Abstract
Objectives. Clinical and experimental observations have suggested that bradykinin, a major activation product of the plasma kallikrein–kinin system, is involved in the pathogenesis of arthritis, but the pathogenic role of bradykinin receptors remains inconclusive. In this study we examined whether bradykinin receptors are important in the pathogenesis of anti-collagen antibody-induced arthritis (CAIA) using double receptor–deficient (B1RB2R−/−) mice.
Methods. CAIA was induced in B1RB2R+/+ and B1RB2R−/− mice by injection of an anti-collagen antibody cocktail on day 0 and lipopolysaccharide on day 3. Severity of disease was evaluated by measurement of joint diameter and histological analysis. The expression of proinflammatory cytokines in joint tissue and peripheral mononuclear cells was determined by ELISA and real-time RT-PCR.
Results. The absent expression of B1R and B2R mRNA in B1RB2R–/– mice was confirmed by RT-PCR. Although B1RB2R+/+ mice developed severe CAIA, the severity of the disease was significantly attenuated in B1RB2R–/– mice. In B1RB2R+/+ mice bearing CAIA, both B1R and B2R mRNA levels were increased in joint tissue and peripheral mononuclear cells. Compared with B1RB2R+/+ mice, the production of IL-1β and IL-6 in joint tissue and their mRNA expression in peripheral mononuclear cells were remarkably reduced in B1RB2R–/– mice.
Conclusion. These observations provide genetic evidence that bradykinin plays an important role in the pathogenesis of CAIA. B1R, whose expression is induced in inflamed joint tissue and peripheral inflammatory cells, is important in the development of CAIA.
Keywords: arthritis, cytokine, bradykinin, plasma kallikrein–kinin system
Introduction
The plasma kallikrein–kinin system (KKS) is important in a variety of (patho)physiological processes, including inflammation, coagulation, angiogenesis and control of vascular tone [1]. The KKS consists of four plasma proteins: prekallikrein, factor XII, factor XI and high molecular weight kininogen (HK) [1]. Upon activation, prekallikrein is converted to the active form, kallikrein, which cleaves HK to release bradykinin. Bradykinin is a potent peptide that participates in inflammation, angio-oedema and vascular dilation [2].
There is increasing evidence suggesting that KKS activation is involved in arthritis [3–7]. In patients with RA, elevated levels of plasma kallikrein and bradykinin are detected in synovial fluid and plasma, and bradykinin receptor expression was also increased in their circulating and synovial neutrophils [3, 5]. Observations in animal models support the important role of the KKS in the pathogenesis of arthritis. Lewis rats have a mutation in HK (S511N) rendering it more susceptible to cleavage by plasma kallikrein [8]. Administration of streptococcal cell wall polymers in Lewis rats induces erosive polyarthritis and an increase in plasma bradykinin [4]. Kininogen-deficient rats exhibit attenuated arthritis [6]. Although these observations suggest that the KKS is important in the pathogenesis of arthritis, the mechanisms by which this system participates in arthritis remains largely unknown. Recently we reported that the inhibition of plasma kallikrein ameliorates arthritis in two Lewis rat models, and the effect of plasma kallikrein is probably through bradykinin [9]. Previous studies have shown that antagonists of B1R and B2R inhibit arthritis, suggesting that both receptors are critical in arthritis. However, a recent study by Song et al. [10] demonstrated in a mouse model of anti-collagen antibody-induced arthritis (CAIA) that B2R deficiency did not protect against arthritis. Thus the role of bardykinin and its receptors in the pathogenesis of arthritis still remains elusive.
In this study we investigated whether bradykinin receptors are required for the pathogenesis of CAIA. Because B1R knockout mice are not commercially available, we used the double bradykinin receptor-deficient mouse model (B1RB2R–/–). We found that the deficiency of B1R and B2R significantly inhibits the development and severity of CAIA and down-regulates IL-1β and IL-6 in joint tissue and circulating inflammatory cells. These observations provide the first genetic evidence showing that bradykinin is critical in the pathogenesis of CAIA.
