Abstract
Vitamin D hormone (1,25-dihydroxyvitamin D) is involved in innate immunity and induces host defense peptides in epithelial cells, suggesting its involvement in mucosal defense against infections. Chlamydia trachomatis is a major cause of bacterial sexually transmitted disease worldwide. We tested the hypothesis that the vitamin D endocrine system would attenuate chlamydial infection. Vitamin D receptor knock-out mice (VDR−/−) and wild-type mice (VDR+/+) were infected with 103 inclusion forming units of Chlamydia muridarum and cervical epithelial cells (HeLa cells) were infected with C. muridarum at multiplicity of infection 5:1 in the presence and absence of 1,25-dihydroxyvitamin D3.VDR−/− mice exhibited significantly higher bacterial loading than wild-type VDR+/+ mice (P<0.01) and cleared the chlamydial infection in 39 days, compared with 18 days for VDR+/+ mice. Monocytes and neutrophils were more numerous in the uterus and oviduct of VDR−/− mice than in VDR+/+ mice (P< 0.05) at d 45 after infection. Pre-treatment of HeLa cells with 10nM or 100nM 1,25-dihydroxyvitamin D3 decreased the infectivity of C. muridarum (P< 0.001). Several differentially expressed protein spots were detected by proteomic analysis of chlamydial-infected HeLa cells pre-treated with 1,25-dihydroxyvitamin D3. Leukocyte elastase inhibitor (LEI), an anti-inflammatory protein, was up-regulated. Expression of LEI in the ovary and oviduct of infected VDR+/+ mice was greater than that of infected VDR−/− mice. We conclude that the vitamin D endocrine system reduces the risk for prolonged chlamydial infections through regulation of several proteins and that LEI is involved in its anti-inflammatory activity.
Keywords: Chlamydial infection; 1,25-Dihydroxyvitamin D3; Vitamin D receptor knock out mouse; HeLa cells; Leukocyte elastase inhibitor
1. Introduction
The vitamin D hormone (1,25-dihydroxyvitamin D, 1,25-(OH)2D)1 is a fat-soluble secosteroid and a major regulator of mineral homeostasis through its actions in the kidney, intestines, bone, and parathyroid glands. The biological effects of 1,25-(OH)2D3 are mediated by the vitamin D receptor (VDR), a member of the super family of nuclear hormone receptors that function as agonist-activated transcription factors. Recent studies indicate that vitamin D hormone regulates the immune system through induction of natural host defense peptides, activation of pattern recognition toll-like receptors, and anti-inflammatory activity [1, 2]. Liu et al. [3] reported that toll-like receptor activation of human macrophages up-regulated expression of VDR and the 25-hydroxyvitamin D-1-hydroxylase (required for synthesis of 1,25-(OH)2D), leading to induction of cathelicidin, an antimicrobial peptide, and killing of intracellular Mycobacterium tuberculosis. Subsequent animal and clinical studies have provided further evidence of the role of the vitamin D endocrine system in innate immunity [4–9]. It has also been reported that 1,25-(OH)2D3/VDR modulates the capacity of antigen presenting cells to induce T cell activation, cytokine secretion, and localized antigen-specific immune responses [10–13].
Chlamydia trachomatis is a major infectious bacterial agent of sexually transmitted disease (STD) in industrialized and developing nations. An estimated three million new cases of chlamydial infection occur annually in the United States [14]. Chlamydial infection, the most common STD in the United States, can cause severe health consequences for women, including pelvic inflammatory disease, ectopic pregnancy, chronic pelvic pain, and infertility [15]. The local mucosal epithelial cells of the genital tract are important in chlamydial infectivity, acting as sentinels to recognize pathogens and send signals to underlying immune cells [16, 17]. We report here on the use of the vitamin D receptor knock-out (VDR−/−) mouse and HeLa cells (human cervical epithelial cells) to test the hypothesis that the vitamin D endocrine system attenuates chlamydial infection.
2. Materials and methods
2.1. Chemicals
1,25-Dihydroxyvitamin D3 was obtained from MP Biomedicals (LLC, Solon, OH). Anti-mouse antibody to leukocyte elastase inhibitor (LEI) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody to Chlamydia conjugated with FITC was obtained from Bio-Rad (Hercules, CA).
2.2. Animals
Female VDR−/−[18, 19] and VDR+/+ mice on a C57BL/6J background (6 week old) were purchased from Jackson Laboratory (Bar Harbor, MA), fed food and water ad libitum, and maintained in laminar flow racks under pathogen-free conditions with a 12 h light and 12 h dark cycle. All VDR−/− and VDR+/+ mice were fed a rescue diet [20] high in calcium, phosphate, and lactose (Harlan Laboratories, Madison, WI) to achieve normal plasma calcium levels in the VDR−/− mice. The mice (n = 6/group) were infected at 8 week old and killed on d 45 after infection by cervical dislocation. The protocols involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) of Morehouse School of Medicine.
2.3. Infectivity assay in VDR−/− and VDR+/+ mice
Female VDR−/− and VDR+/+ mice (n = 6/group, 8 week old) were subcutaneously administered 2 mg of medroxyprogesterone acetate (Sigma-Aldrich Co, St Louis, MO) 7 d prior to infection and infected intra-vaginally with 103 inclusion forming units (IFU) of Chlamydia muridarum in 20 μl of PBS. Bacterial shedding was assessed by performing vaginal swabs on d 3, 6, 9, 12, 15, 18, 25, 32, and 39 after infection. Tissue culture isolation of C. muridarum was by standard procedures and the number of inclusions per group per time point was determined [21].
