Abstract
Background
Assembly of stable light-harvesting complexes (LHCs) in the chloroplast of green algae and plants requires synthesis of chlorophyll (Chl) b, a reaction that involves oxygenation of the 7-methyl group of Chl a to a formyl group. This reaction uses molecular oxygen and is catalyzed by chlorophyllide a oxygenase (CAO). The amino acid sequence of CAO predicts mononuclear iron and Rieske iron-sulfur centers in the protein. The mechanism of synthesis of Chl b and localization of this reaction in the chloroplast are essential steps toward understanding LHC assembly.
Results
Fluorescence of a CAO-GFP fusion protein, transiently expressed in young pea leaves, was found at the periphery of mature chloroplasts and on thylakoid membranes by confocal fluorescence microscopy. However, when membranes from partially degreened cells of Chlamydomonas reinhardtii cw15 were resolved on sucrose gradients, full-length CAO was detected by immunoblot analysis only on the chloroplast envelope inner membrane. The electron paramagnetic resonance spectrum of CAO included a resonance at g = 4.3, assigned to the predicted mononuclear iron center. Instead of a spectrum of the predicted Rieske iron-sulfur center, a nearly symmetrical, approximately 100 Gauss peak-to-trough signal was observed at g = 2.057, with a sensitivity to temperature characteristic of an iron-sulfur center. A remarkably stable radical in the protein was revealed by an isotropic, 9 Gauss peak-to-trough signal at g = 2.0042. Fragmentation of the protein after incorporation of 125I- identified a conserved tyrosine residue (Tyr-422 in Chlamydomonas and Tyr-518 in Arabidopsis) as the radical species. The radical was quenched by chlorophyll a, an indication that it may be involved in the enzymatic reaction.
Conclusion
CAO was found on the chloroplast envelope and thylakoid membranes in mature chloroplasts but only on the envelope inner membrane in dark-grown C. reinhardtii cells. Such localization provides further support for the envelope membranes as the initial site of Chl b synthesis and assembly of LHCs during chloroplast development. Identification of a tyrosine radical in the protein provides insight into the mechanism of Chl b synthesis.
Background
Development of the chloroplast, whether from a proplastid or an etioplast, involves assembly of the abundant thylakoid membrane system, a complex but highly coordinated process that requires chlorophyll (Chl) [1,2]. Within thylakoid membranes, the reactions of photosynthesis are driven by light energy absorbed predominantly by light-harvesting complexes (LHCs). LHCs in plants and green algae contain nearly equal amounts of Chl a and Chl b associated with a family of proteins (Lhcb1 to Lhcb6 for photosystem II and Lhca1 to Lhca4 for photosystem I) that range from about 20 kDa to 30 kDa in mass [3]. These proteins are encoded in the nuclear genome, synthesized on cytosolic ribosomes as precursors with an N-terminal targeting extension, and imported into the plastid [1]. The prototypic apoprotein (Lhcb1) of the major complex, LHCII, binds 3 xanthophyll molecules and 14 Chl molecules, 8 of Chl a and 6 of Chl b [4-7].
Chl b is derived from Chl a by oxygenation of the 7-methyl group to a formyl group [8]. An extensive amount of evidence has been gathered on the requirement of Chl b for accumulation of stable LHCs. For example, Chl b-less strains lack most if not all of the major LHC apoproteins [9-11]. In contrast, over-expression of chlorophyllide (Chlide) a oxygenase (CAO), the enzyme that catalyzes synthesis of Chlide b, in Arabidopsis increased the size of the light-harvesting antenna [12], an indication that the amount of LHCs in plants is controlled by synthesis of Chl b. Eggink et al. [13] proposed that the additional electronegative oxygen atom in Chl b causes further redistribution of the chlorin π electron system towards the periphery of the molecule, thereby increasing the positive point charge on the central Mg atom, which results in an increase in its Lewis acid strength. Stability of LHCs may thus result from strengthening of the coordination bonds between Chl b and electronegative, oxygen-containing ligands because of additional Coulombic attraction. Several of the Chl b molecules are also hydrogen-bonded to amino acid sidechains, which further strengthens the interaction with the protein [7].
The gene and cDNA encoding CAO were isolated and characterized from Chlamydomonas reinhardtii [14]. Subsequently, CAO cDNA was cloned from Arabidopsis thaliana [11], several other plants and algae, and the photosynthetic prokaryotic organisms, Prochloron and Prochlorothrix [15]. Although extant CAO genes vary in sequence and length, the deduced amino acid sequences predict conserved non-heme, mononuclear iron and Rieske iron-sulfur centers [12,15]. The phylogeny of CAO gene sequences, coupled with genetic evidence [11,14,16], indicated that CAO is a single-copy gene and that the ability of photosynthetic systems to synthesize Chl b arose only one time, an important event in the evolution of plants. The absence of Chl b in plants with mutations in the cao locus suggests that orthologous proteins, such as Tic55 and lethal leaf spot (LLS1) proteins [17], do not have CAO activity, although the second-site suppressor mutations found with the Chl b-less mutant strains of C. reinhardtii [18] suggested that similar proteins can possibly acquire CAO activity (see Discussion). To further understand the reaction catalyzed by CAO, we initiated a study of the enzyme. We confirmed that CAO is a membrane-bound protein and determined its intracellular localization. The catalytic subunit, encoded by Arabidopsis CAO cDNA, was expressed in Escherichia coli. During EPR spectroscopic analysis of the recombinant protein, the existence of a stable radical was discovered that is possibly involved in the reaction mechanism.
Results
Intracellular location of CAO
To determine localization of CAO in chloroplasts, leaves of young pea plants were biolistically transformed with the CAO coding sequence fused in frame at the 3' end to the coding sequence of GFP. Twenty-four hours after transient expression from the CaMV35S promoter, green fluorescence of GFP was localized quantitatively to chloroplasts, and appeared throughout the chloroplast (Figure 1A), including thylakoid membranes, which were indicated by red fluorescence of Chl (Figure 1B). As shown by the overlay in Figure 1C, direct correspondence of green and red fluorescence was not found. Particularly at the periphery of the plastids, the green fluorescence of GFP seemed predominant (arrows) while the fluorescence of Chl was predominant within the chloroplast (asterisks). This experiment indicated that CAO was located on envelope and thylakoid membranes in developed chloroplasts, although the relative enrichment seemed greater in the envelope as compared with thylakoid membranes.
Figure 1.
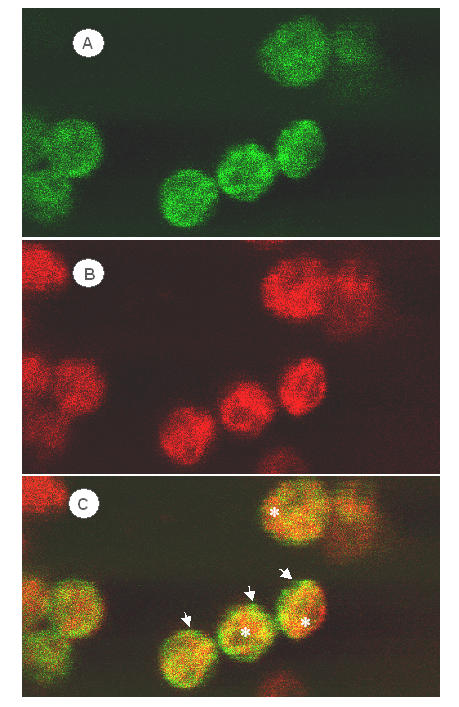
Confocal fluorescence micrographs of a pea leaf 24 h after biolistic transformation and transient expression of CAO-GFP fusion protein. (A), Location of the GFP tag is shown by green fluorescence. (B), Thylakoid membranes are indicated by the red fluorescence of Chl. (C), Overlay of green and red fluorescence signals. Green fluorescence of CAO-GFP was detected only within chloroplasts and was more prominent near the periphery of the organelles (arrows). Examples of regions of the chloroplast where Chl fluorescence predominated are shown by asterisks.
