The emergence of higher cognitive functions stems from the modular architecture of cerebral cortex. Opris and Casanova review evidence from anatomical, electrophysiological and pathological perspectives on the role of cortical minicolumns in normal and disrupted cognitive processing. Inter-laminar microcircuits are required for the processing of executive control signals.
Keywords: prefrontal cortex, interlaminar microcircuit, minicolumn, executive function, autism
Abstract
The prefrontal cortex of the primate brain has a modular architecture based on the aggregation of neurons in minicolumnar arrangements having afferent and efferent connections distributed across many brain regions to represent, select and/or maintain behavioural goals and executive commands. Prefrontal cortical microcircuits are assumed to play a key role in the perception to action cycle that integrates relevant information about environment, and then selects and enacts behavioural responses. Thus, neurons within the interlaminar microcircuits participate in various functional states requiring the integration of signals across cortical layers and the selection of executive variables. Recent research suggests that executive abilities emerge from cortico-cortical interactions between interlaminar prefrontal cortical microcircuits, whereas their disruption is involved in a broad spectrum of neurologic and psychiatric disorders such as autism, schizophrenia, Alzheimer’s and drug addiction. The focus of this review is on the structural, functional and pathological approaches involving cortical minicolumns. Based on recent technological progress it has been demonstrated that microstimulation of infragranular cortical layers with patterns of microcurrents derived from supragranular layers led to an increase in cognitive performance. This suggests that interlaminar prefrontal cortical microcircuits are playing a causal role in improving cognitive performance. An important reason for the new interest in cortical modularity comes from both the impressive progress in understanding anatomical, physiological and pathological facets of cortical microcircuits and the promise of neural prosthetics for patients with neurological and psychiatric disorders.
Introduction
The rich repertoire of cognitive, emotional and sensorimotor behaviours, mastered by humans and animals, is a surprising and still poorly understood outcome of development and learning (Mountcastle et al., 1955; Mountcastle, 1978, 1997; Kaas, 2012). A key anatomical structure, the prefrontal cortex, at the top of executive hierarchy in the primate brain, uses a modular architecture based on minicolumnar aggregates of neurons with afferent and efferent connections distributed across many brain regions, to represent, select, maintain and coordinate thought and behavioural goals (Goldman-Rakic, 1996; Fuster, 2009; Opris et al., 2012a,b). Recent research suggests that executive abilities emerge from cortico-cortical interactions between interlaminar prefrontal microcircuits, the posterior parietal cortex, and cortico-striatal-thalamo-cortical circuits (Opris et al., 2013). These prefrontal microcircuits also play a key role in the perception-to-action cycle that integrates relevant information about the environment, and then selects and enacts behavioural responses. This review focuses on how cortical modularity, as a key organizational principle, embraces both anatomy and physiology in coordinating executive facets of behaviour. Here, we look specifically at evidence from the structural, functional, and pathological perspectives investigating intact as well as disrupted cortical minicolumns.
Anatomical and functional features of cortical modularity
Cortical minicolumns, columns and hypercolumns
Vernon Mountcastle described for the first time the electrophysiological basis of the cortical minicolumn and suggested it as an elemental unit of information processing (Mountcastle, 1957, 1955, 1997). According to this model of cortical organization, neurons and their connections form part of a vertical system that unites the cells of each minicolumn (Fig. 1A and Box 1) into a coordinated functional unit (Mountcastle, 1978, 1997). In this context, the smallest unit of cortical organization is the minicolumn, usually defined in Nissl stained sections by a narrow radial array of pyramidal neurons transversing laminae II–VI (Rakic, 1988; Mountcastle, 1997).
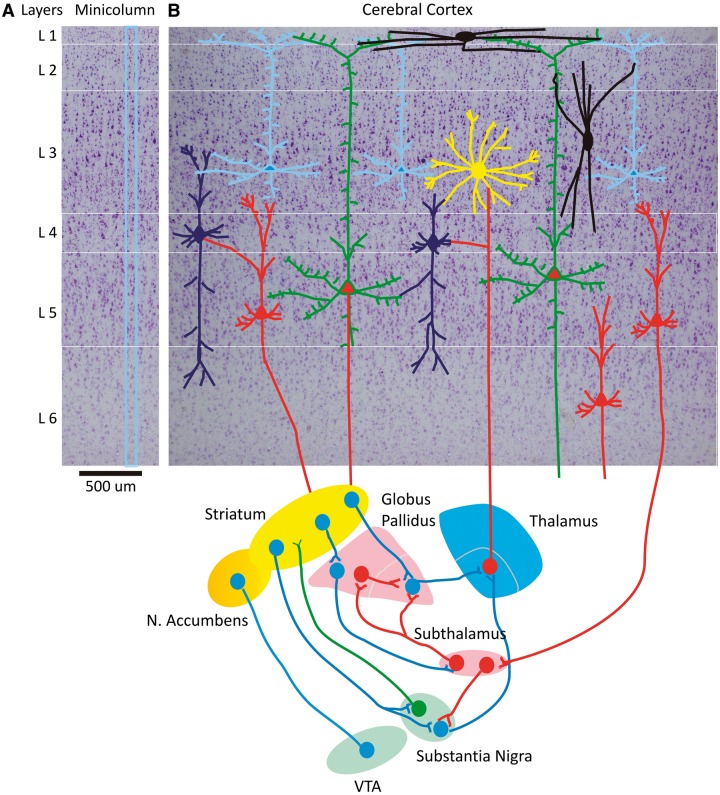
Figure 1.
Prefrontal cortical minicolumn with cortico-striatal-thalamo-cortical circuits. (A) A prefrontal cortical minicolumn in human brain. (B) Laminar and columnar display of prefrontal cortex and its major cell types and connections to basal ganglia and thalamus. The six-layered cerebral cortex with pyramidal neurons and cortical interneurons is presented at the top with a Nissl background. These neurons are connected to the thalamus and the basal ganglia as shown at the bottom of the figure. VTA = ventral tegmental area; N. Accumbens = nucleus accumbens.
Box 1 What is a cortical minicolumn?
The cortical minicolumn, is a radially oriented network of neurons and fibres which constitutes the smallest module capable of information processing (Mountcastle, 1957, 1998; Rakic, 2008). The human neocortex is composed of a large number of minicolumns in parallel vertical arrays (Casanova et al., 2007). Minicolumns are the first step in a nested series of nodes or echelons of increasing complexity. Other levels of modular organization include multiple minicolumns, macrocolumns, and large-scale networks of macrocolumns that are interconnected with the entire brain (Buxhoeveden and Casanova, 2002). The somas of pyramidal cells are not randomly distributed in space; they are organized into both layers and different-sized columns or modules. Similarly, some dendritic and axonal ramifications that begin or end in these somas are wired in parallel groups of fibres (Mountcastle, 1997, 2003). Minicolumns are often considered highly repetitive, even clone-like, units; however, they display considerable heterogeneity between areas and species, perhaps even within a given macrocolumn.
