Abstract
Lipoprotein lipase (LPL) is rate limiting in the provision of triglyceride-rich lipoprotein-derived lipids into tissues. LPL is also present in the brain, where its function has remained elusive. Recent evidence implicates a role of LPL in the brain in two processes: (a) the regulation of energy balance and body weight and (b) cognition. Mice with neuron-specific deletion of LPL have increases in food intake that lead to obesity, and then reductions in energy expenditure that further contribute to and sustain the phenotype. In other mice with LPL deficiency rescued from neonatal lethality by somatic gene transfer wherein LPL in the brain remains absent, altered cognition ensues. Taking into consideration data that associate LPL mutations with Alzheimer’s disease, a role for LPL in learning and memory seems likely. Overall, the time is ripe for new insights into how LPL-mediated lipoprotein metabolism in the brain impacts CNS processes and systems biology.
Keywords: triglycerides, fatty acids, metabolism, energy balance, cognition
INTRODUCTION
Lipoprotein lipase (LPL) is a multifunctional enzyme that plays a major role in the metabolism and transport of lipids in peripheral tissues including heart, skeletal muscle, and white and brown adipose tissue (22, 88). Following synthesis in parenchymal cells, LPL is transported to the endothelium, where it binds to the glycocalyx. At this site the lipase is the rate-limiting enzyme for the hydrolysis of the triglyceride (TG) core of the circulating TG-rich lipoproteins, chylomicrons and very-low-density lipoproteins (VLDLs), to produce chylomicron remnants and intermediate-density lipoproteins, respectively. The fatty acids and monoacylglycerol released from the hydrolysis are in part taken up by the tissues locally, where they are processed in a tissue-specific manner, e.g., stored as neutral lipids in adipose tissue, oxidized or stored in skeletal and cardiac muscle, or as cholesteryl ester and TG in macrophages (46). Exemplary of the tissue-specific effects of LPL are a series of comparative mouse model studies in which skeletal muscle–specific transgenic mice accumulate TG in skeletal muscle, are insulin resistant and relatively lean (18, 27, 30, 35), and skeletal muscle–specific LPL knockout mice have reductions in plasma TG and increases in insulin-mediated glucose uptake in skeletal muscle and subsequently develop obesity (89).
Besides its hydrolytic activity, other functions of LPL include (a) promoting the exchange of lipids between high-density lipoprotein (HDL) subclasses and thus playing a role in the maturation of plasma HDL particles (39, 79); (b) interacting with various lipoproteins such as HDL, low-density lipoprotein (LDL), and VLDL to anchor them to the vessel wall and facilitate lipoprotein particle uptake (1, 15, 16, 50, 61, 75); (c) acting as a ligand for lipoprotein receptors to facilitate lipoprotein uptake (32, 45, 51, 59, 79, 81); and (d) mediating the selective uptake of lipoprotein-associated lipids and lipophilic vitamins without the concomitant uptake of the lipoprotein particles (2, 23, 47, 48, 71, 72, 74). Finally, in some tissues (e.g., liver, muscle, and macrophages) both the active and inactive lipase protein have been shown to enhance the binding, uptake, and degradation of lipoproteins by mechanisms that are both lipoprotein receptor dependent and independent (24, 41, 80). These distinct physiological activities of LPL regulate the partitioning of lipoprotein-derived fatty acids to various peripheral tissues either for storage or oxidation.
LPL is also present throughout the nervous system, including the brain, spinal cord, and peripheral nerve (4, 6, 10, 12, 14, 20, 23, 40, 57, 63, 78, 82, 85, 94). Compared to the numerous in vivo studies in humans, in various tissue culture systems, and in many animal models focusing on the tissue-specific functions of LPL in peripheral tissues (reviewed in 88), relatively limited studies have been published on the function of LPL in the brain and central nervous system (CNS). In this review we focus on LPL gene expression in the brain, with a particular emphasis on recent literature that suggests important functions of LPL in several regulatory processes.
LPL MRNA, PROTEIN, AND ENZYME ACTIVITY IN BRAIN AND CNS
In the brain, LPL mRNA is found predominantly in neurons in the dentate gyrus as well as the CA1-CA4 layers in the hippocampus, pyramidal cells in the striatum and in several cortical areas, the Purkinje cells in the cerebellum, and large motor neurons in the brain stem (Table 1) (4, 6, 22, 63, 85, 94). In the hippocampus, LPL mRNA levels are the highest, at least 2.5-fold higher than in other brain regions (4, 57, 87).
Table 1.
Lipoprotein lipase protein and mRNA in different brain regions
| Brain regions | Protein | Brain regions | mRNA |
|---|---|---|---|
| Telencephalon | Forebrain | ||
| Olfactory bulb | − | Olfactory cortex | |
| Olfactory tubercle (Tu), insulae callfejae | ++ | ||
| Anterior olfactory nucleus, medial part (AOM) | + | ||
| Neocortex layer 1 | − | Neocortex | |
| Neocortex layer II–VI | ++ | Primary motor cortex (M1) | ++ |
| Corpus callosum | + | Secondary motor cortex (M2) | ++ |
| Hippocampal formation | Primary somatosensory cortex, all regions | ++ | |
| Area dentata | Secondary somatosensory cortex (S2) | ++ | |
| Molecular layer | − | Cingulate cortex (CG1, CG2) | ++ |
| Granular layer | ++ | Granular insular cortex (GI) | ++ |
| Outer hilar plexiform layer | − | Dysgranular insular cortex (DI) | ++ |
| Outer hilar cell layer | ++ | Agranular insular cortex (AID, AIV, AIP) | ++ |
| Regio inferior | Ectorhinal cortex (Ect) | ++ | |
| Lacunosum-moleculare | − | Perirhinal cortex (PRh) | ++ |
| Radiatum | ++ | Piriform cortex (Pir), lamina pyramidalis area pyriformis | ++ |
| Pyramidal | + | Secondary auditory cortex, ventral area (AUV) | ++ |
| Oriens | + | Dorsal tenia tecta (DTT) | ++ |
| Alveus | + | Dorsal peduncular cortex (DP) | ++ |
| Regio superior | Infralimbic cortex (IL) | ++ | |
| Lacunosum-moleculare | ++ | Prelimbic cortex (PrL) | ++ |
| Radiatum | − | Primary visual cortex (V1) | ++ |
| Pyramidal | +++ | Secondary visual cortex (V2L, V2ML, V2MM) | ++ |
| Oriens | − | Primary auditory cortex (Au1) | ++ |
| Alveus | +++ | Secondary auditory cortex (AuD, AuV) | ++ |
| Subiculum | ++ | Temporal association cortex (TeA) | ++ |