To localize CAO by a biochemical approach, homogenized Arabidopsis leaves or broken Chlamydomonas cells were centrifuged to separate membrane and soluble proteins. Immunoblot analysis after electrophoresis of proteins in these cell fractions showed that CAO was recovered quantitatively with membranes and was not released when membranes were washed with 1 M NaCl (not shown). Membranes from cells of C. reinhardtii cw15 were resolved on sucrose gradients, followed by immunoblot analysis after electrophoresis of proteins in fractions from the gradients. CAO was recovered in membranes with buoyant densities characteristic of envelope membranes (ρ = 1.10 to 1.13 g cm-3; 0.8 to 1.0 M sucrose) and in thylakoid membranes (ρ ≈ 1.17 g cm-3; about 1.3 M sucrose) [19], consistent with results shown in Figure 1. Because we found previously that the thylakoid marker LHCP was detected in the low-density region of the gradient [20], regions containing envelope were possibly contaminated with thylakoid membranes. Thus advantage was taken of the ability to grow C. reinhardtii cw15 cells in the dark to reduce the amount of thylakoid membranes and determine more clearly whether CAO is present in envelope membranes. When samples from cells grown in the dark for 3 days were applied to sucrose gradients, a yellow, slightly turbid band with a density (ρ ≈ 1.12 g cm-3) typical of the inner envelope membrane was resolved in the middle of the gradient (Figure 2, fractions 3–6). The only immunoreactive polypeptide detected in these fractions with antiserum against CAO was 51 kDa in mass, the size predicted for the CAO gene product [14]. These immunoreactive membranes were clearly separated from residual thylakoid membranes, indicated by their content of Chl and by immunostaining with antibodies against LHCP, which were recovered in fractions 6–9. Intact CAO was not detected in these latter fractions.
Figure 2.
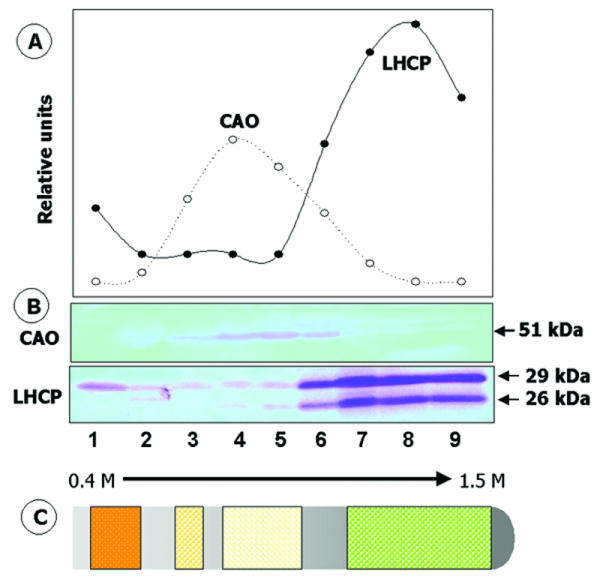
Isopycnic sucrose gradient analysis of the distribution of CAO. Membranes from C. reinhardtii cw15 cells that were grown 3 days in the dark at 28°C were layered on the top of 0.4 to 1.5 M linear sucrose gradients. After centrifugation as described in Methods, fractions were collected, and the proteins in each were precipitated with 10% (w/v) trichloroacetic acid and resolved by electrophoresis. A blot of the gel was probed first with antiserum against CAO and then with anti-LHCP IgG [21]. (A), Graphic presentation of densitometric scans of immunoreactive bands shown in (B). (C), A diagram of the positions of visible amounts of membrane in the gradients. Chl (green), indicating the position of residual thylakoid membranes, was near the bottom, a yellow band was in the middle, and an orange, carotenoid-rich fraction at the top of the gradient.
A low level of mature-sized LHCP was detected in the mid-region and also near the top of the gradient, at a buoyant density of envelope outer membranes [19,20]. Previous work [13,21] showed by immunoelectron microscopy that LHCPs were incorporated initially into membranes of the envelope during chloroplast development, assembled into LHCs, and then transferred to their major site of accumulation, the thylakoid membrane. These results are similar to the small amount of mature-sized LHCP recovered with purified envelope membranes by Bovet et al. [22,23], which was phosphorylated by a membrane-bound kinase and may represent LHCP transiently associated with the envelope during import.
EPR spectrum of recombinant CAO expressed in E. coli
Expression of Arabidopsis CAO cDNA was induced in E. coli at 20°C to maximize recovery of correctly folded enzyme [24]. The initial EPR spectrum of a membrane fraction measured at 125 K revealed the presence of several distinct paramagnetic species (Figure 3A, upper trace). A narrow signal at g = 4.3 was assigned to high-spin, non-heme Fe3+ in a near-maximally rhombic environment (E/D ≈ 1/3). A sextet of small peaks in the region between 3000 and 3600 Gauss was attributed to a low level of contaminating Mn2+. In subsequent experiments, the Mn2+ signals were eliminated by a wash of membranes with 0.1 mM EDTA (Figure 3B). Although the g = 4.3 signal often results from adventitious Fe3+, this signal was not diminished significantly by the wash with EDTA, nor was the signal decreased by addition of reductants such as dithiothreitol or ascorbate (not shown), which should reduce free iron to the EPR-silent Fe2+. Thus the g = 4.3 signal appeared to indicate assembly of the predicted mononuclear iron center, which was possibly protected from the environment or stabilized by the surrounding protein structure. (This conclusion is reinforced by data shown below for membranes solubilized with detergents.)
Figure 3.
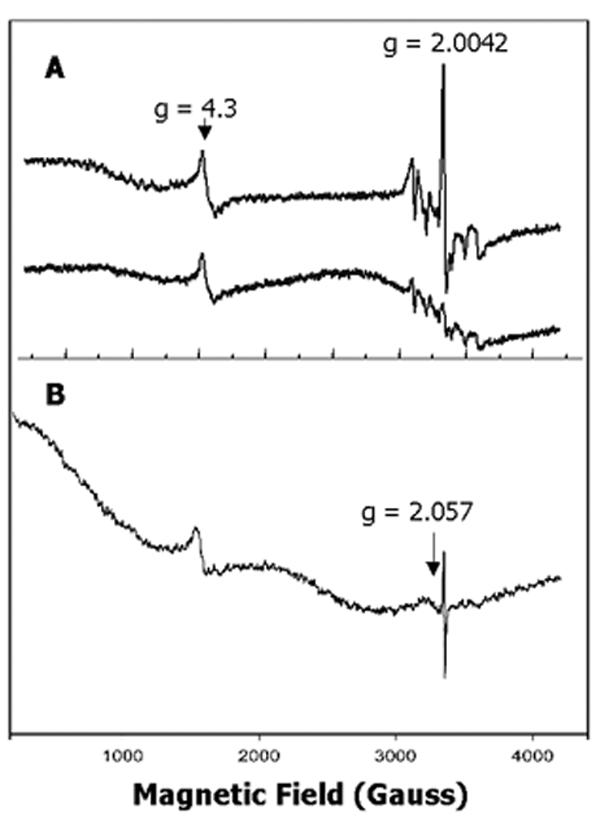
EPR spectra of CAO expressed in E. coli. Membrane fractions from cells induced to over-express CAO cDNA were collected and spectra were determined as described in Methods with microwave power at 5 mW. (A), Upper trace, untreated membrane fraction; 2 scans; lower trace, sample in upper trace after addition of Chl a (5 μM); 1 scan; temperature, 125 K. (B), Membrane fraction after wash with 0.1 mM EDTA; 1 scan; temperature, 14 K. Vertical axis represents first derivative of EPR absorption intensity (in arbitrary units) with respect to magnetic field.
The most striking feature of the spectrum was a signal at g = 2.0042 characteristic of an organic radical species (Figure 3A, upper trace, and Figure 3B). Addition of Chl a to the sample quenched the radical (Figure 3A, lower trace), which suggested that the radical species resided in CAO and is likely involved in catalytic activity. The isotropic feature of the radical spectrum, with a 9 Gauss peak-to-trough linewidth, is shown on an expanded scale in Figure 4A for a sample washed with 0.1 mM EDTA. Free radical species are present in E. coli membranes [25,26], and thus it was important to achieve sufficient over-expression of CAO to facilitate analysis of the EPR spectrum of CAO without interference from endogenous signals. We observed an endogenous radical in the spectrum of membrane samples from uninduced E. coli cells that was approximately equal in amplitude to a strong, endogenous high-spin, Fe3+ signal at g ≈ 6, possibly from a cytochrome [27] (not shown). The g ≈ 6 signal, and by implication that from the endogenous radical, was below detection in samples of over-expressed CAO (Figures 3A and 3B). Electrophoresis of samples used for EPR spectroscopy revealed a 54 kDa polypeptide, the mass expected of the recombinant CAO (see below), that was prominent in fractions from cells in which CAO expression was induced but absent in samples from uninduced cells (see below).
Figure 4.
