Abstract
Genetic ablation of calcium homeostasis modulator 1 (CALHM1), which releases adenosine triphosphate from Type 2 taste cells, severely compromises the behavioral and electrophysiological responses to tastes detected by G protein–coupled receptors, such as sweet and bitter. However, the contribution of CALHM1 to salty taste perception is less clear. Here, we evaluated several salty taste–related phenotypes of CALHM1 knockout (KO) mice and their wild-type (WT) controls: 1) In a conditioned aversion test, CALHM1 WT and KO mice had similar NaCl avoidance thresholds. 2) In two-bottle choice tests, CALHM1 WT mice showed the classic inverted U-shaped NaCl concentration-preference function but CALHM1 KO mice had a blunted peak response. 3) In brief-access tests, CALHM1 KO mice showed less avoidance than did WT mice of high concentrations of NaCl, KCl, NH4Cl, and sodium lactate (NaLac). Amiloride further ameliorated the NaCl avoidance of CALHM1 KO mice, so that lick rates to a mixture of 1000mM NaCl + 10 µM amiloride were statistically indistinguishable from those to water. 4) Relative to WT mice, CALHM1 KO mice had reduced chorda tympani nerve activity elicited by oral application of NaCl, NaLac, and sucrose but normal responses to HCl and NH4Cl. Chorda tympani responses to NaCl and NaLac were amiloride sensitive in WT but not KO mice. These results reinforce others demonstrating that multiple transduction pathways make complex, concentration-dependent contributions to salty taste perception. One of these pathways depends on CALHM1 to detect hypertonic NaCl in the mouth and signal the aversive taste of concentrated salt.
Key words: gustatory electrophysiology, NaCl, salt preference, salt taste
Calcium homeostasis modulator 1 (CALHM1) hexamers form membrane channels that permit the release of adenosine triphosphate (ATP) from Type 2 taste cells (Siebert et al. 2013; Taruno, Matsumoto, et al. 2013; Taruno, Vingtdeux, et al. 2013). CALHM1 is the culminating component of a cascade of intracellular events precipitated by a taste compound interacting with a G protein–coupled receptor (GPCR) and subsequently involving gustducin (or a related G protein), phospholipase C beta 2 (PLCβ2), inositol trisphosphate (IP3), and transient receptor potential subfamily M member 5 (TRPM5) cation channels. In response to TRPM5-mediated membrane depolarization and the initiation of action potentials, CALHM1 channels release ATP (Taruno, Vingtdeux, et al. 2013).
CALHM1 is restricted to Type 2 taste cells (Taruno, Vingtdeux, et al. 2013), which harbor the T1R, T2R, and other classes of GPCRs that are responsible for the detection of sweet, bitter, umami, and calcium compounds. ATP released from Type 2 cells activates P2X2 and P2X3 receptors on nerves that convey taste information to the brain (reviews by Kinnamon and Finger 2013 and Roper 2013). Consistent with this, genetic ablation of CALHM1 either eliminates or severely compromises Type 2 taste cell ATP release, chorda tympani nerve (CT) responses, and behavioral responses produced by GPCR-mediated tastes (Taruno, Vingtdeux, et al. 2013). ATP released from Type 2 cells may also interact with P2Y1 or other receptors on Type 3 taste cells (Huang et al. 2009), which raises the possibility that CALHM1-mediated ATP release from Type 2 cells may modulate the transmission of non-GPCR-mediated taste information originating in Type 3 cells.
The initial report of the taste-related phenotype of CALHM1 knockout (KO) mice focused on sweet and bitter transduction (Taruno, Vingtdeux, et al. 2013), but CALHM1 may also participate in the transduction of other taste qualities, including saltiness. CALHM1 KO mice had compromised responses to high concentrations of NaCl and KCl in brief-access gustometer tests. Moreover, wild-type (WT) control mice showed the classic inverted U-shaped NaCl concentration-response function in two-bottle choice tests, including a preference for 30 and 100mM NaCl over water, whereas CALHM1 KO mice did not prefer any concentration of NaCl over water; they also showed less avoidance of 1000mM NaCl, the highest concentration tested (see Figure S4 of Taruno, Vingtdeux, et al. 2013).
These findings related to salt taste do not reconcile easily with the notion of CALHM1 as an element of the GPCR taste transduction cascade in Type 2 taste cells because sodium, at least at low concentrations, is believed to be transduced by non-GPCR mechanisms probably related to amiloride-sensitive epithelial sodium channels (EnaCs; Brand et al. 1985; DeSimone and Ferrell 1985; Chandrashekar et al. 2010) in Type 1 or 3 taste cells. However, sodium transduction involves multiple pathways in rodents and another pathway involves an amiloride-insensitive mechanism, with TRPV1 channels being 1 candidate (Lyall et al. 2004). The relative contributions of the different sodium transduction mechanisms appear to be complex and concentration dependent. The lowest concentrations of NaCl detected by rodents and humans do not taste salty (Pfaffman et al. 1971; Yamamoto et al. 1994), and responses to threshold and suprathreshold concentrations of NaCl are sometimes independent (Contreras 1977; Colbert et al. 2004). Low concentrations are preferred, but high ones are aversive to mice, perhaps due to the activation of taste receptor cells that are sensitive to sour and bitter compounds (Oka et al. 2013).
Given the theoretical importance of understanding how CALHM1 ablation interferes with NaCl perception, it seemed worthwhile to characterize the salty taste–related phenotype of CALHM1 KO mice. To this end, here, we compared CALHM1 WT and KO mice: We assessed NaCl “recognition” thresholds, confirmed the presence of a behavioral phenotype using two-bottle choice tests of taste-experienced and naive animals, examined the effect of amiloride on brief-access responses to NaCl, and supported these behavioral findings with gustatory electrophysiology.
Methods
Subjects and maintenance
All experiments involved CALHM1 WT and KO mice. The knockout was produced commercially by genOway according to procedures detailed elsewhere (Dreses-Werringloer et al. 2013; Ma et al. 2012). It involved deletion of exon 1 of Calhm1 by homologous recombination in 129Sv embryonic stem cells, which were injected into C57BL/6J blastocysts. The resulting chimeric mice were crossed with C57BL/6J mice to establish germline transmission, and this was maintained by successive backcrossing to the C57BL/6J strain. The mice forming our breeding colony at Monell were from stock generated by Dr Kevin Foskett (University of Pennsylvania) which, in turn, were from stock generated by Dr Philippe Marambaud (Feinstein Institute for Medical Research).
Mice used in experiments described here were derived from parents from at least the 5th backcross generation. CALHM1 heterozygote (+/−) mice were mated brother-to-sister, and the resulting offspring were genotyped commercially (Transnetyx, Inc.). Each experiment involved CALHM1 homozygous (−/−) KO mice derived from several litters, along with homozygous (+/+) WT controls matched for litter and, when available, sex.
The mice were maintained in a vivarium at 23 °C on a 12:12h light/dark cycle with lights off at 7 PM. They were housed in plastic “tub” cages (26.5×17×12cm) with stainless steel grid lids and wood shavings scattered on the floor. The mice had ad libitum access to pelleted AIN-76A diet (Dyets Inc.) and deionized water (except during brief-exposure tests, see below). Pups were weaned at 21–23 days and initially housed in groups of the same sex. Before being tested, all mice were at least 8 weeks old and were individually housed for at least a week. Mice used for gustatory electrophysiology were shipped from Monell to Dr McCaughey’s laboratory at Ball State University in Muncie, Indiana, and allowed at least a week to recover before being tested. All procedures were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center. Gustatory electrophysiological studies were also approved by the Animal Care and Use Committee of Ball State University.
NaCl recognition threshold
Procedures
This experiment was conducted to determine whether the threshold for “recognition” of sodium-specific taste was influenced by genetic ablation of CALHM1. We used procedures described in detail elsewhere (Ishiwatari and Bachmanov 2009, 2012), which are designed to find the lowest concentration of NaCl that tastes salty to mice. We use the term “recognition” to describe this threshold value but only for lack of a better term. It can be considered analogous to recognition thresholds in humans and should be distinguished from the detection threshold (i.e., the lowest concentration that can be distinguished from water). The method takes advantage of the generalization of a conditioned taste aversion: Rodents that drink LiCl become temporarily ill. Because LiCl and NaCl have similar tastes, the association of the salty taste of LiCl with malaise causes the mice to avoid NaCl (Smith and Balagura 1969; Giza and Scott 1991).