| Striatum | Lateral entorhinal cortex (Lent) | ++ | |
| Cadautus putamen | + | Dentral endopiriform nucleus (DEn) | − |
| Globus pallidus | − | Retrosplenial agranular cortex (RSA, RSG) | − |
| Accumbens | − | Primary somatosensory cortex, trunk region (S1Tr) | − |
| Amygdale | + | Lateral and medial parietal association cortex (LPtA, MPtA) | − |
| Septum | − | Basal ganglia | |
| Fimbria | + | Caudate putamen (Cpu) | ++ |
| Diencephalon | Accumbens nucleus, core (AcbC) | ++ | |
| Thalamus | + | Accumbens nucleus, shell (AcbSh) | ++ |
| Hypothalamus | − | Lateral accumbens shell (LacbSh) | ++ |
| Cerebral peduncles | ++ | Interstitial nucleus of the posterior limb of the anterior cocommissure (IPAC) | ++ |
| Optic chiasm | + | Amygdalostriatal transition area (Astr) | ++ |
| Mesencephalon and rhombencephalon | Amygdala, all areas | ++ | |
| Substantia nigra | ++ | Hippocampus | |
| Inferior colliculi | ++ | Polymorph layer of the dentate gyrus (PoDG) | + |
| Cerebellum | Granular layer of the dentate gyrus (GrDG) | + | |
| Molecular layer | + | Pyramidal cell layer (CA1, CA2, CA3) | +++ |
| Purkinje layer | +++ | Fasciola cinereum (EC) | ++ |
| Granular layer | ++ | Thalamus | |
| White matter | − | Reuniens thalamic nucleus (Re) | + |
| Olivocerebellar tract | − | Ventral reuniens thalamic nucleus (VRe) | + |
| Medulla oblongata | +++ | Central medial thalamic nucleus (CM) | + |
| Midbrain | |||
| Pontine nuclei (Pn) | ++ | ||
| Paranigral nucleus (PN) | + | ||
| Interfascicular nucleus (IF) | + | ||
| Hindbrain (Olive) | ++ | ||
| Ventricular system (ependymal cells) | − |
In experiments using 35S methionine, LPL protein is synthesized in the brain (4). By immunohistochemistry, LPL protein is located in the same neurons where LPL mRNA is expressed, but with a strikingly uneven pattern of distribution, i.e., high levels in neuronal cell bodies and sometimes terminal fibers, but very low levels in adjacent areas known to be rich in glial cells (Table 1) (6, 10, 78, 85). Importantly, LPL protein is also associated with vascular endothelial surfaces in brain (85). This discrepancy implicates the possibility of binding of circulating LPL protein (active and/or inactive) to the endothelium (86).
LPL activity has been detected in multiple brain regions in adult rats and mice, with the highest activity detected in the hippocampus (4, 14, 87). In cultured fetal rat hypothalamic cells, de novo synthesis of LPL is maintained and the activity is serum dependent (14). When rats were fasted for 12 hours, LPL activity was modified only in the hippocampus, with a 60% increase in comparison with a fed control group (4). However, prolonged fasting decreased LPL activity in various brain regions (14, 20), with the largest changes occurring in the hypothalamus (14). Refeeding reversed these fasting-related changes in brain LPL activity (20). Rats with streptozotocin-induced diabetes (100 mg streptozotocin per kg body weight intraperitoneally for ten days) showed decreases in cortical LPL activity; hyperthyroidism also appears to reduce brain LPL activity (20). Consistent with the localization of LPL protein to vascular endothelial surfaces throughout the brain, LPL activity is also detected in brain microvessel preparations, and the activity is heparin releasable and dependent on apolipoprotein C-II for activation (10, 78).
The developmental regulation of LPL mRNA and protein and activity levels has been studied in rats. In one study, LPL mRNA levels were higher in fetal brains and then fell 60% during the neonatal period, whereas levels of LPL protein peaked at day 7 after birth before falling 95% by weaning (82). In another study, LPL activity was found to be relatively high in the newborn brain and peaked between day 5 and ~10 after birth, reaching levels five times higher than in the adult brain. LPL also increased sharply during postnatal development in all areas of the brain studied (olfactory bulb, cortex, thalamus, cerebellum, hippocampus, striatum, brain stem, and spinal cord) (57).
PROPOSED FUNCTIONS OF LPL IN THE BRAIN AND CNS
Because LPL is the rate-limiting enzyme for the hydrolysis of the TG core of the circulating TG-rich lipoproteins in peripheral tissues, it is appropriate to ask whether LPL has a similar function in the brain and CNS. Indeed, LPL can mediate the uptake of TGs and their subsequent incorporation into cellular lipids in cultured brain cells (14). Feeding studies with C14-labelled oleic acid have also shown the uptake of TGs in the brain (6). In addition, a recent study using a tail vein injection of radiolabelled triolein-containing chylomicrons also showed an LPL-dependent uptake of chylomicron-derived TGs in the hypothalamus and other brain regions (87).
In neurons, a number of other functions of LPL have been suggested. In cultured neuroblastoma cells, enzymatically active LPL is required to promote VLDL-stimulated differentiation and neurite extension (65), and LPL prevents neurotoxicity when cells are exposed to native or oxidized lipoproteins (64). These in vitro data suggest that LPL can possibly play a role in the differentiation of neurons in vivo and in the response to oxidative stress in several neurodegenerative disorders. Supportive in vivo evidence of the protective role of LPL in the brain comes from studies in which mice are subjected to deafferentation using an entorhinal cortex lesion. The marked induction of LPL mRNA and protein levels in the hippocampus two days postlesion indicates a potential role of LPL in the recycling and/or scavenging of lipids and cholesterol released from the degenerating nerve terminals (8). These observations are particularly relevant in that the dentate gyrus, one of the regions of the hippocampus in which LPL mRNA is abundantly localized, is one of the few regions of the adult brain that undergoes neurogenesis (95).
LPL mRNA levels are also dramatically increased 12 hours after occlusion of the middle cerebral artery, and the increase is sustained at 72 hours after focal cerebral ischemia (63). In a separate study using both immunohistochemical staining and enzyme activity determinations, LPL levels were first found to be decreased in the ischemic cortex at 2 hours, but then increased at 4 to ~6 hours after ischemia (90). These results suggest that LPL may play a role in the pathophysiological response to cerebral ischemia reperfusion.
