Abstract
Two questions regarding sex differences in magical ideation were investigated in this study: (1) whether there are mean level sex differences on the Magical Ideation Scale (MIS), and (2) whether there are quantitative and/or qualitative sex differences in the genetic contributions to variation on this scale. These questions were evaluated using data obtained from a large community sample of adult Australian twins (N=4,355) that included opposite-sex pairs. Participants completed a modified 15-item version of the MIS within a larger assessment battery. Women reported both higher means and variability on the MIS than men; this was also observed within families (in opposite-sex twin pairs). Biometric modeling indicated that the proportion of variation in MIS scores due to genetic influences (indicating quantitative sex differences) and the specific latent genetic contributions to this variation (indicating qualitative sex differences) were the same in men and women. These findings clarify the nature of sex differences in magical ideation and point to avenues for future research.
Keywords: magical ideation, sex differences, twin study, schizotypy, schizotypal personality disorder
Magical ideation is conceptualized as the tendency to accept unconventional forms of causality (Meehl, 1962; Horan et al., 2008). This definition includes a broad range of unconventional thoughts, from relatively common beliefs to delusions (Eckblad & Chapman, 1983; Brugger & Graves, 1997). Magical ideation loads onto the positive factor of schizotypy, or the factor that is characterized by odd beliefs and unusual experiences (Volema & van den Bosch, 1995; Claridge et al., 1996; Venables & Rector, 2000). Magical ideation is a common symptom of schizotypal personality disorder (APA, DSM-IV, 2000). Much of the research regarding magical ideation has relied on the use of psychometric inventories, mainly the Magical Ideation Scale (MIS; Eckblad & Chapman, 1983). The scale includes 30 items designed to tap into beliefs and experiences regarding forms of thinking that, in terms of conventional standards of the predominant culture, are regarded as invalid (Chapman et al., 1994). Magical ideation has been shown to be related to mania (e.g., Kwapil, Barrantes-Vidal, & Silvia, 2008) and the trait of absorption (similar to openness to experiences; Eckblad & Chapman, 1986).
The current study investigated the role of genetic and environmental factors in explaining individual differences in the MIS. Four previous studies have conducted behavioral genetic analyses of the MIS (Kendler & Hewitt, 1992; Miller, 1993; Hay et al., 2001; MacDonald et al., 2001), but they have yielded discrepant findings, with only two of the studies obtaining evidence of a significant genetic component (Kendler & Hewitt, 1992; Miller, 1993). Three of the studies had very small sample sizes, with a combined total of only 271 MZ and 195 DZ twin pairs (Kendler & Hewitt, 1992; Miller, 1993, as cited in MacDonald et al, 2001; Hay et al., 2001). The Hay et al. (2001) study was based on a much larger sample of 614 MZ and 720 DZ twin pairs, but the assessment of MIS was based on an abbreviated 2-item scale.
Investigating sex differences can yield clues about the causes of a disorder, and is especially useful because it permits a “unidirectional interpretation,” because sex cannot be impacted by the disorder (Lewine, 1988; Aleman et al., 2003). In terms of schizophrenia, there has been research regarding sex differences in multiple facets of the disorder (Leung & Chue, 2000). However, the evidence remains inconclusive regarding sex differences in positive schizotypy. Although some investigations have found no differences (Salem & Kring, 1998; Leung & Chue, 2000), the majority of studies examining sex differences in positive schizotypy have found that females on average report more positive symptoms than males (Rawlings et al., 2001; Fossati et al., 2003; Mata et al., 2005). In contrast, the results of a recent meta-analysis of 29 studies comparing mean scores of men and women on the MIS obtained an overall effect size of essentially zero (Miettunen & Jaaskelainen, 2010). Of the four previous behavioral genetic studies of MIS, one reported overall sex differences for a four-item composite perceptual aberration and magical ideation scale score (d=.08, with women having higher mean MIS scores than men). None of the four studies examined sex differences in the sources of individual differences in MIS scores.
The present study extends the small literature on the genetic epidemiology of magical ideation by fitting biometric models to data collected from a large community sample of Australian twins. Because the current study used an abbreviated version of the MIS, it was important to ascertain the construct validity of the abbreviated form before fitting biometric models. The inclusion of opposite-sex twin pairs permitted us to address two major questions concerning sex differences in magical ideation. The first was whether there were mean level sex differences in the MIS. Studying opposite-sex twin pairs allowed for a rigorous test of sex differences while controlling for a host of potential confounds (i.e., environmental factors, such as factors related to familial environment, that would be shared between opposite-sex twin pairs) that could complicate research using unrelated individuals. The second question was whether there were quantitative or qualitative sex differences in the genetic contributions to variation in MIS. After establishing the construct validity of the abbreviated MIS in both men and women, biometric modeling of male and female MZ and DZ twin pairs was used to test whether the proportion of variance in the MIS that was attributable to latent genetic factors differed for men and women (quantitative sex differences). With the inclusion of opposite-sex twin pairs, we were able to extend these models to test the extent to which the latent genetic factors contributing to variation in MIS overlapped or differed in men and women (qualitative sex differences).
Methods
Participants
The participants for this study were members of the national community-based Australian Twin Registry (Slutske et al., 2009). The data were collected from 2004-2007, when participants were between 32-43 years old (M=37.66, SD=2.31). Of the 4,764 participants who completed a telephone interview, 4,355 (91%) returned a personality questionnaire. These 4,355 individual twins included 1,139 monozygotic females (MZF), 761 monozygotic males (MZM), 864 females from same-sex dizygotic pairs (DZF), 576 males from same-sex dizygotic pairs (DZM), 576 females from opposite-sex dizygotic pairs (OSF), and 439 males from opposite-sex dizygotic pairs (OSM). (For more details, see Slutske et al., 2009). This study was approved by the Institutional Review Boards at the University of Missouri and the Queensland Institute of Medical Research. All of the participants provided informed consent.