2.4. Histology and immunohistochemistry
The entire genital tract was collected and fixed in 4% formaldehyde. The samples were embedded in paraffin, cut longitudinally into 4 μm sections, and stained with hematoxylin and eosin. The right and left uterine horns and oviducts were individually evaluated in a blinded method for the presence of acute inflammation (neutrophils), chronic inflammation (monocytes), and plasma cells. A four-tiered semiquantitative scoring system was used to quantitate the inflammation [22]: 0, normal; 1+, rare foci (minimal presence) of inflammatory cells; 2+, scattered (1–4) aggregates or mild diffuse increase in parameter; 3+, numerous aggregates (>4) or moderate diffuse or confluent areas of parameter; 4+, severe diffuse infiltration or confluence of parameter.
Tissue slides from the oviducts were incubated with a mouse anti-LEI (serpinB1a) primary antibody, followed by incubation for 30 min with a horseradish peroxidase-conjugated goat anti-mouse secondary antibody in accordance with the manufacturer’s directions. Images of representative fields were obtained using an Olympus Provis AX70 microscope equipped with a Leica DFC 320 Digital Camera system (Leica Camera AG, Solms, Germany).
2.5. Infectivity in HeLa cells
HeLa cells were pre-treated with 1,25-(OH)2D3 at different concentrations for 24 h. The treated and un-treated cells were washed and infected with C. muridarum at multiplicity of infection (MOI) 5:1 for 48 h. The intensity of infection was measured by staining with a chlamydial antibody conjugated with fluorescein isothiocyanate. The number of inclusions was calculated as previously described [23].
2.6. Proteomic analysis
HeLa cells that had been pre-treated with 1,25-(OH)2D3 (100nM) for 24 h or un-treated were infected with C. muridarum for 2 h. Proteins were extracted with a Bio-Rad protein extraction kit and cleaned with 2-D Clean Up Kit, according to the manufacturer’s protocol. Protein concentration was determined by 2D Quant Kit from GE Healthcare (GE Healthcare, Piscataway, NJ). Samples (80 μg) were labeled with Cy3 or Cy5 fluorescence dyes, mixed in a rehydration buffer, and subjected to two-dimensional fluorescence differential gel electrophoresis analysis (2D-DIGE) [24, 25]. The spots corresponding to differentially expressed proteins were digested and analyzed by nanocapillary LC-MS/MS (Xevo G2 Tof, Waters, Milford, MA). Protein candidates were identified with automated database searching against a NCBI database using MASCOT Daemon software (Matrix Sciences, Boston, MA).
2.7. Western blot analysis
HeLa cells that had been pre-treated with 1,25-(OH)2D3 (100 nM) or un-treated for 24 h were pulsed with Chlamydia muridarum agent (MOI 5:1) for up to 2h. Protein expression of LEI at 0.5, 1, and 2 h after infection with Chlamydia was determined by Western blot analysis. The cell lysates were subjected to electrophoresis for protein separation on 4–20% Mini-PROTEAN TGX Precast Gels (Bio-Rad, Hercules, CA) in running buffer (Tris/glycine/SDS). Proteins were then transferred for 1 h onto nitrocellulose membranes in transfer buffer (Tris/glycine/methanol). Non-specific binding was blocked by incubating the membranes in 5% non-fat dried milk, 0.05% (v/v) Tween 20 in 1X TBS for 1 h at room temperature. After several washes with buffer (TBS Tween 0.05%), membranes were incubated with the primary antibodies overnight at 4°C. The polyclonal antibodies used were: serpinB1 (1:200) (Santa Cruz Biotech. Inc., Santa Cruz, CA); glyceraldehide-3-phosphate dehydrogenase (GAPDH) (1:200) (R&D Systems, Minneapolis, MN). Horseradish peroxidase-conjugated secondary antibodies were utilized in accordance with the manufacturer’s directions (Santa Cruz Biotechnology, Santa Cruz, CA).
2.8. Statistical analysis
A mean ± SD was calculated for each group and statistical significance (P< 0.05) evaluated (SAS 9.2, SAS Institute Inc., Cary, NC). Repeated measures ANOVA was used to compare intensity and duration of infection in VDR−/− and VDR+/+ mice and one way ANOVA to assess the effect of 1,25-(OH)2D3 on intensity of infection in HeLa cells. A t-test was used to compare the pathology scores of VDR−/− and VDR+/+ uterus and oviducts.
3. Results
3.1. Chlamydial infection and inflammatory response in VDR−/−and VDR+/+ mice
VDR−/− mice that were genitally infected with C. muridarum had significantly higher bacteria loads at d 6, 9, 12, 15 post-infection than VDR+/+ mice, indicating a greater intensity of infection (Fig. 1). VDR−/− mice also exhibited a slower rate of clearance of the genital chlamydial infection than VDR+/+ mice. Whereas VDR+/+ mice had cleared the infection by d 18, VDR−/− mice cleared the infection by d 39. The uterus of uninfected VDR+/+ and VDR−/− mice revealed normally observed branched folia with intact epithelial lining composed of both secretory and ciliated cells (Fig. 2). Significantly higher numbers of monocytes (pathology scores: 2.5 ± 0.3 vs. 0.7 ±0.7, P<0.01) and neutrophils (pathology scores: 1.2 ±0.6 vs. 0.2 ±0.2, P<0.05) were observed in the infected uterus of VDR−/−mice, compared with that of infected VDR+/+ mice. No inflammation was observed in the oviduct of un-infected VDR−/− and VDR+/+ mice (Fig. 3). Significantly higher numbers of monocytes (pathology scores: 3.2 ±0.6 vs. 1.7 ±0.7, P<0.01) and neutrophils (pathology scores: 1.5 ± 0.3 vs. 0.3 ± 0.3, P< 0.01) were observed in the oviduct of infected VDR−/− mice, compared with that of infected VDR+/+ mice.