Materials and methods
Animals
B1RB2R−/− mice that have been backcrossed onto C57BL/6 background for more than 10 generations (Jackson Laboratory, Bar Harbor, ME, USA) and their B1RB2R+/+ littermates were used. Mice were maintained in a pathogen-free facility and monitored in accordance with the guidelines from the Institutional Animal Care and Use Committee. Eight-week-old mice with body weight between 20 and 24 g were used.
Induction of CAIA
Mice received a single-dose intraperitoneal (i.p.) injection of anti-collagen II antibody cocktail (6 mg/mouse; Chondrex, Redmond, WA, USA) on day 0 and an i.p. injection of 50 μg of lipopolysaccharide (LPS) on day 3.
Isolation of mouse peripheral blood mononuclear cells
Isolation of mouse peripheral blood mononuclear cells (PBMCs) was carried out as previously described [11].
Extraction of protein and RNA from joint tissue
Joints were frozen in liquid nitrogen and homogenized in ice-cold PBS supplemented with protease inhibitor cocktail (P8849, Sigma-Aldrich, St Louis, MO, USA). Homogenates were centrifuged at 14 000 g for 20 min and the protein concentration of the supernatant was determined by the bicinchoninic acid assay (BCA) method (Bradford). Total RNA was isolated using an RNeasy minikit (Qiagen, Valencia, CA, USA).
Measurement of mRNA expression by RT-PCR and quantitative real-time RT-PCR
One-step RT-PCR (SuperScript One-Step RT-PCR with platinum Taq, Invitrogen, Carlsbad, CA, USA) was performed as previously described [9]. The following primers were used: B2R: forward, 5′-TGTCCTCAGCGTGTTCTTCC-3′, reverse, 5′-GGTCCTGAACACCAACATGG-3′; B1R: forward, 5′-GTTTCAACTGGCCCTTTGGA-3′, reverse, 5′-CACCAGGAAGATGCTGATGA-3′; β-actin: forward, 5′-GTGCTATGTTGCTCTAGACTTCG-3′, reverse, 5′-ATGCCACAGGATTCCATACC-3′. Quantitative real-time PCR was performed using the Maxima SYBR Green/ROX qPCR Master Mix kit (Fermentas, Hanover, MD, USA) according to the manufacturer’s instructions. The mRNA levels were normalized according to levels of β-actin. The following primers were used: B1R: forward, 5′-CCCCTCCCAACATCACCTC-3′, reverse, 5′-GGACAGGACTAAAAGGTTCCCC-3′; B2R: forward, 5′-GGGTTTCTGTCGGTGCATGA-3′, reverse, 5′-TTGTGTGGTGACGTTGAACAT-3′; IL-1β: forward, 5′-TGTGTCTTTCCCGTGGACCT-3′, reverse, 5′-CAGCTCATATGGGTCCGACA-3′; IL-6: forward, 5′-GGTGACAACCACGGCCTTCCC-3′, reverse, 5′-AAGCCTCCGACTTGTGAAGTGGT-3′. In an ABI 7500 system (Applied Biosystems, Carlsbad, CA, USA), real-time PCR was conducted as previously described [9].
Measurement of cytokine levels
Cytokine levels in the joint extract were measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Histological analysis
Images of hind ankle joint sections stained with haematoxylin and eosin were obtained as previously described [9].
Plasma kininogen level measurement
Plasma kininogen levels were functionally assayed using a protocol as previously described [6].
Western blotting
Cleavage of HK in plasma was examined by immunoblotting using an anti-bradykinin antibody (Santa Cruz Biotechnology, Dallas, TX, USA).
Data analysis
The data were calculated as the average (s.e.m.). In one-way analysis of variance (for multiple groups) or Student’s t-test (for comparisons between two groups), differences with P-values <0.05 were considered statistically significant.