The experiment was conducted in 2 identical replications except males were used in one and females in the other. There were a total of 8 WT males, 8 KO males, 7 WT females, and 8 KO females, with a mean age of 11 weeks (range: 8–18 weeks). The males weighed significantly more than did the females, but there was no significant difference in body weight related to genotype (males, WT = 23.6±0.8g, KO = 22.4±0.6g; females, WT = 17.8±0.7g, KO = 18.6±0.8g).
The individually housed mice were first given a choice between 2 bottles of water for 48h in order to familiarize them with having access to 2 drinking spouts. They then received 2 bottles of 150mM LiCl for 24h, 2 bottles of water for 24h to allow recovery, and then a second exposure to 2 bottles of 150mM LiCl for 24h. After a second 48-h test with 2 bottles of water, the mice received 48-h tests with a choice between water and an ascending series of NaCl concentrations (0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, and 150mM NaCl). A final choice between 2 bottles of water was conducted at the conclusion of the experiment. A modification from earlier methods (Ishiwatari and Bachmanov 2009, 2012) was that brand new drinking tubes and spouts were used for each 48-h test to eliminate any possibility of residual contamination in the plastic that might be detected by the mice. The stainless steel drinking spouts were washed 5 times with deionized water before being reused.
Statistical analysis
Results of the 2 replications were combined for data analysis. Student’s t-tests were used to assess differences between the genotypes in intakes during the training trials (i.e., when the mice received LiCl). Preference scores during the ascending concentration series were analyzed using a mixed-design ANOVA with factors of genotype and concentration. One mouse spilled NaCl during the test with 150mM NaCl. To allow inclusion of its data in the within-subject design, the missing value was interpolated. Recognition thresholds were considered to be the lowest NaCl concentration at which each genotype group’s preference scores fell significantly below indifference (50%) according to one-sample t-tests.
Two-bottle choice tests
Procedures
We conducted 2 experiments to assess the voluntary NaCl preferences of CALHM1 KO mice. The first involved replication of the original observations that CALHM1 KO markedly influenced preferences for sweet and bitter tastes and had little-or-no influence on preferences for salty and sour tastes (Taruno, Vingtdeux, et al. 2013). In this experiment, each mouse was tested with ascending concentrations of saccharin, QHCl, NaCl, and HCl. The second experiment was a concentration-response series for NaCl only and thus avoided any possibility that the response to NaCl could be influenced by carryover effects from experience with other taste solutions.
The first experiment included 10 CALHM1 KO mice (9 male, 1 female) and 11 WT controls (5 male, 6 female) aged 59–64 days. The CALHM1 WT mice weighed 19.3±0.7g and the CALHM1 KO mice weighed 19.8±0.6g. They received 4 series of two-bottle choice tests. Each series consisted of an initial 48-h choice between 2 bottles of deionized water and then three or four 48-h tests with a choice between deionized water and ascending concentrations of a taste compound. The taste compounds were chosen as exemplars of the sweet, bitter, salty, and sour taste qualities, and their concentrations (listed in Supplementary Table S1) spanned the range between indifference and marked acceptance or avoidance. The mice had 2 or 3 days with a single bottle of water to drink between each test series.
The second experiment included 8 female CALHM1 KO and 8 female WT mice aged 65–105 days. The KO mice weighed significantly less than did the WT mice (WT = 20.3±0.6g, KO = 17.8±0.3g, t(14) = 3.32, P = 0.0051). They received 48-h two-bottle choice tests, first with 2 bottles of water, then with water and each of 5 ascending concentrations of NaCl, and finally with water and 3.2mM saccharin (to confirm that the phenotype was present).
Specifics of construction of the drinking tubes and details of the measurement procedures are available online (Monell Mouse Taste Phenotyping Project website; Tordoff and Bachmanov 2001b). At the beginning of each 48-h test, the mice were presented with 2 drinking tubes with stainless steel spouts. These were placed to the (mouse’s) right of the food hopper. Their tips were 15mm apart and extended into the cage ~25mm (described in Bachmanov et al. 2002 and Tordoff and Bachmanov 2001a). Initially, the spout on the left provided water and the spout on the right provided taste solution. The level of fluid in each drinking tube was read volumetrically (to the nearest 0.1mL) when the tube was placed on the cage, 24h later when the position of the tubes was switched to control for side preferences, and at 48h, the end of the test. The change in fluid level was considered to be the mouse’s fluid intake (Spillage and evaporation using these procedures are < 0.2mL/day; Tordoff and Bachmanov 2003).
Statistical analysis
Intakes during each 48-h test were divided by 2 to provide average daily intakes of each fluid. Total fluid intake was calculated as the sum of intakes from the 2 bottles. Solution preference was calculated as the intake of taste solution divided by total fluid intake, and this ratio was expressed as a percentage.
Results were analyzed by mixed-design analyses of variance with factors of group (WT or KO) and concentration. Differences between the groups in consumption of specific concentrations of taste solution were determined using Tukey’s tests. One-sample t-tests were used to determine whether a group of mice had a preference score for a particular concentration of a taste compound that was significantly different from indifference (i.e., 50% preference).
Brief-exposure (gustometer) tests
The purpose of this experiment was to determine how genetic ablation of CALHM1 influenced the short-term acceptance of NaCl and related salts. We conducted 4 experiments using 3 batches of mice. One batch of 10 WT and 10 KO males (aged 49–105 days) was used to test NaCl (without and then with amiloride). One batch of 10 WT and 12 KO males (aged 49–69 days) was used to test KCl (without and then with amiloride). The third batch of 10 WT and 10 KO females (aged 52–83 days) was used to test first NH4Cl and then sodium lactate.
Each mouse was weighed (to the nearest 0.1g) daily, immediately before it was placed into a gustometer. There were no statistically significant differences in body weight between the WT and KO groups.
Training
In order to train the mice to sample taste solutions, they were first water deprived for 22.5h and then placed in a gustometer (MS160-Mouse gustometer, DiLog Instruments) with a motorized shutter that controlled access to a taste solution. Details of gustometer construction and operation are available elsewhere (Glendinning et al. 2002).
During the first training session, each mouse had continuous access to water for 30min from the time it first licked the drinking spout. It was then returned to its home cage and given water for 1h. On the following 2 days, this procedure was repeated except the shutter allowing access to water was closed 5 s after the mouse began to lick, and it was reopened after a 7.5-s interval. After 20min, the mouse was returned to its home cage and given water for 1h. By the 3rd test using these procedures, all mice had learned to obtain water during the 5-s access periods.
Testing
Each mouse received a total of 6 test sessions, one a day: For the first batch of mice there were 3 test sessions with NaCl followed by 3 test sessions with NaCl mixed with 10 µM amiloride (NaCl + amiloride). For the second batch of mice, there were 3 test sessions with KCl followed by 3 test sessions with KCl + amiloride. For the third batch, there were 3 test sessions with NH4Cl followed by 3 test sessions with sodium L-lactate (NaLac; Sigma, L7022). During each test session, each mouse received repeated blocks of 5 salt concentrations (deionized water (0), 56, 178, 562, and 1000mM), with the presentation order randomized for each block. When the solutions were dissolved in amiloride, the water stimulus was also 10 µM amiloride. Five-second “washout” trials with deionized water (never containing amiloride) were interposed between each solution presentation. Thus, a mouse received access to a salt solution for 5 s followed by 7.5 s with the shutter closed, then access to water for 5 s followed by 7.5 s with the shutter closed, followed by the next salt solution for 5 s, and so on. The washout trials with water were included to help maintain the mouse’s performance by “cleaning its palate” and preventing it from quitting because it expected only bad-tasting solutions. After each session, each mouse received water for 1h in its home cage and was then water deprived in preparation for the next session.