LPL also appears to regulate vitamin E levels in the brain, a process that may help the survival, plasticity, and regeneration of neuronal processes. In a study using a porcine in vitro blood-brain barrier model, LPL facilitated the uptake and transcytosis of LDL and LDL-associated α-tocopherol (23). In a rat model of epilepsy, LPL expression in the hippocampus area was increased with reduced levels of vitamin E (77). In a mouse model of LPL deficiency that was rescued from neonatal lethality by somatic gene transfer, exogenous and endogenous LPL expression remained absent in the brain (57). These mice demonstrated increased levels of plasma vitamin E, whereas the vitamin E in the hippocampus was dramatically decreased, supporting a role of LPL in the transport of vitamin E from the periphery to the CNS (93).
LPL binds apolipoprotein E (apoE)–containing lipoprotein particles and a major apoE receptor, LDL receptor–related protein (LRP). Of interest, both apoE and LRP have gene polymorphisms associated with an increased risk for Alzheimer’s disease (AD). LPL is found in AD amyloid plaques along with at least six other LRP-binding proteins (70). The relationship of LPL mutations and/or polymorphisms to AD risk has been inconsistent (3, 19, 42, 52, 53). However, when clustered with other cholesterol-related gene single polymorphisms, i.e., the cholesterol catabolite 24S-hydroxycholesterol, LPL clearly increases the susceptibility for AD (62). Recent studies also show that the H+ allele of LPL HindIII intronic polymorphism increases the risk for sporadic late-onset AD (73), and a common LPL polymorphism, a PvuII single-nucleotide polymorphism (SNP) (rs285; referred to as the P+ allele), also increases the risk for sporadic AD in the eastern Canadian population (7). The potential role of LPL in AD is consistent with the proposed protective functions of LPL in brain injury models, acting to modify the synaptic loss/remodeling process and adult neurogenesis (9). Most recently, LPL has also been shown to bind to amyloid beta protein (Aβ) and promote cell-surface association and uptake of Aβ in mouse primary astrocytes, providing an alternative mechanism of how LPL might play a role in AD (54).
LPL MRNA, PROTEIN, ENZYME ACTIVITY, AND FUNCTIONS IN THE SPINAL CORD AND PERIPHERAL NERVOUS SYSTEM
LPL mRNA, protein, and enzyme activity have been identified in the lower spinal cord and cauda equina, with much of the immunostaining signal in the spinal cord seen in white matter (6). It is interesting to point out that the levels of LPL activity and mRNA in the caudal spinal cord were 5–10 times higher than those found in any other area of the brain, making the levels in the spinal cord similar to those found in adipose tissue or skeletal muscle (6, 57).
LPL-mediated hydrolysis of exogenous triacylglycerol is an important source of free fatty acids (FFAs) for phospholipid synthesis in cultured Schwann cells, and thus the enzyme may play a role in myelin biosynthesis in the peripheral nervous system (PNS) (25). LPL is also expressed in the sciatic nerve, and in response to crush injury, the levels of LPL protein and activity are increased in the distal portion of the injured nerve (26). Similar to the role of LPL in response to brain injury, the role of LPL in response to peripheral nerve injury could also involve reutilization of lipids from degenerating myelin.
Transthyretin (TTR), a plasma protein that functions as the transporter of thyroxine and retinol, is mainly synthesized by the liver and choroid plexus of the brain. Several TTR mutations are associated with familial amyloid polyneuropathy, an autosomal dominant lethal disorder characterized by extracellular deposition of TTR amyloid fibrils, particularly in the PNS, and leading to axonal and neuronal loss. TTR knockout mice display impaired nerve regeneration and increased levels of LPL in both CNS and PNS (55). Following crush injury, the LPL activities in both Schwann cells and the dorsal root ganglion neurons were further increased, along with an increased content of sphingolipid, suggesting that the up-regulation of LPL in TTR mice is a compensatory mechanism for the decreased capacity for nerve regeneration (56).
It is interesting to note that LPL activity in sciatic nerve is reduced in rats with streptozotocin-induced diabetes after just two days of streptozotocin treatment, and it remained reduced for at least 35 days. The decrease in LPL activity coincided temporally with a drop in motor nerve conduction velocity, and this reduction was reversed by treatment with insulin for four days (17). These results suggest a possible role of LPL in the PNS in the pathophysiology of diabetic neuropathy.
NOVEL FUNCTION OF LPL IN CNS: LEARNING AND MEMORY
The expression of LPL mRNA occurs throughout the brain, but most impressively in the CA1-CA4 layers of the hippocampus. This area is well recognized to be the learning center of the brain, where hippocampal neurons contribute to memory by rapidly assimilating information about the perceptual and behavioral structure of experience (76). The process by which this occurs is called long-term potentiation (LTP), whereby a series of conditioned impulses potentiates the size of synaptic potentials (5). In the mouse model of LPL deficiency rescued from neonatal lethality by somatic gene transfer, altered cognition was found (93). Two commonly used cognitive tests were conducted in 12-month-old mice to assess hippocampus-related spatial learning and memory. In a water maze test, increased latency to escape the platform and increased mistake frequency were observed, and in the step-down inhibitory avoidance test, decreased latency to the platform ensued. Transmission electron microscopy revealed a decrease in the number of presynaptic vesicles in the hippocampus of LPL-deficient mice, with levels of the presynaptic marker synaptophysin reduced and with no change in the levels of a postsynaptic marker, postsynaptic density protein 95. When these preclinical data are considered along with those from humans wherein LPL mutations relate to AD, it appears likely that LPL plays an important role in learning and memory.
NOVEL FUNCTION OF LPL IN CNS: ENERGY BALANCE AND BODY WEIGHT REGULATION
A novel neuron-specific LPL-deficient mouse model has been created recently to facilitate the study of LPL physiology in the brain and CNS. In brief, LPL loxP mice were crossed with transgenic mice with CNS neuron–specific expression of cre recombinase driven by the regulatory sequences of NEX, a gene that encodes a neuronal basic helix-loop-helix protein. Control breeding into different lines of lacZ-indicator mice demonstrated that cre activity was expressed in the neocortex, hippocampus, mid- and hindbrain, pyramidal neurons, and dentate gyrus mossy and granule cells in the dorsal telencephalon, and was absent from proliferating neural precursors of the ventricular zone, interneurons, oligoden-drocytes, and astrocytes (21). The expression pattern of NEX in the brain and CNS was quite similar to that of LPL, making it a good promoter to drive the selective deletion of LPL in CNS neurons by cre recombinase.