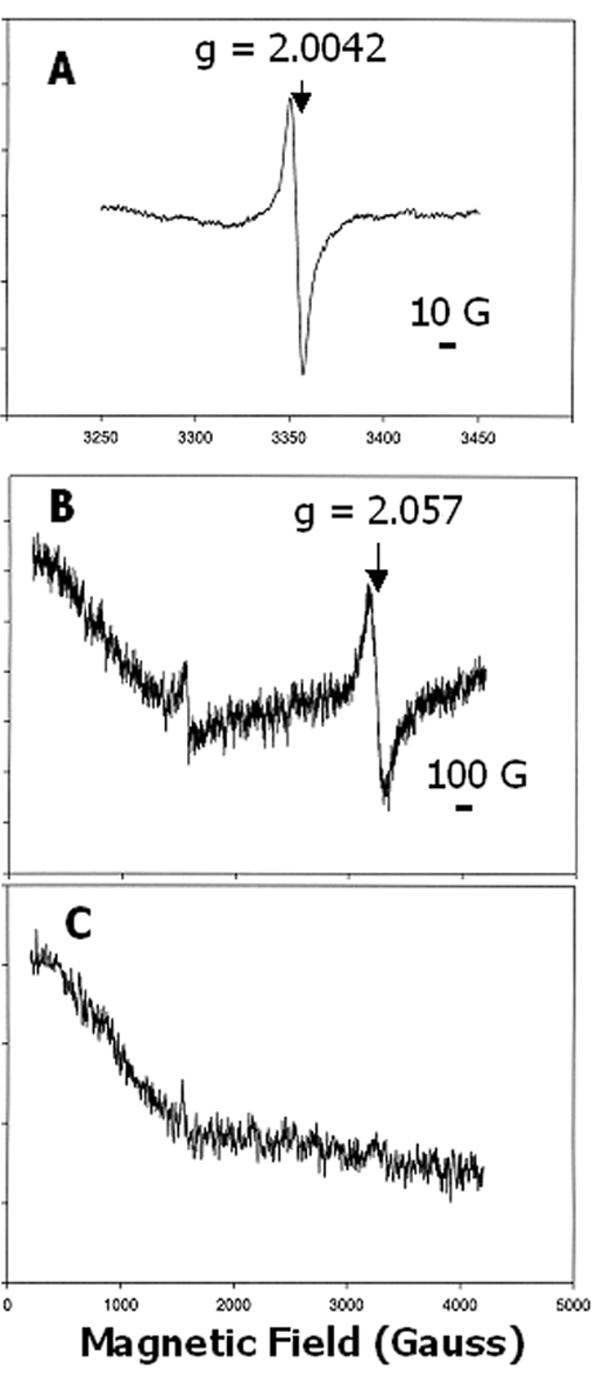
EPR spectra of CAO expressed in E. coli. (A), Spectrum of a membrane fraction on an expanded x-axis to indicate the isotropic character of the g = 2.0042 radical; 12 scans; 14 K. (B), Spectrum of a membrane fraction after wash with 0.1 mM EDTA and addition of Chl a (5 μM), which revealed the broad, isotropic character of the g = 2.057 signal; 4 scans; 7 K. (C), Sample as in (B) after addition of mercaptoethanol (0.5%, v/v); 1 scan; 7 K. The bar in panels (A) and (B) indicate the magnetic field scale. The y-axis of the spectra was expanded by a factor of 5 for (B) and 10 for (C), relative to the spectra in Figure 3A, for presentation of the data.
When the temperature at which EPR spectra were measured was lowered below 15 K, a broad signal appeared at g = 2.057 (Figure 3B). The nearly symmetrical nature of this signal, with a peak-to-trough linewidth of about 100 Gauss, is shown in Figure 4B after addition of Chl to quench the radical. This signal weakened dramatically as the temperature was increased above 20 K (not shown). Interestingly, the addition of reducing agents such as dithiothreitol or dithionite to the ambient-oxidized samples did not significantly affect the intensity of this signal. However, the signal disappeared after addition of mercaptoethanol (Figure 4C). We have no obvious explanation for the differing effects of reducing agents other than the possibility that the EPR-active center was more accessible to the smaller, less polar mercaptoethanol. Although the shape and position of this signal is similar to the EPR spectrum of Cu2+ [28-30], its sensitivity to temperature and resistance to reductants, except for mercaptoethanol, did not support this assignment. The large stability constant of Cu2+ with EDTA [31] also argues against the metal being retained after the sample was washed with EDTA.
EPR spectrum of immunopurified CAO
To determine whether the spectrum of the recombinant protein was the same as the native enzyme, proteins were obtained by immunoprecipitation with antiserum raised against a C-terminal fragment of CAO. This region of the protein was selected as antigen to avoid cross-reaction with other redox active proteins that contain conserved Rieske or mononuclear iron domains. The EPR spectrum of CAO recovered from detergent-solubilized extracts from Arabidopsis leaves (Figure 5A) was similar to that obtained with the recombinant protein. Strong signals were found at g = 4.3 for the mononuclear iron center and at g = 2.057, which overlapped the spectrum of the radical. We did not detect a signal predicted for a Rieske iron-sulfur center up-field from the radical spectrum after addition of dithionite or ascorbate. The spectrum was not significantly different in shape when the microwave power used for the measurement was raised from 2 mW to 100 mW at 7 K (not shown). Immunoprecipitated CAO from C. reinhardtii cw15 cells (Figure 5B) provided a spectrum similar to that obtained with the sample from Arabidopsis. Preparations from Chlamydomonas cells were often contaminated with Mn2+ (not shown), but as illustrated in Figure 5B, samples washed with 0.1 mM EDTA prior to the analysis lacked Mn2+ signals. The treatment with EDTA did not significantly affect the magnitude of the g = 4.3 signal, which indicated that the latter did not arise from contaminating Fe3+. Iron has a slightly higher stability constant with EDTA than manganese [31], which supports this treatment as an appropriate control for adventitious transition metals. Furthermore, these samples were prepared from detergent-solubilized membranes, which should preclude simply trapping iron within a membrane pellet.
Figure 5.
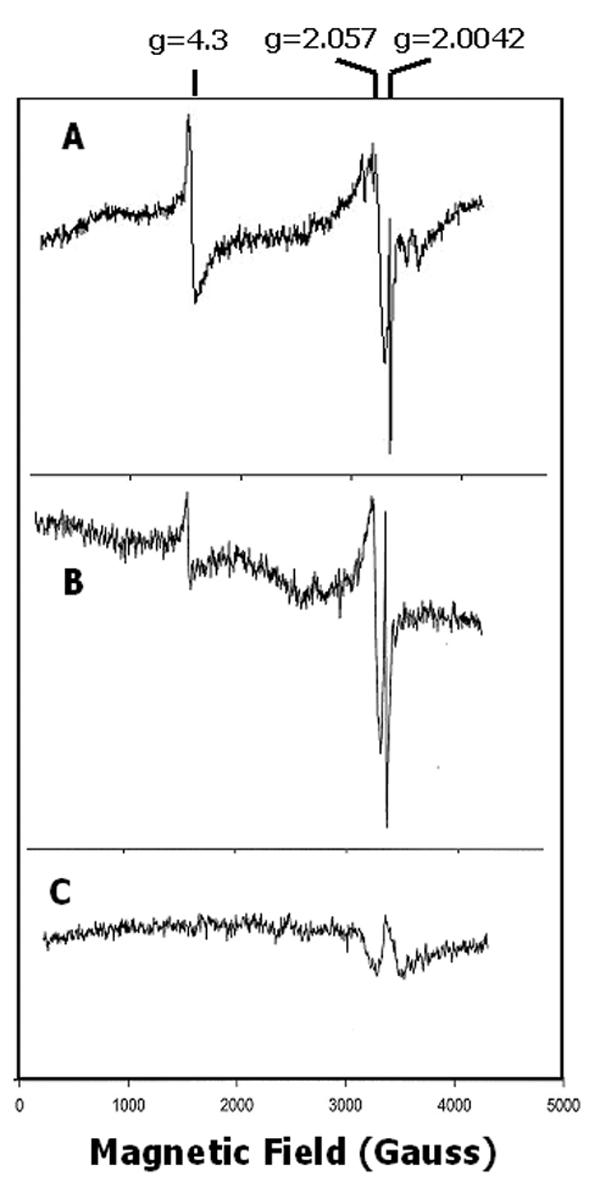
EPR spectra of native, immunoprecipitated CAO after wash with 0.1 mM EDTA. (A), CAO from Arabidopsis; 4 scans; 5 K; (B), CAO from Chlamydomonas; 2 scans; 5 K; (C), sample from Chlamydomonas treated as in (A) but with preimmune serum; 2 scans; 10 K. The shape of the weak spectrum in the preimmune control was out of phase with the g = 2.057 signal and thus did not contribute significantly to the spectrum of CAO. Microwave power was 2 mW. The y-axis was the same for all spectra.