Statistical analysis
Separate analyses were conducted for each of the 4 salts tested. The mean number of licks in response to each concentration made by each mouse was obtained by averaging together the results from identical exposures. Mice initiated on average ~4.5 exposures with each concentration over the 3 days of tests (irrespective of the concentration and test compound available); all mice responded at least once to each concentration except for 1 WT mouse that never drank 562mM NH4Cl (this mouse was excluded from statistical analyses). The mean values for individual mice were used in mixed-design ANOVAs with factors of group (WT or KO), concentration, and (if relevant) amiloride absence or presence. Post hoc LSD tests were used to assess differences between the groups in consumption of specific concentrations of taste solution and differences in response of each group to individual concentrations of each taste compound (Statistica 10, Stat Soft Inc.).
Gustatory electrophysiology
Surgery
Measurements of CT activity were made in 6 male CALHM1 WT and 6 male CALHM1 KO mice. Each was anesthetized with a mixture of ketamine, xylazine, and acepromazine (90, 20, and 3mg/kg, respectively; i.p.), with further doses as necessary. A cannula was placed in the trachea to prevent suffocation, a fistula was placed in the esophagus to prevent ingestion of solutions, and the animal was placed supine with the head secured in a nontraumatic head holder. The nerve was accessed through the right ear by puncturing the tympanic membrane and exposing the right CT adjacent to the malleus (Cheal 1977). An electrode made of platinum/iridium wire was placed on the nerve, and the multiunit signal was amplified, filtered, rectified, and integrated with a time constant of 1.0 s.
Stimuli and delivery
Taste stimuli, which were mixed in distilled water (except where indicated), included the following: NaCl at 56, 178, 562, and 1000mM, each mixed in either water or 10 µM amiloride; 178mM sodium lactate (NaLac) mixed in water or 10 µM amiloride; 2mM quinine hydrochloride (QHCl); 10mM HCl; 100mM NH4Cl; 500mM sucrose; and deionized water. We focused on high concentrations of NaCl because of the clear effects of CALHM1 KO on licking for them in the behavioral studies. KCl at 100mM was also applied at regular intervals (usually after every 4–5 other stimuli) throughout the entire process to serve as a reference stimulus. We selected 100mM KCl based on previously published data showing similar licking to 32–316mM KCl by WT and CALHM1 KO mice (Taruno, Vingtdeux, et al. 2013). We also further evaluated its suitability as a reference stimulus by using constant amplifier settings throughout the whole experiment and then comparing the WT and KO mice on the mean absolute sizes (i.e., areas under the curve) of their raw responses to KCl (see Frank and Blizard 1999 for discussion); we found no difference between the WT and KO mice in the sizes of their raw responses to KCl, confirming its appropriateness as a reference stimulus. For each mouse, the relative response size across all presentations of a stimulus was then averaged in order to obtain a single relative response size. We also calculated the Pearson product moment correlation between the response sizes of the first and second applications for all 74 instances in which a stimulus was applied twice for an animal. The result was r = +0.90, indicating that our preparations were stable.
In order to apply taste solutions, the tongue was extended slightly and placed in a flow chamber. Room temperature deionized water was used as a rinse, and room temperature stimulus solutions were applied by continuous flow at a rate of 0.3mL/s. Rinse and stimulus were separated by a small air bubble that allowed for a distinct, sharp onset, but that minimized interruption to the flow. In prior experiments, this method has resulted in no responses to weak concentrations of taste stimuli that are thought to be undetectable (Cherukuri et al. 2011), and thus responses to higher concentrations can be taken as purely gustatory in nature, without a tactile component. We also tested this assumption directly in the current experiment by using water as a taste stimulus. Each stimulus presentation lasted for 20 s and was followed by at least 60 s of rinse. Separate concentration series of NaCl mixed in water or in amiloride were typically applied in ascending order, but otherwise the order of presentation for different stimuli was random.
Statistical analysis
Response sizes for stimulus applications were based on the area under the curve of the integrated voltage for 10 s after stimulus onset (evoked) minus the area for 10 s before onset (baseline). Relative response sizes were calculated for each stimulus application based on the size of the KCl reference. The relative responses to NaCl mixed in water served as the “overall response” to this compound; the relative responses when NaCl was mixed in amiloride composed the “amiloride-insensitive portion” of the response. The latter was then subtracted from the former for each mouse to yield an estimate of the “amiloride-sensitive portion” of its response to NaCl.
Data were analyzed using repeated measures ANOVAs with genotype (CALHM1 WT or KO) as a between-subjects factor and amiloride, concentration, and/or stimulus as within-subjects factors. A 3-way ANOVA was used to analyze responses to NaCl with and without amiloride (effects of genotype, amiloride, and concentration). In addition, the effects of amiloride within each genotype group were evaluated using 2-way ANOVAs with amiloride and concentration as factors. Separate 2-way ANOVAs were used to analyze the amiloride-sensitive component of NaCl responses (with factors of genotype and concentration), responses to NaLac (with factors of genotype and amiloride), and nonsodium stimuli (with factors of genotype and stimulus). Post hoc t-tests were used when appropriate to pinpoint differences between specific concentrations or stimuli. The absence of a significant response to a particular stimulus was tested by examining whether the group mean plus and minus a 95% confidence interval overlapped with zero. The statistical analyses were performed using the Systat software package.
Results
Values presented in the text, tables, and figures are means ± standard errors of the mean. All statistical tests were made using a criterion for significance of P < 0.05.
NaCl recognition threshold
Training
The CALHM1 WT and KO mice drank similar volumes of water before training (WT = 5.7±0.3, KO = 6.2±0.4mL). They drank equally from each tube (preference scores for “solution tube,” WT = 45±4%, KO = 51±3%). The KO mice drank significantly more 150mM LiCl during both exposures (First exposure, WT = 2.2±0.2mL, KO = 3.2±0.3, t(29) = 3.16, P = 0.0036; second exposure, WT = 2.0±0.1mL, KO = 3.0±0.1mL, t(29) = 4.67, P < 0.0001). Relative to initial water intakes, WT mice drank significantly more water during the test between the 2 LiCl exposures (WT = 9.8±0.7mL, KO = 7.3±0.5mL).
Test
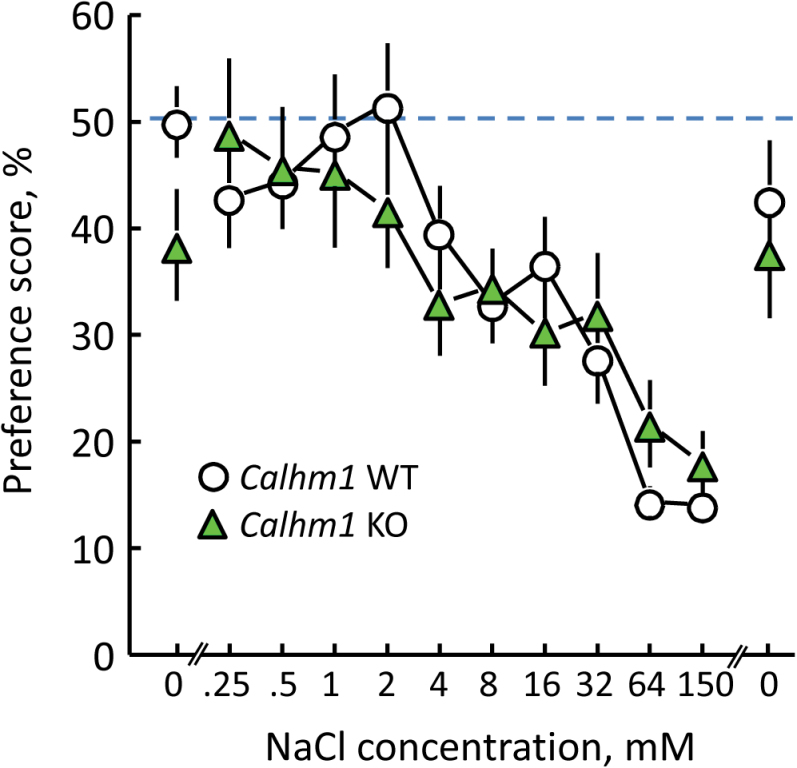
There were no significant differences in preference scores between CALHM1 WT and KO mice (Group × NaCl concentration interaction, F(11, 319) = 1.35, P = 0.20). The threshold for avoidance of NaCl by both groups was 4mM NaCl; that is, both groups were indifferent to 2mM NaCl and both avoided 4mM NaCl and all higher concentrations tested (Figure 1).