Fig. 1.
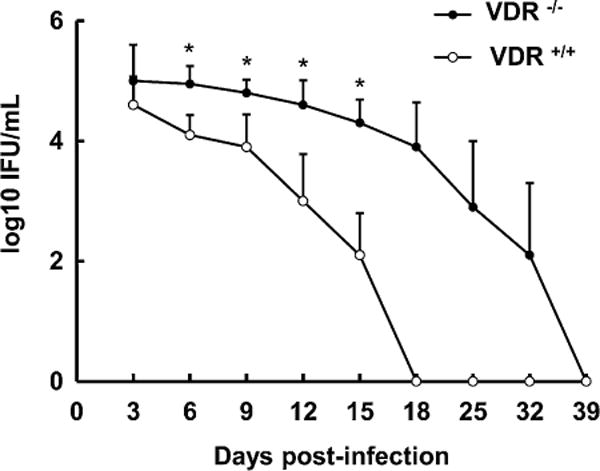
VDR−/− mice infected intra-vaginally with Chlamydia muridarum exhibit greater bacteria loads and slower rates of bacterial clearance than VDR+/+ mice. VDR−/− and VDR+/+ mice were infected intra-vaginally with 103 inclusion forming units (IFU) of C. muridarum as described in Section 2.3. The course of infection was monitored by isolation of C. muridarum from cervicovaginal swabs [21]. Values are mean ± SD, n = 6. Overall intensity different from VDR+/+, P< 0.01. Rate of clearance different from VDR+/+, P< 0.001. Intensity of infection different from VDR+/+, *P< 0.001.
Fig. 2.
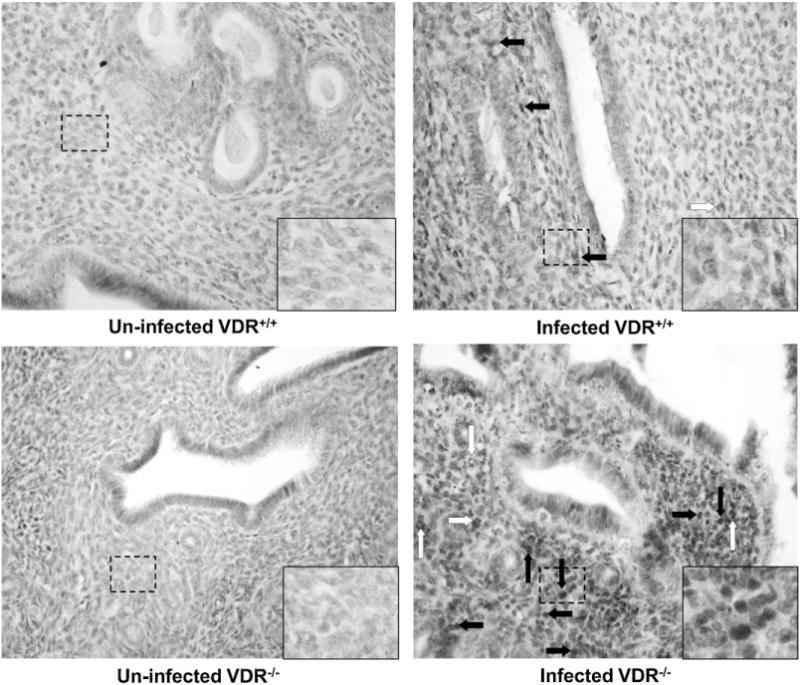
More monocytes and neutrophils are observed in the uterus of infected VDR−/− mice, compared with that of infected VDR+/+ mice. The uterus horns of each mouse were removed at d 45 post-infection with Chlamydia muridarum and stained with hematoxylin and eosin (HE), as described in Section 2.4. HE-stained sections are at 40× magnification. Open arrows point to scattered neutrophils; closed arrows indicate monocytes/lymphocytes.
Fig. 3.
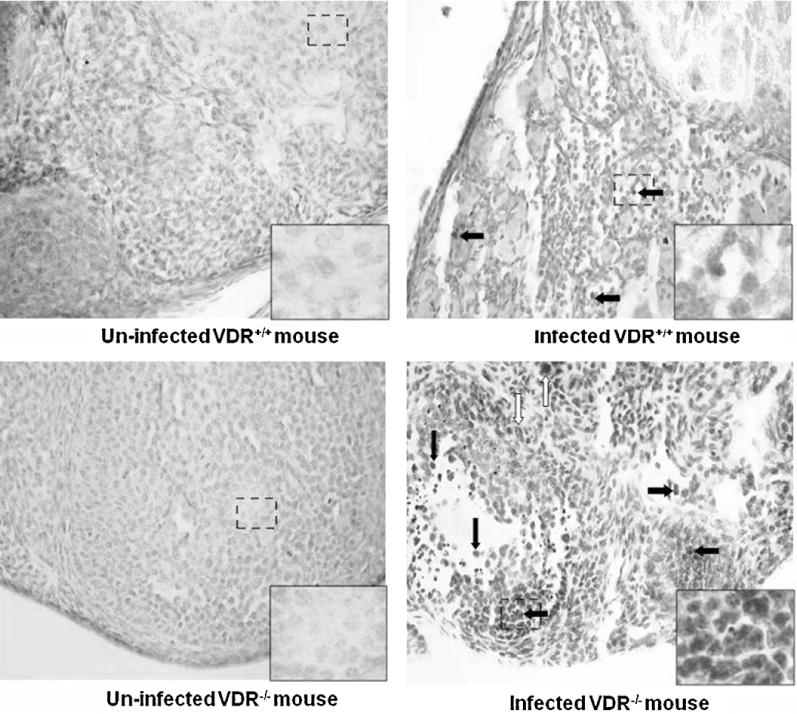
More monocytes and neutrophils are observed in the oviducts of infected VDR−/− mice, compared with that of infected VDR+/+ mice. The oviducts of each mouse were removed at d 45 post-infection with Chlamydia muridarum and stained with hematoxylin and eosin (HE), as described in Section 2.4. HE-stained sections are at 40× magnification. Open arrows point to scattered neutrophils; closed arrows indicate monocytes/lymphocytes.