Minicolumns are arranged within larger columns or macrocolumns (e.g. barrel somatosensory cortex of the rodent) bound together by short-range horizontal connections (Jones, 2000; Zhang and Alloway, 2006; Jones and Rakic, 2010; DeFelipe et al., 2012). The different echelons are semi-independent of each other, a function of the limited number of information channels between them (Casanova, 2005). According to Buxhoeveden and Casanova (2002), and in agreement with Favorov and coworkers (Favorov et al., 1987; Favorov and Diamond, 1990) and Mountcastle (1957, 1997), the estimated width of cortical macrocolumns is 350–600 μm. Hubel and Wiesel (1974) found that optimal orientation tuning changes systematically through 180° with an electrode advance between 0.5 and 1.0 μm. The term ‘hypercolumn’ refers to a complete rotation of columns (e.g. 0°, 10°, 20°, … , 180°; Hubel and Wiesel, 1974; Wiesel and Hubel, 1974).
Recently, Opris et al. (2011, 2012a,b) suggested that interlaminar interaction in prefrontal cortical minicolumns take an active role in sensorimotor integration and the selection of behaviourally relevant targets. Rinkus (2010) proposed a computational model for visual cortex hypothesizing that: (i) a macrocolumn’s function is to store sparse distributed representations of its inputs and to recognize those inputs; and (ii) the generic function of the minicolumn is to enforce macrocolumnar code sparseness. Such distributed representations of inputs flowing from visual cortex to the higher association areas in prefrontal cortex appear to be part of distributed networks, named ‘cognits’ by Fuster and Bressler (2012). However, the specific role of prefrontal macrocolumns in prospective coding and representation storage is yet to be demonstrated (Bastos et al., 2012).
A sparse distributed representation is one where items are encoded by activation of a small set of the available representing units. Sparse encoding does not reduce to a straight majority vote scheme. Anatomically, sparse encoding may be enforced by variability between minicolumns, which themselves suggest differences in their internal architecture (Casanova, 2008; Rinkus, 2010). This variability among components of a minicolumn may contribute to the fault tolerance of larger networks such as macrocolumns. McCulloch (1959/1965) has shown that when failure of individual components occur under a certain threshold (e.g. cell loss at the beginning stages of Alzheimer’s disease) redundant networks of unstable nets could be designed for greater reliability than redundant systems of stable nets of the same size.
Cortical modules and maps
Early studies by Mountcastle (1955, 1957, 1997) and Hubel and Wiesel (Hubel and Wiesel, 1974; Wiesel and Hubel, 1974; Gilbert and Wiesel, 1989) showed that neurons with similar response properties are grouped in vertical columns, ∼0.5–1 mm in diameter, with each column oriented perpendicular to the surface of the cortex and spanning its thickness. Correspondingly, anatomical studies (using histological staining for the enzyme cytochrome oxidase) showed a modular organization in the primate visual cortex with periodically spaced patches ∼350 μm apart (Horton and Adams, 2005). In the primate prefrontal cortex the diameter of columns vary between 300 and 500 μm, but it does not differ significantly in size between brains with over three orders of magnitude difference in volume (Bugbee and Goldman-Rakic, 1983). This common periodicity means that any block of cortex, approximately the size of a single hypercolumn, contains cells tuned to all values of every receptive field variable (Swindale, 2000). Hubel (1982) and Katz et al., (1989) applied the term ‘module’ to this tissue block comprising multiple, overlapping hypercolumns. Mountcastle (1997) has used the term ‘module’ interchangeably with ‘column’. The most salient feature of cortical organization is the presence of an orderly topographic map of visual space that is remapped sequentially as information flows from visual cortex to prefrontal cortex (Salinas, 2004). Neighbouring neurons tend to have receptive fields in similar positions in visual space, and these positions change predictably as a function of position on the cortex (Swindale, 2000). The receptive fields are large enough that the fields of adjacent neurons overlap, but receptive fields of cells ∼1–2 mm apart, although near each other, will not overlap.
In addition, visual neurons vary in their preference to: i) the orientation of bar or edge stimuli, ii) the direction of motion of the oriented bar or edge, iii) stimuli delivered to one eye or the other (ocular 95 dominance), and for iv) low versus high spatial frequencies in the visual image (Swindale, 1998). All of these properties have been found to vary in an orderly way with position on the cortical surface, so that typically, a complete set of values occurs at least once every millimetre or so.
Utility of modular approach
Compartmentalizing neurons into modules can reduce the metabolic cost of wiring. This approach allows a large number of cells to be connected by fewer axons (Hofman, 2001). The resulting small-world anatomical network is then poised to participate in tasks requiring high discrimination and time-critical responses. Minicolumns are the first step in a nested series of echelons of increasing complexity (Buxhoeveden and Casanova, 2002; Casanova et al., 2007). This arrangement of interacting modules provides for a heterogeneous system that makes it difficult to judge the effects of singular pathological or physiological perturbations. No single aspect of pathology or physiology can therefore be treated individually, and changes in characteristics at lower levels may have a substantial impact at superior echelons of organization and higher levels of function.
Functional role of cortical minicolumns in executive function
Integration and selection within interlaminar microcircuits
The functional role of the cortical minicolumn is a continuing source of research and debate (Bugbee and Goldman-Rakic, 1983) more than half a century after it was identified as a component of brain organization (Mountcastle et al., 1955; Mountcastle 1957, 1997). Nevertheless, in agreement with the optimal principle of design (Leise, 1990), segregating neurons into modules can reduce the cost of wiring. This allows a large number of cells to be connected by fewer axons (Hofman, 2001; see above). The resulting short-range connections are then poised to participate in behavioural tasks requiring integration and selection of neural signals during executive control (attention, working memory and decision-making), sensory discrimination and time-critical responses.
The integrative role of cortical minicolumns (Fig. 2) as a module (Leise, 1990) stems from connecting the horizontal and vertical components of the cortex within the same columnar space. The supragranular layers L2/3, which are the major source of corticocortical projections, also receive sensory information, whereas the infragranular layer L5 is the output to subcortical structures involved in behaviour. Thus, interlaminar connections form microcircuits that bind sensory-related signals with behaviour/movement related outputs (Opris et al., 2011). This sensorimotor integration was demonstrated by Opris et al. (2011, 2013) by means of interlaminar correlated firing between supragranular layers that carry perceptual/visual spatial information and the infragranular layers that carry action related information. Such transformations of neural signals may likely reduce the output degrees of freedom within the cortical minicolumn by selecting only the relevant signals for action/behaviour. This integrative process that occurs in canonical microcircuits binds/segregates parallel streams of minicolumnar processing within the ‘executive cognit’ network (Fuster and Bressler, 2012).