Measures
Three of the measures for this study were included in the personality questionnaire [MIS, Chapman Infrequency Scale, and Multidimensional Personality Questionnaire (MPQ; Tellegen, 1982; 1985], and a mania screen was included in the structured diagnostic telephone interview.
Magical Ideation Scale
Participants completed an abbreviated 15-item version of the MIS (Eckblad & Chapman, 1983). The abbreviated MIS consisted of 15 true-false items designed to measure “beliefs in forms of causation that by conventional standards are invalid” (Eckblad & Chapman, p. 215). The original MIS has good test-retest reliabilities among both women and men (r=.82 and .80 respectively; Chapman et al., 1982), and good construct validity in that it predicts future psychosis (Gooding et al., 2005), and is also associated with measures of schizotypal personality disorder (Cicero & Kerns, 2010). In the present study, the 15 MIS items had adequate internal consistency reliability (for women, α=.77; for men, α=.73).
Chapman Infrequency Scale
The 11-item Chapman Infrequency Scale (Chapman & Chapman, 1983) was included in order to exclude those participants who were responding in a random or invalid manner. Participants who endorsed 3 or more infrequency items were dropped from further study (Chapman & Chapman, 1983). On the basis of Chapman Infrequency Scale scores, 54 (0.01%) participants were excluded from all analyses.
Multidimensional Personality Questionnaire
The MPQ (Tellegen, 1982; 1985) is a self-report personality inventory of normal personality. The personality questionnaire included the MPQ Absorption Scale. The Absorption Scale is associated with fantasy and openness to experience (Glisky et al., 1991). This measure was included in order to investigate the construct validity of the abbreviated MIS included in the personality questionnaire.
Mania Screen
The mania screen categorized participants into three categories: no evidence of mania, possible mania, and probable mania. Individuals were assigned a diagnosis of possible mania if they endorsed experiencing a week or more of heightened energy and felt unusually good, and had experienced a week or more of rapid speech and impulsivity. Individuals were assigned a diagnosis of probable mania if they met criteria for possible mania and had been hospitalized or been treated with medication for their symptoms. Seventy-five participants (1.7%) were classified as having a history of possible mania, and 44 participants (1.0%) were classified as having a history of probable mania. Possible mania and probable mania were combined for analyses. The mania screen was also used to establish the construct validity of the abbreviated 15-item MIS used in the present study.
Data Analysis
Prior to conducting behavioral genetic analyses of the MIS, it was first necessary to establish that the MIS scale was measurement invariant across sex and an equally valid measure among both men and women. These psychometric analyses were an essential step for guiding and interpreting the behavior genetic analyses.
Factor analyses were initially conducted in order to ensure that the fifteen items of the abbreviated MIS yielded a unifactorial measure. Following these initial analyses, CFAs were conducted to test whether there was evidence of cross-sex measurement invariance of the MIS, that is, whether the MIS items function the same in men and women (Reise et al., 1993; Byrne, 2008). This was accomplished by comparing a model in which the measurement parameters for each of the items (i.e., factor loadings and thresholds) were freely estimated for men and women to a model in which the parameters were constrained to be equal for men and women. The relative fit of the constrained model to the fit of the freely estimated model provided a test of cross-sex measurement invariance. These analyses were conducted in Mplus (Muthen & Muthen, 1998) in which the twin pair data were treated as clustered observations.
Two methods were used to examine mean sex differences in MIS. The first method examined the between-family mean and variances differences using data from all of the men and women in the sample. Models in which either the means or variances (or both) were allowed to vary between men and women were tested in Mplus. The fits of pairs of nested models were compared via a chi-square difference test to determine whether there were significant sex differences in the means or variances.
The second method used data from the DZO twin pairs to determine whether there were significant within-family mean and variance sex differences in MIS. This method controls for all between family differences that might contribute to or obscure a mean sex difference in MIS. For these DZO twin pairs, mean sex differences were investigated using a matched pairs t-test. The variances differences were tested the same way as in the between-family tests described above except that the analyses were restricted to the DZO twin pairs.