A control sample prepared with preimmune serum and washed with 0.1 mM EDTA lacked the radical spectrum at g = 2.0042 and the g = 4.3 signal (Figure 5C). The preimmune control in some experiments contained a low amplitude signal that overlapped the g = 2.057 signal, but the background structure did not match the peak-to-trough positions of signals in the immunopurified CAO, nor was it present in other samples (see Figure 4C). Analysis of immunoprecipitates from Chlamydomonas by electrophoresis revealed the heavy and light chains of IgG and three additional polypeptides between 14 kDa and 20 kDa in mass (not shown). These polypeptides were possibly functionally associated with the enzyme but did not react with antibodies against CAO. Preliminary evidence obtained by mass spectrometry of tryptic fragments provided a tentative identification of the 14 kDa component as a thioredoxin, while the analysis suggested that the 20 kDa polypeptide was a minor Lhcb. CAO migrated near the heavy chain of IgG.
Reaction with iodide
The isotropic g = 2.0042 signal indicated the existence of a stable radical in the protein, characteristic of the phenolate radical of tyrosine. To confirm that the radical occurred on an amino acid residue, membrane fractions from E. coli, Arabidopsis and Chlamydomonas were incubated with Na125I, which was reported to react specifically with tyrosine radicals [32,33], and the proteins were resolved by electrophoresis. Radioautographic analysis of the dried gel revealed labeling of a protein, 51 kDa in membranes from Chlamydomonas (Figure 6A, lanes 1 and 2) and 56 kDa from Arabidopsis membranes (Figure 6A, lanes 3 and 4). Other proteins also became labeled, in particular a 32 kDa protein, probably the reaction center protein D2, whose tyrosine radical is known to react with iodide [32].
Figure 6.
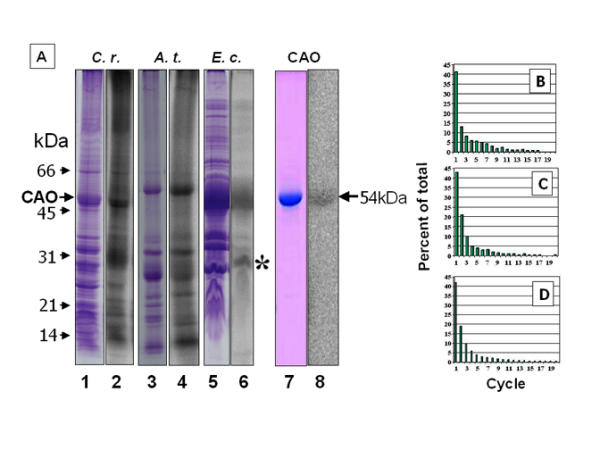
Incorporation of 125I- into membrane proteins from Chlamydomonas (lanes 1, 2), Arabidopsis (lanes 3, 4), and E. coli (lanes 5, 6). CAO was electroeluted from a gel as shown in lane 5 and re-run (lanes 7, 8). Lanes 2, 4, 6 and 8 are radioautograms of Coomassie blue-stained lanes 1, 3, 5 and 7, respectively. Lanes 1 to 6 were from the same gel. A 26 kDa fragment of CAO in lane 6 is labeled with an asterisk. (B), CAO was purified by immunoprecipitation from detergent-solubilized extracts of dark-grown, yellow cells of C. reinhardtii cw15, labeled with 125I-, digested with trypsin and subjected to N-terminal degradation as described in Methods. (C), CAO was purified from light-grown, green cells of C. reinhardtii and treated as under (B). (D), 125I-Labeled CAO from Arabidopsis was analyzed as described in (B). Radioactivity in each fraction was plotted as a percent of the total released in the 20 cycles.
CAO expressed in E. coli, either as the crude membrane sample (Figure 6A, lanes 5 and 6) or after purification (Figure 6A, lanes 7 and 8), migrated as a 54 kDa polypeptide. From N-terminal sequence analysis, the terminal two amino acids were derived from the pQE70 vector, which were followed by the CAO sequence starting at Lys-51. The smaller, labeled polypeptide in lane 6, 26 kDa in mass (marked with an asterisk), was a C-terminal fragment of CAO beginning at Pro-298. This fragment possibly resulted from a trypsin-like cleavage at Lys-296 and removal of the subsequent N-terminal Met-297. The N-terminal sequences of the other two major polypeptides in Figure 6A, lane 6, 39 kDa and 35 kDa in mass, corresponded to the abundant porins OmpC and OmpA, respectively, which were not significantly labeled. Recovery of radioactivity with the protein after electrophoresis indicated that the radical species was associated with the protein and not a cofactor.
The labeled amino acid was identified based on its position relative to a Lys or Arg residue. A tyrosine residue occurs in 9 of the first 15 positions downstream of these trypsin cleavage sites. CAO from dark-grown C. reinhardtii cells was immunopurified and 125I-labeled. The sample was digested with trypsin and the mixture was subjected to successive cycles of N-terminal degradation. Most of the radioactivity was released in cycle 1 (Figure 6B). The amount of radioactivity in cycle 2 was likely the result of incomplete transfer of the sample from the reaction vial to the fraction collector. Essentially the same result was obtained with CAO prepared from light-grown cells (Figure 6C). Only Tyr-422 in CAO from C. reinhardtii cw15 should be N-terminal after trypsin digestion. Similar results were obtained with immunopurified CAO from Arabidopsis (Figure 6D), with Tyr-518 as the corresponding residue. When the Arabidopsis CAO expressed in E. coli was analyzed, data similar to those in Figure 6D were obtained (not shown). The low levels of radioactivity released in remaining cycles indicated that labeling with 125I- was highly specific and digestion with trypsin was essentially complete.
Discussion
Role of Chl b in LHC assembly
Assembly of thylakoid membranes, and in particular of LHCII, has been studied during initiation of membrane formation in strains of the alga C. reinhardtii that are unable to synthesize Chl in the dark. The ability to deplete the chloroplast of thylakoid membranes by growth in the dark, with subsequent synthesis of Chl a and Chl b and formation of these complexes at linear rates when cells are exposed to light, are major advantages of this system [34-36]. Results from these experiments showed that thylakoid membranes emanated from the chloroplast envelope, with generation of small vesicles [34]. Extensive evidence for invagination and vesiculation of the inner membrane of the envelope during chloroplast development has also been obtained with other systems [2,37-39]. LHCPs, the major proteins of the membrane, were initially detected – within minutes after initiation of chloroplast development by exposure of dark-grown cells to light – on envelope-associated membranes by immunoelectron microscopy [21]. Transfer of energy from Chl b to Chl a indicated assembly of LHCII during this time [21]. In the absence of Chl synthesis, LHCPs were detected in the cytosol but not in the chloroplast by immunoelectron microscopy [40]. This information, coupled with the requirement of Chl b for accumulation of LHCPs [9-11], led to the conclusion that initial events required for import of LHCPs and assembly of LHCII occur in the chloroplast envelope.
Localization of CAO
CAO was recovered from dark-grown Chlamydomonas cells in membranes with a buoyant density less than that of thylakoid membranes (Figure 2), characteristic of the envelope inner membrane [19,41]. The mass of the CAO protein in Chlamydomonas, estimated by electrophoresis, was the value predicted from the DNA sequence [14]. Thus, CAO seems not to be processed after integration of the protein into the envelope membrane in the alga, which is supported by the lack of similarity of the N-terminal sequence to a typical transit sequence such as encoded by the gene in Arabdopsis. These results indicate that synthesis of Chl b, an important step in LHC assembly, occurs in the envelope during the first few minutes of greening of these cells [34,35]. This conclusion is consistent with the requirement of Chl, and in particular Chl b, for retention of LHCPs in the chloroplast [1]. Reinbothe et al. [42] also reported a CAO ortholog on the inner membrane of barley etiochloroplast envelopes. Additional support for envelope localization was the EPR spectrum of purified chloroplast envelope membranes reported by Jäger-Vottero et al. [43], which was essentially identical to the EPR spectrum of recombinant CAO and of CAO recovered from Arabidopsis and Chlamydomonas cells with antibodies (Figures 3, 4 and 5). Other mononuclear iron/iron-sulfur proteins, such as Tic55, occur in the inner envelope [44,45], which may provide the Rieske center detected by Jäger-Vottero et al. [43] with whole envelope membranes. A western blot of C. reinhardtii cw15 proteins with antibodies to Tic55 did not stain a protein near the expected size of this protein (55 kDa) but instead stained polypeptides of about 42 and 35 kDa (not shown). The Tic55 antibodies possibly cross-react with other iron-sulfur proteins in Chlamydomonas.