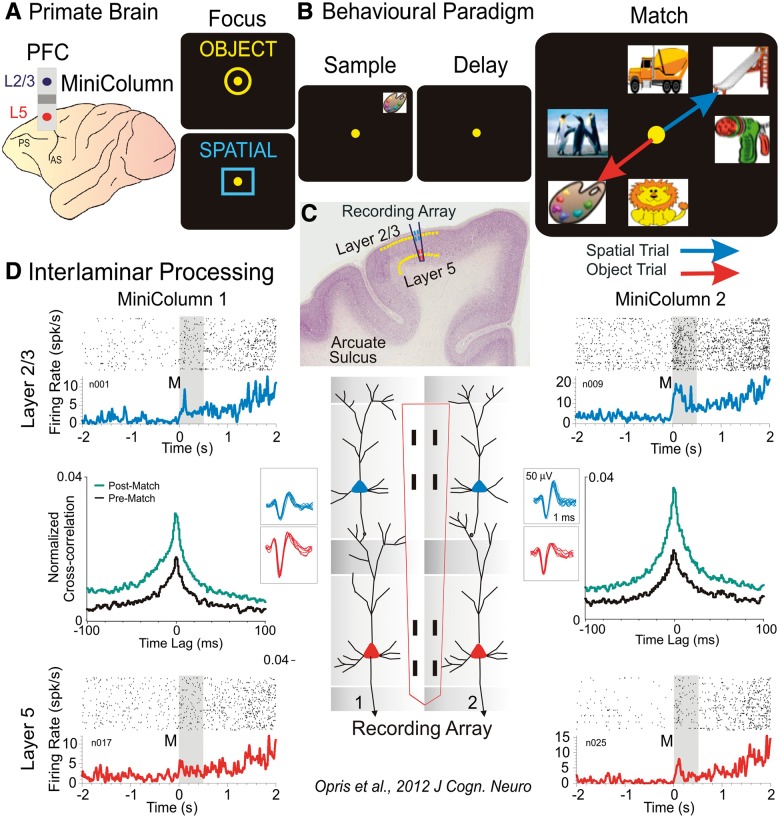
Figure 2.
Illustration of columnar laminar neural activity during a cognitive task. (A) Primate brain with prefrontal cortex (PFC) cortical minicolumn and the layers L2/3 and 5. (B) Behavioural delayed match to sample task with the sequence of events (focus, sample presentation, delay period, match array and the arrows representing target selections in object and spatial trials). (C) Interlaminar display of recording array. (D) Minicolumnar interlaminar processing of target selection during the match phase of the task. Perievent and cross-correlation (post- versus pre-match) histograms depict the functional role in integration/selection of prefrontal cortical layers and minicolumns. Adapted from Opris et al., 2012a.
The available evidence suggests that the prefrontal cortical minicolumn might be the first stage bottleneck in the cortical-striatal-palidal-thalamo-cortical loop (Alexander et al., 1986). It is obvious that cell layers 2/3 and 4 integrate a lot of inputs from virtually all of the brain, whereas the number of outputs from layer 5/6 pyramidal cells to subcortical structures participating in behaviour is much less. Also, a key role in selection is played by the GABAergic interneurons of minicolumns (Raghanti et al., 2010) that shape the tuning for preferred direction/location by means of lateral inhibition. The minicolumnar role in plasticity is associated not only with the tuning (Fig. 3) of synaptic activity states, but also with optimal selection among alternate subnetworks of microcircuits developing within a given context. Such parallel subnetworks may process complementary submodalities within a defined receptive/memory field; alternatively they may provide overlapping response characteristics to a common input. Competition among networks allows for circuit optimization (selection), in particular by means of learning.
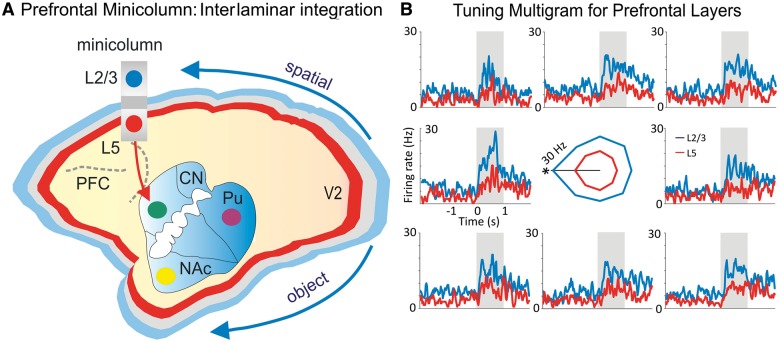
Figure 3.
Role of prefrontal cortical minicolumn in integration and selection of information. (A) The laminar structure of the neocortex is depicted in blue (layer 2/3), grey (layer 4) and red (layer 5/6). A prefrontal cortical minicolumn is depicted with the projection to striatum. The two arrows show the flow of visual spatial/object information on the dorsal and ventral streams, respectively. (B) Tuning multigram for a pair of prefrontal cortical neurons from layers 2/3 and 5. Overlay mean firing rate depict the preferred activity of layer 2/3 (blue) and layer 5 (red) for the target located on the left (180°). The tuning vectors are in the centre of the multigram. NAc = nucleus accumbens; Pu = putamen; PFC = prefrontal cortex; CN = caudate nucleus. Adapted from Opris et al., 2012a and 2013.
We hypothesize that minicolumnar diversity provides the substrate for this competition and the basis for adapting learned behaviour to context. During development, neurogenetic programs interact with epigenetic factors to regulate formation of cortical microcircuit templates (Rakic, 1988; Jones, 2000; Jones and Rakic, 2010; Kaas, 2012), which are then shaped and pruned by differential patterns of sensory activity. Thus, increased minicolumnar diversity may give rise to greater potential for combinatorial activity of microcircuits within overlapping networks, resulting in enhanced learning and behavioural flexibility (Casanova, 2008). Cortical minicolumns may therefore play a crucial role in behavioural selection (Box 2) that is in fact the substrate of executive function (e.g. attention, decision making).
Box 2 Is the cortical minicolumn a decision module?
A decision circuit is defined as a closed neural network that measures the probable value of a signal element and makes an output signal based on the value of the input signal and a predetermined criterion or threshold (Ratcliff et al., 2003). Minicolumns in the prefrontal cortex are interconnected to each other through horizontal ‘long range’ projections in layer 2/3 (Kritzer and Goldman-Rakic, 1995; Rao et al., 1999) and interlaminar mini-loops (Weiler et al., 2008; Takeuchi et al., 2011). The loop is then closed (‘reverberatory loops’) through projections to the subcortical basal ganglia nuclei and thalamus (Alexander et al., 1986; Swadlow et al., 2002). Such ‘reverberatory loops’ may be regarded as the ‘basic functional unit’ of cognitive/executive mechanism because they:
Combine incoming signals of the different input layers (Casanova et al., 2007);
Store mnemonic information through feedback connections in ‘persistent’ spiking activity (Wang, 2012); and
Compare input signals to a threshold criterion triggering an output response (selection), which constitutes the ability to make a decision (Ratcliff et al., 2003). Thus, a cortical minicolumn with integrative, selective and threshold abilities can play the role of a decision module.
Interlaminar interactions within prefrontal cortical microcircuits
The relevance of minicolumnar activity to executive function has been investigated with different approaches under several conditions (Hirata and Sawaguchi, 2008; Opris et al., 2011, 2012a,b; Hampson et al., 2012; Opris et al., 2013). Our recent results in non-human primates show for the first time interlaminar processing in the prefrontal cortex (Fig. 2A, C and D) during target selection (Opris et al., 2011, 2012a, b) and sensorimotor integration (Opris et al., 2011). An example of this interlaminar interaction during target selection (Opris et al., 2012a, b) in delay match to sample task is shown in Fig. 2 for two cell pairs with rasters and peri-event histograms bracketing the temporal interval of image presentation (match phase onset) and completion of the target selection match response (0–2 s). The cell pairs were recorded on appropriate sets of adjacent pads (minicolumns 1 and 2) in the conformal multiple electrode array shown in the illustration (Opris et al., 2011, 2012a, b) of both interlaminar cell pairs in L2/3 and L5 (Fig. 2D). Neurons in both layers showed significant increases in mean firing in supra- and infragranular layers as a function of match presentation (post-match: 0 to +2 s) and during subsequent movements associated with target selection. Demonstration of precise functional connections between individual cells within each minicolumn was provided by cross correlation histograms (Opris et al., 2011, 2012a, b); constructed for individual L2/3 and L5 cell pairs recorded on vertically positioned pads of the multiple electrode array. Normalized cross correlation histograms for both minicolumn cell pairs are shown in Fig. 2 for cell firing in the displayed perievent histograms: (i) before match phase onset (2 s to 0, pre); or (ii) after match phase onset (0 to +2 s, post) for the same cell pairs. Both cross correlation histograms show significantly correlated firing (Opris et al., 2012a).