3.2. Effect of 1,25-(OH)2D3 on chlamydial infection in HeLa cells
Chlamydial infectivity of HeLa cells pre-treated with 10nM or 100nM 1,25-(OH)2D3 for 24h was significantly less than chlamydial infectivity of un-treated HeLa cells or HeLa cells pre-treated with 1.0nM 1,25-(OH)2D3(Fig. 4). Vitamin D hormone treatment of chlamydial-infected HeLa cells resulted in numerous differentially expressed protein spots, of which 25 have been identified, using LC-MS/MS ion search software and associated databases (Table 1). Fewer differentially expressed protein spots were observed for pre-treated un-infected HeLa cells (Supplemental Fig. 1).
Fig.4.
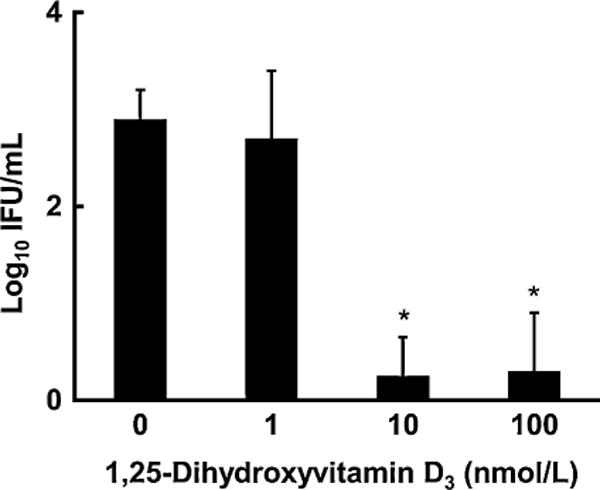
Chlamydial infectivity of HeLa cells pre-treated with 1,25-(OH)2D3 is significantly less than chlamydial infectivity of un-treated HeLa cells. HeLa cells were pre-treated with 1,25-(OH)2D3 at different concentrations for 24h. Treated and un-treated cells were washed and infected with Chlamydia muridarum for 48 h as described in Section 2.5. The intensity of infection was measured by staining with a chlamydial antibody and determining the number of inclusions [23]. Values are mean ± SD, n = 6. Significant effect of 1,25-(OH)2D3 treatment, P< 0.001. Intensity of infection different from un-treated, *P< 0.001.
Table 1.
Several spots were up-regulated in chlamydial-infected HeLa cells pre-treated with 1,25-dihydroxyvitamin D3.
| Spota | Protein name | Accession IDb | MW(Da)c | pIc | Scored | Matched peptidese | Sequence coverage (%) |
|---|---|---|---|---|---|---|---|
| 10 | Protein S100-A6 | S10A6_RABIT | 10,147 | 5.30 | 54 | 1 | 20 |
| 13 | Tropomyosin alpha-3 chain | TPM3_HUMAN | 32,799 | 4.68 | 113 | 3 | 11 |
| 18 | Tubulin beta chain | TBB5_HUMAN | 49639 | 4.78 | 178 | 7 | 17 |
| 19 | Hsc70-interacting protein | F10A1_HUMAN | 41,305 | 5.18 | 91 | 2 | 6 |
| 20 | ATP synthase subunit beta | ATPB_HUMAN | 56,525 | 5.26 | 384 | 11 | 33 |
| 21 | Vimentin | VIME_HUMAN | 53,619 | 5.06 | 282 | 7 | 18 |
| 22 | 60 kDa heat shock protein | CH60_HUMAN | 61,016 | 5.70 | 488 | 12 | 31 |
| 23 | 60 kDa heat shock protein | CH60_HUMAN | 61,016 | 5.70 | 111 | 4 | 11 |
| 24 | T-complex protein 1 subunit epsilon | TCPE_HUMAN | 59,633 | 5.45 | 271 | 10 | 23 |
| 25 | Heat shock cognate 71 kDa protein | HSP7C_HUMAN | 70,854 | 5.37 | 393 | 11 | 22 |
| 26 | Serum albumin precursor | ALBU_BOVIN | 69,248 | 5.82 | 58 | 3 | 5 |
| 30 | Protein disulfide-isomerase A3 precursor | PDIA3_HUMAN | 56,747 | 5.98 | 223 | 8 | 21 |
| 34 | T-complex protein 1 subunit beta | TCPB_HUMAN | 57,452 | 6.01 | 183 | 4 | 9 |
| 36 | Proliferation-associated protein 2G4 | PA2G4_HUMAN | 43,759 | 6.13 | 157 | 4 | 15 |
| 37 | Proliferation-associated protein 2G4 | PA2G4_HUMAN | 43,759 | 6.13 | 41 | 1 | 7 |
| 39 | Leukocyte elastase inhibitor | ILEU_HUMAN | 42,715 | 5.90 | 103 | 3 | 8 |
| 42 | 60S acidic ribosomal protein P0 | RLA0_HUMAN | 34,252 | 5.71 | 173 | 4 | 13 |
| 46 | Peroxiredoxin-6 | PRDX6_HUMAN | 25,019 | 6.00 | 130 | 4 | 20 |
| 48 | Heat-shock protein beta-1 (HspB1) | HSPB1_HUMAN | 22,768 | 5.98 | 122 | 3 | 18 |
| 49 | Thioredoxin-dependent peroxide reductase | PRDX3_HUMAN | 27,675 | 7.67 | 142 | 3 | 13 |
| 54 | Peroxiredoxin-2 | PRDX2_HUMAN | 21,878 | 5.66 | 122 | 3 | 19 |
| 57 | Tumor protein D54 (hD54) | TPD54_HUMAN | 22,224 | 5.26 | 42 | 2 | 11 |
| 58 | Transitional endoplasmic reticulum ATPase | TERA_HUMAN | 89,266 | 5.14 | 144 | 7 | 9 |
| 64 | Vinculin (Metavinculin) | VINC_HUMAN | 123,722 | 5.50 | 60 | 3 | 2 |
As indicated in Supplemental Fig. 1. Down-regulated protein spots were not identified fortechnical reasons.