The presence of CAO in thylakoid membranes of mature chloroplasts, as indicated by the fluorescence of the GFP-fusion protein (Figure 1) suggested that CAO is incorporated into envelope membranes after synthesis in the cytosol and subsequently transferred to thylakoid membranes along with other material as membrane vesicles [2,34,37,38]. However, we cannot exclude the possibility that the presence of the CAO-GFP fusion protein in thylakoid membranes is the result of mislocalization caused by over-expression from the CaMV35S promoter. Other enzymes involved in Chl biosynthesis, such as S-adenosyl-L-methionine:Mg-protoporphyrin IX methyltransferase, show a dual distribution in mature chloroplasts [46,47]. These enzymes have higher specific activities in envelope than thylakoid membranes, and vesicle traffic from envelope to thylakoid would provide a simple explanation for these locations.
The EPR spectrum of CAO
Jäger-Vottero et al. [43] detected a g = 2.057 signal in the spectrum of envelope membranes and showed that it disappeared as the temperature was raised to 40 K, an observation that we confirmed in our work. The g = 2.057 signal apparently was not caused by Cu2+ in the sample, although similar, because in contrast to the signal in the EPR spectrum of CAO, Cu2+ can be measured at room temperature. Moreover, the wash with EDTA should have removed Cu2+. Jäger-Vottero et al. [43] suggested that the sensitivity of the g = 2.057 signal to temperature was indicative of an unusual iron-sulfur center, which may be a modified Rieske center. The identity of this signal remains to be confirmed.
Jäger-Vottero et al. [43] also detected a remarkably stable radical in envelope membranes. In their experiments, the amplitude of the radical was decreased by NADPH under argon but increased several-fold by oxygenation of the sample, which led to the conclusion that the radical resulted from oxidation of a quinol cofactor to the semiquinone form. The incorporation of 125I- into the protein in our experiments (Figure 6) instead demonstrated that the signal emanated from the protein itself. Likely candidates for bearing a radical are tyrosine and tryptophan residues [48]. We attempted to identify the specific residue by its distance from a trypsin cleavage site within the protein. As shown in Figures 6B, 6C and 6D, most radioactivity was released from the tryptic digest in cycle 1 with the protein sequencer. The only tyrosine residue that should be released in cycle 1 is Tyr-422 in the Chlamydomonas CAO (Tyr-518 in Arabidopsis), which resides within an arginine-rich sequence that is conserved from the prokaryotic Protochlorothrix hollandica to higher plants (Figure 7A). The sequence downstream of the conserved region appears random, which highlights the apparent importance of the tyrosine and surrounding residues. The Pro0890 gene in the Chl b-containing prokaryote Prochlorococcus marinus SS120 encodes a protein with predicted non-heme iron and Rieske domains [49] that is distantly related to genes in the CAO clade [Figure 7B]. Although this protein has low homology to CAO in plants and algae, a tyrosine-containing sequence – R308VIVRHYRKFMKNK321-, rich in basic amino acids, occurs C-terminal to the mononuclear iron center and possibly has properties similar to that indicated in Figure 7A. Putative CAO proteins in Prochlorococcus marinus MED4 and MIT9313 are phylogenetically more related to CAO than to Tic55 [50].
Figure 7.
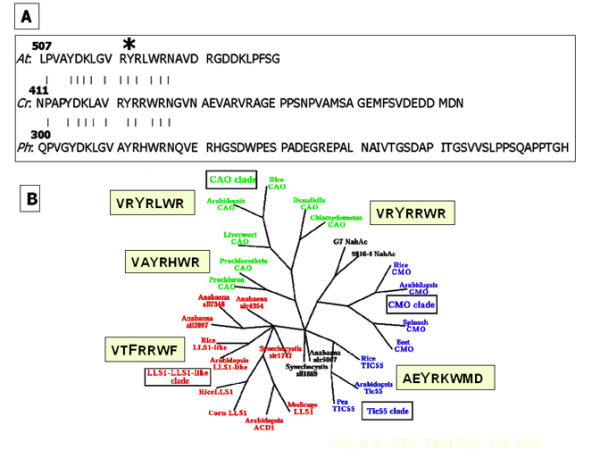
(A), C-Terminal sequences of CAO of Arabidopsis, Chlamydomonas and Prochlorothrix. Vertical lines indicate conserved residues. The conserved tyrosine (Y) residue, identified in Figure 6 as labeled with 125I-, is marked with an asterisk. At, Arabidopsis thaliana; Cr, Chlamydomonas reinhardtii; Ph, Prochlorothrix hollandica. (B), The evolutionary relationship between CAO and other proteins that contain non-heme iron and Rieske domains. Examples of the sequence surrounding the site of the conserved tyrosine, indicated in (A), are illustrated for a representative protein in each clade. (Cladogram from ref. [17], used with permission).
The stability of the radical is impressive and does not seem to require substrate for its formation. The relative magnitude of the radical signal with the protein expressed in E. coli was greater than that found with immunoprecipitated CAO from Chlamydomonas or Arabidopsis. Moreover, CAO eluted from a gel after electrophoresis and subjected to conditions for reconstitution of iron-sulfur centers [51,52] generated a small radical signal (not shown). The location of the putative radical-bearing Tyr-422 within a sequence remarkably rich in arginine residues suggests that the highly positively charged environment may be a factor in stabilization of the radical. Orthologous proteins such as Tic55 contain the conserved tyrosine but have an adjacent acidic glutamate, while the lethal leaf spot protein LLS1 has the tyrosine residue replaced with phenylalanine [see ref. [17] and Figure 7B]. Second-site revertants of Chl b-less mutants of C. reinhardtii [18] raise the possibility that these proteins could attain CAO activity by mutations in this region.
Specificity of antiserum
Antigen used for preparation of the antiserum against Arabidopsis CAO was a gel-purified, C-terminal fragment from Ser-361 to the C-terminal Gly-536 (see Methods). The Rieske and mononuclear iron domains, which are conserved among orthologous proteins and other redox proteins, are in the N-terminal two-thirds of the protein and were excluded from this fragment in order to prevent cross-reaction with these other proteins. Immunoblots of E. coli in which CAO cDNA was expressed revealed reaction only with full-length CAO and, when present, the 26 kDa fragment of CAO shown in Figure 6A. Blots with intact or broken chloroplasts from Arabidopsis yielded a single band corresponding to the expected size of mature, full-length CAO (not shown). Immunoblots of fractions from sucrose gradients that contained envelope inner membranes from Chlamydomonas also provided a single immunoreactive protein (Figure 2). When used as a preparative tool, the EPR spectra of the immunoprecipitated proteins were essentially the same as the spectrum of CAO expressed in E. coli, which was prepared without use of the antiserum. Also, the 125I-labeling pattern of immunoprecipitated proteins was the same as that of the protein expressed from CAO cDNA in E. coli. These data indicate that the antiserum used in these experiments is specific for CAO.
Enzymatic activity of CAO
Bednarik and Hoober [53] observed conversion of Pchlide a to Chlide b in the presence of NADPH and phenanthroline with membranes from yellow cells of C. reinhardtii y1. An explanation for the requirement of phenanthroline in this reaction has not been found. Pchlide b was detected in green plants [54] and in etiolated barley seedlings when a 7-formyl reductase activity was inhibited [55]. Pchlide b and Chl b were also detected in Synechocystis cells after transformation with CAO cDNA [56]. Oster et al. [57] reported that Chlide b is synthesized from Chlide a rather than from Pchlide, with 7-hydroxymethyl Chlide as an intermediate, in a reaction catalyzed in vitro by recombinant CAO. However, further work is required to establish optimal conditions for an assay in vitro and the active substrate for CAO in vivo.
As indicated by disappearance of the radical signal upon addition of Chl a (Figure 3A), the radical species in CAO possibly abstracts a hydrogen atom from the substrate. This type of reaction is well-known with free radicals and is thought to initiate P450-catalyzed monooxygenation [58]. A tyrosine radical (Em = 0.94 V) [48] would establish a sufficiently strong redox center at the catalytic site for oxidation of Chl(ide) a (E1 = 0.86 V) [59]. The resulting Chl radical might then react with an oxygen complex at the mononuclear iron center to generate the 7-hydroxymethyl Chl intermediate. We have been unable to detect sustained activity with the recombinant protein, which suggested that it may be unable to perform more than a single-turnover event and that essential subunits for catalytic activity were missing. Oxygenases, whether they contain a heme, diiron or mononuclear iron in the catalytic center, are generally multi-subunit complexes that contain additional proteins for transfer of electrons from a reductant such as NADPH to the site of reaction [e.g., references [60-62]]. In this respect, our tentative identification of a thioredoxin and a minor Lhcb species in immunoprecipitates prepared with antibodies against CAO suggests possible avenues to achieve catalytic activity in vitro. The possible association of Lhcb is particularly interesting, because Chl b did not accumulate when synthesis of LHCPs was inhibited [35]. Experiments in progress include developing an assay based on our EPR data to understand the mechanism of this reaction.