Causal relationships to executive control in prefrontal cortex microcircuits
Interlaminar regulated microstimulation
The unique properties of biomorphic multiple electrode arrays (Moxon et al., 2004) together with previous microstimulation work (Opris et al., 2001, 2005) has provided a basis for showing functional relationships to executive function in prefrontal cortex of non-human primates. The conformal multiple electrode arrays also provide the basis for applying a system-specific model to control firing of cells through application of electrical stimulation (Hampson et al., 2012; Opris et al., 2012b) to the same loci in which columnar firing has been detected and analysed with respect to delay match to sample task performance (Hampson et al., 2012; Opris et al., 2012b). This same model was implemented to test whether it could facilitate performance on trials that show a distinctive difference in correct performance (Hampson et al., 2012) as a function of the previous instructions as to type of response to make in the match phase (i.e. object versus spatial trials). Figure 4 shows the integration of a multi-input multi-output (MIMO) non-linear mathematical model to assess the patterns of firing in L2/3 and L5 cells recorded in the columnar manner with the multiple electrode array shown with adjacent vertical pads (Opris et al., 2011, 2012a; Hampson et al., 2012). Figure 4B illustrates the input and output firing patterns recorded and analyzed by the MIMO model. This figure also shows the output pattern of L5 cell firing applied through a multichannel stimulator that is capable of delivering predetermined patterns of pulses to the same L5 pads to mimic firing on correct trials. The advantage of the MIMO model is that the online recording provides the means to detect when the inappropriate L2/3 firing pattern occurs and triggers the delivery of the appropriate L5 stimulation pattern. This provides the means to override errors and to enhance performance (Hampson et al., 2012). Stimulation consisted of 1.0 ms bipolar pulses (20–50 μA) delivered to L5 recording locations after presentation of the match phase screen and before the completion of the match response.
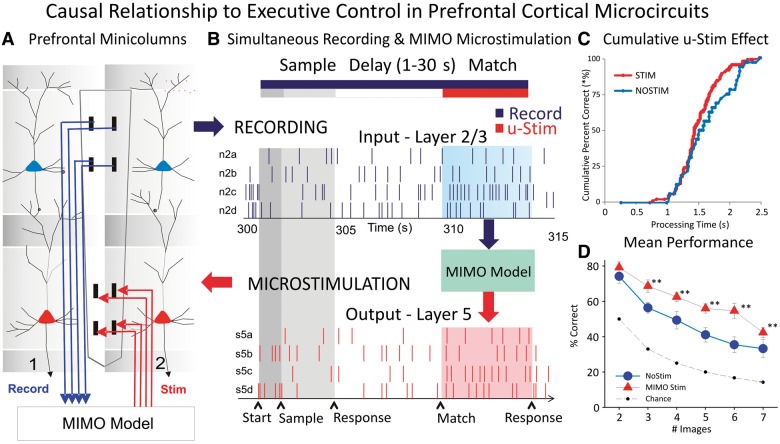
Figure 4.
Causal relationship of prefrontal cortex interlaminar microcircuit to executive control. (A) Diagram shows the interfacing of MIMO model with conformal multiple electrode arrays shown in Fig. 2 between L2/3 and L5 during task performance. Electrical stimulation delivered to multiple electrode array pads in L5 through patterns of pulses (biphasic) recorded and derived from the same L5 locations on successful trials by the MIMO model. (B) Firing of L2/3 and L5 located columnar neurons as shown in Fig. 2 recorded on line and fed to MIMO model shown in A. Shaded areas indicate time of match response execution during delay match to sample trial, and the illustrated firing in L5 which is the same pattern as the delivered stimulation on trials with inappropriate L2/3 firing. (C) Changes in cumulative performance as a function of processing time from match phase onset (‘0’) during trials with stimulation delivered in the manner shown in A and B. (D) Increase in performance across trials with increasing difficulty as a function of the number of match phase distracter images on trials that received MIMO stimulation in the manner shown in A. Asterisk indicates data points of statistical significance p<0.001, ANOVA. Adapted from Opris et al., 2012b and 2013.
The novel results of MIMO microstimulation are shown in Fig. 4C and D, in which the effects on performance are compared to trials in which stimulation was not delivered, irrespective of trial type. Figure 4C compares the change in % correct performance as a function of processing time (reaction time + movement time) on stimulation trials with respect to the no stimulation case. Figure 4D showed for the first time the increase in correct performance on trials as a function of the number of distracter images in the match phase. The results indicate that MIMO-derived stimulation induces enhanced cognitive processing (Hampson et al., 2012; Opris et al., 2012b, 2013) required to retrieve the ‘rule’ for successful selection of the appropriate item.
Dysfunction of cortical minicolumns in cognitive disease
A growing amount of evidence points to the dorsolateral prefrontal cortex as the main region for executive control (Brennan, Arnsten, 2008). Studies suggest that the monitoring mechanism of the dorsolateral prefrontal cortex (that provides a representation for action) and its direct connections to the anterior cingulate cortex (that monitors conflict/errors) and basal ganglia (that implement a corrective action) is abnormal in a significant number of psychiatric conditions. Thus, functional gradients emerging on rostral–ventral to caudal–dorsal part of the medial prefrontal cortex (Nee et al., 2011) turn into error negativity maps in autistic children (Sokhadze et al., 2012). The monitoring mechanism of errors detects the conditions under which errors are likely to occur (Carter et al., 1998), by comparing actual versus expected outcomes (Jessup et al., 2010) as distinct from actual versus intended outcomes (Gehring and Fencsig, 2001).
Disordered brain function involving disruptions of minicolumnar processing have been linked to a range of neurological/psychiatric conditions (Box 3) such as autism spectrum disorders that are characterized by the inability to consistently and accurately monitor ongoing behaviours (Mundy, 2003). Recent reports indicate that children and adult patients with autism show reduced error processing and deficient behavioural correction after an error has been committed (Thakkar et al., 2008; Vlamings et al., 2008; Sokhadze et al., 2010, 2012). There is solid evidence that dysfunctions of error/conflict monitoring are also present in depression (Georgiadi et al., 2011), anxiety (Aarts and Pourtois, 2010), attention-deficit/hyperactivity disorder (De Voorde, 2010), obsessive compulsive disorder (Endrass, 2008), addiction (Hampson et al., 2011), schizophrenia (Perez et al., 2012), and dementia (Bettcher, 2008). Several psychiatric conditions that involve aberrant aspects of social intercourse, high-level perception, and predicting consequences of actions have abnormalities in the conformation of minicolumns.
Box 3 Minicolumnar alterations in autism, schizophrenia and drug addiction.