SwissProt protein accession number.
Relative molecular mass of proteins and isoelectric point.
Probability based mowse score that indicates the quality of the MS/MS peptide fragment ion matches. Ion score is−10 × log(P),where P is the probability that the observed match is a random event. Protein scores are derived from ions scores as a non-probabilistic basis for ranking protein hits.
Number of peptides that match the theoretical digest of the primary protein identified.
Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/jjsbmb.2012.11.002.
3.3. Leukocyte elastase inhibitor expression
Western blots indicated significantly more LEI in infected HeLa cells pre-treated with 1,25-(OH)2D3 than in un-treated infected HeLa cells (Fig. 5). Pre-treatment with 1,25-(OH)2D3 did not, however, significantly increase LEI in un-infected HeLa cells. LEI was detected in the oviduct and ovary of infected VDR+/+ mice by immunohistochemistry, but not in the oviduct and ovary of infected VDR−/− mice (Fig. 6).
Fig. 5.
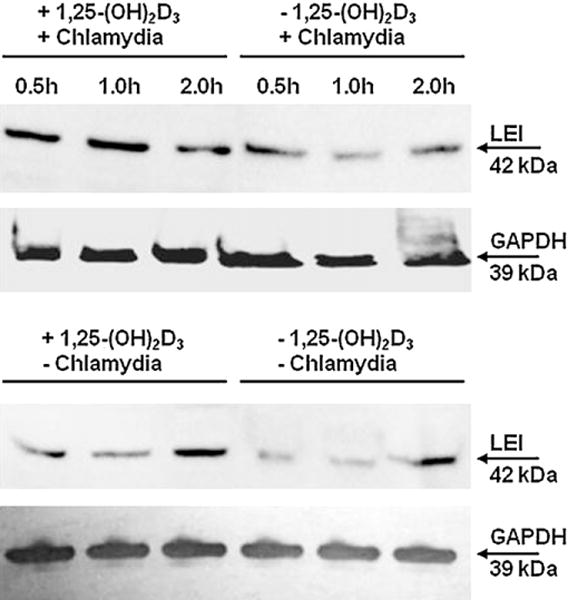
Leukocyte elastase inhibitor is up-regulated in infected HeLa cells pre-treated with 1,25-(OH)2D3, but not in pre-treated un-infected HeLa cells. HeLa cells that had been pre-treated with 1,25-(OH)2D3 (100nM) or un-treated for 24h were pulsed with Chlamydia muridarum (MOI 5:1) for up to 2 h. Total protein was extracted, subjected to SDS-PAGE, transferred onto nitrocellulose membrane, probed with primary and secondary antibodies, and imaged as described in Section 2.7.
Fig. 6.
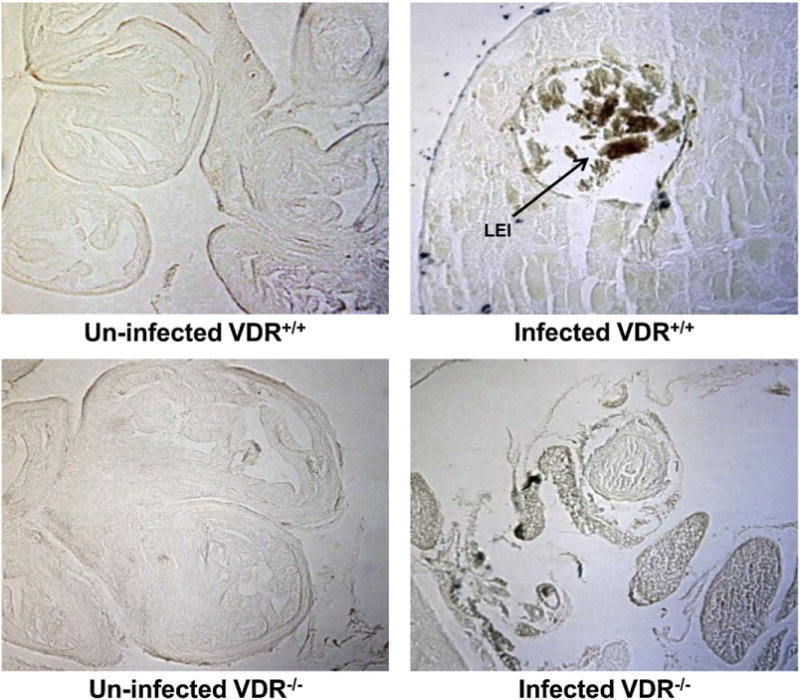
Leukocyte elastase inhibitor is detectable in the ovaries of infected VDR+/+ mice, but not in the ovaries of infected VDR−/− mice. VDR−/− and VDR+/+ mice were infected intra-vaginally with Chlamydia muridarum as described in Section 2.3. Tissue slides of ovaries from the mice were incubated with a mouse anti-leukocyte elastase inhibitor primary antibody and imaged as described in Section 2.4.
