Abstract
Objectives
Recent research has found abnormalities in reward-related neural activation in bipolar disorder (BD), during both manic and euthymic phases. However, reward-related neural activation in currently depressed individuals with BD and that in currently depressed individuals with major depressive disorder (MDD) have yet to be directly compared. Here, we studied these groups, examining the neural activation elicited during a guessing task in fronto-striatal regions identified by previous studies.
Methods
We evaluated neural activation during a reward task using fMRI in two groups of depressed individuals, one with bipolar I disorder (BD-I) (n = 23) and one with MDD (n = 40), with similar levels of illness severity, and a group of healthy individuals (n = 37).
Results
Reward expectancy-related activation in the anterior cingulate cortex was observed in the healthy individuals, but was significantly reduced in depressed patients (BD-I and MDD together). Anticipation-related activation was increased in the left ventrolateral prefrontal cortex in the BD-I depressed group compared with the other two groups. There were no significant differences in prediction error-related activation in the ventral striatum across the three groups.
Conclusions
The findings extend previous research which has identified dysfunction within the ventrolateral prefrontal cortex in BD, and show that abnormally elevated activity in this region during anticipation of either reward or loss may distinguish depressed individuals with BD-I from those with MDD. Altered activation of the anterior cingulate cortex during reward expectancy characterizes both types of depression. These findings have important implications for identifying both common and distinct properties of the neural circuitry underlying BD-I and MDD.
Keywords: anticipation, bipolar disorder, functional magnetic resonance imaging, mood disorders, prediction error, prefrontal cortex, reward, ventral striatum
Bipolar disorder (BD) is one of the six most debilitating of all non-communicable illnesses in the developed world (1). Misdiagnosis of the illness, however, as major depressive disorder (MDD) occurs in approximately 60% of individuals with BD seeking treatment for depression who do not have a clear history of mania, leading to inadequate treatment and a possible worsening of course (2). The correct diagnosis of BD is made in only 20% of cases within the first year of seeking treatment (2) and it takes on average 7.5 ± 9.8 years for individuals with BD to obtain a correct diagnosis (3). It is, therefore, critical that objective markers of BD are identified to help distinguish BD from MDD as early as possible in depressed individuals (4). Identifying such biological markers will not only help facilitate more accurate differential diagnosis, but will also inform our understanding of the patho-physiology of depression and BD (4).
Reward-related processes are a promising area in which to look for such markers for theoretical reasons. Several lines of research distinguish two components of reward processing: the encoding and evaluation of the hedonic properties of rewarding or reward-predictive stimuli; and the arousing or non-specific motivational properties of such stimuli (5–7). Both responsiveness to reward and arousal are dimensions highlighted in the recent National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiative (8), and can be examined in individuals with mood disorders. Evidence suggests that individuals with BD show increased sensitivity to reward (9), and also to stimuli which evoke arousal (10). A hypersensitivity to reward cues may lead to an excessive increase in approach or goal-directed motivation to stimuli or life events involving reward pursuit. In the extreme, this excessive increase in motivation is reflected in manic symptoms. By contrast, MDD is characterized by reduced sensitivity to reward cues, which has been related to anhedonic symptoms (11). BD and MDD may, therefore, be associated with distinct patterns of neural and behavioral response to reward, and reward sensitivity may be a fruitful area in which to identify neuroimaging measures that can act as biomarkers to help differentiate BD and MDD. Specifically, an increased neural response to rewarding or arousing stimuli, which is maintained through the depressive phase of BD but is absent in MDD depression, may serve as an effective biomarker.
There is relatively consistent literature emerging from studies of reward processing in healthy individuals. This consistency is dependent, in part, upon a convergence of the methods of modeling variation in neural activation. For example, across a variety of different paradigms, the ventral striatum (VS) is typically activated by unexpected changes in predicted or obtained rewards, in accordance with temporal difference models (12–14). In parallel, midline regions including the ventrome-dial prefrontal cortex, anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC) are frequently reported to be activated in studies of reward-related learning and decision making, often during choice or passive reward expectancy (15, 16). These activations may reflect decision value or hedonic evaluation.
The influence of mood disorders on reward-related brain activation has received some attention, particularly in patients with MDD (17–22). To our knowledge, however, no neuroimaging studies have examined reward-related activation in currently depressed adults with BD. We previously reported elevated ventral striatal, left ventrolateral prefrontal cortex (VLPFC), and right central orbitofrontal cortex (OFC) activation during reward anticipation in euthymic adults with BD relative to healthy adults (23). One study also reported abnormally elevated left central OFC activation during anticipation of reward-relevant cues in adults with manic BD versus healthy adults (24), while another study reported increased activation in the VS coupled with reward omission in manic versus healthy adults (25). These findings accord with the widely observed dysfunction of VS and the ventral prefrontal cortex during processing and regulation of emotionally salient material (26–28) and abnormally elevated reward sensitivity (29–31) in individuals with BD.