Conclusions
Previous immunoelectron microscopic localization of initial integration of LHCPs into membranes, and the kinetics of LHC assembly, identified envelope membranes as the site of these processes during initiation of chloroplast development. Localization of CAO on the envelope inner membrane provides further support for the envelope as the primary site for LHC assembly and thylakoid biogenesis. Discovery of a stable tyrosine radical in this enzyme, which is quenched by Chl a, suggests that synthesis of Chl b is initiated by a radical-mediated reaction. The inability of recombinant CAO to demonstrate significant activity suggests that additional accessory proteins are required for optimal catalytic oxygenase activity. These novel observations provide a foundation on which further investigations of these processes can be based.
Methods
cDNA and organisms
The Arabidopsis CAO cDNA [14] was ligated into pQE70 and expressed in E. coli strain M15/pREp4, as described by Oster et al. [57]. Cells in 50 mM Hepes buffer, pH 7.5, were broken by passage two times through a French pressure cell at 15 k psi. Cells of C. reinhardtii cw15, a cell-wall-less strain that does not synthesize Chl in the dark, were grown as described previously [63]. Primary leaves of two-week old Arabidopsis thaliana (var. Col-0) were chopped with razor blades, homogenized with a Waring blender and filtered through cheesecloth. Samples were centrifuged at 3 kgav and supernatant fractions were centrifuged at 200 kgav for 30 min to obtain membrane pellets.
Transient expression system and confocal microscopy
CaMV35S promoter, TMV Omega sequence and AtCAO-GFP gene were introduced into the multi-cloning site of pGreenII0029. For transient expression in pea guard cells, 25 μl of gold particles (1 μm diameter, 30 mg ml-1 in 50% glycerol) were mixed with 5 μl of plasmid DNA (~5 μg). After addition of 25 μl of 2.5 M CaCl2 and 10 μl of 0.1 M spermidine, the DNA was precipitated on the gold particles at room temperature for 3 min with continuous shaking. The gold pellet was washed once in 70% ethanol before being suspended in 30 μl of 100% ethanol. Aliquots of gold were spotted on macrocarriers and used to transform pea guard cells at 1.3 k psi using a PDS 1000HE biolistic device (Bio-Rad Laboratories, Hercules, CA). The bombarded leaf was mounted on a plastic plate 24 h after transformation and fluorescence images were recorded on a MRC 1024 system (Bio-Rad) and an Axioplan fluorescent microscope (Carl Zeiss, Jena, Germany) equipped with a Plan-Fluar100 × objective lens. A krypton-argon laser was used as excitation source at 488 nm and fluorescence intensities at 522 and 680 nm were recorded.
Sucrose gradient centrifugation
Membrane pellets from Chlamydomonas were suspended in 50 mM Hepes, pH 7.5, and applied to linear sucrose gradients, 0.4 to 1.5 M containing 10 mM Hepes, pH 7.5, and 1 mM EDTA. Gradients in a Beckman SW41 rotor were centrifuged at 250 kgav for 15 h. Fractions were collected from gradients, and after electrophoresis on 10 to 20% polyacrylamide gradient gels [64], proteins were transblotted onto Immobilon-P membranes (Millipore, Bedford, MA) and immunostained as described previously [21].
Preparation of antibodies
Antiserum was produced against a fragment of Arabidopsis CAO that had been fused to the maltose binding protein using the pMAL fusion system (New England Biolabs, Beverly, MA). A partial CAO cDNA clone (103D24) was amplified with the following primers, 5'-GGAATTCAGTGTCCCAAGTTTGGTGAA-3' and 5'-GCTCTAGATTAGCCGGAGAAAGGTAGTTT-3', which cover the region spanning Ser-381 to the C-terminal Gly-536. The PCR product was ligated into the pMAL-c2 vector using XbaI and EcoRI, and the resulting plasmid insert was sequenced. The recombinant protein (4.3 mg) was purified according to manufacturer's instructions, cleaved with Factor Xa, and the antigen fragment was purified by SDS-PAGE. Coomassie blue-stained gel slices were used to produce polyclonal antiserum in rabbits by Antibodies Inc., Davis, CA.
Immunoprecipitation
Arabidopsis leaves (10 g) were homogenized in 50 mM Hepes buffer, pH 7.5, and filtered through cheese cloth. n-Dodecyl-β-D-maltoside was added to 2% (w/v) and the sample was centrifuged at 3 kgav for 5 min to remove particulate material. The supernatant fraction (30 ml) was incubated with 0.5 ml of antiserum against CAO for 90 min with gentle shaking. Sufficient protein A-agarose (Sigma Chemical Co., St. Louis, MO) was added to bind all IgG in the antiserum, and the immunocomplex was recovered after incubation for an additional 90 min by centrifugation at 3 kgav for 5 min. An immunocomplex was also obtained from broken, log-phase cells of C. reinhardtii cw15 as described for Arabidopsis leaves. The precipitates were washed 3 times with 50 mM Hepes buffer, pH 7.5, and one time with 0.1 mM EDTA, pH 7.5, which was necessary to remove adventitious Mn2+.
EPR spectroscopy
Continuous wave EPR spectra were obtained with a Bruker E580 spectrometer equipped with an Oxford ESR900 liquid helium flow cryostat and Bruker TE102 rectangular standard cavity, at temperatures from 6 to 14 K. Other typical measurement conditions were: magnetic field modulation amplitude, 10 Gauss; modulation frequency, 100 kHz; microwave power, 5 mW for Figures 3 and 4 and 2 mW for Figure 5; microwave frequency, 9.40 GHz; horizontal resolution, 1024 points; total signal averaging time per spectrum, about 22 min; gain, 60 dB; time constant, 0.082 seconds. Chl a was purified from acetone extracts of Chlamydomonas cells by preparative HPLC with methanol as the elution solvent [35], evaporated to dryness with a stream of N2 and dissolved in a small volume of ethanol. Immediately prior to EPR analysis, samples were mixed at room temperature and then frozen in liquid N2.
Reaction with iodide
Membrane pellets (3 to 5 mg protein) from E. coli, C. reinhardtii and A. thaliana were suspended in 50 mM Hepes, pH 7.5, containing 50 mM NaCl, incubated with 55 kBq carrier-free Na125I (Amersham Biosciences, Piscataway, NJ) for 20 min at 25°C (50 μl final volume). Proteins were precipitated by addition of 10 volumes of acetone, washed 2 times with acetone, and resolved by electrophoresis [64]. Gels were dried between sheets of BioDesignGelWrap™ (BioDesign, Inc., New York, NY) and radioautograms were obtained with a Storm 840 PhosphorImager (Molecular Dynamics, Piscataway, NJ). Immunoprecipitates prepared as described above from Chlamydomonas and Arabidopsis were suspended in 200 to 400 μl 50 mM Hepes, pH 8.0, containing 50 mM NaCl, and incubated with 3 MBq Na125I for 15 min at 25°C. Samples were then precipitated by adding 5 vol acetone and washed two times with acetone. Protein was recovered from agarose beads by extraction with 1% SDS in 50 mM Hepes, pH 8.0, for 1 min in a boiling water bath followed by 5 washes with buffer. Protein was recovered by precipitation with 5 volumes of acetone and dried under a stream of N2. Approximately 1 mg protein in 200 to 300 μl 20 mM NH4HCO3 was digested with 200 μg trypsin (Worthington, Lakewood, NJ) for 20 h at 38°C, dried at 50°C and subjected to N-terminal Edman degradation with a gas phase protein sequencer (model 2090, Beckman/Porton, Fullerton, CA). The amino acid derivatives released in each sequencing cycle were collected, spotted onto Whatman 3 MM paper, and quantitated with the Phosphorimager and ImageQuant software (Molecular Dynamics).