4. Discussion
In this study, vitamin D receptor knock-out mice and control mice (VDR+/+) were used to evaluate the 1,25-(OH)2D3/VDR effect on genital chlamydial infection. Homozygous VDR−/− mice are phenotypically normal at birth and survive for at least six months [18]. These mice exhibit hypocalcemia, hyperparathyroidism, rickets and osteomalacia. One feature seen in VDR−/− mice, but not seen in mice made vitamin D deficient by dietary means, is alopecia, which develops progressively from the age of four weeks [18]. Feeding VDR−/− mice a rescue diet high in calcium, phosphorus, and lactose [20] normalized all of the symptoms above except alopecia [19]. In the present study, VDR−/− mice fed the rescue diet suffered a more intense and prolonged genital chlamydial infection compared with VDR+/+ mice, suggesting that attenuation of genital chlamydial infectivity in the VDR+/+ mice is not an indirect effect of maintaining normal calcium, but is a process which requires a functional VDR.
Intensive chlamydial infection and a prolonged infection course trigger inflammatory responses that are thought to be largely res,ponsible for the chlamydial induced pathology of the genital tract. Inflammation was not observed in the uterus and oviduct of un-infected VDR−/− and VDR+/+ mice, but higher numbers of monocytes and neutrophils were observed in the infected uterus and oviduct of VDR−/− mice, compared with that of VDR+/+ mice. The more intense and prolonged chlamydial infection in the VDR−/−mice was thus accompanied by a more prolonged inflammatory response.
Genital epithelial cells are the first line of defense against microbial invasion, so we conducted chlamydial infection assays with HeLa cells, a genital epithelial cell line. Pre-treatment of HeLa cells with 1,25-(OH)2D3 dramatically inhibited intracellular replication of C. muridarum and inclusion development. It is possible that 1,25-(OH)2D3 mediates the protective response of epithelial cells to bacterial replication [26]. HeLa cells pre-treated with 1,25-(OH)2D3 and infected with C. muridarum exhibited differential expression of 65 protein spots, whereas fewer differentially expressed protein spots were detected in un-infected HeLa cells pre-treated with 1,25-(OH)2D3. Several categories of proteins were differentially expressed: cytoskeleton proteins that affect microfilament network and cell-cell junctions (tubulin, vimentin, tropomyosin chain, vinculin); stress response proteins related to protein folding and chaperones (heat shock proteins, protein disulfide isomerase, T-complex protein-1); proteins involved in oxidation/reduction (peroxiredoxin-2 and -6, thioredoxin-dependent peroxide reductase); proteins involved in growth and regulation of cell proliferation (tumor protein D54, 60S acidic ribosomal protein P0, proliferation-associated protein 2G4); proteins involved with ATP (ATP synthase subunit beta, transitional endoplasmic reticulum ATPase); calcium binding protein (protein S100-A7); anti-inflammatory protein (LEI); serum albumin precursor. A caveat to be considered in conducting complementary experiments with HeLa cells is that human monocytes and macrophages show vitamin D hormone induced expression of antimicrobial proteins (e.g. cathelicidin, beta-defensin), but these genes are not regulated by 1,25-(OH)2D3 in mice.
We selected for further study LEI, an anti-inflammatory protein that was up-regulated in chlamydial infected HeLa cells pre-treated with 1,25-(OH)2D3, compared with un-treated cells. LEI is a 42 kDa member of the serine protease inhibitor (serpin) superfamily [27] and is also known as monocyte/neutrophil elastase inhibitor (MNEI) and serpinB1. It inhibits neutrophil proteases (elastase, cathepsin G, proteinase-3) that are involved in killing phagocytosed microbes by forming irreversible covalent complexes with them. LEI is abundant in the cytoplasm of monocytes, neutrophils, and macrophages and attenuates the inflammatory response to microbial infection by limiting the activity of the proteases, thus limiting degradation of host defense and matrix proteins [28]. Recombinant MNEI administered daily by aerosolization to rats previously inoculated with Pseudomonas aeruginosa decreased the inflammatory injury and enhanced the clearance of bacteria from the infected rat lungs [29]. SerpinB1−/− mice exhibited higher mortality relative to wild type mice, associated with late-onset failed clearance of P. aeruginosa. Co-administration of recombinant serpinB1 with the P. aeruginosa inoculum normalized bacterial clearance in serpinB1−/− mice [30]. Gong et al. [31] reported that serpinB1−/− mice died earlier and in greater numbers than wild-type mice when infected with high-dose surfactant protein-D-sensitive influenza A/Philadelphia/82(H3N2). They concluded that serpinB1 plays a critical role in mitigating inflammation and restricting pro-inflammatory cytokine production in influenza infection. L-DNase II, an endonuclease involved in the degradation of genomic DNA during apoptosis, is derived from LEI by an acidic-dependent post-translational modification or by digestion with elastase [32–36]. Appearance of the endonuclease activity results in loss of the anti-protease activity [32]. Benarafa et al. [37] have indicated that human MNEI is encoded by a single SERPINB1 gene, whereas four murine genes have been identified and fully sequenced, with one of them, EIA (SERPINB1a), being the mouse ortholog of MNEI.