Results
B1RB2R deficiency attenuates anti-CAIA
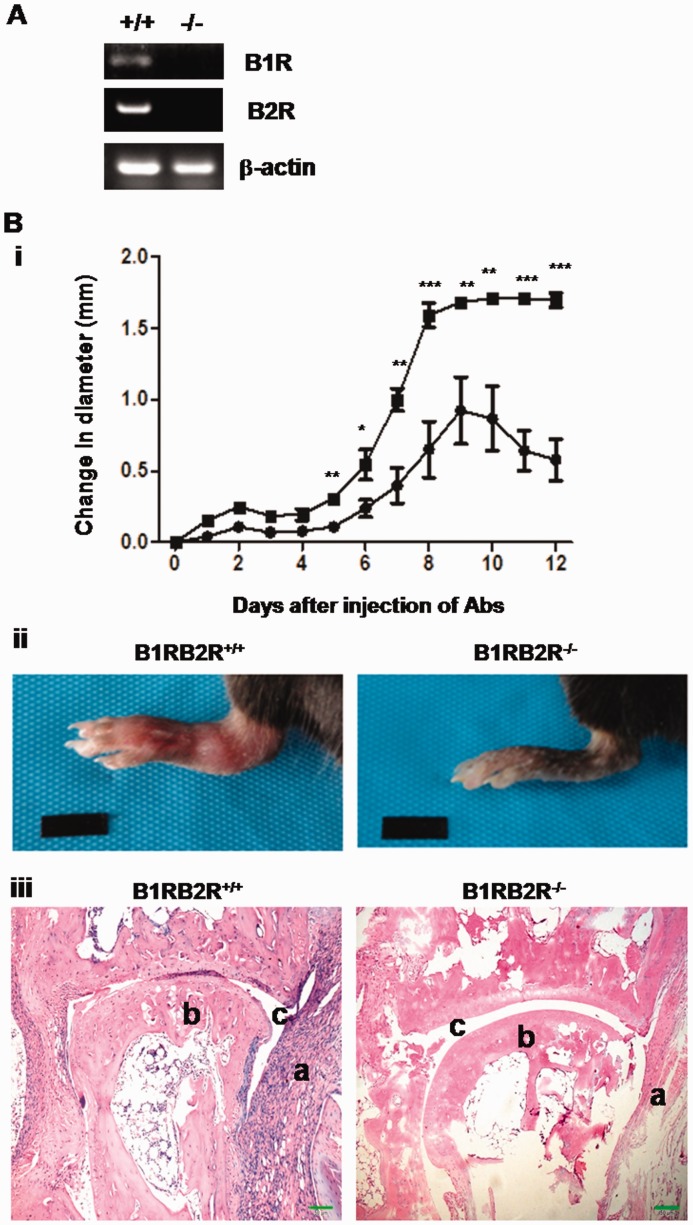
In this study we investigated the role of bradykinin receptors in the pathogenesis of CAIA using the B1RB2R double-deficient (B1RB2R–/–) mouse model. The absence of B1R and B2R mRNA in B1RB2R–/– mice was verified by RT-PCR (Fig. 1A). In a CAIA model, 6 days after injection of anti-collagen antibodies, the B1RB2R+/+ mice began to develop joint swelling and exhibited severe stable joint swelling after day 8 (Fig. 1B). In contrast, the development of arthritis was delayed and the severity of their arthritis was significantly reduced in B1RB2R–/– mice, and the joint swelling started to resolve after day 9 (Fig. 1B). On day 12, although B1RB2R+/+ mice exhibited severe joint swelling and redness, these signs of joint inflammation were remarkably attenuated in B1RB2R–/– mice (Fig. 1C). Histological analysis of joint sections revealed severe synovial hyperplasia, inflammatory cell infiltration and erosive bone damage in B1RB2R+/+ mice; in contrast, B1RB2R–/– mice exhibited minimal arthropathy (Fig. 1D).
Fig. 1.
B1RB2R deficiency attenuates CAIA in mice
(A) B1R and B2R mRNA are absent in B1RB2R−/− mice. Total RNA isolated from B1RB2R+/+ (+/+) and B1RB2R−/− (−/−) mice was reversely transcribed into cDNA and subsequently analysed by PCR. The PCR products of B1R and B2R were identified by agarose gel electrophoresis. Expression of β-actin mRNA serves as the control. (B) To induce CAIA, B1RB2R+/+ and B1RB2R−/– mice received i.p. injections of anti-collagen antibodies on day 0, followed by i.p. injection of LPS on day 3 (n = 6). (i) The severity of arthritis was assessed by triplicate measurement of hind paw thickness with digital callipers (Ultra-Call Mark III, F.V. Fowler, Newton, MA, USA) every day. The change in joint diameter in millimetres from the baseline on day 0 was recorded and indicated as mean (s.e.m.). Closed box: B1RB2R+/+ mice; closed circle: B1RB2R–/– mice. *P < 0.01, **P < 0.005, ***P < 0.001. (ii) On day 12 the hind paw was photographed. (iii) On day 12 the mice were euthanized and the hind ankle joints were removed. After the joints were fixed and decalcified, they were embedded in paraffin and the paraffin sections were stained with haematoxylin and eosin. The sections were viewed and photographed under a microscope. A representative stained section shows the histopathological features of arthritis, including synovial hyperplasia, bone or cartilage erosions and mononuclear cell infiltration (original magnification 100×). a: inflamed synovial tissue; b: bone; c: joint space. Scale bar represents 50 µm. i.p.: intraperitoneal; CAIA: anti-collagen antibody-induced arthritis; LPS: lipopolysaccharide.