The neuropathology of the ‘basic functional unit’ of cognitive/executive mechanism shows four different psychiatric and neurological conditions:
Decrease minicolumnar width in autism (Casanova et al., 2002b, 2006b);
Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex and planum temporale in schizophrenia (Chance et al., 2008; Di Rosa et al., 2009);
Disruption in the inhibitory architecture of the cell minicolumn in autism (Casanova et al., 2003); and
Disruption of interlaminar processing in schizophrenia and addiction (Casanova et al., 2008; Opris et al., 2012a).
Minicolumnar pathology in autism
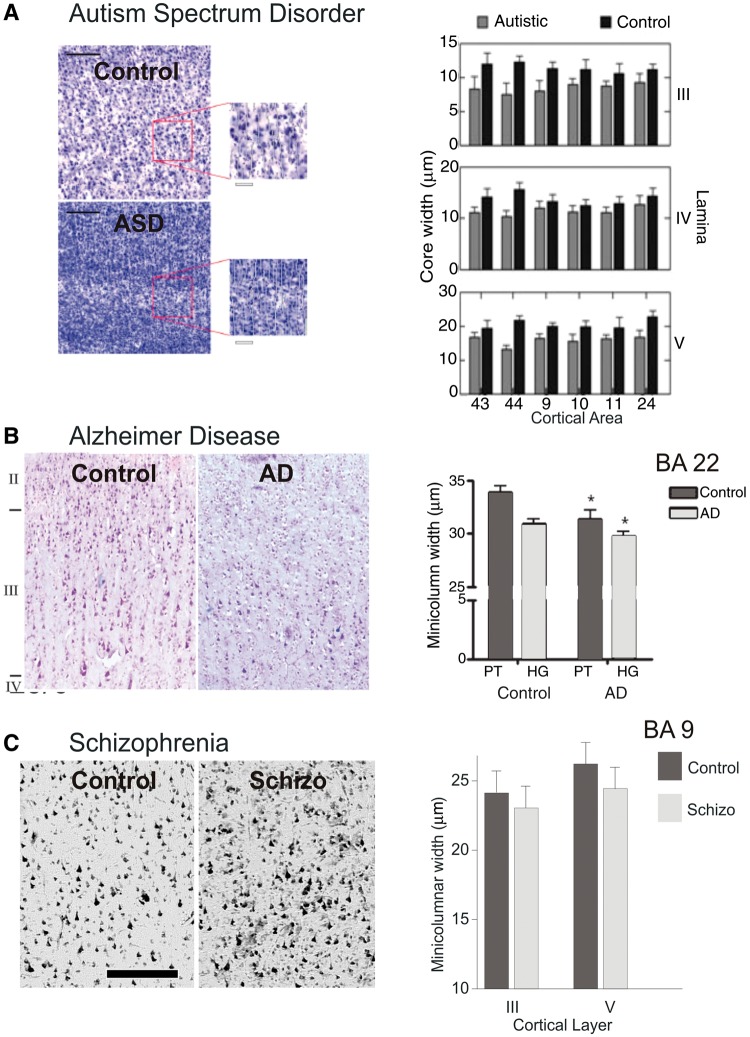
Computerized image analyses of pyramidal cell arrays have shown minicolumnar abnormalities in the brains of autistic patients (Casanova et al., 2002a, 2006a, b). These studies have shown reduced horizontal spacing in-between minicolumns, which is most salient within their peripheral neuropil space (Casanova et al., 2002a, 2003a). Evidence for this minicolumnopathy has been reproduced using the grey level index (Casanova et al., 2002b). The specificity of the findings has been investigated in independent populations, some of which exhibit an autism phenotype, e.g. Asperger syndrome, Rett syndrome, dyslexia, Down’s syndrome (Buxhoeveden et al., 2002; Casanova et al., 2002c; 2003b). These findings seem to hold when controlling for possible confounds such as mental retardation.
Using the Delaunay triangulation method to parcellate the position of pyramidal cells within the cortex reveals the presence of intra- and inter-cluster distances that correspond to the separation of neurons within and between minicolumns (Casanova et al., 2006a). The result from this study indicates that in autism the total number of neurons per minicolumn appears normal but the distance between minicolumns is reduced (Fig. 5A). The reduction, primarily within the peripheral neuropil space, seems to involve all layers of the minicolumn. The findings suggest the possibility that in autism an anatomical element in-common to all laminae is affected. Given the preponderance of inhibitory elements within the peripheral neuropil space and the increase in total number of minicolumns, it has been proposed that heterochronic divisions of periventricular germinal cells may lead to a desynchronization in the maturation of excitatory and inhibitory cells during corticogenesis (Casanova, 2012; Casanova et al., 2013).
Figure 5.
The pathology of cortical minicolumn in disease. (A) Example of prefrontal cortical minicolumn in an autistic patient compared with a normal control subject (left). Comparison of the minicolumnar width in autism versus normal control subjects for layers III, IV and V, across prefrontal Brodmann areas 9, 10, 11, 24, 43 and 44 (Casanova et al., 2002a, 2003a, 2010). (B) Example of prefrontal cortical minicolumn in a patient with Alzheimer's disease (AD) as compared to a neurotypical subject (left). Comparison of the minicolumnar width in Alzheimer's disease versus normal control subjects for planum temporale (PT) and primary auditory region of Heschl’s gyrus (HG), of prefrontal Brodmann areas 22 (Chance et al., 2011). (C) Example of prefrontal cortical minicolumn in schizophrenic patient compared to normal control (left). Comparison of the minicolumnar width in schizophrenic versus normal control subjects for layers III and V, across prefrontal Brodmann area 9 (Casanova et al., 2008). Bar length was 250 µm. Adapted from Casanova et al., 2008, 2010; and Chance et al., 2011.
Minicolumnar studies that have used neuronal morphometry for pyramidal cells have shown a diminished size for both the cell soma and nucleoli in autism (Casanova et al., 2006a). Both size of the soma and nuclei help define the total metabolic capacity of a cell. Their reduction in autism suggests a bias in connectivity that favours short connections at the expense of longer ones. In autism, the findings may help explain the significant increase in the outer radiate white matter compartment at the expense of a smaller corpus callosum (Herbert et al., 2004). Altered minicolumnar circuits may thus be related to changes in transcortical and callosal white matter pathways linking together regional networks of minicolumns. Changes to the blue print of connectivity may help explain a weak drive for central coherence and peculiarities of information processing favouring local (detail focused) over global information (Brosnan et al., 2004; Happé and Frith, 2006).
Disruption of cortical modularity in Alzheimer’s disease
Degenerative changes (e.g. senile plaques, neurofibrillary tangles) provide the diagnostic features of Alzheimer’s disease. According to disease severity, neurofibrillary tangles populate the cortex in small clusters that register across the supra- and infragranular layers (Pearson et al., 1985). The resultant disposition of neurofibrillary tangles defines a minicolumnopathy wherein both the intralaminar circuitry and corticopetal fibres are selectively impaired (Chance et al., 2006; Esiri and Chance, 2006). Computerized image analysis of minicolumnar morphometry in monkeys has shown how changes in this modular structure bear a direct correlation to declining cognitive abilities (Cruz et al., 2004). Not surprisingly, minicolumnar thinning (Fig. 5B) is a concomitant of normal human ageing and is an early change noted in mild cognitive impairment. Further, or more severe, thinning is seen in cases of Alzheimer’s disease (Chance et al., 2011). A recent study on minicolumnar morphometry has shown a direct relationship between the width of this modular structure and IQ decline. This correlation seems to hold for the dorsolateral prefrontal cortex, but not for the parahippocampal gyrus (van Veluw et al., 2012). These observations have given rise to a two-stage model for Alzheimer’s disease, whereby minicolumnar thinning in normal ageing precedes the minicolumnar degeneration that accompanies the onset of dementia (Chance, 2006). Loss of a significant number of minicolumns may disrupt information processing among distributed networks thus propitiating cognitive decline.