In the present study, Western blots indicated up-regulation of LEI in infected HeLa cells pre-treated with 1,25-(OH)2D3, but not in pre-treated un-infected HeLa cells (confirming the proteomics results). A greater amount of LEI was detected by immunohistochemistry in the oviduct and ovary of infected VDR+/+ mice than in the oviduct and ovary of infected VDR−/− mice, although monocytes and neutrophils (the primary cellular sources of LEI) were more numerous in the oviduct of infected VDR−/− mice than in the oviduct of infected VDR+/+ mice. The association of more prolonged chlamydial infection and inflammatory response with lesser LEI expression in the oviduct and ovary of VDR−/− mice, compared with VDR+/+ mice, would suggest a role for LEI in countering the inflammatory response and for vitamin D hormone in LEI expression. Previous studies have demonstrated a role for 1,25-(OH)2D3 in LEI expression. Protiva et al. [38] demonstrated, via global gene array analysis, activation of the VDR pathway in rectal mucosal biopsies from estradiol treated postmenopausal women and up-regulation of SERPINB1. Kovalenko et al. [39] identified numerous transcript changes, including up-regulation of SERPINB1, in response to 1,25-(OH)2D3 treatment of the immortalized, non-transformed prostate epithelial cell line, RWPE1. Unlike the anti-microbial proteins, LEI appears to be regulated by 1,25-(OH)2D3 in mice, as well as in cells of human origin. Wang et al. [40] have demonstrated that multiple DR3 elements in the serpinB1 gene are conserved between mouse and human. Consequently, the mouse can serve as a model for examining the relationship between the vitamin D endocrine system and LEI, a protein with anti-inflammatory effects.
In summary, we demonstrate here that genital chlamydial infectivity was more intense and prolonged in VDR−/− mice than in VDR+/+ mice. The inflammatory response to chlamydial infection was also more prolonged in VDR−/− mice than in VDR+/+ mice. The more prolonged chlamydial infection and inflammatory response in the VDR−/− mice coincided with lesser expression of LEI (an antiinflammatory protein) in the oviduct and ovary of VDR−/− mice, compared with VDR+/+ mice, suggesting a role for LEI in countering the inflammatory response to chlamydial infection and for vitamin D hormone in LEI expression.
We conclude that the VDR is involved in immunoregulation of chlamydial infection. LEI, the protein examined in this study, was only one of numerous proteins differentially expressed in infected HeLa cells pre-treated with 1,25-(OH)2D3. Additional studies are necessary to determine other mechanisms by which vitamin D hormone attenuates chlamydial infection and the anti-inflammatory response.
Supplementary Material
Acknowledgments
This work was funded by Georgia Research Alliance Collaboration Planning Grant GRA VAC10.C, the Centers for Disease Control and Prevention, and Morehouse School of Medicine. Facilities and support services were partially funded by National Institutes of Health/National Center for Research Resources grants G12-RR03034, 1 C06 RR18386, 1 U54 RR026137 (to Morehouse School of Medicine) and RR03062 and 08247 (to Clark Atlanta University).
Abbreviations
- 1,25-(OH)2D3
1,25-dihydroxycholecalciferol
- 1,25-(OH)2D
1,25-dihydroxyvitamin D
- 2D-DIGE
two-dimensional differential in gel electrophoresis
- HE
hematoxylin and eosin
- HeLa cells
human cervical epithelial cells
- IFU
inclusion forming units
- LEI
MNEI or serpinB1, leukocyte elastase inhibitor
- MOI
multiplicity of infection
- SERPINB1
leukocyte elastase inhibitor gene
- VDR
vitamin D receptor
- VDR−/−
vitamin D receptor knock out mouse
- VDR+/+
C57BL/6J mouse
References
- 1.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection and Immunity. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewison M. Antibacterial effects of vitamin D. Nature Reviews Endocrinology. 2011;7(6):337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptortriggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 4.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. Journal of Clinical Investigation. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zügel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. A single dose of vitamin D enhances immunity to mycobacteria. American Journal of Respiratory and Critical Care Medicine. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 8.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. American Journal of Clinical Nutrition. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 9.Martineau AR, Honecker FU, Wilkinson RJ, Giffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 10.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Imazeki I, Matsuzaki J, Tsuji K, Nishimura T. Immunomodulating effect of vitamin D3 derivatives on type-1 cellular immunity. Biomedical Research. 2006;27:1–9. doi: 10.2220/biomedres.27.1. [DOI] [PubMed] [Google Scholar]
- 12.Do JE, Kwon SY, Park S, Lee ES. Effects of vitamin Don expression of toll-like receptors of monocytes from patients with Behçet’s disease. Rheumatology. 2008;47:840–848. doi: 10.1093/rheumatology/ken109. [DOI] [PubMed] [Google Scholar]
- 13.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. Journal of the American Society of Nephrology. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers DS, Halvorson H, Luckhaupt S. Screening for chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2007;147:135–142. doi: 10.7326/0003-4819-147-2-200707170-00173. [DOI] [PubMed] [Google Scholar]