Figure 1.
NaCl avoidance in 48-h choice tests by CALHM1 WT and KO mice that had been poisoned with LiCl. There were no significant differences between the 2 groups.
Two-bottle choice: 4 basic tastes
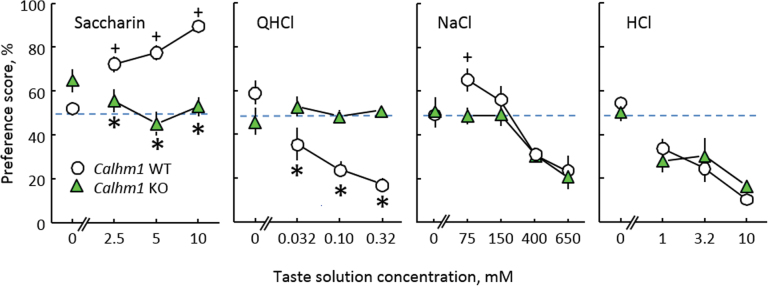
WT mice showed the expected avidity for saccharin, inverted U-shaped function for NaCl, and avoidance of QHCl and HCl. Unlike these controls, the CALHM1 KO mice were indifferent to all concentrations of saccharin and QHCl, and they did not prefer any NaCl concentration above indifference (50%; Figure 2; Supplementary Tables S1 and S2). The CALHM1 WT preferred 75mM NaCl to water (indifference), whereas the KO mice did not. The significance of the difference between the CALHM1 WT and KO mice at this concentration was ambiguous. A simple comparison of the 2 group means using a t-test was significant, t(19) = 2.46, P = 0.0239, but more conservative post hoc tests were not. The 2 groups showed similar avoidance of high concentrations of NaCl and HCl.
Figure 2.
Two-bottle choice preferences for concentration series of saccharin, QHCl, NaCl, and HCl by CALHM1 KO mice (n = 10) and their WT littermates (n = 11). *P < 0.05 relative to WT group. + P < 0.05 above indifference.
Two-bottle choice: NaCl concentration series
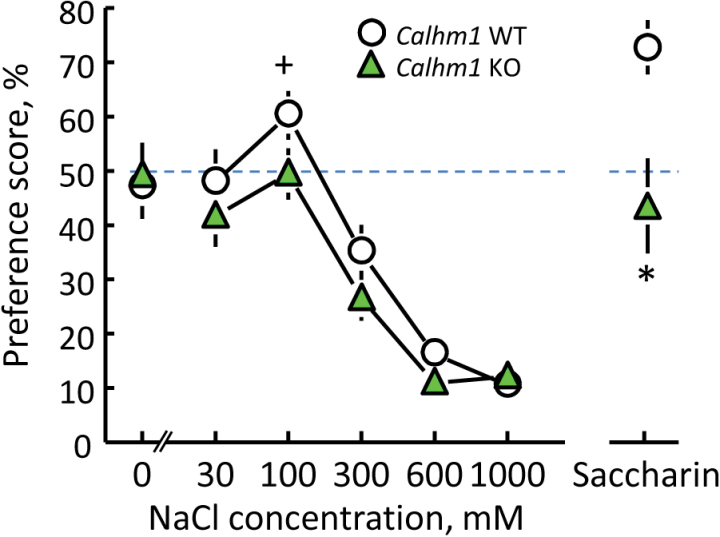
There were no statistically significant differences between WT and KO mice in response to NaCl (Figure 3; Supplementary Tables S3 and S4). Most pertinently, 300, 600, and 1000mM NaCl were avoided by both groups equally. Consistent with the previous experiment, WT mice had a preference score significantly greater than 50% (indifference) for 100mM NaCl, t(7) = 2.66, P = 0.03, but KO mice did not. The KO group did not prefer any concentration of NaCl above indifference.
Figure 3.
Two-bottle choice preferences for an ascending concentration series of NaCl and 3.2mM saccharin of CALHM1 KO mice (n = 8) and their WT controls (n = 8). *P < 0.05 relative to WT group. + P < 0.05 above indifference.
The WT mice showed strong preferences for saccharin, but the KO mice did not; they drank significantly less saccharin and had a preference score that did not differ significantly from indifference (Figure 3; Supplementary Tables S3 and S4).
Brief-access tests
NaCl
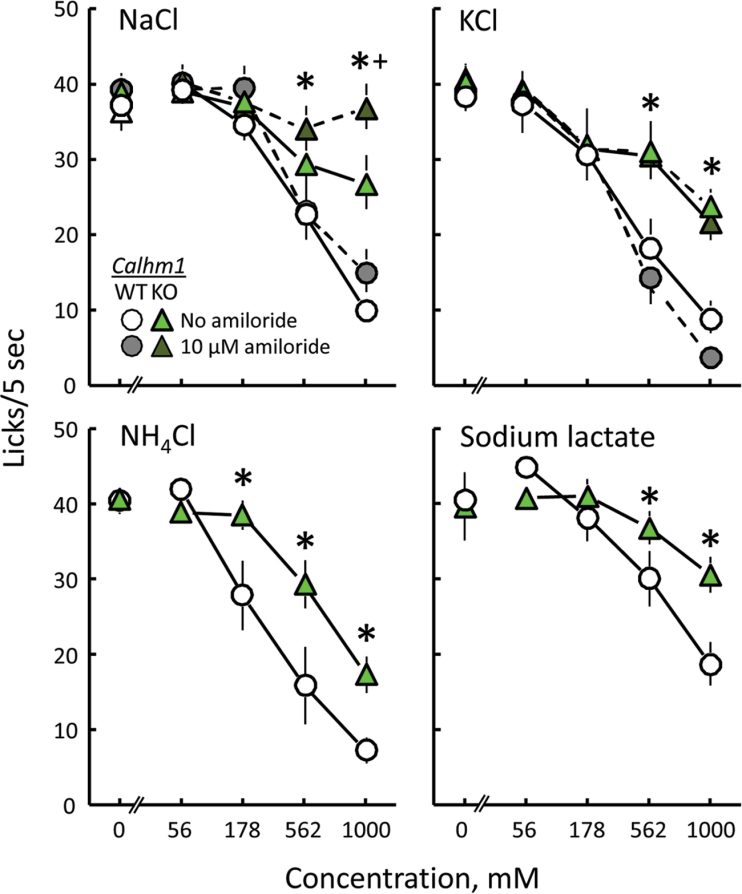
The CALHM1 WT and KO mice licked similarly for water and 56 and 178mM NaCl, and the presence of amiloride did not affect licking rates for these 3 fluids. However, at higher NaCl concentrations, there were differences due to both genotype and amiloride (Figure 4; Supplementary Table S5). The CALHM1 KO mice licked more 562 and 1000mM NaCl than did the WT mice. CALHM1 KO mice but not CALHM1 WT mice licked more 1000mM NaCl in amiloride than 1000mM NaCl in water. The net result was that the WT mice showed a NaCl concentration–related decrease in licking that was unaffected by amiloride. The KO mice showed a less pronounced concentration-related decrease in licking of NaCl in water. Moreover, unlike the results with the WT mice, the CALHM1 KO mice treated high concentrations of NaCl in amiloride no differently than if they were water.
Figure 4.
Lick rates of CALHM1 WT and KO mice presented with water and 4 concentrations of NaCl, KCl, NH4Cl, or sodium lactate. NaCl and KCl were tested dissolved either in water or in 10 µM amiloride. *P < 0.05 relative to WT group(s). + P < 0.05 relative to tests without amiloride.
KCl
Both the CALHM1 WT and KO groups of mice licked less to high concentrations (562 and 1000mM) of KCl than they did to water or low concentrations (56 or 178mM) of KCl. However, the KO mice licked more to 562 and 1000mM KCl than did the WT mice. Neither group was influenced by the addition of amiloride to KCl (Figure 4; Supplementary Table S5).
NH4Cl
The CALHM1 WT and KO groups responded similarly to 0 and 56mM NH4Cl. However, the KO mice licked 178, 562, and 1000mM NH4Cl more than did the WT mice. The WT mice licked significantly less 178, 562, and 1000mM NH4Cl than water; the KO mice licked significantly less 562 and 1000mM NH4Cl than water (Figure 4; Supplementary Table S5).