B1RB2R deficiency reduces proinflammatory cytokine levels in joint tissue and PBMCs
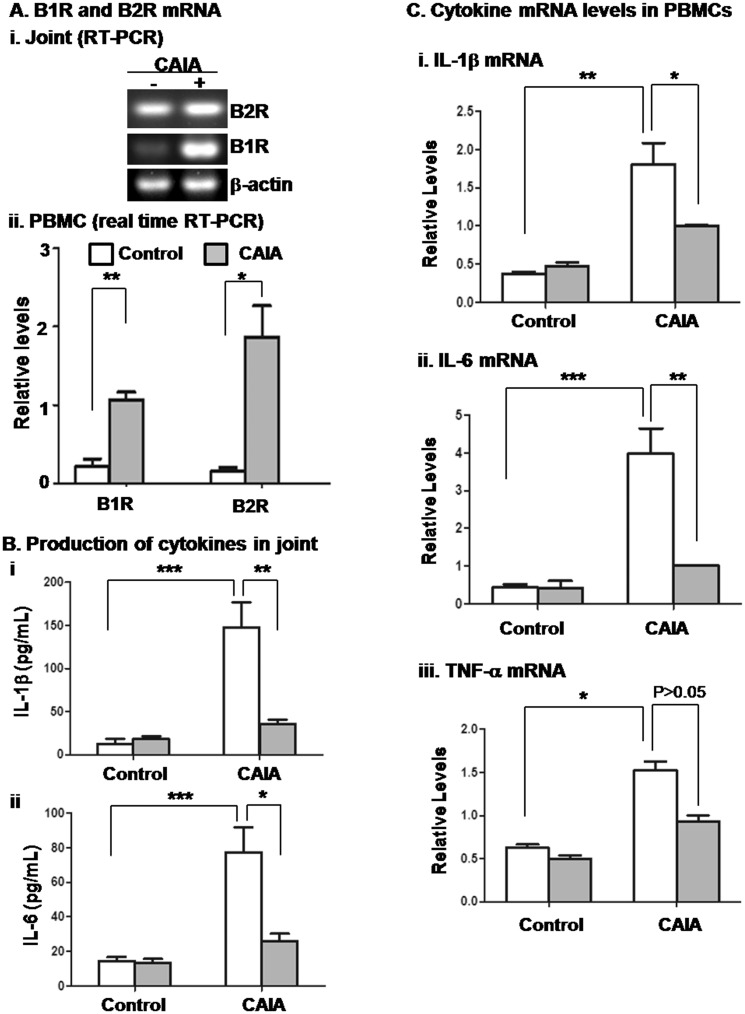
The suppressed development of CAIA in B1RB2R–/– mice suggests that bradykinin receptors are involved in the inflammatory response. To determine the altered expression of B1R and B2R in inflamed joint tissue and circulating PBMCs, total RNA was isolated and analysed by RT-PCR. As shown in Fig. 2A(i), both B1R and B2R mRNA levels were increased in the joints of diseased mice, especially that of B1R. Moreover, compared with non-diseased mice, both B1R and B2R mRNA expression in PBMCs was markedly up-regulated in the mice bearing CAIA [Fig. 2A(ii)]. These results suggest that both of these receptors participate in joint and systemic inflammation.
Fig. 2.