- 15.Cohen CR, Gichui J, Rukaria R, Sinei SS, Gaur LK, Brunham RC. Immunogenetic correlates for Chlamydia trachomatis-associated tubal infertility. Obstetrics and Gynecology. 2003;101:438–444. doi: 10.1016/s0029-7844(02)03077-6. [DOI] [PubMed] [Google Scholar]
- 16.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. Journal of Reproductive Immunology. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 17.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–749. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 18.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 20.Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. Journal of Nutrition. 2007;137:2608–2615. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Q, Eko FO, Lyn D, Ananaba GA, Bandea C, Martinez J, Joseph K, Kellar K, Black CM, Igietseme JU. Involvement of LEK1 in dendritic cell regulation of T cell immunity against Chlamydia. Journal of Immunology. 2008;181:4037–4042. doi: 10.4049/jimmunol.181.6.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scurlock AM, Frazer LC, Andrews CW, Jr, O’Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infection and Immunity. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Q, Moore TT, Eko FO, Lyn D, Ananaba GA, Martin A, Singh S, James L, Stiles J, Black CM, Igietseme JU. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. Journal of Immunology. 2005;174:4860–4869. doi: 10.4049/jimmunol.174.8.4860. [DOI] [PubMed] [Google Scholar]
- 24.Ye Y, Mar EC, Tong S, Sammons S, Fang S, Anderson LJ, Wang D. Application of proteomics methods for pathogen discovery. Journal of Virological Methods. 2010;163(1):87–95. doi: 10.1016/j.jviromet.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arruda SC, Barbosa Hde S, Azevedo RA, Arruda MA. Two-dimensional difference gel electrophoresis applied for analytical proteomics: fundamentals and applications to the study of plant proteomics. Analyst. 2011;136(20):4119–4126. doi: 10.1039/c1an15513j. [DOI] [PubMed] [Google Scholar]
- 26.Sun J. Vitamin D and mucosal immune function. Current Opinion in Gastroenterology. 2010;26:591–595. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remold-O’Donnell E, Chin J, Alberts M. Sequence and molecular characterization of human monocyte/neutrophil elastase inhibitor. Proceedings of the National Academy of Sciences. 1992;89:5635–5639. doi: 10.1073/pnas.89.12.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitch PM, Roghanian A, Howie SE, Sallenave JM. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochemical Society Transactions. 2006;34:279–282. doi: 10.1042/BST20060279. [DOI] [PubMed] [Google Scholar]
- 29.Woods DE, Cantin A, Cooley J, Kenney DM, Remold-O’Donnell E. Aerosol treatment with MNEI suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Pediatric Pulmonology. 2005;39:141–149. doi: 10.1002/ppul.20167. [DOI] [PubMed] [Google Scholar]
- 30.Benarafa C, Priebe GP, Remold-O’Donnell E. The neutrophil serine protease inhibitorserpinB1 preserves lung defense functions in Pseudomonas aeruginosa infection. Journal of Experimental Medicine. 2007;204:1901–1909. doi: 10.1084/jem.20070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong D, Farley K, White M, Hartshorn KL, Benarfa C, Remold-O’Donnell E. Critical role of serpinB1 in regulating inflammatory responses in pulmonary influenza infection. Journal of Infectious Diseases. 2011;204:592–600. doi: 10.1093/infdis/jir352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torriglia A, Perani P, Brossas JY, Chaudun E, Treton J, Courtois Y, Counis MF. L-DNase II, a molecule that links proteases and endonucleases in apoptosis, derives from the ubiquitous serpin leukocyte elastase inhibitor. Molecular and Cellular Biology. 1998;18:3612–3619. doi: 10.1128/mcb.18.6.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leprêtre C, Fleurier Y, Martin E, Torriglia A. Nuclear export of LEI/L-DNase II by Crm1 is essential for cell survival. Biochimica et Biophysica Acta. 2008;1783:1068–1075. doi: 10.1016/j.bbamcr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Torriglia A, Leprêtre C, Padrón-Barthe L, Chahory S, Martin E. Molecular mechanisms of L-DNase II activation and function as a molecular switch in apoptosis. Biochemical Pharmacology. 2008;76:1490–1502. doi: 10.1016/j.bcp.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 35.Padrón-Barthe L, Courta J, Leprêtre C, Nagbou A, Torriglia A. Leukocyte elastase inhibitor, the precursor of L-DNase II, inhibits apoptosis by interfering with caspase-8 activation. Biochimica et Biophysica Acta. 2008;1783:1755–1766. doi: 10.1016/j.bbamcr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Leprêtre C, Sidoli G, Scovassi AI, Torriglia A. Leukocyte elastase inhibitor: a new regulator of PARP-1. Annals of the New York Academy of Sciences. 2009;1171:25–31. doi: 10.1111/j.1749-6632.2009.04701.x. [DOI] [PubMed] [Google Scholar]
- 37.Benarafa C, Cooley J, Zeng W, Bird PI, Remold-O’Donnell E. Characterization of four murine homologs of the human ov-serpin monocyte neutrophil elastase inhibitor MNEI (SERPINB1) Journal of Biological Chemistry. 2002;277:42028–44233. doi: 10.1074/jbc.M207080200. [DOI] [PubMed] [Google Scholar]
- 38.Protiva P, Cross HS, Hopkins ME, Kallay E, Bises G, Dreyhaupt E, Augenlicht L, Lipkin M, Lesser M, Livote E, Holt PR. Chemoprevention of colorectal neoplasia by estrogen: potential role of vitamin D activity. Cancer Prevention Research. 2009;2(1):43–51. doi: 10.1158/1940-6207.CAPR-08-0103. [DOI] [PubMed] [Google Scholar]
- 39.Kovalenko PL, Zhang Z, Cui M, Clinton SK, Fleet JC. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T-T, Travera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Molecular Endocrinology. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