Homozygous LPL knockout mice (NEXLPL−/−) are hyperphagic before obesity and start to gain significantly more body weight by week 16 on standard chow. Energy expenditure is also modified in these mice after obesity develops and drives the further obesity development after food intake resumes at the normal level. Importantly, reduced uptake of TG-rich lipoprotein fatty acids and reduction in the levels of essential fatty acids [n-3 polyunsaturated fatty acids (PUFAs)] are observed in the hypothalamus of NEXLPL−/− mice three months before obesity development (87). Of added interest, the up-regulation of several genes of fatty acid chain length elongation and desaturation indicates that the deficiency of the n-3 PUFAs appears to be sensed in the hypothalamus. Overall, these results suggest that TG-rich lipoprotein metabolism is regulated by LPL in CNS neurons and provides lipid signals for energy balance and body weight regulation.
The hypothalamus plays a critical role in monitoring the nutritional status of the body and initiating cogent behavioral and metabolic responses. Pharmacological and molecular manipulations of hypothalamic nutrient sensing affect appetite, disrupt energy balance, and contribute in a substantial manner to body weight regulation. In NEXLPL−/− mice, LPL mRNA and enzyme activity are mostly reduced in the hippocampus, but LPL mRNA is also reduced to 50% in the hypothalamus with no detectable change in heparin releasable enzyme activity. There is not enough evidence at this point to link the reduction of LPL expression in the hypothalamus directly to the obesity and other related phenotypes. Nevertheless, the expression of the hypothalamus-specific orexigenic neuropeptide agouti-related protein (AgRP) is up-regulated in both NEXLPL−/− and heterozygous NEXLPL+/− mice before obesity. And this elevation persists after obesity development, making AgRP up-regulation the likely major driving force for obesity development in NEXLPL mice.
The mechanism by which neuronal LPL deficiency leads to hypothalamic AgRP up-regulation is not clear at this point. Because LPL mRNA and activity are mostly reduced in the hippocampus in NEXLPL mice, one cannot rule out the possibility that modification of LPL gene expression in the hippocampus is somewhat involved in body weight regulation. There are precedent studies to suggest such a role for the hippocampus (13), especially in the case of brain-derived neurotrophic factor (BDNF) that not only is synthesized in the hippocampus (83) but also is known to play an important role in activity-dependent synaptic plasticity in the adult brain (34). However, BDNF levels are normal in both the hippocampus and hypothalamic areas of the brain in NEXLPL mice. Taking into consideration the n-3 PUFA deficiency observed in the hypothalamus before obesity and the fact that the uptake of TG-rich lipoprotein fatty acids is reduced only in the hypothalamus, our current hypothesis is that LPL regulates TG-rich lipoprotein metabolism in CNS neurons, providing important lipid-derived regulatory signals such as n-3 PUFAs; these dietary essential PUFAs in turn regulate AgRP expression in the hypothalamus and thus energy balance and body weight. It is worthwhile to point out that the proximal promoter region of AgRP has a 21 nucleotide sequence that is 100% identical to the sequence found in the promoter of the neuron-derived orphan receptor (NOR-1) (11), and NOR-1 action is preferentially regulated by n-3 and n-6 fatty acids (44).
Fatty acid availability in the hypothalamus appears to be very important to the regulation of energy balance. But how the brain regulates the de novo synthesis versus the transport of different classes of fatty acids into the brain remains unclear. Lipids are major constituents of the brain. Different classes of lipids (such as cholesterol, fatty acids) and lipid derivatives (such as endocannabinoids) have been shown to be essential for basic brain function and the CNS regulation of important physiological processes. In earlier studies wherein FFAs were infused into the brain and food intake was inhibited (58), it was proposed that fatty acids and their metabolism in the brain play an important role in regulating energy balance (33, 38, 49, 68, 92). Paradoxically, the time when plasma FFAs are highest is during fasting and starvation, not during or after feeding. Thus, brain lipids—specifically, hypothalamic fatty acids—might be regulated differently and independent of the circulating plasma FFA concentration. The delivery of fatty acids to the brain, in particular in the form of TG-rich lipoproteins, has not been extensively examined in the CNS. NEXLPL mice thus become the first physiologically relevant model to demonstrate the role of TG-rich lipoprotein metabolism in CNS functions.
LPL-ASSOCIATED PROTEINS AND THEIR POTENTIAL ROLES IN REGULATING LPL FUNCTION IN BRAIN AND CNS
In the periphery, the tissue-specific expression of LPL is regulated at transcriptional, posttranscriptional, translational, and posttranslational levels, by nutrition and hormonal signals, and by a number of interacting proteins including lipase maturation factor 1, angiopoietin-like protein 3 and 4 (Angptl3, 4), and receptor-associated protein (RAP) (reviewed in 88). Lipase maturation factor 1 is involved in the posttranslational modification of LPL to help form and stabilize the active LPL dimer and is also expressed in the brain, making it a possible regulator of LPL function in the brain (66, 67). Angptl3 and 4 are members of the Angptl family proteins that inhibit LPL activity in peripheral tissues. Both Angptl3 and 4 are expressed in the brain (43), and increased hypothalamic Angptl4 levels can suppress food intake and body weight gain and enhance energy expenditure (29). RAP is a recognized chaperone/escort protein for members of the LDL receptor gene family and plays a role in LPL maturation (84). RAP can also inhibit the LRP-mediated LPL degradation in adipocytes (60). Both RAP and LRP are expressed in the brain, with RAP mostly localized to cortical neurons including large pyramidal and smaller interneurons and some glial cells (69), and they are also colocalized with LRP on neuronal soma (70). Furthermore, reduction of RAP protein is found in AD brain (69). In addition to these LPL-interacting proteins, a new protein called sortilin and sortilin-related receptor (SorLA) are emerging as new players in the regulation of lipoprotein metabolism (91). SorLA regulates the activity of LPL by mediating intracellular trafficking, and in primary neuronal cells, SorLA binds to LPL and regulates the vesicle-like localization of LPL (31).
LRP is a multifunctional receptor that binds and rapidly internalizes several ligands, including apolipoprotein E and LPL, into senile plaques in AD (70). In neurons, LRP1 has been shown to function in a cooperative manner with heparin sulfate proteoglycans to regulate cellular Aβ binding and uptake (28). Interestingly, LPL is also anchored to the vascular endothelium by heparin sulfate proteoglycans, and deletion of LRP1 in forebrain neurons leads to phenotypes such as a global defect in brain lipid metabolism, progressive and age-dependent synapse loss, memory loss, and neurodegeneration (36), a phenotype that is somewhat similar to that characterized in rescued LPL-deficient mice (93). Even more interesting is the observation that conditional deletion of LRP1 in the brain, and particularly in the hypothalamus, results in obesity characterized by increased food intake and decreased energy expenditure (37). These phenotypes are very much similar to those observed in neuronal LPL-deficient mice (87), raising speculation that LPL and LRP1 in some way interact in CNS lipoprotein metabolism, with a deficiency in either playing a role in the regulation of energy balance and cognitive function.