Abbreviations
CAO, Chlide a oxygenase; Chl, chlorophyll; Chlide, chlorophyllide; EPR, electron paramagnetic resonance; GFP, green fluorescent protein; LHC, light-harvesting complex; LHCII, light-harvesting complex associated with photosystem II; LHCP, apoprotein of LHC; mW, milliwatts; psi, pounds per square inch
Authors' Contributions
LLE performed biochemical studies, immunoblots, and expression of recombinant CAO. LLE and RL carried out the EPR studies. DCB performed peptide sequencing and mass spectrometry. JB prepared antiserum against CAO and carried out characterization of the antiserum. AY and AT performed transient expression in pea leaves and analysis by confocal fluorescence microscopy. AT constructed the CAO plasmid used for expression of the protein in E. coli. JKH carried out experiments with 125I- and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Drs. P. Mulineaux and R. Hellens, John Innes Centre and the Biotechnology and Biological Sciences Reseaarch Council, for their gift of pGreenII0029 and Dr. Jürgen Soll, Department für Biologie I, Ludwig-Maximilians-Universität-München for the antibodies against Tic55. L.L. Eggink was supported by Graduate Training Grant DGE9553456 from the National Science Foundation. This is publication 587 from the Arizona State University Center for the Study of Early Events in Photosynthesis.
Contributor Information
Laura L Eggink, Email: leggink@asu.edu.
Russell LoBrutto, Email: russell.lobrutto@asu.edu.
Daniel C Brune, Email: daniel.brune@asu.edu.
Judy Brusslan, Email: bruss@csulb.edu.
Akihiro Yamasato, Email: yamasato@snowbell.lowtem.hokudai.ac.jp.
Ayumi Tanaka, Email: ayumi@pop.lowtem.hokudai.ac.jp.
J Kenneth Hoober, Email: khoober@asu.edu.
References
- Hoober JK, Eggink LL. Assembly of light-harvesting complex II and biogenesis of thylakoid membranes in chloroplasts. Photosynth Res. 1999;61:197–215. doi: 10.1023/A:1006313703640. [DOI] [Google Scholar]
- Vothknecht UC, Westhoff P. Biogenesis and origin of thylakoid membranes. Biochim Biophys Acta. 2001;1541:91–101. doi: 10.1016/S0167-4889(01)00153-7. [DOI] [PubMed] [Google Scholar]
- Jansson S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 1999;4:236–240. doi: 10.1016/S1360-1385(99)01419-3. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W, Wang DN, Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Croce R, Weiss S, Bassi R. Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J Biol Chem. 1999;274:29613–29623. doi: 10.1074/jbc.274.42.29613. [DOI] [PubMed] [Google Scholar]
- Das SK, Frank HA. Pigment compositions, spectral properties, and energy transfer efficiencies between the xanthophylls and chlorophylls in the major and minor pigment-protein complexes of photosystem II. Biochemistry. 2002;41:13087–13095. doi: 10.1021/bi0204802. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature. 2004;428:287–292. doi: 10.1038/nature02373. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Schäfer W, Cmiel E, Katheder I, Scheer H. The derivation of the formyl-group of chlorophyll b in higher plants from molecular oxygen. Eur J Biochem. 1994;219:671–679. doi: 10.1111/j.1432-1033.1994.tb19983.x. [DOI] [PubMed] [Google Scholar]
- Król M, Spangfort MD, Huner NPA, Öquist G, Gustafsson P, Jansson S. Chlorophyll a/b-binding proteins, pigment conversions, and early light-induced proteins in a chlorophyll b-less barley mutant. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossmann B, Grimme LH, Knoetzel J. Protease-stable integration of Lhcb1 in thylakoid membranes is dependent on chlorophyll b in allelic chlorina-f2 mutants of barley (Hordeum vulgare L.) Planta. 1999;207:551–558. doi: 10.1007/s004250050517. [DOI] [Google Scholar]
- Espineda CE, Linford AS, Devine D, Brusslan JA. The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:10507–10511. doi: 10.1073/pnas.96.18.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Koshino Y, Sawa S, Ishiguro S, Okada K, Tanaka A. Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J. 2001;26:365–373. doi: 10.1046/j.1365-313X.2001.2641034.x. [DOI] [PubMed] [Google Scholar]
- Eggink LL, Park H, Hoober JK. The role of chlorophyll b in photosynthesis: hypothesis. BMC Plant Biology. 2001;1:2. doi: 10.1186/1471-2229-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA. 1998;95:12719–12723. doi: 10.1073/pnas.95.21.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomitani A, Okada K, Miyashita H, Matthijs HCP, Ohno T, Tanaka A. Chlorophyll b and phycobilins in the common ancestor of cyanobacteria and chloroplasts. Nature. 1999;400:159–162. doi: 10.1038/22101. [DOI] [PubMed] [Google Scholar]
- Chunaev AS, Mirnaya ON, Maslov VG, Boschetti A. Chlorophyll b- and loroxanthin-deficient mutants of Chlamydomonas reinhardtii. Photosynthetica. 1991;25:291–301. [Google Scholar]
- Gray J, Janick-Buckner D, Buckner B, Close PS, Johal GS. Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol. 2002;130:1894–1907. doi: 10.1104/pp.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoulina KV, Chekunova EM, Rüdiger W, Chunayev AS. Genetic analysis of revertants of chlorophyll b -deficient mutants of Chlamydomonas reinhardtii. Russian J Genet. 1997;33:474–479. [Google Scholar]
- Block MA, Dorne A-J, Joyard J, Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983;258:13281–13286. [PubMed] [Google Scholar]
- Eggink LL, Park H, Hoober JK. The role of the envelope in assembly of light-harvesting complexes in the chloroplast: distribution of LHCP between chloroplast and vacuoles during chloroplast development in Chlamydomonas reinhardtii. In: Argyroudi-Akoyunoglou JH, Senger H, editor. In The Chloroplast: From Molecular Biology to Biotechnology. Dordrecht: Kluwer; 1999. pp. 161–166. [Google Scholar]
- White RA, Wolfe GR, Komine Y, Hoober JK. Localization of light-harvesting complex apoproteins in the chloroplast and cytoplasm during greening of Chlamydomonas reinhardtii at 38°C. Photosynth Res. 1996;47:267–280. doi: 10.1007/BF02184287. [DOI] [PubMed] [Google Scholar]
- Bovet L, Müller MO, Siegenthaler PA. The 26- and 14-kDa phosphoproteins associated with spinach chloroplast envelope membranes are distinct membrane-bound poosl of the light-harvesting complex of photosystem II and of the small subunit of the ribulose-1,5-bisphosphate carboxylase-oxygenase. Planta. 1995;195:563–569. [Google Scholar]
- Bovet L, L'Eplattenier B, Siegenthaler PA. In vitro and in organello phosphorylation of envelope proteins and phosphoglucomutase in spinach chloroplasts. Plant Sci. 1997;128:169–180. doi: 10.1016/S0168-9452(97)00160-X. [DOI] [Google Scholar]
- Wingfield PT. Production of recombinant proteins. In: Coligan JE, Dunn BM, Speicher DW, Wingfield PT, Ploegh HL, editor. In Current Protocols in Protein Science. Vol. 1. New York: John Wiley & Sons; 2000. pp. 5.0.1–5.3.18. [Google Scholar]
- Schmidt CL, Hatzfeld OM, Petersen A, Link TA, Schäfer G. Expression of the Solfolobus acidocaldarius Rieske iron sulfur protein II (SOXF) with the correctly inserted [2Fe-2S] cluster in Escherichia coli. Biochem Biophys Res Commun. 1997;234:283–287. doi: 10.1006/bbrc.1997.6599. [DOI] [PubMed] [Google Scholar]
- Hägerhäll C, Magnitsky S, Sled VD, Schröder I, Gunsalus RP, Cecchini G, Ohnishi T. An Escherichia coli mutant quinol:fumarate reductase contains an EPR-detectable semiquinone stabilized at the proximal quinone-binding site. J Biol Chem. 1999;274:26157–26164. doi: 10.1074/jbc.274.37.26157. [DOI] [PubMed] [Google Scholar]
- Basu P, Katterle B, Andersson KK, Dalton H. The membrane-associated form of methane mono-oxygenase from Methylococcus capsulatus (Bath) is a copper/iron protein. Biochem J. 2003;369:417–427. doi: 10.1042/BJ20020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles PF, Brown RD, III, Koenig SH, Wang S, Scott RA, McGuirl MA, Brown DE, Dooley DM. Spectroscopic studies of the active site of galactose oxidase. Inorg Chem. 1995;34:3895–3902. [Google Scholar]
- Klinman JP. Mechanisms whereby mononuclear copper proteins functionalize organic substrates. Chem Rev. 1996;96:2541–2561. doi: 10.1021/cr950047g. [DOI] [PubMed] [Google Scholar]
