions of 3.2–12.8% Intralipid condition significant flavor preferences (Sclafani and Glendinning 2005; Sclafani, Ackroff, et al. 2007; Sclafani et al. 2013; Ackroff and Sclafani 2014). Yet, although the KO mice are similar in their experience-induced fat preference, they differ in their fat acceptance. That is, in Test 2, the P2X DoKO and WT mice consumed comparable amounts of 2.5–5% Intralipid, whereas experienced Trpm5 KO and CD36 KO mice consumed less Intralipid at these concentrations than their WT controls. The reason for this discrepancy is not known, but as noted above, it may be related to background strain differences.
CD36, GPR120, and GPR40 have been implicated as fat taste receptors, and individual deletion of these receptors substantially attenuates fatty acid preferences in mice (Laugerette et al. 2005; Cartoni et al. 2010). However, we recently reported that GPR40 KO, GPR120 KO, and GPR40/120 DoKO mice, unlike CD36 KO mice, did not differ from WT mice in their preferences for low-to-high Intralipid concentrations (Sclafani, Ackroff, et al. 2007; Sclafani et al. 2013). The GPR40 KO and, to a greater degree, GPR40/120 DoKO mice were deficient, however, in their flavor conditioning response to IG Intralipid infusions (Sclafani et al. 2013). These findings suggest that GPR40 and GPR120 are more essential for the postoral than oral detection of fats. The involvement of other GPR fatty acid receptors and fatty acid sensitive potassium channels in fat taste requires further study (Gilbertson and Khan 2014).
In summary, this study extends prior reports of severe taste deficits in P2X DoKO mice and their ability to use postoral chemosensory cues to guide food/fluid selection (Finger et al. 2005; Eddy et al. 2009; Stratford and Finger 2011; Ohkuri et al. 2012). Whereas prior work focused on the 5 “primary” tastes, the present results implicate ATP neurotransmission in taste buds in the detection and preference for maltodextrin and fat. Recent studies suggest that maltodextrin and fat taste receptors exist in humans and, although not evoking strong sensations, they may influence exercise, metabolism, and perhaps food selection (Gant et al. 2010; Jeukendrup and Chambers 2010; Mattes 2011; Pepino et al. 2011; Keller et al. 2012).
Funding
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant [DK-031135]. The P2X2/P2X3 DoKO and WT mice were supplied by the Rocky Mountain Taste & Smell Center that is supported by the National Institute on Deafness and Other Communication Disorders Grant [P30 DC004657].
Acknowledgements
We thank D.A. Cockayne and Roche Palo Alto (Palo Alto, CA) for graciously permitting use of the P2X2/P2X3 DoKO and WT mice and T. Finger for providing the mice. We thank M. Riad and M. Bahrani for their expert technical assistance.
References
- Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA. 2013. Ca2+ signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie. 196:8–13 [DOI] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. 2010. Oral and post-oral determinants of dietary fat appetite. In: Montmayeur J-P, le Coutre J, editors. Fat detection: taste, texture, and post ingestive effects. Boca Raton (FL): Taylor & Francis; p. 295–321 [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. 2014. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav. 129:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. 2001. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 72:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. 2001. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 26:905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 30:8376–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. 2008. Food reward in the absence of taste receptor signaling. Neuron. 57:930–941 [DOI] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. 2009. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 34:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. 1990. Flavor preferences conditioned by intragastric Polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 47:63–77 [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. 2005. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310:1495–1499 [DOI] [PubMed] [Google Scholar]
- Gaillard D, Laugerette F, Darcel N, El Yassimi A, Passilly-Degrace P, Hichami A, Akhtar Khan N, Montmayeur JP, Besnard P. 2008. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 22:1458–1468 [DOI] [PubMed] [Google Scholar]
- Gant N, Stinear CM, Byblow WD. 2010. Carbohydrate in the mouth immediately facilitates motor output. Brain Res. 1350:151–158 [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Khan NA. 2014. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res. 53C:82–92 [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR, Sclafani A, Antonucci RF. 1991. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res. 555:1–9 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Feld N, Goodman L, Bayor R. 2008. Contribution of orosensory stimulation to strain differences in oil intake by mice. Physiol Behav. 95:476–483 [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Chambers ES. 2010. Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care. 13:447–451 [DOI] [PubMed] [Google Scholar]
- Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, Driggin E, Tepper BJ, Lanzano PC, Deng L, et al. 2012. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity Res. 20:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC, Finger TE. 2013. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 7:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 115:3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Shah BP, Croasdell S, Gilbertson TA. 2011. Transient receptor potential channel type M5 Is essential for fat taste. J Neurosci. 31:8634–8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes R. 2003. Fat: the sixth taste—implications for health. Food Aust. 55:510–514 [Google Scholar]
- Mattes RD. 2011. Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol Behav. 105:27–35 [DOI] [PubMed] [Google Scholar]
- Montmayeur J-P, le Coutre J, editors. 2010. Fat detection: taste, texture, and post ingestive effects. Boca Raton (FL): Taylor & Francis; [PubMed] [Google Scholar]
- Ohkuri T, Horio N, Stratford JM, Finger TE, Ninomiya Y. 2012. Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (P2X2/P2X3 double-KO) mice. Chem Senses. 37:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passilly-Degrace P, Chevrot M, Bernard A, Ancel D, Martin C, Besnard P. 2014. Is the taste of fat regulated? Biochimie. 96:3–7 [DOI] [PubMed] [Google Scholar]
- Pepino MY, Love-Gregory L, Klein S, Abumrad NA. 2011. The fatty acid translocase gene, CD36, and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 153:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. 1994. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 56:741–745 [DOI] [PubMed] [Google Scholar]
- Sclafani A. 1987. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev. 11:131–153 [PubMed] [Google Scholar]
- Sclafani A. 2004. The sixth taste. Appetite. 43:1–3 [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2006. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav. 87:745–756 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2012. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 106:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, Abumrad N. 2007. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 293:R1823–R1832 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. 2005. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 289:R712–R720 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Marambaud P, Ackroff K. 2014. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 126:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Ackroff K. 2013. GPR40 and GPR120 fatty acid sensors are critical for post-oral but not oral mediation of fat preferences. Am J Physiol Regul Integr Comp Physiol. 305:R1490–R1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. 2007. Fat and carbohydrate preferences in mice: the contribution of α-gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol. 293:R1504–R1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somenarain L, Jakinovich W., Jr 1990. Antagonism of the gerbil’s sweetener and Polycose gustatory responses by copper chloride. Brain Res. 522:83–89 [DOI] [PubMed] [Google Scholar]
- Stratford JM, Finger TE. 2011. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 31:9101–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495:223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde G, Spector AC. 2009. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 296:R855–R865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, Spector AC. 2011. Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci. 31:13527–13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. 2012. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 303:R218–R235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2013. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav. 109:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. 2009. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 296:R866–R876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. 2013. Impact of deleting T1r3 or Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses. 38:421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Touzani K, Margolskee RF, Sclafani A. 2009. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 34:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]