NaLac
The CALHM1 WT and KO mice responded similarly to 0, 56, and 178mM NaLac. However, the KO mice licked more to 562 and 1000mM NaLac than did the WT mice. The WT mice licked significantly less for 562 and 1000mM NaLac than water; the KO mice licked significantly less for only 1000mM NaLac than water (Figure 4; Supplementary Table S5).
Gustatory electrophysiology
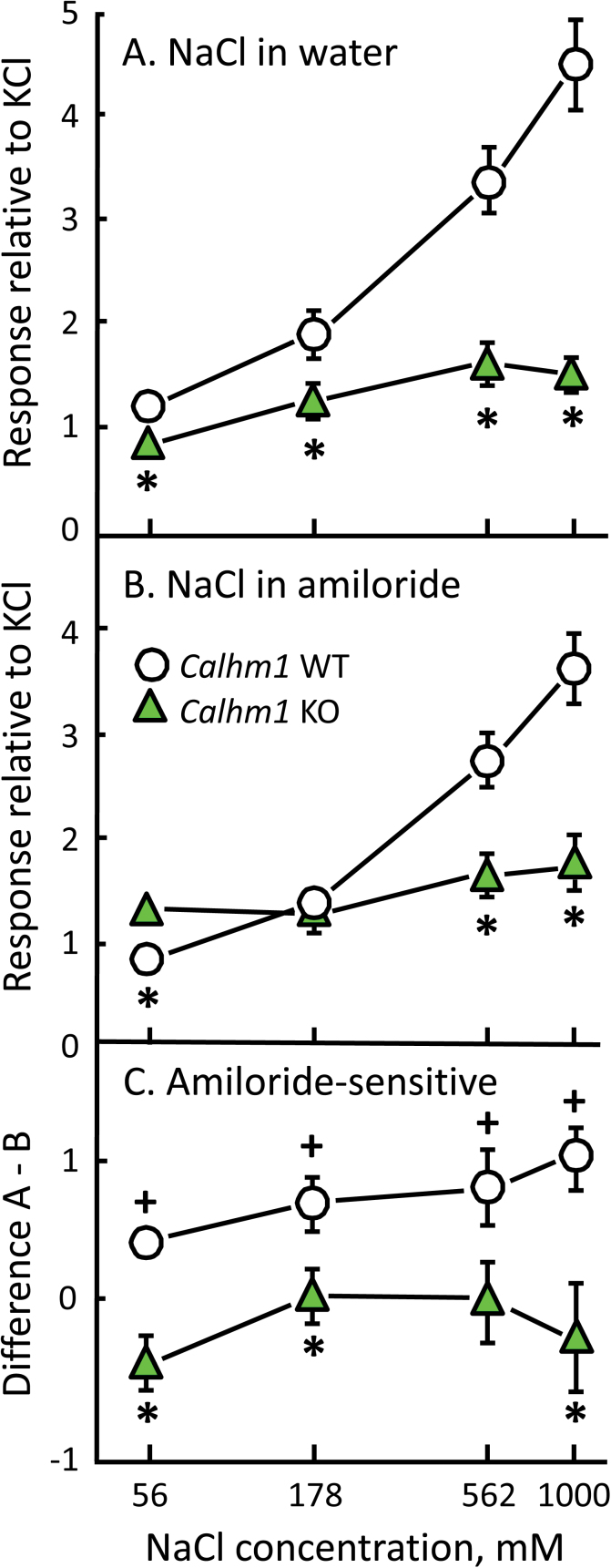
Multiunit CT responses to NaCl were larger in CALHM1 WT than KO mice, especially at 562 and 1000mM, the 2 highest concentrations tested (Figures 5 and 6). Consistent with this, there was a significant overall effect of genotype on NaCl response magnitude, F(1,10) = 23.5, P = 0.001, and a significant genotype × concentration interaction, F(3,30) = 38.0, P < 0.001. All 4 concentrations of NaCl mixed in water evoked significantly larger responses in WT than in KO mice (Figure 6A; P < 0.03 in all cases).
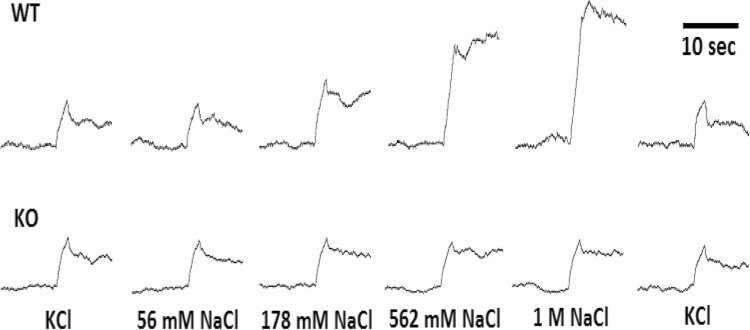
Figure 5.
Representative neural recordings showing changes in the integrated voltage over time for a CALHM1 WT (top) and KO (bottom) mouse. For each animal, 1 concentration series of applications of NaCl mixed in water is shown, along with preceding and following reference KCl presentations for each series (on the left and right, respectively). Ten seconds before and after stimulus onset are shown.
Figure 6.
Mean (±SEM) chorda tympani responses to applications of NaCl in CALHM1 WT and KO mice. Responses are shown for NaCl mixed in water (A) or in 10 μM amiloride (B). (C) The amiloride-sensitive NaCl component for each strain was calculated by subtracting values for NaCl mixed in amiloride from those for NaCl mixed in water. *P < 0.05, WT versus KO; + P < 0.05, effect of amiloride within WT mice.
The presence of 10 μM amiloride reduced responses evoked by NaCl (main effect of amiloride, F(1,10) = 5.8, P = 0.04), particularly those of the CALHM1 WT group (genotype × amiloride interaction, F(1,10) = 19.1, P = 0.001). With amiloride present, WT mice evoked larger responses at only 562 and 1000mM NaCl, with the 2 groups showing no difference at 178mM and a significant difference in the opposite direction (i.e., smaller responses in WT mice) at 56mM (Figure 6B; P < 0.006 in post hoc tests). This pattern of differences between the CALHM1 WT and KO groups reflected the fact that amiloride did not affect NaCl responses in KO mice (main effect of amiloride and amiloride × concentration interaction, n.s.), whereas it did in WT mice (effect of amiloride, F(1,5) = 41.0, P = 0.001). Amiloride suppressed NaCl responses significantly (P < 0.04 in post hoc tests) and to a similar extent (amiloride × concentration interaction, n.s.) for all 4 NaCl concentrations in WT mice (Figure 2C). The amiloride-sensitive response component differed between the WT and KO groups: This was significant for 56, 178, and 1000mM NaCl (effect of genotype, F(1,10) = 19.1, P = 0.001, P < 0.02 in post hoc tests), and the difference at 562mM approached significance (P = 0.053).