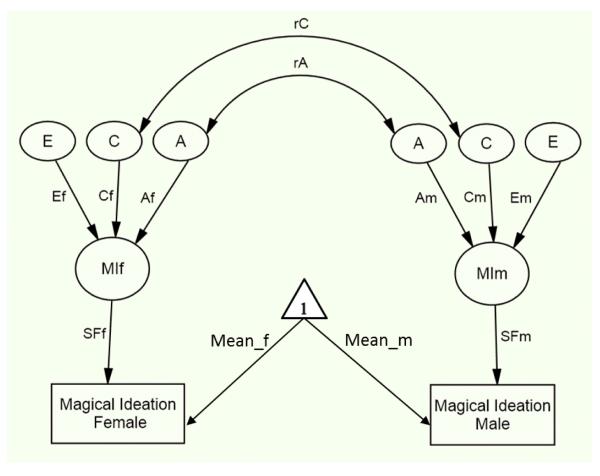
The genetic analysis for the MIS partitioned the variance into additive genetic (A), shared environmental (C) or non-additive genetic (D), and nonshared environment (E) influences. The models were fit directly to the raw twin data by the method of maximum likelihood using data from incomplete as well as complete twin pairs. For each of four models created, the estimates of A, C or D, and E were either constrained to be equal between men and women or allowed to vary, and the estimate of the genetic correlation (rA) for opposite-sex twin pairs was either fixed to 0.5 (because same-sex dizygotic twin pairs share on average half of their segregating genes) or allowed to vary. The univariate ACE sex differences twin model for an opposite-sex twin pair is shown in Figure 1. The figure displays a model in which the causal paths leading to the latent MIS trait (MIf, MIm) were freely estimated for women (Af, Cf, and Ef) and men (Am, Cm, and Em). Scaling factors from the latent MIS trait to the measured MIS trait were included for women and men (SFf and SFm, respectively) to allow for sex differences in the variances in MIS. Means were included in the model for women and men (Mean_f and Mean_m, respectively) to allow for mean sex differences in MIS. A more restricted model was fitted in which the ACE parameters were constrained to be equal for men and women, that is, Af=Am, Cf=Cm, and Ef=Em. Comparing the fit of the constrained model to that of the freely estimated model indicated whether the etiologic structure of MIS was equivalent for men and women (a test of quantitative sex differences). To test for qualitative sex differences, we compared the fits of models in which the estimates of the genetic (rA) correlation between the latent additive genetic factors contributing to MIS in opposite-sex DZ pairs were either free to vary or fixed to 0.5. Scores on the MIS were not significantly associated with age [r(4269)=−.01, p=.49] so it was not necessary to include age in any of the biometric models. Models that included age yielded results that were identical to the results from models that did not include age.
Figure 1.
The univariate sex differences twin model of magical ideation. The model is one in which the additive genetic (A), shared environmental (C), and nonshared environmental (E) influences are allowed to differ for males (m) and females (f). The genetic (rA) and shared environmental (rC) correlation among opposite-sex twins are freely estimated. [In (same-sex) monozygotic twins, rA and rC are fixed at 1.0 and 1.0, respectively, and in same-sex dizygotic twins rA and rC are fixed at 0.5 and 1.0, respectively.] The scalar factor (SF) accommodated possible sex differences in variances in magical ideation by being constrained to 1 for men and allowed to vary for women. The ADE model would be similar to the ACE model, except the ‘C’ would be replaced with ‘D’.
Results
Factor Analyses
The 15 MIS items were subjected to an EFA using principal-axis factor analysis (using the WLSMV estimation technique) followed by an oblique rotation. We selected a one-factor solution based on the scree plot, eigenvalues, and previous literature (Chapman et al., 1982; Venables & Rector, 2000). In order to confirm these results, a CFA was also performed. The CFA confirmed the appropriateness of the one-factor structure (RMSEA=0.03, CFI=0.96, TLI=0.96). Measurement invariance analyses were conducted in order to examine whether every MIS question functioned the same in men and women (see Table 1). The results of the measurement invariance analyses indicated that items 6 and 8 were sex specific, and therefore a 13-item cross-sex-invariant version of the MIS was used for all subsequent analyses. The results were virtually the same regardless of whether we used the 13 or 15-item MIS scale.
Table 1.
Factor Loadings for Measurement Invariance Models of a 15-item Magical Ideation Scale.
| Free | Partial Invariance | |||
|---|---|---|---|---|
|
| ||||
| Women | Men | Women | Men | |
| Magical Ideation Question | ||||
| 1. Some people can make me aware of them just by thinking about me |
1.00 | 1.00 | 1.00 | 1.00 |
| 2. I have sometimes been fearful of stepping on sidewalk cracks |
0.67 | 0.69 | 0.69 | 0.69 |
| 3. I think I could learn to read other people’s minds if I wanted to |
1.11 | 1.12 | 1.14 | 1.14 |
| 4. Horoscopes are right too often for it to be a coincidence |
1.02 | 1.15 | 1.017 | 1.02 |
| 5. Numbers like 13 and 7 have no special powers (R) |
0.88 | 0.93 | 0.87 | 0.87 |
| 6. The government refuses to tell us the truth about flying saucers |
0.83 | 0.86 | 0.84 | 0.78 |
| 7. I have felt that there were messages for me in the way things were arranged, like in a store window |
1.26 | 1.17 | 1.25 | 1.25 |
| 8. Good luck charms don’t work (R) | 1.07 | 0.95 | 1.08 | 0.86 |
| 9. I almost never dream about things before they happen (R) |
0.83 | 0.86 | 0.84 | 0.84 |
| 10. It is not possible to harm others merely by thinking bad thoughts about them (R) |
0.84 | 0.91 | 0.86 | 0.86 |
| 11. If reincarnation were true, it would explain some unusual experiences I havehad |
1.21 | 1.22 | 1.22 | 1.22 |
| 12. At times I perform certain little rituals to ward off negative influences |
1.09 | 0.95 | 1.09 | 1.09 |
| 13. I have felt that I might cause something to happen just by thinking too muchabout it |
1.17 | 1.29 | 1.20 | 1.20 |
| 14. I have wondered whether the spirits of the dead can influence the living |
1.25 | 1.28 | 1.26 | 1.26 |
| 15. I have sometimes felt that strangers were reading my mind |
1.12 | 1.08 | 1.12 | 1.12 |
Note.Free= All parameters are allowed to be sex-specific. Partial Invariance= All parameters are constrained to be equal across sex except questions 6 and 8. FL= factor loading. R=reverse coded. Note that thresholds are indicative of the endorsement prevalence of an item in standardized z-score units. A higher item threshold parameter indicates that fewer individuals endorsed an item and a lower item threshold indicates that more individuals endorsed an item. For example, the threshold for Item 8 was substantially lower among women (0.42) than among men (0.88), indicating that this item was more frequently endorsed among women than among men.