Minicolumnar pathology in schizophrenia
It has been postulated that the prefrontal cortices of schizophrenic patients have significant alterations in their neuropil space (Casanova et al., 2008). One study has examined this finding by analysing the distance between pyramidal cells both within and between minicolumns (Casanova, 2007; Casanova et al., 2008). No significant differences (Fig. 5C) were reported for either minicolumnar width or the vertical orientation of minicolumns. However, measures of cell density, and inter-cellular distances within and between cell minicolumns indicate alterations in the neuropil of schizophrenic patients according to both the lamina and cortical area examined. The lack of alterations in core parameters of the minicolumn alongside abnormalities of mean cell-spacing suggested to investigators a disturbance of lamination (Akil et al., 1999; Casanova et al., 2007b). In these studies lack of abnormalities for the total number of pyramidal cells per minicolumn as well the total number of minicolumns argues in favour of a defect that postdates the formation of this modular structure.
Perpendicular to the vertical axis of the minicolumn, monoaminergic projections serve to modulate information processing in a laminar and area-specific manner. Lamination arises subsequent to the formation of minicolumns during the second trimester of gestation. According to epidemiological studies the time window during which lamination is formed coincides with the action of other risk factors for schizophrenia (Akil et al., 1999). The plurality of monoaminergic neurotransmitters may help explain, in part, the multiplicity of neurochemicals involved in the mechanism of action of atypical antipsychotic agents.
In more recent studies, Chance et al., (2008) and Di Rosa et al. (2009) have added that in schizophrenia the spacing of minicolumns is altered in a sex-dependent manner as a result of the absence of age-related minicolumnar thinning. This was interpreted as a failure of adult neuroplasticity to maintain the neuropil space.
Cognitive abilities are based on the capacity to bind together multiple features of perception. Impaired cognition in schizophrenia is more incapacitating and refractory to treatment than the more salient positive symptoms. The ability to bind together different features of perception together is a distributed feature of the cortex coded by gamma frequencies. EEG studies in schizophrenia have shown reduced power and coherence at gamma frequencies (Yeragani et al., 2006). These abnormalities, usually demonstrated on working memory tasks and recordings from the dorsolateral prefrontal cortex, correlate to observed cognitive deficits in schizophrenic patients (Light et al., 2006). Monoamines serve to modulate signal processing within minicolumnar circuits. The findings are in agreement with the idea that in schizophrenia the basic modulation of minicolumns by monoaminergic systems is abnormal but the circuitry intrinsic to this module remains unaltered (Casanova, 2007).
Disruption of interlaminar processing in drug addiction
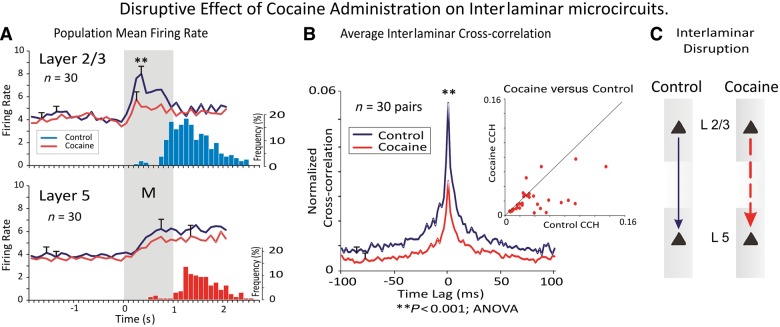
A study of antenatal exposure to cocaine in rats suggest its ability to modify the maturation of the ontogenetic minicolumn (Buxhoeveden et al., 2006). Rats antenatally exposed to cocaine were sacrificed at post-natal Day 21. The distance between both minicolumns and apical dendritic bundles were found to be narrower in all cortical areas examined.
Administration of cocaine has a dramatic effect in the interlaminar processing of cognitive information. Figure 6 shows that the loss in correlation produced by cocaine is most extreme for pairs of cells that exhibit high correlation values in the control (saline) half of the session whereas cell pairs with low normalized correlations (<0.04) were relatively unaffected by cocaine administration. These findings are in close agreement with previous studies showing marked influences of acute administered cocaine in altering task-related neural firing (Bradberry, 2000; Nestler, 2004; Beveridge et al., 2008; Opris et al., 2009; Hampson et al., 2011; Porrino et al., 2013) and support the notion that dopaminergic modulation of prefrontal cortex neuron firing may be responsible for regulating columnar processing in a manner that controls decision-making and target selection in cognitive tasks (Opris et al., 2005; Tomasi et al., 2010; Porter et al., 2011).
Figure 6.
Disruption of interlaminar interaction in cocaine addiction. (A) Population perievent histograms contrasting neural firing in prefrontal cortical layers before and after cocaine administration. (B) Interlaminar cross-correlation showing the decrease in interlaminar interaction induced by cocaine administration (Opris et al., 2012a). (C) Diagram depicting interlaminar disruption. Asterisk indicates data points of statistical significance p<0.001, ANOVA. Adapted from Opris et al., 2012a.
Summary
The cell minicolumn is a basic architectural motif of the cerebral cortex. A generic circuit within the cell minicolumn provides the elements necessary for redundancy and plasticity. Thalamic afferent terminating in its middle layers are joined by narrow connections to cells in more superficial and deeper cortical regions all of which are stimulated with only small latency differences. A large number of studies implicate minicolumnopathies in different psychiatric conditions. Recent work on prefrontal minicolumns has shown how they bind perception to executive control. Technological progress in developing multi-electrode arrays has allowed the simultaneous recording in cortical layers on adjacent minicolumns and the microstimulation of the deep cortical layers. The implementation of the MIMO model (Hampson et al., 2012; Opris et al., 2013; Opris, 2013) has allowed the extraction of the code from supragranular layers to determine the optimal pattern of spikes that will be fed into the infragranular layers. It was thus demonstrated that microstimulation of deep cortical layers led to increased cognitive performance, with the prefrontal interlaminar microcircuits playing a causal role in the improvement of cognitive performance. An important reason for the new interest in cortical modularity comes from the impressive progress in understanding anatomical, physiological and pathological facets of cortical microcircuits.
Funding
Financial support for this work was derived from NIH grant R01 HD-65279 (MFC).