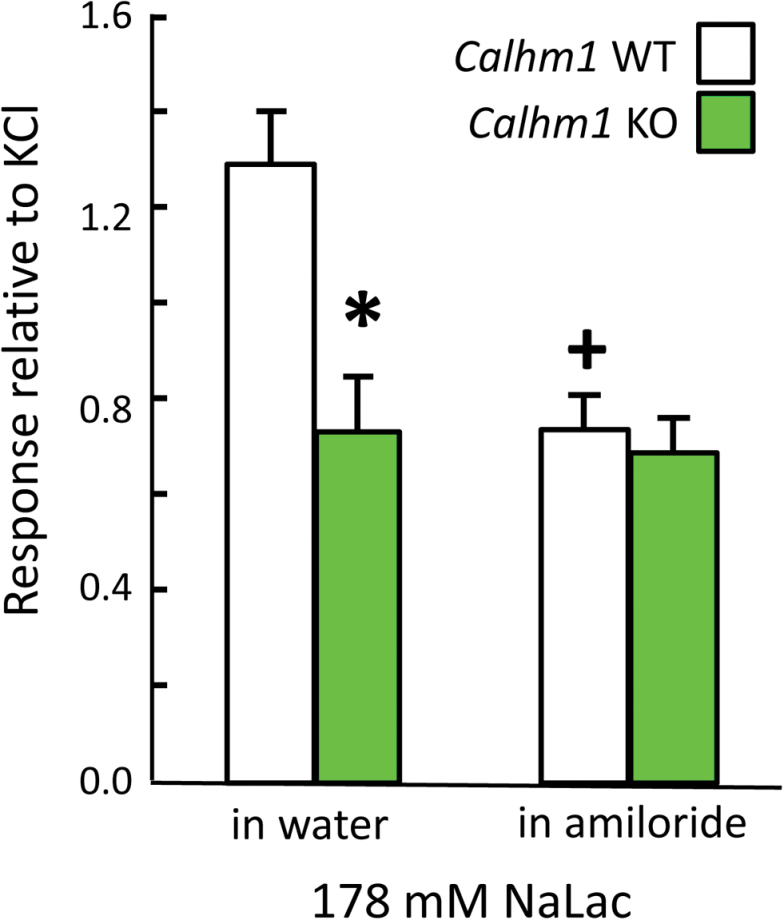
Responses to 178mM NaLac (Figure 7) paralleled those observed with the same concentration of NaCl. That is, WT mice had significantly larger responses than did KO mice to NaLac in water (main effect of genotype, F(1,10) = 17.0, P = 0.002, P = 0.004 in post hoc test), but the WT and KO groups did not differ in response to NaLac mixed with 10 μM amiloride. Amiloride significantly suppressed the NaLac response in WT mice (effect of amiloride, F(1,10) = 7.0, P = 0.03; amiloride × genotype interaction, F(1,10) = 5.5, P = 0.04; P = 0.02 in post hoc test) but had no effect in KO mice.
Figure 7.
Mean (±SEM) chorda tympani responses of CALHM1 WT and KO mice to 178mM sodium lactate (NaLac) mixed in water or in 10 μM amiloride. *P < 0.05, WT versus KO; + P < 0.05, effect of amiloride within WT mice.
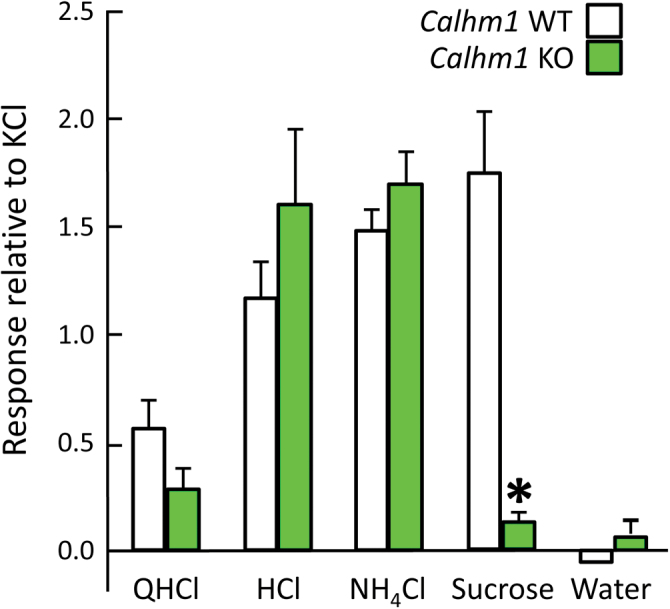
Figure 8 shows mean CT responses to nonsodium stimuli in WT and KO mice. There was a significant genotype × stimulus interaction, F(4,40) = 13.0, P < 0.001, due to responses to 500mM sucrose being significantly larger in WT than in KO mice (P < 0.001 in post hoc test); although the mean response of the KO group to sucrose was quite small, it was significantly greater than zero. The WT and KO groups did not differ in their responses to QHCl, HCl, NH4Cl, or water. Mean responses to the latter did not differ from zero in either genotype group, indicating that water was not effective at changing neural firing rates in the CT, either due to a touch component or any other factors.
Figure 8.
Mean (±SEM) chorda tympani responses to nonsodium stimuli in CALHM1 WT and KO mice. QHCl = 2mM quinine hydrochloride; HCl = 10mM hydrochloric acid; NH4Cl = 100mM ammonium chloride; Sucrose = 500mM sucrose. *P < 0.05, WT versus KO.
Discussion
Each of the basic taste qualities is mediated by multiple transduction pathways (Herness and Gilbertson 1999; Hacker et al. 2008; Ohkuri et al. 2009; Yasumatsu et al. 2009, 2012), and salty taste is no exception. NaCl is the prototypical stimulus for this basic taste quality, and at first glance it appears to be a simple compound from a taste perspective. However, a complex sequence of events follows the sampling of NaCl. Its transduction involves amiloride-sensitive (ENaC-mediated) and amiloride-insensitive components (review by McCaughey and Scott 1998), and each of these may involve several mechanisms. There are also nonsalty components to NaCl taste, perhaps due in part to the anion.
Investigation of salty taste is particularly challenging in animals such as mice because they cannot give verbal reports of their perceptions. Rather, we must make inferences based on their behavior or the activity of their cells, and behavioral measurements are affected not only by perceived taste quality but also by intensity and palatability, along with nongustatory factors such as postingestive effects and irritation. These complications mean that there is no single test that can provide a complete picture of how NaCl tastes to mice. Thus, we employed several techniques, each of which provides partial insight into how CALHM1 mediates sodium taste transduction. We found that genetic ablation of CALHM1 had no effect on NaCl recognition thresholds, measured using a conditioned aversion procedure; it had a fairly subtle influence on the response to moderate NaCl concentrations, measured using two-bottle choice tests and electrophysiology; and it had substantial effects on the response to high NaCl concentrations, measured using brief-access tests and gustatory electrophysiology. Although each assay has its advantages and caveats, the broad implication of our results is that in intact mice, CALHM1 contributes to the perception of moderate and intense saltiness but not threshold or mild saltiness.
CALHM1 mediation of gustatory transduction of sodium is concentration dependent
CALHM1 WT and KO mice had similar taste recognition thresholds (of 4mM NaCl), and the 2 groups equally avoided 8–150mM NaCl after they had been poisoned with 150mM LiCl. There was also no difference between unconditioned (naive) WT and KO mice in two-bottle preferences for 30mM NaCl. These findings suggest that CALHM1 does not participate in the transduction of low sodium concentrations. The lowest concentration supporting a CALHM1-dependent effect was 56mM NaCl, for which there were significant differences in CT responses between the CALHM1 WT and KO groups. However, at this concentration, CALHM1 did not influence brief-access licking; both groups treated 56mM NaCl as if it were water. We note that 1) indifferent licking may reflect a neutral taste, not necessarily a lack of a taste, and 2) we would expect 56mM NaCl to be hedonically preferred, but our use of thirsty mice for gustometry most likely caused ceiling effects that bias against obtaining increases in licking behavior.
There were indications that 75 and 100mM NaCl activated a CALHM1-dependent pathway. WT mice preferred NaCl in two-bottle choice tests at these concentrations, resulting in a broad peak of the inverted U-shaped preference-aversion function that is often observed in rodents tested with progressively ascending concentrations of NaCl (Bachmanov et al. 2002; Tordoff et al. 2007). Intriguingly, the CALHM1 KO mice did not show the “preference” component of this function. This phenotype, albeit subtle, was observed in both of the two-bottle choice experiments conducted here as well as in the original report of Taruno, Vingtdeux, et al. (2013) although, admittedly, the CALHM1 WT and KO groups did not differ significantly using conservative statistical tests. We conclude that CALHM1 participates in the response to these palatable NaCl concentrations, but additional work will be needed to determine whether the modest differences we observed are due to only perfunctory activation of the CALHM1-dependent pathway by 75–100mM NaCl or to a lack of sensitivity of the two-bottle test, specifically the problem of observing reduced preferences of the KO mice relative to only modest NaCl preferences of the WT control mice.