Construct Validity
We examined the construct validity of the abbreviated MIS to ensure that it was a valid measure of psychosis proneness among both men and women. The construct validity of the 13-item abbreviated MIS was evaluated by testing whether it significantly predicted a history of mania or the MPQ absorption scale. Higher scores on the MIS significantly predicted a history of mania in the full sample (χ2(1)=52.17, p<.01) and for women (χ2(1)=27.30, p<.01) and men (χ2(1)=29.21, p<.01). Furthermore, the MIS scores were significantly correlated with MPQ absorption scale scores in the full sample (r=.58, p<.01) and among women (r=.60, p<.01) and men (r=.56, p<.01). The strong associations with history of mania and the personality trait of absorption provide convincing evidence that the abbreviated MIS is functioning much like the original scale (Chapman et al., 1994; Harkness & McNulty, 1994), and is an equally valid indicator of psychosis proneness in women as in men.
Mean Differences
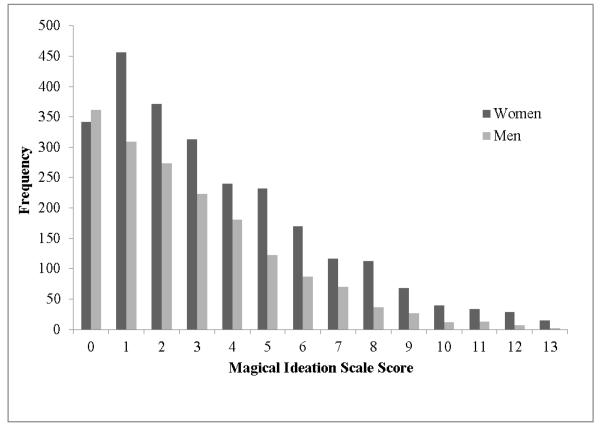
There were significant between-family mean sex differences in MIS scores, Δχ2(1)=39.77, p<.01, d=0.26 with women (n=1968, M=2.18) having a higher mean than men (n=1302, M=1.71). The distribution of MIS scores for women and men can be seen in Figure 2. There were also significant between-family variance differences in MIS scores, Δχ2(1)=17.00, p<.01, with women (σ2=3.93) having a greater variance than men (σ2=3.01). When variances were allowed to differ, there were still significant mean between-family sex differences for the MIS score.
Figure 2.
Distribution of Magical Ideation Scale scores for women and men.
A matched pairs t-test indicated that there was a significant within-family mean difference between men and women on the total MIS score in DZO twins (t(323)=3.94, p<.01; women: M=2.36, SD=2.04; men: M=1.80, SD=1.73; d=0.29).There were also significant differences in the variances in MIS scores for men and women in DZO twins (Δχ2(1)=7.62, p<.01) with women (σ2=4.10) having a greater variance than men (σ2=2.07).
Genetic Analyses
Twin correlations for the total MIS score for each of the zygosity groups are presented in Table 2. For men and women, the MZ twin correlations were larger than the DZ twin correlations, and the correlations among male twins, both MZ and DZ were smaller than among female twins. Furthermore, there was a non-significant trend indicating a smaller twin correlation for DZO than same-sex DZ twins.
Table 2.
Twin correlations for the 13-item Magical Ideation Scale (omitting items 6 and 8).
| Zygosity group | Pairs | Correlation | 95% Confidence Interval |
χ 2 | p-value |
|---|---|---|---|---|---|
| MZf | 472 | 0.40 | 0.32-0.46 | <.001 | |
| MZm | 284 | 0.31 | 0.20-0.41 | <.001 | |
| DZf | 325 | 0.26 | 0.16-0.36 | <.001 | |
| DZm | 160 | 0.12 | −0.04-0.27 | 0.13 | |
| DZo | 323 | 0.09 | −0.02-0.20 | 0.10 | |
| χ2 test of differences in twin correlations (df=1) | |||||
| MZ vs DZ | 7.09 | <0.01 | |||
| DZsvsDZo | 2.40 | 0.12 | |||
| MZmvsMZf | 4.10 | 0.04 | |||
| DZmvsDZf | 5.04 | 0.02 | |||
| MZfvsDZf | 2.53 | 0.11 | |||
| MZmvsDZm | 4.75 | 0.03 | |||
| DZfvsDZo | 6.03 | 0.01 | |||
| DZmvsDZo | 0.00 | 0.96 | |||
Note. MZ= monozygotic, DZ= dizygotic, s= same sex twin pairs, o=opposite sex twin pairs, m= male, f=female. The correlations remain the same when age was co-varied out of the MIS scores.
Based upon the results demonstrating mean and variance differences between men and women on the MIS scale, all of the biometric models allowed for mean and variance differences across sex. Based on the relative fits of the ACE (Δχ2(19)=59.04, p<.01) and ADE models (Δχ2(19)=60.33, p<.01), ACE models were used in all analyses presented below (since the ACE model provided better fit indices). An ADE/ACE model (ADE in men, ACE in women) was not used because it would have precluded examining qualitative sex differences.