B1RB2R deficiency reduces the levels of cytokines in joint tissue and circulating PBMCs
As indicated in the legend for Fig. 1, B1RB2R+/+ and B1RB2R–/– mice received injections of PBS (control) and anti-collagen antibodies plus LPS (CAIA). At the end of the experiments (day 12), the mice were euthanized, followed by removal of the ankle joints and collection of blood. (A) The RNA was purified from (i) joint tissue and (ii) PBMCs of B1RB2R+/+ mice that received injection of PBS (CAIA negative) and anti-collagen antibodies plus LPS (CAIA positive). The mRNA expression of B1R and B2R was evaluated by RT-PCR and real-time RT-PCR, respectively. (B) The levels of (i) IL-1β and (ii) IL-6 in joint tissue were measured using ELISA. *P < 0.05, **P < 0.01, ***P < 0.001. (C) The cytokine mRNA levels in PBMCs were quantitated by real-time RT-PCR (i–iii). The results were normalized against mRNA of β-actin and relative levels of quantification were calculated. Open column: B1RB2R+/+ mice; filled column: B1RB2R–/– mice. *P < 0.05, ** P < 0.01, ***P < 0.001. PBMCs: peripheral blood mononuclear cells; LPS: lipopolysaccharide; CAIA: anti-collagen antibody-induced arthritis.
To determine whether these bradykinin receptors are required for the production of proinflammatory cytokines in joints, we measured the levels of IL-1β and IL-6, which have been shown to be important for the development of CAIA. Both IL-1β and IL-6 levels were markedly increased in the joint tissue of wild-type (WT) mice with CAIA compared with non-diseased mice (Fig. 2B). However, the production of IL-1β and IL-6 was significantly reduced in joint tissue of B1RB2R–/– mice (Fig. 2B). In contrast, TNF-α production was not significantly decreased in the joint tissue of B1RB2R–/– mice (data not shown).
Since arthritis is a systemic disease, we tested whether circulating inflammatory cells are also regulated by bradykinin receptors. In the CAIA model, on day 12, PBMCs were isolated and their cytokine mRNA levels were quantified by real-time RT-PCR. mRNA expression of IL-1β (i), IL-6 (ii) and TNF-α (iii) in PBMCs from WT mice was significantly increased (Fig. 2C) compared with cells from non-diseased mice. In contrast, mRNA levels of IL-1β and IL-6, but not TNF-α, in B1RB2R–/– cells were significantly attenuated (Fig. 2C). Also, we could not detect the difference in TNF-α level in plasma between B1RB2R+/+ mice and B1RB2R–/– mice on day 12 (data not shown).
Discussion
Experimental and clinical observations have demonstrated that the KKS plays a critical role in the pathogenesis of arthritis, however, the molecular and cellular mechanisms by which activation of the KKS mediates arthritis remain unknown. We recently reported that plasma kallikrein mediates synovial inflammation in rat models of arthritis [9]. In this study we further characterized a downstream event of plasma kallikrein and determined the role of receptors for bradykinin, which is a product of kallikrein cleavage of HK. Using double-receptor knockout mice, we found that deficiency of B1RB2R attenuates CAIA and inhibits the production of proinflammatory cytokines IL-1β and IL-6 in joint tissue and circulating PBMCs. Thus these observations provide the first genetic evidence that bradykinin plays an important role in the pathogenesis of CAIA.
The inflammatory response in arthritis is composed of an acute phase with oedema, pain and neutrophil migration, all of which are known to be associated with the effect of bradykinin. A decrease in plasma HK level was observed at an early stage of CAIA (see supplementary Fig. S1, available at Rheumatology Online), suggesting activation of the KKS and generation of bradykinin at an acute phase of the disease. The function of bradykinin receptors in arthritis seems universal, including local joint tissue and circulating inflammatory cells. First, the innate immune system plays an important role in CAIA, as evidenced by the expression in joint tissue of monocyte/macrophage-derived cytokines such as IL-1β and IL-6 [12]. The KKS components can enter the synovial joint space either by transudation from the plasma or from degranulating neutrophils chemotactically attracted into the synovium [13]. Up-regulated expression of bradykinin receptors in joints may increase cytokine production. Excessive release of kinins in synovial tissues may release neurotransmitters (substance P, acetylcholine) and stimulate the synthesis of a second wave of proinflammatory mediators (IL-1β, IL-6, prostaglandins, leukotrienes and histamine) to produce inflammatory joint disease [3, 14]. Second, up-regulated bradykinin receptor expression in circulating PBMCs may enhance systemic inflammation and their homing to inflamed synovial tissue. Expression of B1R in CAIA can be induced by proinflammatory cytokines such as IL-1β and IL-6, and the increase in B1R expression (Fig. 2A) probably occurs as part of a proinflammatory feedback mechanism. B1RB2R deficiency significantly inhibited IL-1β and IL-6 mRNA expression, but not that of TNF-α mRNA (Fig. 2), suggesting that bradykinin receptors differently regulate expression of these cytokines and their role in the pathogenesis of CAIA is, at least in part, through regulation of IL-1β and IL-6.