The strongest support for CALHM1’s involvement in sodium transduction was present for concentrations of 178mM and higher. Both NaCl and NaLac at this concentration evoked significantly smaller CT responses in CALHM1 KO than in WT mice. The effects of CALHM1 KO were especially dramatic at 562 and 1000mM. For these concentrations, the KO group showed not only smaller CT responses relative to the WT animals, but also less aversion in brief-access licking tests of NaCl and NaLac. The effects we found were larger and less idiosyncratic than those reported in a similar experiment by Taruno, Vingtdeux, et al. (2013); but in both cases, WT mice licked significantly less 1000mM NaCl than did KO mice. The more robust differences observed here may reflect our use of naive mice, whereas Taruno, Vingtdeux, et al. used mice that had previously been tested with sucrose, quinine, and HCl. Despite the robust group differences in brief-access and electrophysiological measures for hypertonic NaCl, there were no differences in two-bottle preferences at these concentrations. Perhaps this can be explained by two-bottle preferences being influenced by postingestive effects, which may curb excessive intake of concentrated NaCl by CALHM1 KO mice, even if this is not perceived as being intense or aversive immediately upon ingestion.
Our conclusion that the GPCR → CALHM1 transduction cascade participates in sodium transduction raises the question of whether knockout of intermediate members of this cascade can also influence responses to oral sodium. Our finding that CALHM1 KO mice do not show a preference for moderate concentrations of NaCl in two-bottle tests is also apparent (with at least a trend) in mice with genetic ablation of gustducin (Wong et al. 1996; Ruiz et al. 2003; He et al. 2004), ITPR3 (Hisatsune et al. 2007), or TRPM5 (Damak et al. 2006); mice with PLCβ2 KO do not appear to have been tested with NaCl in two-bottle choice tests. Our observation that CALHM1 KO mice show reduced avoidance of high concentrations of NaCl in brief-access tests is also present with ablation of gustducin (Glendinning et al. 2005), PLCβ2 (Dotson et al. 2005; but see Zhang et al. 2003), or TRPM5 (Damak et al. 2006; but see Zhang et al. 2003 and Oka et al. 2013). Mice with ITPR3 or TRPM5 KO also show reduced avoidance of high NaCl concentrations in long-term preference tests (Damak et al. 2006), and this has also been observed in CALHM1 KO mice (Taruno, Vingtdeux, et al. 2013 but not this study). Some of these deficits in behavior are paralleled by the results of gustatory electrophysiological recordings. PLCβ2 KO and TRPM5 KO mice have CT responses to some concentrations of NaCl that are reduced relative to controls (Damak et al. 2003; Oka et al. 2013), and recent data indicate that TRPM5 mediates the amiloride-insensitive portion of the CT response in rats and mice (Ren et al. 2013). CT and glossopharyngeal responses to NaCl were normal in gustducin KO mice, but high NaCl concentrations were not tested (Wong et al. 1996). Thus, the data are not entirely consistent, but there are sufficient positive findings to implicate several components of the GPCR → CALHM1 cascade in salt taste transduction.
Participation of the GPCR → CALHM1 cascade in sodium transduction does not exclude other CALHM1-mediated mechanisms from participating as well. In particular, there may be an indirect action of CALHM1-released ATP on activity of Type 3 cells, which harbor other transduction mechanisms (see Introduction). There is also the potential for CALHM1 in other sites to influence NaCl ingestion. The gene is not expressed in other taste tissue (Taruno, Vingtdeux, et al. 2013). There is one report of the presence of CALHM1 in cells from mouse cortex and hippocampus (Ma et al. 2012); another could not detect CALHM1 mRNA in mouse brain (Wu et al. 2012; although it was present in human brain; Dreses-Werringloer et al. 2008). As far as we know, there have been no studies attempting to identify CALHM1 in other organs of mice, in large part because a satisfactory CALHM1 antibody has not yet been developed. Our findings that CALHM1 KO influences NaCl-elicited brief-access test licking responses and CT responses are tell-tale signs of a disruption of taste transduction, but it remains possible that unknown postoral actions of CALHM1 influenced the results of the two-bottle choice tests.
ENaC-mediated (amiloride-sensitive) gustatory transduction of sodium
One component of salty taste in rodents involves sodium ions passing through amiloride-sensitive ENaC channels in a subset of taste receptor cells (Heck et al. 1984; Chandrashekar et al. 2010). Our results confirm the general importance of this mechanism. Amiloride had dramatic effects on the CT responses of WT mice, with significant suppression of the response size for concentrations ranging from 56 to 1000mM NaCl, as well as for 178mM NaLac. These data are not surprising given that the CALHM1 KO line has a mixed genetic background derived from the C57BL/6J and 129/Sv strains; both of which are amiloride sensitive (Ohkuri et al. 2006; Cherukuri et al. 2013). The amiloride-sensitive component of the CT response in WT mice was of similar magnitude across the entire range of NaCl concentrations. These data are consistent with a prior finding that the amiloride-sensitive transduction pathway is activated maximally by moderate concentrations of sodium, with the transduction of concentrated NaCl occurring largely through amiloride-insensitive mechanisms (Chandrashekar et al. 2010).
Relationship between CALHM1 and amiloride-sensitive ENaCs
ENaCs are thought to reside in Type 1 taste cells (although this is unproven; Roper 2013), but CALHM1 is expressed exclusively in Type 2 taste cells (Taruno, Vingtdeux, et al. 2013). Thus, a direct interaction between ENaC channels and CALHM1 is unlikely. But even so, communication between receptor cells could allow for interactions between ENaC- and CALHM1-mediated sodium transduction.
There was a discrepancy between our brief-access and electrophysiological data involving the response of CALHM1 KO mice with amiloride. For the electrophysiology, we observed no effect of amiloride on the size of CT responses to NaCl in CALHM1 KO mice, which suggests that ENaCs and CALHM1 are part of the same transduction pathway. However, for the brief-access tests, amiloride increased licking to 1000mM NaCl in CALHM1 KO mice, which is consistent with CALHM1 being associated with an amiloride-insensitive (i.e., non-ENaC) transduction pathway. We are not sure how to reconcile these results. One possibility is that electrophysiological responses reflect only the fungiform and anterior foliate papillae that are innervated by the CT (Hill 2004), whereas the behavior reflects the involvement of the entire mouth. Perhaps the blunted avoidance of 1000mM NaCl mixed in water by CALHM1 KO mice was mediated by parts of the mouth innervated by the greater superficial petrosal nerve, which conveys amiloride-sensitive NaCl responses in rats (see Sollars and Hill 1998; but also Harada et al. 1997 for conflicting results). However, behavior toward NaCl is thought to be dominated by the CT in rodents, which argues against this interpretation (Blonde et al. 2010). Other possibilities include differences caused by the mixed genetic background of the mice and by experience because the behavioral tests with NaCl in amiloride were conducted only after exposing the animals to NaCl in water, whereas the electrophysiology was conducted in naive animals. Additional work is needed to resolve this.
CALHM1 mediation of transduction for nonsodium compounds
We observed CALHM1 involvement in nonsalty taste qualities that was largely consistent with prior work (Taruno, Vingtdeux, et al. 2013). In contrast to WT controls, our CALHM1 KO mice were completely indifferent to saccharin in two-bottle tests and had minimal (but not absent) sucrose-elicited CT responses, confirming the importance of CALHM1 for the transduction of sweetness. We also replicated the observation that CALHM1 KO eliminates the avoidance of bitter compounds (Taruno, Vingtdeux, et al. 2013) in our two-bottle tests with quinine. Unlike Taruno, Vingtdeux, et al. (2013), we did not observe an effect of CALHM1 KO on electrophysiological responses to quinine. In both studies, the CT responses of CALHM1 KO mice were about half the magnitude of those in the WT group; but in our case, there was too much variability for the difference to be statistically significant. The mismatch between the weak electrophysiological and solid behavioral results for quinine may simply relate to the relatively unimportant role played in bitter taste by the CT, which is less sensitive to bitter stimuli than is the glossopharyngeal nerve (Tanimura et al. 1994).
Consistent with the prior study (Taruno, Vingtdeux, et al. 2013), CALHM1 ablation did not affect two-bottle preferences for HCl, and our CT data with HCl match the earlier electrophysiological data with citric acid in that neither of these sour stimuli evoked different responses in the CALHM1 WT and KO groups. We also replicated the original observations that CALHM1 KO influences licking to high concentrations of the mineral salts, KCl and NH4Cl.