Prior to examining sex differences, tests were conducted to determine the overall significance of each of the components of A, C, and E. The fit of a model that did not include the A parameter was compared to a full model. The relative fits of these two models, (Δχ2(3)=21.58, p<.01) indicated that dropping A resulted in a substantial and significant deterioration in model fit. Next, the fit of a model that did not include the C parameter was compared to a full model. The relative fits of these two models, (Δχ2(2)=2.07, p=.12) indicated that C could be dropped from the model without a significant reduction in model fit. (A model that did not include the E parameter could not be fit to the data.) Although we could have proceeded with a more parsimonious “best-fitting” AE model, we took a more conservative approach by testing sex differences within full models. The use of best-fitting models can sometimes yield misleading results because, for example, an AE model is a reduced model that provides estimates of A and E that would be obtained under the strict assumption that C has zero influence.
Quantitative sex differences were investigated by comparing a model in which Af=Am, Cf=Cm, and Ef=Em (Table 3, Model 3) to a model that allowed the estimates to vary Af≠Am, Cf≠Cm, and Ef≠Em (Table 3, Model 1). The relative fits of these two models (Δχ2(3)=31.09, p<.0001) indicated that there were quantitative sex differences in the sources of variation in the MIS. Next, qualitative sex differences were tested by comparing the fit of a model that fixed the rA estimate of DZO at 0.5 to a model that allowed it to vary between 0.00 and 0.50 (Table 3, Model 2 versus Model 1). The model in which rA was fixed to 0.5 for DZO did not provide a significantly worse fit compared to the model in which it was allowed to take on a value less than 0.5 (Δχ2(1)=0.04, p=.84). The results of this test of qualitative sex differences suggest that there are not significant differences in the latent genetic factors contributing to variation in MIS in men and women. Based on finding quantitative sex differences but not qualitative sex differences, Model 2 was selected as the final model (see Table 3).
Table 3.
Standardized Estimates (and 95% Confidence Intervals) of Additive Genetic, Shared Environmental, and Non-shared Environmental Influence for Men and Women for the 13-item Magical Ideation Scale
| χ 2 | DF | p- value |
rA in DZO pairs |
Am | Cm | Em | Af | Cf | Ef | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 ACE free, DZO free |
16.51 | 15 | 0.35 | 0.18 (0.00-0.50) |
0.29 (0.08-0.49) |
0.14 (−0.06-0.30) |
0.60 (0.53-0.66) |
0.31 (0.18-0.45) |
0.00 (−0.09-0.10) |
0.68 (0.60-0.79) |
| Model 2 ACE free, DZO fixed |
16.55 | 16 | 0.42 | 0.50 (fixed) |
0.28 (0.08-0.47) |
0.13 (−0.04-0.29) |
0.60 (0.54-0.66) |
0.29 (0.19-0.40) |
0.02 (−0.06-0.10) |
0.69 (0.60-0.77) |
| Model 3 ACE fixed, DZO free |
47.60 | 18 | <0.01 | 0.01 (0.00-0.15) |
0.28 (0.17-0.38) |
0.10 (0.02-0.18) |
0.63 (0.58-0.68) |
0.28 (0.17-0.38) |
0.10 (0.02-0.18) |
0.63 (0.58-0.68) |
| Model 4 ACE fixed, DZO fixed |
52.58 | 19 | <0.01 | 0.50 (fixed) |
0.37 (0.32-0.42) |
0.00 (0.00-0.00) |
0.63 (0.59- 0.68) |
0.37 (0.32-0.42) |
0.00 (0.00-0.00) |
0.63 (0.59- 0.68) |
Note. – rA = estimate of genetic correlation for DZO twins. A= additive genetic influence. C= shared environmental influence. E= non-shared environmental. f = female. m = male.
After the selection of Model 2 as the final model, follow-up analyses were conducted to probe the quantitative sex difference in the sources of variation in MIS. A model in which ACE were allowed to vary between men and women was compared to a model in which CE were allowed to vary (and A was fixed), Δχ2(1)=0.02, p=.89. This indicated that there were not significant differences in the contribution of genetic influences on variation in MIS between men and women. Likewise, a model in which ACE were allowed to vary was compared to a model in which AE were allowed to vary (and C was fixed), Δχ2(1)=3.90, p<.05. This indicated that there were significant differences in the contribution of shared environmental influences on variation in MIS between men and women. Lastly, a model in which ACE were allowed to vary was compared to a model in which AC were allowed to vary (and E was fixed), Δχ2(1)=0.02, p=.89. This indicated that there were also not significant differences in the contribution of the non-shared environmental influences on variation in MIS between men and women.
Discussion
Research on the causes of sex differences in positive schizotypy (Claridge & Hewitt, 1987; Del Giudice et al., 2010; Macare et al., 2012) can provide important clues to better understand the phenomenon more generally (Rutter et al., 2003; Weisberg et al., 2011). The present study represents an investigation of sex differences in one aspect of positive schizotypy, magical ideation, as indicated by scores on the MIS. We found that men and women differed in mean levels, variability, and causes of variation in magical ideation. Each of these findings is elaborated on below.
Women scored 0.26 standard deviations higher than men on the MIS in the full sample (i.e. in between-family comparisons). These results were confirmed in a more stringent within-family comparison among opposite-sex twin pairs (d = 0.29). Opposite-sex twin pairs provide the ideal comparison because they represent matched pairs that are equated on age, childhood family socioeconomic status, parental and neighborhood characteristics, religion, and all other between-family characteristics (even including those unforeseen) that might contribute to or obscure the mean level sex difference in MIS. These twin pairs also partially control for genetic differences. In these male-female pairs, both the mean levels and variability of MIS scores were greater in women than in men. Not only were women more likely to endorse items from the MIS, but they were more likely to be in the extremes of the MIS distribution. Finding both higher means and variances in women in magical ideation supports previous research indicating that women have higher levels of positive schizotypy. One explanation for this sex difference is the potential role of estrogen in the development of positive schizotypy. Specifically, there is evidence of higher rates of psychosis among women with lowered estrogen production (e.g., Jacobs & D’Esposito, 1994). Estrogen level may constitute a risk factor for psychosis that is specific to women, pointing to a potential explanation for increased levels of positive schizotypy in women. Furthermore, there is evidence of higher rates of schizophrenia spectrum disorders among women with both lowered estrogen production and variations at the COMT gene locus, which is considered an estrogen metabolism gene (Min et al., 2012). Higher levels of magical ideation variation in women may be in part explained by individual differences in estrogen levels. Thus, there are several pieces of evidence indicating sex-specific factors that may contribute to higher means and variances in women.