In summary, despite the decades of evidence that LPL in adipose tissue and muscle plays an important role in lipid partitioning, insulin action, and energy balance, the original speculation (14) that LPL in the brain could be important to energy balance and body weight regulation has proven to be true with the phenotypic characterization of NEXLPL−/− mice. In addition, a potential role of the LPL-dependent lipoprotein metabolism in the brain in cognitive function has been suggested by work in the rescued general LPL-deficient mice and LRP1-deficient mice. Taken together, the data strongly indicate that dietary TG-rich lipoprotein metabolism in the brain plays a more important role than previously realized.
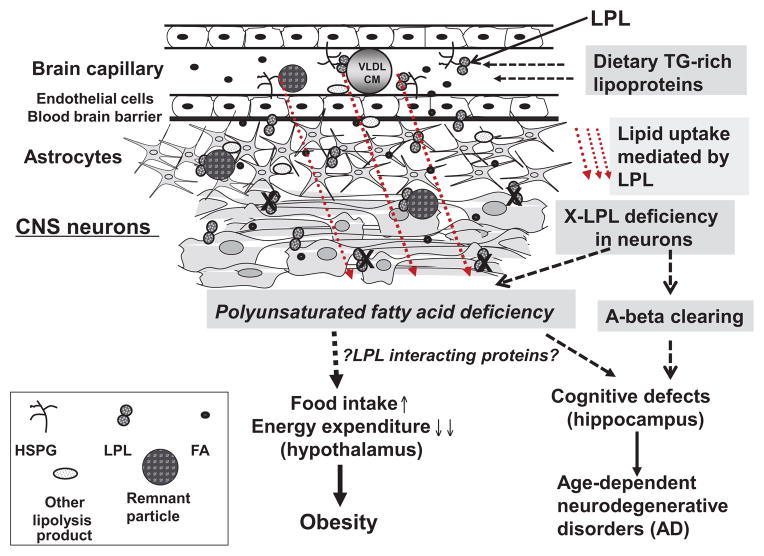
The sequential pathway is outlined in Figure 1:
Figure 1.
A schematic of lipoprotein lipase (LPL) biology in the brain. AD, Alzheimer’s disease; CM, central medial; CNS, central nervous system; FA, fatty acid; HSPG, heparin sulfate proteoglycan; TG, triglyceride; VLDL, very-low-density lipoprotein.
Dietary TG-rich lipoproteins do enter the brain in a regulated manner.
The uptake of dietary TG-rich lipoprotein-derived lipids into the brain is regulated by an LPL-dependent mechanism.
Neuronal LPL deficiency leads to specific deficiency of long-chain n-3 PUFAs, with no change in saturated and monounsaturated fatty acids.
The deficiency in long-chain n-3 PUFAs provides a signal to modulate neuropeptides that are important to body weight regulation in the hypothalamus and related brain areas.
Neuronal LPL deficiency, most likely in the hippocampus, contributes to cognitive defects.
LPL-interacting proteins may also contribute to LPL-deficiency-related phenotypes, including alterations in energy balance, obesity, and cognitive impairment.
Of course, many studies are needed before all the dotted lines in Figure 1 can be verified and fully established. Nevertheless, we are left with the need to address a plethora of unanswered questions, for example:
How does LPL in the brain access TG-rich lipoproteins?
Is the enzymatic activity of LPL necessary for the physiological function of the protein in the brain?
Does LPL deficiency in the hippocampus contribute to both the development of obesity and the cognitive impairment, or do these phenotypes relate differentially to reductions of LPL in the hypothalamus and hippocampus, respectively?
By what process does the reduction of LPL in the neuron modify energy balance and cognition?
The time is ripe for new insights into how lipoprotein metabolism in the brain impacts CNS processes.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Auerbach BJ, Bisgaier CL, Wolle J, Saxena U. Oxidation of low density lipoproteins greatly enhances their association with lipoprotein lipase anchored to endothelial cell matrix. J Biol Chem. 1996;271(3):1329–35. doi: 10.1074/jbc.271.3.1329. [DOI] [PubMed] [Google Scholar]
- 2.Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, et al. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89(4):939–50. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 3.Baum L, Wiebusch H, Pang CP. Roles for lipoprotein lipase in Alzheimer’s disease: an association study. Microsc Res Tech. 2000;50(4):291–96. doi: 10.1002/1097-0029(20000815)50:4<291::AID-JEMT8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Ben Zeev O, Doolittle MH, Singh N, Chang CH, Schotz MC. Synthesis and regulation of lipoprotein lipase in the hippocampus. J Lipid Res. 1990;31(7):1307–13. [PubMed] [Google Scholar]
- 5.Bennett MR. The concept of long term potentiation of transmission at synapses. Prog Neurobiol. 2000;60(2):109–37. doi: 10.1016/s0301-0082(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 6.Bessesen DH, Richards CL, Etienne J, Goers JW, Eckel RH. Spinal cord of the rat contains more lipoprotein lipase than other brain regions. J Lipid Res. 1993;34(2):229–38. [PubMed] [Google Scholar]
- 7.Blain JF, Aumont N, Theroux L, Dea D, Poirier J. A polymorphism in lipoprotein lipase affects the severity of Alzheimer’s disease pathophysiology. Eur J Neurosci. 2006;24(5):1245–51. doi: 10.1111/j.1460-9568.2006.05007.x. [DOI] [PubMed] [Google Scholar]
- 8.Blain JF, Paradis E, Gaudreault SB, Champagne D, Richard D, Poirier J. A role for lipoprotein lipase during synaptic remodeling in the adult mouse brain. Neurobiol Dis. 2004;15(3):510–19. doi: 10.1016/j.nbd.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Blain JF, Poirier J. Could lipoprotein lipase play a role in Alzheimer’s disease? Sci World J. 2004;4:531–35. doi: 10.1100/tsw.2004.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brecher P, Kuan HT. Lipoprotein lipase and acid lipase activity in rabbit brain microvessels. J Lipid Res. 1979;20(4):464–71. [PubMed] [Google Scholar]
- 11.Brown AM, Mayfield DK, Volaufova J, Argyropoulos G. The gene structure and minimal promoter of the human agouti related protein. Gene. 2001;277(1–2):231–38. doi: 10.1016/s0378-1119(01)00705-3. [DOI] [PubMed] [Google Scholar]
- 12.Chajek T, Stein O, Stein Y. Pre- and post-natal development of lipoprotein lipase and hepatic triglyceride hydrolase activity in rat tissues. Atherosclerosis. 1977;26(4):549–61. doi: 10.1016/0021-9150(77)90122-8. [DOI] [PubMed] [Google Scholar]
- 13.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–16. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel RH, Robbins RJ. Lipoprotein lipase is produced, regulated, and functional in rat brain. Proc Natl Acad Sci USA. 1984;81(23):7604–7. doi: 10.1073/pnas.81.23.7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest. 1992;90(5):2013–21. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Borja M, Bellido D, Vilella E, Olivecrona G, Vilaro S. Lipoprotein lipase-mediated uptake of lipoprotein in human fibroblasts: evidence for an LDL receptor-independent internalization pathway. J Lipid Res. 1996;37(3):464–81. [PubMed] [Google Scholar]