Summary of gustatory transduction of sodium
Our results, along with those published by Taruno, Vingtdeux, et al. (2013), indicate that sampling of NaCl initiates multiple events with their relative contributions depending on the concentration of sodium. At very low sodium concentrations, there is no involvement of CALHM1, but there is permeation of sodium through ENaCs and also at least one other type of channel found in taste buds, with the relative contributions of the 2 varying among different mouse strains (Ishiwatari and Bachmanov 2012; Cherukuri et al. 2013). At concentrations of ~56mM or more, sodium (and probably KCl and NH4Cl) activates Type 2 cells containing CALHM1, which contribute slightly to the overall response to NaCl. At progressively higher sodium concentrations, CALHM1 plays an increasingly important role in the neural response. Additional work will be needed to determine whether this is due to the activation of a high-threshold sodium (or salt) receptor found in Type 2 cells, whether it arises due to other sodium-responsive cell types communicating with Type 2 cells, or whether both of these mechanisms (and perhaps others as well) are involved.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by National Institutes of Health (NIH) [grant DC-010393 to M.G.T. and DC-012538 to J.K.F]. A.D. was funded by an NIH-National Institute on Deafness and Other Communication Disorders (NIDCD) supplement to [grant DC-010393] and the Monell Science Apprenticeship Program. Mouse gustometry was performed at the Monell Phenotyping Core, which is supported, in part, by funding from the NIH-NIDCD P30 Core [Grant DC011735].
Supplementary Material
Acknowledgements
Expert technical assistance was provided by L. Alarcón and S. Valmeki. We thank Drs. A. Spector and J. Glendinning for their guidance suggesting improvements to the procedures used for gustometry.
References
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 32(6):435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonde G, Jiang E, Garcea M, Spector AC. 2010. Learning-based recovery from perceptual impairment in salt discrimination after permanently altered peripheral gustatory input. Am J Physiol Regul Integr Comp Physiol. 299(4):R1027–R1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JG, Teeter JH, Silver WL. 1985. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 334(2):207–214 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 464(7286):297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheal M. 1977. Taste responses of the chorda tympani nerve in the mouse. Physiol Behav. 19(1):175–177 [DOI] [PubMed] [Google Scholar]
- Cherukuri CM, Bachmanov AA, McCaughey SA. 2013. A/J and C57BL/6J mice differ in chorda tympani responses to NaCl. Neurosci Res. 75:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri CM, McCaughey SA, Tordoff MG. 2011. Comparison of differences between PWD/PhJ and C57BL/6J mice in calcium solution preferences and chorda tympani nerve responses. Physiol Behav. 102(5):496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CL, Garcea M, Spector AC. 2004. Effects of selective lingual gustatory deafferentation on suprathreshold taste intensity discrimination of NaCl in rats. Behav Neurosci. 118(6):1409–1417 [DOI] [PubMed] [Google Scholar]
- Contreras RJ. 1977. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 121(2):373–378 [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, et al. 2006. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 31(3):253–264 [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853 [DOI] [PubMed] [Google Scholar]
- DeSimone J, Ferrell F. 1985. Analysis of amiloride inhibition of chorda tympani raste response of rat to NaCl. Am J Physiol Regul Integ Comp Physiol. 249:R52–R61 [DOI] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. 2005. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses. 30(7):593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, et al. 2008. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 133(7):1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreses-Werringloer U, Vingtdeux V, Zhao H, Chandakkar P, Davies P, Marambaud P. 2013. CALHM1 controls the Ca2+-dependent MEK, ERK, RSK and MSK signaling cascade in neurons. J Cell Sci. 126(5):1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Blizard DA. 1999. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol Behav. 67(2):287–297 [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR. 1991. The effect of amiloride on taste-evoked activity in the nucleus tractus solitarius of the rat. Brain Res. 550(2):247–256 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. 2005. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 30(4):299–316 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. 2002. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 27(5):461–474 [DOI] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. 2008. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 99(3):1503–1514 [DOI] [PubMed] [Google Scholar]
- Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. 1997. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 22(2):133–140 [DOI] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. 2004. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 24(35):7674–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA. 1984. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 223(4634):403–405 [DOI] [PubMed] [Google Scholar]
- Herness MS, Gilbertson TA. 1999. Cellular mechanisms of taste transduction. Annu Rev Physiol. 61:873–900 [DOI] [PubMed] [Google Scholar]
- Hill DL. 2004. Neural plasticity in the gustatory system. Nutr Rev. 62:S208–S217; discussion S224–S241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. 2007. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 282(51):37225–37231 [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. 2009. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 29(44):13909–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, Bachmanov AA. 2009. A high-throughput method to measure NaCl and acid taste thresholds in mice. Chem Senses. 34(4):277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, Bachmanov AA. 2012. NaCl taste thresholds in 13 inbred mouse strains. Chem Senses. 37(6):497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC, Finger TE. 2013. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 7:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. 2004. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 558(Pt 1):147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. 2012. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci USA. 109(28):E1963–E1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey SA, Scott TR. 1998. The taste of sodium. Neurosci Biobehav Rev. 22(5):663–676 [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. 2009. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 296(4):R960–R971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Shigemura N, Yoshida R, Ninomiya Y. 2006. Amiloride inhibition on NaCl responses of the chorda tympani nerve in two 129 substrains of mice, 129P3/J and 129X1/SvJ. Chem Senses. 31(6):565–572 [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. 2013. High salt recruits aversive taste pathways. Nature. 494(7438):472–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffman C, Bartoshuk LM, McBurney DH. 1971. Taste psychophysics. In Beidler LM, editor. Handbook of sensory physiology; Chemical Senses Vol IV, Section 2. New York (NY): Springer; p. 75–101 [Google Scholar]
- Ren Z, Rhyu MR, Phan TH, Mummalaneni S, Murthy KS, Grider JR, DeSimone JA, Lyall V. 2013. TRPM5-dependent amiloride- and benzamil-insensitive NaCl chorda tympani taste nerve response. Am J Physiol Gastrointest Liver Physiol. 305(1):G106–G117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. 2013. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 24(1):71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. 2003. Behavioral evidence for a role of alpha-gustducin in glutamate taste. Chem Senses. 28(7):573–579 [DOI] [PubMed] [Google Scholar]
- Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. 2013. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem. 288(9):6140–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Balagura S. 1969. Role of oropharyngeal factors in LiCl aversion. J Comp Physiol Psychol. 69(2):308–310 [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. 1998. Taste responses in the greater superficial petrosal nerve: substantial sodium salt and amiloride sensitivities demonstrated in two rat strains. Behav Neurosci. 112(4):991–1000 [DOI] [PubMed] [Google Scholar]
- Tanimura S, Shibuya T, Ishibashi T. 1994. Neural responses of the glossopharyngeal nerve to several bitter stimuli in mice. Comp Biochem Physiol Comp Physiol. 108(2-3):189–194 [PubMed] [Google Scholar]
- Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK. 2013. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays. 35(12):1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495(7440):223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. 2001a. Food intakes, water intakes, and spout side preferences Available from: http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&id=63
- Tordoff MG, Bachmanov AA. 2001b. Monell mouse taste phenotyping project Available from: www.monell.org/MMTPP
- Tordoff MG, Bachmanov AA. 2003. Mouse taste preference tests: why only two bottles? Chem Senses. 28(4):315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. 2007. Forty mouse strain survey of water and sodium intake. Physiol Behav. 91(5):620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. 1996. Transduction of bitter and sweet taste by gustducin. Nature. 381(6585):796–800 [DOI] [PubMed] [Google Scholar]
- Wu J, Peng S, Wu R, Hao Y, Ji G, Yuan Z. 2012. Generation of Calhm1 knockout mouse and characterization of calhm1 gene expression. Protein Cell. 3(6):470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. 1994. Some critical factors involved in formation of conditioned taste aversion to sodium chloride in rats. Chem Senses. 19(3):209–217 [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Horio N, Murata Y, Shirosaki S, Ohkuri T, Yoshida R, Ninomiya Y. 2009. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr. 90(3):747S–752S [DOI] [PubMed] [Google Scholar]
- Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. 2012. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol. 590(Pt 5):1155–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112(3):293–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.