The results of the present study are inconsistent with the previous meta-analysis (Miettunen & Jaaskelainen, 2010) that obtained an effect size near zero of the difference between men and women on the MIS. The major difference between the present and previous studies included in the meta-analysis is that the present study was based on a middle-aged community sample and the meta-analysis was primarily based on young adult college student samples. Thus, one logical explanation may be that the magnitude of the sex difference increases with age; however, this explanation was tested and ruled out (Miettunen & Jaaskelainen, 2010). Another possibility is that there are systematic differences (other than age) between college students and community residents that also vary in men versus women (Chmielewski et al., 1995). College student samples are inherently selective compared to general community samples and the forces that select one into college may differ in men and women. However, Miettunen & Jaaskelainen (2010) found no significant differences between student and non-student samples, and therefore this explanation was tentatively tested (with far fewer community than student samples and individuals). Interestingly, Miettunen & Jaaskelainen (2010) did report a significant difference between the student and non-student samples on another measure of positive schizotypy, perceptual aberration, with women obtaining significantly higher scores than men in non-student samples, but not in student samples. The bulk of the evidence suggests that there might be systematic differences between college and community samples that might obscure mean differences between men and women on the MIS (and other indicators of positive schizotypy) when using a college student sample, as has been reported for other characteristics (e.g. Gladstone & Koenig, 1994; Tolin & Foa, 2006).
This study improves upon the weaknesses of the previous studies, with a sample size that far exceeds the total number of participants included in all four of the previous twin studies of MIS, and convincingly demonstrates the importance of genes in contributing to individual differences in MIS scores. The previous studies obtained far-ranging estimates of heritability, accounting for between zero to 56% of the variation in MIS scores; only two of the four studies obtained evidence for significant genetic influences. In the present study, we obtained a heritability estimate of 28% for men and 29% for women, with no evidence for sex differences. The heritability estimate for this psychosis proneness scale is low compared to the high heritabilities obtained for psychosis per se, that is, schizophrenia and bipolar disorder, which have heritability estimates of at least 80% (Cardno & Gottesman, 2000; McGuffin et al., 2003).
It is worth considering whether differences across the extant studies might be explained by age differences because three of the four previous studies were based on younger adult samples. There is accumulating evidence from the behavioral genetic literature that as individuals age the proportion of phenotypic variation that is explained by genetic influences increases, potentially through the strengthening of gene-environment correlations in which people seek out environments to which they are genetically predisposed (Bergen, Gardner, & Kendler, 2007). One especially interesting comparison is with the previous study of Hay et al. (2001), because it was based on an overlapping sample from the Australian Twin Registry. Many of the individuals who participated in the Hay et al (2001) study as young adults (18-25 years of age) also participated in the present study some 15 years later (when they were 32-43 years of age). The assessments of MIS were quite different in the two studies and included only one item in common (item #6 in Table 1). Yet the results were remarkably similar, with a heritability of 33% in young adulthood (Hay et al, 2001) and 28-29% in middle adulthood (present study). The scant evidence available suggests that the heritability of scores on the MIS may be relatively stable from early to mid-adulthood.
There were significant differences between men and women in the proportion of variation in MIS scores that was due to shared environmental influences even though the shared environment did not significantly contribute to variation in MIS scores, either overall or in men or women considered individually. Furthermore, although there were not statistically significant qualitative sex differences, when the genetic correlation for opposite-sex twin pairs was freely estimated in a biometric twin model, it was estimated at 0.08, compared to the assumption of a correlation of 0.50 among same-sex DZ pairs. In fact, a genetic correlation of zero between men and women could not be statistically ruled out. Future research might profit from following up on these intriguing clues suggesting that the shared environment may make a stronger contribution to variation in MIS in men than in women, and that the genetic risk factors for MIS may differ in men and women.
Conclusions
In sum, we found that men and women differed in mean levels and variability, but not necessarily in the causes of variation in magical ideation. On average, women scored higher on a measure of magical ideation and their distribution of scores on magical ideation showed more variability than among men. The proportion of variation in magical ideation scores due to genetic influences and the specific latent genetic factors contributing to MIS variation did not differ between men and women.
These results have implications for future research. First, the within-family comparisons of men and women convincingly demonstrated a mean-level sex difference in magical ideation scores. These findings raise doubts about the use of college student samples for making broader inferences about mean-level sex differences (in magical ideation as well as other traits) in the general population. The mean and variance differences indicate that women are not only endorsing more MIS items, but also occupy more of the extremes of the distribution. This supports previous research indicating higher rates of positive schizotypy in women, and indicates the possibility of sex-specific risk factors (i.e., estrogen) contributing to increased magical ideation in women. Second, the biometric analyses convincingly demonstrated that genetic factors explain variation in magical ideation in both men and women. However, much work is left to do in examining whether there are differences in the contributions of genetic and environmental factors to variation in magical ideation across development, or whether there are certain environmental contexts in which genetic influences on magical ideation are amplified. An obvious next step will also be to identify the specific genes that are associated with magical ideation. Because magical ideation is a quantitative trait that can be easily measured in large surveys of the general population, it might serve as a useful endophenotype in the search for susceptibility genes for the psychotic disorders.