- 17.Ferreira LD, Huey PU, Pulford BE, Ishii DN, Eckel RH. Sciatic nerve lipoprotein lipase is reduced in streptozotocin-induced diabetes and corrected by insulin. Endocrinology. 2002;143(4):1213–17. doi: 10.1210/endo.143.4.8723. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira LD, Pulawa LK, Jensen DR, Eckel RH. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes. 2001;50(5):1064–68. doi: 10.2337/diabetes.50.5.1064. [DOI] [PubMed] [Google Scholar]
- 19.Fidani L, Compton D, Hardy J, Petersen RC, Tangalos E, et al. No association between the lipoprotein lipase S447X polymorphism and Alzheimer’s disease. Neurosci Lett. 2002;322(3):192–94. doi: 10.1016/s0304-3940(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 20.Gavin LA, Cavalieri RR, Moeller M, McMahon FA, Castle JN, Gulli R. Brain lipoprotein lipase is responsive to nutritional and hormonal modulation. Metabolism. 1987;36(10):919–24. doi: 10.1016/0026-0495(87)90124-7. [DOI] [PubMed] [Google Scholar]
- 21.Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44(12):611–21. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg IJ, Soprano DR, Wyatt ML, Vanni TM, Kirchgessner TG, Schotz MC. Localization of lipoprotein lipase mRNA in selected rat tissues. J Lipid Res. 1989;30(10):1569–77. [PubMed] [Google Scholar]
- 23.Goti D, Balazs Z, Panzenboeck U, Hrzenjak A, Reicher H, et al. Effects of lipoprotein lipase on uptake and transcytosis of low density lipoprotein (LDL) and LDL-associated alpha-tocopherol in a porcine in vitro blood-brain barrier model. J Biol Chem. 2002;277(32):28537–44. doi: 10.1074/jbc.M203989200. [DOI] [PubMed] [Google Scholar]
- 24.Heeren J, Niemeier A, Merkel M, Beisiegel U. Endothelial-derived lipoprotein lipase is bound to postprandial triglyceride-rich lipoproteins and mediates their hepatic clearance in vivo. J Mol Med. 2002;80(9):576–84. doi: 10.1007/s00109-002-0351-5. [DOI] [PubMed] [Google Scholar]
- 25.Huey PU, Marcell T, Owens GC, Etienne J, Eckel RH. Lipoprotein lipase is expressed in cultured Schwann cells and functions in lipid synthesis and utilization. J Lipid Res. 1998;39(11):2135–42. [PubMed] [Google Scholar]
- 26.Huey PU, Waugh KC, Etienne J, Eckel RH. Lipoprotein lipase is expressed in rat sciatic nerve and regulated in response to crush injury. J Lipid Res. 2002;43(1):19–25. [PubMed] [Google Scholar]
- 27.Jensen DR, Schlaepfer IR, Morin CL, Pennington DS, Marcell T, et al. Prevention of diet-induced obesity in transgenic mice overexpressing skeletal muscle lipoprotein lipase. Am J Physiol. 1997;273(2 Pt 2):R683–89. doi: 10.1152/ajpregu.1997.273.2.R683. [DOI] [PubMed] [Google Scholar]
- 28.Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci. 2011;31(5):1644–51. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HK, Youn BS, Shin MS, Namkoong C, Park KH, et al. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59(11):2772–80. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98(13):7522–27. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klinger SC, Glerup S, Raarup MK, Mari MC, Nyegaard M, et al. SorLA regulates the activity of lipoprotein lipase by intracellular trafficking. J Cell Sci. 2011;124(Pt. 7):1095–105. doi: 10.1242/jcs.072538. [DOI] [PubMed] [Google Scholar]
- 32.Kounnas MZ, Chappell DA, Strickland DK, Argraves WS. Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro. J Biol Chem. 1993;268(19):14176–81. [PubMed] [Google Scholar]
- 33.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11(3):320–27. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 34.Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci. 2006;126–127:30–38. doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, et al. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest. 1995;96(2):976–86. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30(50):17068–78. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, et al. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9(1):e1000575. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288(5475):2379–81. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 39.Long S, Tian Y, Zhang R, Yang L, Xu Y, et al. Relationship between plasma HDL subclasses distribution and lipoprotein lipase gene HindIII polymorphism in hyperlipidemia. Clin Chim Acta. 2006;366(1–2):316–21. doi: 10.1016/j.cca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Lorent K, Overbergh L, Moechars D, De Strooper B, Van Leuven F, Van den Berghe H. Expression in mouse embryos and in adult mouse brain of three members of the amyloid precursor protein family, of the alpha-2-macroglobulin receptor/low density lipoprotein receptor-related protein and of its ligands apolipoprotein E, lipoprotein lipase, alpha-2-macroglobulin and the 40,000 molecular weight receptor-associated protein. Neuroscience. 1995;65(4):1009–25. doi: 10.1016/0306-4522(94)00555-j. [DOI] [PubMed] [Google Scholar]
- 41.Makoveichuk E, Castel S, Vilaro S, Olivecrona G. Lipoprotein lipase-dependent binding and uptake of low density lipoproteins by THP-1 monocytes and macrophages: possible involvement of lipid rafts. Biochim Biophys Acta. 2004;1686(1–2):37–49. doi: 10.1016/j.bbalip.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Rehrmann MD, Cho HS, Rebeck GW. Lack of association of two lipoprotein lipase polymorphisms with Alzheimer’s disease. Neurosci Lett. 2002;328(2):109–12. doi: 10.1016/s0304-3940(02)00511-6. [DOI] [PubMed] [Google Scholar]
- 43.Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta. 2011;1821(5):782–89. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medh JD, Bowen SL, Fry GL, Ruben S, Andracki M, et al. Lipoprotein lipase binds to low density lipoprotein receptors and induces receptor-mediated catabolism of very low density lipoproteins in vitro. J Biol Chem. 1996;271(29):17073–80. doi: 10.1074/jbc.271.29.17073. [DOI] [PubMed] [Google Scholar]