- Roberts AG, Bowman MK, Kramer DM. Certain metals are inhibitors of cytochrome b6f complex 'Rieske'iron-sulfur protein domain movements. Biochemistry. 2002;41:4070–4079. doi: 10.1021/bi015996k. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Elliott DC, Elliott WH, Jones KM. Data for Biochemical Research. 3. Oxford: Clarendon Press; 1986. [Google Scholar]
- Takahashi Y, Satoh K. Identification of the photochemically iodinated amino-acid residue on D1-protein in the photosystem II core complex by peptide mapping analysis. Biochim Biophys Acta. 1989;973:138–146. [Google Scholar]
- Proshlyakov DA, Pressler MA, DeMaso C, Leykam JF, DeWitt DL, Babcock GT. Oxygen activation and reduction in respiration: involvement of redox-active tyrosine 244. Science. 2000;290:1588–1591. doi: 10.1126/science.290.5496.1588. [DOI] [PubMed] [Google Scholar]
- Hoober JK, Boyd CO, Paavola LG. Origin of thylakoid membranes in Chlamydomonas reinhardtii y-1 at 38°C. Plant Physiol. 1991;96:1321–1328. doi: 10.1104/pp.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney MA, Hoober JK, Marks DB. Kinetics of chlorophyll accumulation and formation of chlorophyll-protein complexes during greening of Chlamydomonas reinhardtii y-1 at 38°C. Plant Physiol. 1989;91:1100–1106. doi: 10.1104/pp.91.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober JK, Park H, Wolfe GR, Komine Y, Eggink LL. Assembly of light-harvesting systems. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editor. In The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Dordrecht: Kluwer; 1998. pp. 363–376. [Google Scholar]
- Morré DJ, Selldén G, Sundqvist C, Sandelius AS. Stromal low temperature compartment derived from the inner membrane of the chloroplast envelope. Plant Physiol. 1991;97:1558–1564. doi: 10.1104/pp.97.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll D, Meierhoff K, Bechtold N, Kinoshita M, Westphal S, Vothknecht UC, Soll J, Westhoff P. VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc Natl Acad Sci USA. 2001;98:4238–4242. doi: 10.1073/pnas.061500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S, Soll J, Vothknecht UC. A vesicle transport system inside chloroplasts. FEBS Lett. 2001;506:257–261. doi: 10.1016/S0014-5793(01)02931-3. [DOI] [PubMed] [Google Scholar]
- Park H, Hoober JK. Chlorophyll synthesis modulates retention of apoproteins of light-harvesting complex II by the chloroplast in Chlamydomonas reinhardtii. Physiol Plant. 1997;101:135–142. doi: 10.1034/j.1399-3054.1997.1010118.x. [DOI] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Reinbothe C, Buhr F, Pollmann S, Reinbothe S. In vitro reconstitution of light-harvesting POR-protochlorophyllide complex with protochlorophyllides a and b. J Biol Chem. 2003;278:807–815. doi: 10.1074/jbc.M209738200. [DOI] [PubMed] [Google Scholar]
- Jäger-Vottero P, Dorne A-J, Jordanov J, Douce R, Joyard J. Redox chains in chloroplast envelope membranes: spectroscopic evidence for the presence of electron carriers, including iron-sulfur centers. Proc Natl Acad Sci USA. 1997;94:1597–1602. doi: 10.1073/pnas.94.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe A, Grimm R, Kaiser G, Lübeck J, Soll J, Heins L. The chloroplastic protein import machinery contains a Rieske-type iron-sulfur cluster and a mononuclear iron-binding protein. EMBO J. 1997;16:7342–7350. doi: 10.1093/emboj/16.24.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchler M, Decker S, Hörmann F, Soll J, Heins L. Protein import into chloroplasts involves redox-rgulated proteins. EMBO J. 2002;21:6136–6145. doi: 10.1093/emboj/cdf621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J, Teyssier E, Miège C, Berny-Seigneurin D, Maréchal E, Block MA, Dorne A-J, Rolland N, Ajlani G, Douce R. The biochemical machinery of plastid envelope membranes. Plant Physiol. 1998;118:715–723. doi: 10.1104/pp.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block MA, Tewari AK, Albrieux C, Maréchal E, Joyard J. The plant S-adenosyl-L-methionine:MG-protoporphyrin IX methyltransferase is located in both envelope and thylakoid chloroplast membranes. Eur J Biochem. 2002;269:240–248. doi: 10.1046/j.0014-2956.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- Stubbe J, van der Donk W. Protein radicals in enzyme catalysis. Chem Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- Dufresne A, Salanoubat M, Partensky F, Artiguenave F, Axmann IM, Barbe V, Duprat S, Galperin MY, Koonin EV, Le Gall F, Makarova KS, Ostrowski M, Oztas S, Robert C, Rogozin IB, Scanlan DJ, de Marsac NT, Weissenbach J, Wincker P, Wolf YI, Hess WR. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc Natl Acad Sci USA. 2003;100:10020–10025. doi: 10.1073/pnas.1733211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Rocap G, Ting CS, Larimer F, Stilwagen S, Lamerdin J, Chisholm SW. The photosynthetic apparatus of Prochlorococcus: insights through comparative genomics. Photosynth Res. 2001;70:53–71. doi: 10.1023/A:1013835924610. [DOI] [PubMed] [Google Scholar]
- He WZ, Malkin R. Reconstitution of iron-sulfur center B of photosystem I damaged by mercuric chloride. Photosynth Res. 1994;41:381–388. doi: 10.1007/BF02183040. [DOI] [PubMed] [Google Scholar]
- Jung YS, Yu L, Golbeck JH. Reconstitution of iron-sulfur center FB results in complete restoration of NADP+ photoreduction in Hg-treated photosystem I complexes from Synechococcus sp. PCC6301. Photosynth Res. 1995;46:249–255. doi: 10.1007/BF00020437. [DOI] [PubMed] [Google Scholar]
- Bednarik DP, Hoober JK. Synthesis of chlorophyllide b from protochlorophyllide in Chlamydomonas reinhardtii y-1. Science. 1985;230:450–453. doi: 10.1126/science.230.4724.450. [DOI] [PubMed] [Google Scholar]
- Shedbalkar VP, Ioannides IM, Rebeiz CA. Chloroplast biogenesis: detection of monovinyl protochlorophyll(ide) b in plants. J Biol Chem. 1991;266:17151–17157. [PubMed] [Google Scholar]
- Reinbothe S, Pollmann S, Reinbothe C. In situ conversion of protochlorophyllide b to protochlorophyllide a in barley. J Biol Chem. 2003;278:800–806. doi: 10.1074/jbc.M209737200. [DOI] [PubMed] [Google Scholar]
- Xu H, Vavilin D, Vermaas W. The presence of chlorophyll b in Synechocystis sp. PCC 6803 disturbs tetrapyrrole biosynthesis and enhances chlorophyll degradation. J Biol Chem. 2002;277:42726–42732. doi: 10.1074/jbc.M205237200. [DOI] [PubMed] [Google Scholar]
- Oster U, Tanaka R, Tanaka A, Rüdiger W. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 2000;21:305–310. doi: 10.1046/j.1365-313x.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- Hata M, Hirano Y, Hoshino T, Tsuda M. Monooxygenation mechanism by cytochrome P-450. J Am Chem Soc. 2001;123:6410–6416. doi: 10.1021/ja000908p. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kobayashi M. Electrochemistry of chlorophylls. In: Scheer H, editor. In Chlorophylls. Boca Raton: CRC Press; 1991. pp. 287–315. [Google Scholar]
- Pikus JD, Studts JM, Achim C, Kauffmann KE, Münck E, Steffan RJ, McClay K, Fox BG. Recombinant toluene-4-monooxygenase: catalytic and Mössbauer studies of the purified diiron and Rieske components of a four-protein complex. Biochemistry. 1996;35:9106–9119. doi: 10.1021/bi960456m. [DOI] [PubMed] [Google Scholar]
- Gassner GT, Lippard SJ. Component interactions in the soluble methane monooxygenase system from Methylococcus capsulatus (Bath) Biochemistry. 1999;38:12768–12785. doi: 10.1021/bi990841m. [DOI] [PubMed] [Google Scholar]
- Mitchell KH, Studts JM, Fox BG. Combined participation of hydroxylase active site residues and effector protein binding in a para to ortho modulation of toluene 4-monooxygenase regiospecificity. Biochemistry. 2002;41:3176–3188. doi: 10.1021/bi012036p. [DOI] [PubMed] [Google Scholar]
- Wolfe GR, Park H, Sharp WP, Hoober JK. Light-harvesting complex apoproteins in cytoplasmic vacuoles in Chlamydomonas reinhardtii (Chlorophyta) J Phycol. 1997;33:377–386. [Google Scholar]
- Hoober JK, Millington RH, D'Angelo LP. Structural similarities between the major polypeptides of thylakoid membranes from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1980;202:221–234. doi: 10.1016/0003-9861(80)90424-5. [DOI] [PubMed] [Google Scholar]