Acknowledgements
This work was supported by National Institutes of Health Grant (grant number MH66206).We thank Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for the twins, and David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
This work was supported by National Institutes of Health Grant (grant number MH66206).
References
- Aleman A, Kahn RS, Selten J. Sex differences in the risk of schizophrenia: Evidence from meta-analysis. Archives of General Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. doi:10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. text rev. [Google Scholar]
- Brugger PP, Graves RE. Right hemispatial inattention and magical ideation. European Archives of Psychiatry and Clinical Neuroscience. 1997;247:55–57. doi: 10.1007/BF02916254. doi:10.1007/BF02916254. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Testing for multigroup equivalence of a measuring instrument: A walk through the process. Psicothema. 2008;20:872–882. [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. American Journal of Medical Genetics. 2000;97:12–17. [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency Scale. 1983. Unpublished test.
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. doi:10.1037/0021-843X.103.2.171. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Miller EN. Reliabilities and intercorrelations of eight measures of proneness to psychosis. Journal of Consulting and Clinical Psychology. 1982;50:187–195. doi: 10.1037//0022-006x.50.2.187. doi:10.1037/0022-006X.50.2.187. [DOI] [PubMed] [Google Scholar]
- Chmielewski PM, Fernandes LL, Yee CM, Miller GA. Ethnicity and gender in scales of psychosis proneness and mood disorders. Journal of Abnormal Psychology. 1995;104:464–470. doi: 10.1037//0021-843x.104.3.464. doi:10.1037/0021-843X.104.3.464. [DOI] [PubMed] [Google Scholar]
- Cicero DC, Kerns JG. Multidimensional factor structure of positive schizotypy. Journal of Personality Disorders. 2010;24:327–343. doi: 10.1521/pedi.2010.24.3.327. doi:10.1521/pedi.2010.24.3.327. [DOI] [PubMed] [Google Scholar]
- Claridge GG, Hewitt JK. A biometrical study of schizotypy in a normal population. Personality and Individual Differences. 1987;8:303–312. doi:10.1016/0191-8869(87)90030-4. [Google Scholar]
- Claridge GG, McCreery CC, Mason OO, Bentall RR, Boyle GG, Slade PP, Popplewell DD. The factor structure of ‘schizotypal’ traits: A large replication study. British Journal of Clinical Psychology. 1996;35:103–115. doi: 10.1111/j.2044-8260.1996.tb01166.x. doi:10.1111/j.2044-8260.1996.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Craddock NN, Owen MJ, O’Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: Evidence and lessons. Molecular Psychiatry. 2006;11:446–458. doi: 10.1038/sj.mp.4001808. doi:10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Romina A, Adelina B, Marco ER. The evolution of autistic-like and schizotypal traits: A sexual selection hypothesis. Frontiers in Psychology. 2010;41:1664–1078. doi: 10.3389/fpsyg.2010.00041. doi: 10.3389/fpsyg.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisi LE, Friedrich U, Wahlstrom J, Boccio-Smith A, Forsman A, Eklund K, Crow TJ. Schizophrenia and sex chromosome anomalies. Schizophrenia Bulletin. 1994;20:495–505. doi: 10.1093/schbul/20.3.495. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. doi:10.1037/0022-006X.51.2.215. [DOI] [PubMed] [Google Scholar]
- Fossati A, Raine A, Carretta I, Leonardi B, Maffei C. The three-factor model of schizotypal personality: Invariance across age and gender. Personality and Individual Differences. 2003;35:1007–1019. doi:10.1016/S0191-8869(02)00314-8. [Google Scholar]
- Fonseca-Pedrero E, Paino M, Lemos-Giráldez S, Sierra-Baigrie S, Muñiz J. Factor structure and measurement invariance of the Wisconsin Schizotypy Scales across gender and age. Spanish Journal of Psychology. 2010;13:941–950. doi: 10.1017/s1138741600002584. [DOI] [PubMed] [Google Scholar]
- Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Langstrom N, Hultman CM. Advanced paternal and grandpaternal age and schizophrenia: A three-generation perspective. Schizophrenia Research. 2011;133:120–124. doi: 10.1016/j.schres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone TR, Koenig LJ. Sex differences in depression across the high school to college transition. Journal of Youth and Adolescence. 1994;23:643–669. doi:10.1007/BF01537634. [Google Scholar]
- Glisky ML, Tataryn DJ, Tobias BA, Kihlstrom JF, McConkey KM. Absorption, openness to experience, and hypnotizability. Journal of Personality and Social Psychology. 1991;60:263–272. doi: 10.1037//0022-3514.60.2.263. doi:10.1037/0022-3514.60.2.263. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Seidman LJ, Petryshen TL, Fitzmaurice G, Tsuang MT, Buka SL. Sex-specific rates of transmission of psychosis in the New ngland high-risk family study. Schizophrenia Research. 2011;128:150–155. doi: 10.1016/j.schres.2011.01.019. doi:10.1016/j.schres.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical Status of At-Risk Individuals 5 Years Later: Further Validation of the Psychometric High-Risk Strategy. Journal of Abnormal Psychology. 2005;114:170–175. doi: 10.1037/0021-843X.114.1.170. doi:10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Harkness A, McNulty J. Differentiating normal and abnormal personality. Springer Publishing Co; New York (NY): 1994. The Personality Psychopathology Five (PSY-5): Issues from the pages of a diagnostic manual instead of a dictionary; pp. 291–315. [Google Scholar]