Glossary
Abbreviation
- MIMO
multi-input multi-output
References
- Aarts K, Pourtois G. Anxiety not only increases, but also alters early error-monitoring functions. Cogn Affect Behav Neurosci. 2010;10:479–92. doi: 10.3758/CABN.10.4.479. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–9. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong ME, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–15. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Giovannetti T, Macmullen L, Libon DJ. Error detection and correction patterns in dementia: a breakdown of error monitoring processes and their neuropsychological correlates. J Int Neuropsychol Soc. 2008;14:199–208. doi: 10.1017/S1355617708080193. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–66. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–45. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan MJ, Scott FJ, Fox S, Pye J. Gestalt processing in autism: failure to process perceptual relationships and the implications for centextual understanding. J Child Psychol Psychiatry. 2004;45:459–69. doi: 10.1111/j.1469-7610.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Bugbee NM, Goldman-Rakic PS. Columnar organization of corticocortical projections in squirrel and rhesus monkeys: similarity of column width in species differing in cortical volume. J Comput Neurol. 1983;220:355–64. doi: 10.1002/cne.902200309. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Casanova MF. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–51. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D, Fobbs A, Roy E, Casanova MF. Quantitative comparison of radial cell columns in children with Down syndrome and controls. J Intellect Disabil Res. 2002;46:76–81. doi: 10.1046/j.1365-2788.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF. Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Dev Neurosci. 2006;24:335–41. doi: 10.1016/j.ijdevneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casanova MF. An apologia for a paradigm shift in neurosciences. In: Casanova MF, editor. Neocortical modularity and the cell minicolumn. New York: Nova Biomedical Publishers; 2005. pp. 33–55. [Google Scholar]
- Casanova MF. Schizophrenia seen as a deficit in the modulation of cortical minicolumns by monoaminergic systems. Int Rev Psychiatry. 2007;19:361–72. doi: 10.1080/09540260701486738. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The significance of minicolumnar size variability in autism: a perspective from comparative anatomy. In: Zimmerman A, editor. Autism current theories and evidence, current clinical neurology, chapter 16. New York: Humana Press; 2008. pp. 349–60. [Google Scholar]
- Casanova MF. The minicolunopathy of autism. In: Joseph D Buxbaum, Patrick R Hof., editors. The neuroscience of autism spectrum disorder. Amsterdam: Academics Pres; 2012. pp. 327–34. [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002a;58:428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Neuronal density and architecture (gray level index) in the brains of autistic patients. J Child Neurol. 2002b;17:515–21. doi: 10.1177/088307380201700708. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Cohen M, Switala AE. Minicolumnar pathology in dislexia. Ann Neurol. 2002c;52:108–10. doi: 10.1002/ana.10226. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2003a;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Rett síndrome as a minicolumnopathy. Clin Neuropathol. 2003b;22:163–8. [PubMed] [Google Scholar]
- Casanova MF, van Kooten I, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006a;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Van Kooten I, Switala AE, Van Engeland H, Heinsen H, Steinbusch HW, et al. Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clin Neurosci Res. 2006b;6:127–33. [Google Scholar]
- Casanova MF, Trippe JT, II, Switala AE. A temporal continuity to the vertical organization of the human neocortex. Cereb Cortex. 2007;17:130–37. doi: 10.1093/cercor/bhj134. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Kreczmanski P, Trippe J, II, Switala A, Heinsen H, Steinbusch HW, et al. Neuronal distribution in the neocortex of schizophrenic patients. Psychiatry Res. 2008;158:267–77. doi: 10.1016/j.psychres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Ayman El-Baz A, Vanbogaert E, Narahari P, Switala A. A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol. 2010;20:451–8. doi: 10.1111/j.1750-3639.2009.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz AS, Switala AE. Laws of conservation as related to brain growth, aging, and evolution: symmetry of the minicolumn. Front Neuroanat. 2011;5:66. doi: 10.3389/fnana.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Baruth JM, El-Baz AS, Tasman A, Sears L, Sokhadze EM. Repetitive TMS (rTMS) modulates ERP indices of attention in autism. Trans Neurosci. 2012;3:170–80. doi: 10.2478/s13380-012-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz AS, Kamat SS, Dombroski BA, Khalifa F, Elnakib A, et al. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol Commun. 2013;1:67. doi: 10.1186/2051-5960-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA. Subtle changes in the aging human brain. Nutr Health. 2006;18:217–24. doi: 10.1177/026010600601800303. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ, Esiri MM. Minicolumn thinning in temporal lobe association cortex but not primary auditory cortex in normal human ageing. Acta Neuropathol. 2006;111:459–64. doi: 10.1007/s00401-005-0014-z. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain. 2008;131:3178–92. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Clover L, Cousijn H, Currah L, Pettingill R, Esiri MM. Microanatomical correlates of cognitive ability and decline: normal ageing, MCI, and Alzheimer's disease. Cereb Cortex. 2011;21:1870–8. doi: 10.1093/cercor/bhq264. [DOI] [PubMed] [Google Scholar]
- Cruz L, Roe DL, Urbaric B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci USA. 2004;101:15846–51. doi: 10.1073/pnas.0407002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Markram H, Rockland KS. The neocortical column. Front Neuroanat. 2012;6:22. doi: 10.3389/fnana.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa E, Crow TJ, Walker MA, Black G, Chance SA. Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res. 2009;166:102–15. doi: 10.1016/j.psychres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychol. 2008;46:1877–87. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Chance SA. Vulnerability to Alzheimer's pathology in neocortex: the roles of plasticity and columnar organization. J Alzheimers Dis. 2006;9(Suppl 3):79–89. doi: 10.3233/jad-2006-9s310. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Diamond ME. Demonstration of discrete place-defined columns-segregates-in the cat SI. J Comput Neurol. 1990;298:97–112. doi: 10.1002/cne.902980108. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Diamond ME, Whitsel BL. Evidence for a mosaic representation of the body surface in area 3b of the somatic cortex of cat. Proc Natl Acad Sci USA. 1987;84:6606–10. doi: 10.1073/pnas.84.18.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–72. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bressler SL. Cognit activation: a mechanism enabling temporal integration in working memory. Trends Cogn Sci. 2012;16:207–18. doi: 10.1016/j.tics.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Fencsig DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–7. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadi E, Liotti M, Nixon NL, Liddle PF. Electrophysiological evidence for abnormal error monitoring in recurrent major depressive disorder. Psychophysiol. 2011;48:1192–202. doi: 10.1111/j.1469-8986.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989;9:2432–42. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil. Trans. R. Soc. Lond. 1996;351:1445–53. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Gerhardt GA, Marmarelis V, Song D, Opris I, Santos L, et al. Facilitation and restoration of cognitive function in primate prefrontal cortex by a neuroprosthesis that utilizes minicolumn-specific neural firing. J Neural Eng. 2012;9:056012. doi: 10.1088/1741-2560/9/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Porrino LJ, Opris I, Stanford TR, Deadwyler SA. Effects of cocaine rewards on neural representations of cognitive demand in nonhuman primates. Psychopharmacol. 2011;213:105–18. doi: 10.1007/s00213-010-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detailed-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Sawaguchi T. Functional columns in the primate prefrontal cortex revealed by optical imaging in vitro. Neurosci Res. 2008;61:1–10. doi: 10.1016/j.neures.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hofman MA. Brain evolution in hominids: are we at the end of the road? In: Falk D, Gibson KR, editors. Evolutionary anatomy of the primate cerebral cortex. Cambridge: Cambridge University Press; 2001. pp. 113–27. [Google Scholar]
- Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360:837–62. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH. Cortical neurobiology: a slanted historical perspective. Annu Rev Neurosci. 1982;5:363–70. doi: 10.1146/annurev.ne.05.030182.002051. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974;158:267–93. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. J Neurosci. 2010;30:3467–72. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Microcolumns in the cerebral cortex. Proc Natl Acad Sci USA. 2000;97:5019–21. doi: 10.1073/pnas.97.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Rakic P. Radial columns in cortical architecture: it is the composition that counts. Cereb Cortex. 2010;20:2261–4. doi: 10.1093/cercor/bhq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Evolution of columns, modules, and domains in the neocortex of primates. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10655–60. doi: 10.1073/pnas.1201892109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Gilbert CD, Wiesel TN. Local circuits and ocular dominance columns in monkey striate cortex. J Neurosci. 1989;9:1389–99. doi: 10.1523/JNEUROSCI.09-04-01389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comput Neurol. 1995;359:131–43. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Leise EM. Modular construction of nervous systems: a basic principle of design for invertebrates and vertebrates. Brain Res Brain Res Rev. 1990;15:1–23. doi: 10.1016/0165-0173(90)90009-d. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenic patients. Biol Psychiatry. 2006;60:1231–40. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- McCulloch WS. Agatha Tyche of nervous nets–the lucky reckoners. In: Warren S, McCulloch, editors. Embodiments of mind. Cambridge: MIT Press; 1965. pp. 203–15. Reprint of National Physical Laboratory, Mechanisation of thought processes; London, H. M. Stationery Office; 1959, pp. 611–25. [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–34. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. An organizing principle for cerebral function: the unit module and the distributed system. In: Edelman GM, Mountcastle VB, editors. The mindful brain. Massachusetts: MIT Press; 1978. pp. 7–50. [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–22. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Perceptual neuroscience: the cerebral cortex. Cambridge: Harvard University Press; 1998. [Google Scholar]
- Mountcastle VB. Introduction. Cereb Cortex. 2003;13:2–4. doi: 10.1093/cercor/13.1.2. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Berman A, Davies P. Topographic organization and modality representation in the first somatic area of cat's cerebral cortex by method of single unit analysis. Am J Physiol. 1955;183:464. [Google Scholar]
- Mountcastle VB, Berman AL, Davies P. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957;20:374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Leiser SC, Gerhardt GA, Barbee KA, Chapin JK. Ceramic-based multisite electrode arrays for chronic single-neuron recording. IEEE Trans Biomed Eng. 2004;51:647–56. doi: 10.1109/TBME.2003.821037. [DOI] [PubMed] [Google Scholar]
- Mundy P. The neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 2011;54:528–40. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–8. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Opris I. Inter-laminar microcircuits across the neocortex: repair and augmentation. Front Syst Neurosci. 2013;7:80. doi: 10.3389/fnsys.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Barborica A, Ferrera VP. A gap effect during microstimulation in the prefrontal cortex of monkey. Exp. Brain Res. 2001;138:1–7. doi: 10.1007/s002210100686. [DOI] [PubMed] [Google Scholar]
- Opris I, Barborica A, Ferrera VP. Microstimulation of dorsolateral prefrontal cortex biases saccade target selection. J Cogn Neurosci. 2005;17:893–904. doi: 10.1162/0898929054021120. [DOI] [PubMed] [Google Scholar]
- Opris I, Fuqua JL, Huettl PF, Gerhardt GA, Berger TW, Hampson RE, et al. Closing the loop in primate prefrontal cortex: inter-laminar processing. Front Neural Circuits. 2012b;6:88. doi: 10.3389/fncir.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations. Neuroscience. 2009;163:40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Gerhardt GA, Berger TW, Deadwyler SA. Columnar processing in primate pFC: evidence for executive control microcircuits. J Cogn Neurosci. 2012a;24:2334–47. doi: 10.1162/jocn_a_00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Hampson RE, Stanford TR, Gerhardt GA, Deadwyler SA. Neural activity in frontal cortical cell layers: evidence for columnar sensorimotor processing. J Cogn Neurosci. 2011;23:1507–21. doi: 10.1162/jocn.2010.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Santos LM, Song D, Gerhardt GA, Berger TW, Hampson RE, et al. Prefrontal cortical microcircuits bind perception to executive control. Sci Rep. 2013;3:2285. doi: 10.1038/srep02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4531–4. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, et al. Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2012;121:372–87. doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Hampson RE, Opris I, Deadwyler SA. Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacol. 2013;225:105–14. doi: 10.1007/s00213-012-2798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–34. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Spocter MA, Butti C, Hof PR, Sherwood CC. A Comparative perspective on minicolumns and inhibitory GABAergic interneurons in the neocortex. Front Neuroanat. 2010;4:3. doi: 10.3389/neuro.05.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Confusing cortical columns. Proc Natl Acad Sci USA. 2008;105:12099–100. doi: 10.1073/pnas.0807271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–16. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol. 2003;90:1392–407. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- Rinkus GJ. A cortical sparse distributed coding model linking mini- and macrocolumn-scale functionality. Front Neuroanat. 2010;4:17. doi: 10.3389/fnana.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E. Fast remapping of sensory stimuli onto motor actions on the basis of contextual modulation. J Neurosci. 2004;24:1113–8. doi: 10.1523/JNEUROSCI.4569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, et al. Impaired error monitoring and correction function in autism. J Neurother. 2010;14:79–95. doi: 10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Casanova MF. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback. 2012;37:91–102. doi: 10.1007/s10484-012-9182-5. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci. 2002;22:7766–7773. doi: 10.1523/JNEUROSCI.22-17-07766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindale NV. Cortical organization: modules, polymaps and mosaics. Curr Biol. 1998;8:R270–3. doi: 10.1016/s0960-9822(98)70170-8. [DOI] [PubMed] [Google Scholar]
- Swindale NV, Shoham D, Grinvald A, Bonhoeffer T, Hübener M. Visual cortex maps are optimized for uniform coverage. Nat Neurosci. 2000;3:822–6. doi: 10.1038/77731. [DOI] [PubMed] [Google Scholar]
- Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science. 2011;331:1443–7. doi: 10.1126/science.1199967. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Robert M, Joseph RM, David S, Tuch DS, Hadjikhani N, Barton JJS, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–78. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Voorde S, Roeyers H, Wiersema JR. Error monitoring in children with ADHD or reading disorder: an event-related potential study. Biol Psychol. 2010;84:176–85. doi: 10.1016/j.biopsycho.2010.01.011. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Sawyer EK, Clover L, Cousijn H, De Jager C, Esiri MM, et al. Prefrontal cortex cytoarchitecture in normal aging and Alzheimer’s disease: a relationship with IQ. Brain Struct Funct. 2012;217:797–808. doi: 10.1007/s00429-012-0381-x. [DOI] [PubMed] [Google Scholar]
- Vlamings P H, Jonkman L M, Hoeksma M R, van Engeland H, Kemner C. Reduced error monitoring in children with autism spectrum disorder: an ERP study. Euro J Neurosci. 2008;28:399–406. doi: 10.1111/j.1460-9568.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neural dynamics and circuit mechanisms of decision-making. Curr Opin Neurobiol. 2012;22:1039–46. doi: 10.1016/j.conb.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci. 2008;11:360–6. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974;158:307–18. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Cashmere D, Miewald J, Tancer M, keshavan MS. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: a preliminary report. Psychiatry Res. 2006;141:53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang M, Alloway KD. Intercolumnar synchronization of neuronal activity in rat barrel cortex during patterned airjet stimulation: a laminar analysis. Exp Brain Res. 2006;169:311–25. doi: 10.1007/s00221-005-0152-5. [DOI] [PubMed] [Google Scholar]