- 46.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res. 2002;43(12):1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 47.Merkel M, Heeren J, Dudeck W, Rinninger F, Radner H, et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem. 2002;277(9):7405–11. doi: 10.1074/jbc.M107914200. [DOI] [PubMed] [Google Scholar]
- 48.Merkel M, Kako Y, Radner H, Cho IS, Ramasamy R, et al. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc Natl Acad Sci USA. 1998;95(23):13841–46. doi: 10.1073/pnas.95.23.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem. 2004;279(30):31139–48. doi: 10.1074/jbc.M400458200. [DOI] [PubMed] [Google Scholar]
- 50.Mulder M, Lombardi P, Jansen H, Van Berkel TJ, Frants RR, Havekes LM. Heparan sulphate proteoglycans are involved in the lipoprotein lipase-mediated enhancement of the cellular binding of very low density and low density lipoproteins. Biochem Biophys Res Commun. 1992;185(2):582–87. doi: 10.1016/0006-291x(92)91664-c. [DOI] [PubMed] [Google Scholar]
- 51.Mulder M, Lombardi P, Jansen H, Van Berkel TJ, Frants RR, Havekes LM. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparan sulfate proteoglycans via lipoprotein lipase. J Biol Chem. 1993;268(13):9369–75. [PubMed] [Google Scholar]
- 52.Myllykangas L, Polvikoski T, Sulkava R, Notkola IL, Rastas S, et al. Association of lipoprotein lipase Ser447Ter polymorphism with brain infarction: a population-based neuropathological study. Ann Med. 2001;33(7):486–92. doi: 10.3109/07853890109002098. [DOI] [PubMed] [Google Scholar]
- 53.Myllykangas L, Polvikoski T, Sulkava R, Verkkoniemi A, Tienari P, et al. Cardiovascular risk factors and Alzheimer’s disease: a genetic association study in a population aged 85 or over. Neurosci Lett. 2000;292(3):195–98. doi: 10.1016/s0304-3940(00)01467-1. [DOI] [PubMed] [Google Scholar]
- 54.Nishitsuji K, Hosono T, Uchimura K, Michikawa M. Lipoprotein lipase is a novel amyloid beta (Abeta)-binding protein that promotes glycosaminoglycan-dependent cellular uptake of Abeta in astrocytes. J Biol Chem. 2011;286(8):6393–401. doi: 10.1074/jbc.M110.172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nunes AF, Saraiva MJ, Sousa MM. Transthyretin knockouts are a new mouse model for increased neuropeptide Y. FASEB J. 2006;20(1):166–68. doi: 10.1096/fj.05-4106fje. [DOI] [PubMed] [Google Scholar]
- 56.Nunes AF, Sousa MM. Transthyretin knockout mouse nerves have increased lipoprotein lipase and sphingolipid content following crush. Neurosci Lett. 2008;446(2–3):83–87. doi: 10.1016/j.neulet.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 57.Nunez M, Peinado-Onsurbe J, Vilaro S, Llobera M. Lipoprotein lipase activity in developing rat brain areas. Biol Neonate. 1995;68(2):119–27. doi: 10.1159/000244227. [DOI] [PubMed] [Google Scholar]
- 58.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51(2):271–75. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 59.Obunike JC, Edwards IJ, Rumsey SC, Curtiss LK, Wagner WD, et al. Cellular differences in lipoprotein lipase-mediated uptake of low density lipoproteins. J Biol Chem. 1994;269(18):13129–35. [PubMed] [Google Scholar]
- 60.Obunike JC, Sivaram P, Paka L, Low MG, Goldberg IJ. Lipoprotein lipase degradation by adipocytes: receptor-associated protein (RAP)-sensitive and proteoglycan-mediated pathways. J Lipid Res. 1996;37(11):2439–49. [PubMed] [Google Scholar]
- 61.Panzenboeck U, Wintersberger A, Levak-Frank S, Zimmermann R, Zechner R, et al. Implications of endogenous and exogenous lipoprotein lipase for the selective uptake of HDL3-associated cholesteryl esters by mouse peritoneal macrophages. J Lipid Res. 1997;38(2):239–53. [PubMed] [Google Scholar]
- 62.Papassotiropoulos A, Wollmer MA, Tsolaki M, Brunner F, Molyva D, et al. A cluster of cholesterol-related genes confers susceptibility for Alzheimer’s disease. J Clin Psychiatry. 2005;66(7):940–47. [PubMed] [Google Scholar]
- 63.Paradis E, Clavel S, Julien P, Murthy MR, de Bilbao F, et al. Lipoprotein lipase and endothelial lipase expression in mouse brain: regional distribution and selective induction following kainic acid-induced lesion and focal cerebral ischemia. Neurobiol Dis. 2004;15(2):312–25. doi: 10.1016/j.nbd.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Paradis E, Clement S, Julien P, Ven Murthy MR. Lipoprotein lipase affects the survival and differentiation of neural cells exposed to very low density lipoprotein. J Biol Chem. 2003;278(11):9698–705. doi: 10.1074/jbc.M208452200. [DOI] [PubMed] [Google Scholar]
- 65.Paradis E, Julien P, Ven Murthy MR. Requirement for enzymatically active lipoprotein lipase in neuronal differentiation: a site-directed mutagenesis study. Brain Res Dev Brain Res. 2004;149(1):29–37. doi: 10.1016/j.devbrainres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Peterfy M. Lipase maturation factor 1: a lipase chaperone involved in lipid metabolism. Biochim Biophys Acta. 2011;1821(5):790–94. doi: 10.1016/j.bbalip.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE, et al. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet. 2007;39(12):1483–87. doi: 10.1038/ng.2007.24. [DOI] [PubMed] [Google Scholar]
- 68.Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116(4):1081–91. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Provias J, Jeynes B. Immunohistochemical detection of receptor-associated protein in normal human brain and Alzheimer’s disease. Patholog Res Int. 2010;2010:173496. doi: 10.4061/2010/173496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rebeck GW, Harr SD, Strickland DK, Hyman BT. Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor-related protein. Ann Neurol. 1995;37(2):211–17. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 71.Rinninger F, Brundert M, Brosch I, Donarski N, Budzinski RM, Greten H. Lipoprotein lipase mediates an increase in selective uptake of HDL-associated cholesteryl esters by cells in culture independent of scavenger receptor BI. J Lipid Res. 2001;42(11):1740–51. [PubMed] [Google Scholar]