- Hay DA, Martin NG, Foley D, Treloar SA, Kirk KM, Heath AC. Phenotypic and genetic analyses of a short measure of psychosis-proneness in a large-scale Australian twin study. Twin Research and Human Genetics. 2001;4:30–40. doi: 10.1375/1369052012128. [DOI] [PubMed] [Google Scholar]
- Horan WP, Reise SP, Subotnik KL, Ventura J, Nuechterlein KH. The validity of Psychosis Proneness Scales as vulnerability indicators in recent-onset schizophrenia patients. Schizophrenia Research. 2008;100:224–236. doi: 10.1016/j.schres.2007.12.469. doi:10.1016/j.schres.2007.12.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. The Journal of Neuroscience. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. doi:10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Hewitt J. The structure of self-report schizotypy in twins. Journal of Personality Disorders. 1992;6:1–17. doi:10.1521/pedi.1992.6.1.1. [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta psychiatricaScandinavica. Supplementum. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lewine RJ. Gender and schizophrenia. In: Nasrallah HA, editor. Handbook of Schizophrenia. Amsterdam; Elsevier: 1988. pp. 379–397. [Google Scholar]
- Macare C, Bates TC, Heath AC, Martin NG, Ettinger U. Substantial genetic overlap between schizotypy and neuroticism: A twin study. Behavior Genetics. 2012;42:732–742. doi: 10.1007/s10519-012-9558-6. doi:10.1007/s10519-012-9558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: A community-based twin study. Schizophrenia Bulletin. 2001;27:47–58. doi: 10.1093/oxfordjournals.schbul.a006859. [DOI] [PubMed] [Google Scholar]
- Mata I, Mataix-Cols D, Peralta V. Schizotypal Personality Questionnaire-Brief: Factor structure and influence of sex and age in a nonclinical population. Personality and Individual Differences. 2005;38:1183–1192. doi:10.1016/j.paid.2004.08.001. [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of general psychiatry. 2003;60:497. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. doi:10.1037/h0041029. [Google Scholar]
- Miettunen J, Jääskeläinen E. Sex differences in Wisconsin Schizotypy Scales—A meta-analysis. Schizophrenia Bulletin. 2010;36:347–358. doi: 10.1093/schbul/sbn075. doi:10.1093/schbul/sbn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB. Unpublished Ph.D. dissertation. University of Wisconsin; Madison: 1993. A study of schizotypal traits in young male twins. [Google Scholar]
- Min J, Kim J, Pae C, Kim K, Lee C, Lee C, Paik I. Association of estrogen receptor genes and schizophrenia: A preliminary study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36:1–4. doi: 10.1016/j.pnpbp.2011.09.012. doi:10.1016/j.pnpbp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Muthén &Muthén; Los Angeles (CA): 1998. [Google Scholar]
- Rawlings D, Claridge G, Freeman JL. Principal components analysis of the Schizotypal Personality Scale (STA) and the Borderline Personality Scale (STB) Personality and Individual Differences. 2001;31:409–419. doi:10.1016/S0191-8869(00)00146-X. [Google Scholar]
- Reise SP, Widaman KF, Pugh RH. Confirmatory factor analysis and item response theory: Two approaches for exploring measurement invariance. Psychological Bulletin. 1993;114:552–566. doi: 10.1037/0033-2909.114.3.552. doi:10.1037/0033-2909.114.3.552. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: Unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Salem JE, Kring AM. The role of gender differences in the reduction of etiologic heterogeneity in schizophrenia. Clinical Psychology Review. 1998;18:795–819. doi: 10.1016/s0272-7358(98)00008-7. doi:10.1016/S0272-7358(98)00008-7. [DOI] [PubMed] [Google Scholar]
- Seeman M, Lang M. The role of estrogens in schizophrenia gender differences. Schizophrenia Bulletin. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian twin study of gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12:63–78. doi: 10.1375/twin.12.1.63. doi:10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Content categories: Absorption Items (Revised) University of Minnesota; 1982. Unpublished manuscript. [Google Scholar]
- Tellegen A. Structure of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuman AJ, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale (NJ); Erlbaum: 1985. pp. 681–706. [Google Scholar]
- Tellegen A, Atkinson G. Openness to absorbing and self-altering experiences (‘absorption’), a trait related to hypnotic susceptibility. Journal of Abnormal Psychology. 1974;83:268–277. doi: 10.1037/h0036681. doi:10.1037/h0036681. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin. 2006;132:959. doi: 10.1037/0033-2909.132.6.959. doi:10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Venables PH, Rector NA. The content and structure of schizotypy: A study using confirmatory factor analysis. Schizophrenia Bulletin. 2000;26:587–602. doi: 10.1093/oxfordjournals.schbul.a033480. [DOI] [PubMed] [Google Scholar]
- Vollema MG, van den Bosch RJ. The multidimensionality of schizotypy. Schizophrenia Bulletin. 1995;21:19–31. doi: 10.1093/schbul/21.1.19. [DOI] [PubMed] [Google Scholar]
- Weisberg YJ, DeYoung CG, Hirsh JB. Gender differences in personality across the ten aspects of the Big Five. Frontiers in Psychology. 2011;2:1–11. doi: 10.3389/fpsyg.2011.00178. doi: 10.3389/fpsyg.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]