It has been proposed that bradykinin receptors are therapeutic targets for the treatment of arthritis [15]. Although both B1R and B2R belong to the G protein-coupled receptors and are activated by bradykinin, their biological functions and effect are distinct in many respects. For example, B2R is ubiquitously and constitutively expressed in vascular cells and responds to stimulation with a short, strong signal but also immediately undergoes rapid desensitization of functional responses [16]. B1R expression, undetectable in physiological conditions, is induced following tissue injury and inflammation on lymphocytes, monocytes and vascular endothelium [16]. Although a B2R antagonist has been shown to inhibit acute inflammation and arthritis [7], other studies have demonstrated that the effect of B2R antagonists is very limited in the treatment of arthritis [17, 18]. Correspondingly, B2R deficiency did not inhibit CAIA [10], thus the role of B2R in CAIA is probably dispensable. We found that, unlike the phenotype of B2R-deficient mice, the mice lacking both B1R and B2R display attenuated arthritis (Fig. 1), indicating that B1R is probably a key receptor that mediates the proinflammatory effect of bradykinin in CAIA. If this is the case, B1R might be a better target for the treatment of inflammatory arthritis. Kaufman et al. [19] also found that B1R antagonist prevents OA. B1R might be a main receptor for bradykinin in the inflammatory response and widely involved in different joint inflammation. On the other hand, overexpression of B1R up-regulates B2R expression [20] and an increase in both B1R and B2R is often observed in the same pathological settings [16]. Their relative functional roles, spatially and temporally, in arthritis is more complex than originally believed [16], and their reciprocal expression and synergistic effects in the progression of arthritis need to be investigated.
Taken together, this study demonstrates for the first time that bradykinin receptors are required for CAIA, which underlies an important role of KKS activation in the pathogenesis of arthritis. This study not only provides novel insight into the mechanisms by which KKS activation mediates arthritis, but also leads to a new concept that B1R is a better therapeutic target for anti-arthritic treatment. Further understanding of the cellular and molecular mechanisms for its specific role should improve our understanding of the pathogenesis of arthritis.
Rheumatology key messages.
Bradykinin receptors in joint tissue and peripheral blood mononuclear cells (PBMCs) are up-regulated in anti-collagen antibody-induced arthritis (CAIA).
Bradykinin receptor deficiency attenuates CAIA.
Bradykinin receptor deficiency reduces cytokine levels in joint tissue and PBMCs in CAIA.