- 72.Rinninger F, Kaiser T, Mann WA, Meyer N, Greten H, Beisiegel U. Lipoprotein lipase mediates an increase in the selective uptake of high density lipoprotein-associated cholesteryl esters by hepatic cells in culture. J Lipid Res. 1998;39(7):1335–48. [PubMed] [Google Scholar]
- 73.Scacchi R, Gambina G, Broggio E, Moretto G, Ruggeri M, Corbo RM. The H+ allele of the lipoprotein lipase (LPL) HindIII intronic polymorphism and the risk for sporadic late-onset Alzheimer’s disease. Neurosci Lett. 2004;367(2):177–80. doi: 10.1016/j.neulet.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 74.Schorsch F, Malle E, Sattler W. Selective uptake of high density lipoprotein-associated cholesterylesters by differentiated Ob1771 adipocytes is modulated by endogenous and exogenous lipoprotein lipase. FEBS Lett. 1997;414(3):507–13. doi: 10.1016/s0014-5793(97)01061-2. [DOI] [PubMed] [Google Scholar]
- 75.Seo T, Al-Haideri M, Treskova E, Worgall TS, Kako Y, et al. Lipoprotein lipase-mediated selective uptake from low density lipoprotein requires cell surface proteoglycans and is independent of scavenger receptor class B type 1. J Biol Chem. 2000;275(39):30355–62. doi: 10.1074/jbc.M910327199. [DOI] [PubMed] [Google Scholar]
- 76.Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9(4):365–84. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 77.Shi X, Yao BZ, Liu D. Lipoprotein lipase expression in the hippocampus and its effects on vitamin E levels in rats with epilepsy. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12(5):377–81. [PubMed] [Google Scholar]
- 78.Shirai K, Saito Y, Yoshida S, Matsuoka N. Existence of lipoprotein lipase in rat brain microvessels. Tohoku J Exp Med. 1986;149(4):449–50. doi: 10.1620/tjem.149.449. [DOI] [PubMed] [Google Scholar]
- 79.Strauss JG, Frank S, Kratky D, Hammerle G, Hrzenjak A, et al. Adenovirus-mediated rescue of lipoprotein lipase-deficient mice. Lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J Biol Chem. 2001;276(39):36083–90. doi: 10.1074/jbc.M104430200. [DOI] [PubMed] [Google Scholar]
- 80.Tacken PJ, Beer FD, Vark LC, Havekes LM, Hofker MH, Willems VD. Very-low-density lipoprotein binding to the apolipoprotein E receptor 2 is enhanced by lipoprotein lipase, and does not require apolipoprotein E. Biochem J. 2000;347(Pt. 2):357–61. doi: 10.1042/0264-6021:3470357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, et al. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270(26):15747–54. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- 82.Tavangar K, Murata Y, Patel S, Kalinyak JE, Pedersen ME, et al. Developmental regulation of lipoprotein lipase in rats. Am J Physiol. 1992;262(3 Pt 1):E330–37. doi: 10.1152/ajpendo.1992.262.3.E330. [DOI] [PubMed] [Google Scholar]
- 83.Tongiorgi E, Domenici L, Simonato M. What is the biological significance of BDNF mRNA targeting in the dendrites? Clues from epilepsy and cortical development. Mol Neurobiol. 2006;33(1):17–32. doi: 10.1385/MN:33:1:017. [DOI] [PubMed] [Google Scholar]
- 84.van Vlijmen BJ, Rohlmann A, Page ST, Bensadoun A, Bos IS, et al. An extrahepatic receptor-associated protein-sensitive mechanism is involved in the metabolism of triglyceride-rich lipoproteins. J Biol Chem. 1999;274(49):35219–26. doi: 10.1074/jbc.274.49.35219. [DOI] [PubMed] [Google Scholar]
- 85.Vilaro S, Camps L, Reina M, Perez-Clausell J, Llobera M, Olivecrona T. Localization of lipoprotein lipase to discrete areas of the guinea pig brain. Brain Res. 1990;506(2):249–53. doi: 10.1016/0006-8993(90)91258-i. [DOI] [PubMed] [Google Scholar]
- 86.Wallinder L, Peterson J, Olivecrona T, Bengtsson-Olivecrona G. Hepatic and extrahepatic uptake of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1984;795(3):513–24. doi: 10.1016/0005-2760(84)90181-4. [DOI] [PubMed] [Google Scholar]
- 87.Wang H, Astarita G, Taussig MD, Bharadwaj KG, DiPatrizio NV, et al. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab. 2011;13(1):105–13. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297(2):E271–88. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Knaub LA, Jensen DR, Young JD, Hong EG, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58(1):116–24. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Sun W, Xu E. The expression and activity of brain lipoprotein lipase is increased after acute cerebral ischemia-reperfusion in rats. Neuropathology. 2010;30(2):131–39. doi: 10.1111/j.1440-1789.2009.01061.x. [DOI] [PubMed] [Google Scholar]
- 91.Willnow TE, Kjolby M, Nykjaer A. Sortilins: new players in lipoprotein metabolism. Curr Opin Lipidol. 2011;22(2):79–85. doi: 10.1097/MOL.0b013e3283416f2b. [DOI] [PubMed] [Google Scholar]
- 92.Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, et al. Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. J Neurochem. 2008;105(4):1550–59. doi: 10.1111/j.1471-4159.2008.05255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xian X, Liu T, Yu J, Wang Y, Miao Y, et al. Presynaptic defects underlying impaired learning and memory function in lipoprotein lipase-deficient mice. J Neurosci. 2009;29(14):4681–85. doi: 10.1523/JNEUROSCI.0297-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yacoub LK, Vanni TM, Goldberg IJ. Lipoprotein lipase mRNA in neonatal and adult mouse tissues: comparison of normal and combined lipase deficiency (cld) mice assessed by in situ hybridization. J Lipid Res. 1990;31(10):1845–52. [PubMed] [Google Scholar]
- 95.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]