Funding: This work was supported in part by the National Institute of Health (AR057542 and AR063290), the National Natural Science Foundation of China (81270592, 30971491, 31201058 and 81301534) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–43. [PubMed] [Google Scholar]
- 2.Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599–607. doi: 10.2174/138161206777698710. [DOI] [PubMed] [Google Scholar]
- 3.Cassim B, Shaw OM, Mazur M, et al. Kallikreins, kininogens and kinin receptors on circulating and synovial fluid neutrophils: role in kinin generation in rheumatoid arthritis. Rheumatology. 2009;48:490–6. doi: 10.1093/rheumatology/kep016. [DOI] [PubMed] [Google Scholar]
- 4.Dela Cadena R, Stadnicki A, Uknis A, et al. Inhibition of plasma kallikrein prevents peptidoglycan-induced arthritis in the Lewis rat. FASEB J. 1995;9:446–52. doi: 10.1096/fasebj.9.5.7896018. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez CC, Donadi EA, Reis ML. Kallikreins and kininogens in saliva and plasma of patients presenting with rheumatoid arthritis. Scand J Rheumatol. 2002;31:38–40. doi: 10.1080/030097402317255354. [DOI] [PubMed] [Google Scholar]
- 6.Sainz IM, Isordia-Salas I, Castaneda JL, et al. Modulation of inflammation by kininogen deficiency in a rat model of inflammatory arthritis. Arthritis Rheum. 2005;52:2549–52. doi: 10.1002/art.21202. [DOI] [PubMed] [Google Scholar]
- 7.Sainz IM, Uknis AB, Isordia-Salas I, et al. Interactions between bradykinin (BK) and cell adhesion molecule (CAM) expression in peptidoglycan-polysaccharide (PG-PS)-induced arthritis. FASEB J. 2004;18:887–9. doi: 10.1096/fj.03-0835fje. [DOI] [PubMed] [Google Scholar]
- 8.Isordia-Salas I, Pixley RA, Parekh H, et al. The mutation Ser511Asn leads to N-glycosylation and increases the cleavage of high molecular weight kininogen in rats genetically susceptible to inflammation. Blood. 2003;102:2835–42. doi: 10.1182/blood-2003-02-0661. [DOI] [PubMed] [Google Scholar]
- 9.Dai J, Agelan A, Yang A, et al. Role of plasma kallikrein-kinin system activation in synovial recruitment of endothelial progenitor cells in experimental arthritis. Arthritis Rheum. 2012;64:3574–82. doi: 10.1002/art.34607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JJ, Hwang I, Cho KH, et al. Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis. J Clin Invest. 2011;121:3517–27. doi: 10.1172/JCI46387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai JH, Iwatani Y, Ishida T, et al. Glycyrrhizin enhances interleukin-12 production in peritoneal macrophages. Immunology. 2001;103:235–43. doi: 10.1046/j.1365-2567.2001.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong PKK, Campbell IK, Egan PJ, Ernst M, Wicks IP. The role of the interleukin-6 family of cytokines in inflammatory arthritis and bone turnover. Arthritis Rheum. 2003;48:1177–89. doi: 10.1002/art.10943. [DOI] [PubMed] [Google Scholar]
- 13.Bhoola KD, Elson CJ, Dieppe PA. Kinins—key mediators in inflammatory arthritis? Rheumatology. 1992;31:509–18. doi: 10.1093/rheumatology/31.8.509. [DOI] [PubMed] [Google Scholar]
- 14.Palacios FA, Novaes GS, Guzzo ML, Laurindo IM, de Mello SB. Interrelationship of the kinin system, nitric oxide and eicosanoids in the antigen-induced arthritis in rabbits. Mediators Inflamm. 1999;8:245–51. doi: 10.1080/09629359990414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma JN, Buchanan WW. Pathogenic responses of bradykinin system in chronic inflammatory rheumatoid disease. Exp Toxicol Pathol. 1994;46:421–33. doi: 10.1016/S0940-2993(11)80053-9. [DOI] [PubMed] [Google Scholar]
- 16.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92:1699–775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 17.Meßlinger K, Schepelmann K, Pawlak M, Schmidt RF. Bradykinin B1 and B2 receptor antagonists do not change the ongoing activity of slowly conducting articular afferents in the inflamed knee joint of the cat. Neurosci Lett. 1993;164:21–4. doi: 10.1016/0304-3940(93)90847-e. [DOI] [PubMed] [Google Scholar]
- 18.Griesbacher T, Legat FJ. Effects of the non-peptide B2 receptor antagonist FR173657 in models of visceral and cutaneous inflammation. Inflamm Res. 2000;49:535–40. doi: 10.1007/s000110050628. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman GN, Zaouter C, Valteau B, Sirois P, Moldovan F. Nociceptive tolerance is improved by bradykinin receptor B1 antagonism and joint morphology is protected by both endothelin type A and bradykinin receptor B1 antagonism in a surgical model of osteoarthritis. Arthritis Res Ther. 2011;13:R76. doi: 10.1186/ar3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues ES, Silva RF, Martin RP, et al. Evidence that kinin B2 receptor expression is upregulated by endothelial overexpression of B1 receptors. Peptides. 2013;42:1–7. doi: 10.1016/j.peptides.2013.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.