In the present study, we aimed to compare reward-related neural activation in depressed individuals with BD versus that in depressed individuals with MDD. We focused on bipolar I disorder (BD-I) rather than bipolar II disorder (BD-II) or BD not otherwise specified (NOS), given that BD-I may be more readily identified and be a more stable long-term diagnosis. We employed a guessing task examining reward and loss (23, 32, 33), and fitted regional changes in blood oxygenation level-dependent (BOLD) signals, measured using functional magnetic resonance imaging (fMRI), to parameters representing variations in expected value and prediction error. This simple approach, employed previously in the context of guessing tasks (34), in some ways approximates formal models which have already been applied to examine differences in neural activation in depressed individuals with MDD relative to healthy individuals (17, 19).
In line with a recent meta-analysis of reward expectancy (15), we hypothesized that neural activation coupled to the expected value of a predictive cue (reward expectancy) would be present in the ACC and VS. Given that depression is associated with diminished behavioral and neural responses to reward, we hypothesized that activation in these regions would be significantly reduced in both groups of depressed individuals relative to healthy individuals. On the other hand, enhanced activation in the BD group compared to the other two groups would provide evidence in favor of a trait-like dysfunction in reward responsiveness in BD.
We also examined the effect of collapsing anticipation of both reward and loss into a single regressor. Given that our regression model included a term reflecting the difference between reward and punishment, a collapsed regressor may reflect anticipation per se, independent of anticipated value. A similar approach has been used in previous neuroimaging studies of emotion, often yielding activation in the VLPFC (35), particularly in the left hemisphere (36, 37). Such activation has been interpreted in terms of arousal (35) or salience (38). In addition, while the region has been implicated in the regulation of affect (39), the effect of emotion on memory encoding (40) and the flexible control of task performance (41–43), VLPFC activation is not thought to be related to positive hedonic evaluation (44). Consistent with this view, although activation in this region was previously observed to be enhanced during reward compared to loss anticipation in individuals with BD (23), a general positive association between the region's activation and both win and loss anticipation was also observed. The region has also been observed to be dysfunctional in individuals with BD relative to healthy individuals across a variety of paradigms (45, 46). We therefore evaluated whether anticipation per se would be associated with elevated VLPFC activation in BD depressed individuals versus either MDD depressed individuals or healthy individuals.
Activation coupled to reward prediction errors is frequently observed within the VS, elicited both by errors of reward expectancy and delivery (12, 13, 47). There is some prior evidence that such activation is disrupted in MDD (17, 19). Equivalent investigations have not been performed in BD depression. We examined prediction error-related activation in the VS, testing the hypothesis that there would be blunted activation in both groups of depressed patients relative to healthy individuals. It should also be noted that there are several prior observations of attenuated VS reward- or prediction error-related activations following administration of antipsychotic medications compared to placebo (e.g., 48–50), or in patients taking these medications compared to those who are not (51). As a substantial proportion of the patients were medicated with antipsychotics, we compared the VS prediction error activation in these patients with that in those not taking the medications, in addition to comparing the other brain variables.
To test these hypotheses, we employed a region-of-interest (ROI) approach, using defini tions derived from the aforementioned meta-analysis (15) in the dorsal ACC and VS, and a region of the left VLPFC defined by a recent anatomical parcellation of the human orbitofrontal/ventral prefrontal cortex (52) which closely reflected the coordinates of the left ventrolateral PFC region identified by Nusslock and colleagues (23) and also previous studies of positively and negatively valenced stimuli (35, 36, 53). We extracted parameter estimates from these regions, reflecting activation coupled to reward expectancy and parameter estimates from the left VLPFC, reflecting activation coupled to anticipation per se. Following previous studies, examination of reward prediction errors was focused on the VS. In addition, due to the suggestion that the amygdala is dysfunctional in patients with BD (e.g., 54, 55), we performed a series of focused analyses to establish whether task and/or group differences would be observed in this region. Amygdala ROIs were based on the definitions of Amunts and colleagues (56).
Methods
Participants
Twenty-four currently depressed adults with BDI, and 42 currently depressed adults with MDD participated in the study. BD depressed and MDD depressed individuals were diagnosed according to DSM-IV criteria using the Structured Clinical Interview for DSM-IV-Research Version (SCID-P) (57). At the time of scanning, all individuals with BD and MDD were in a major depressive episode, as determined by SCID-P criteria. Nineteen individuals with BD and 30 individuals with MDD had at least one lifetime comorbid anxiety and/or substance use disorder, also determined by the SCID-P. These rates of lifetime comorbidity are consistent with existing epidemio-logical research on lifetime comorbidity rates in mood disorders (58, 59). All patients meeting SCID-P criteria for a depressive episode had a Hamilton Rating Scale for Depression (HRSD-25) score ≥10 and a Young Mania Rating Scale (YMRS) score ≤10 on the day of the scan. Five individuals with BD and one individual with MDD had an HRSD-25 score between 11 and 17, while the remaining participants had scores >17. All individuals also completed the Spielberger State Anxiety Inventory (60) on the scanning day. Individuals with BD and MDD were free from alcohol/ substance abuse or dependence for a minimum of three months prior to the study [range: 3–233 months; MDD mean = 85.80, standard devi ation (SD) = 68.92; BD mean = 59.69, SD = 67.32]. The two depressed groups did not differ significantly on the majority of these clinical measures (Table 1).
Table 1.
Demographic information for all three groups, representing only the individuals included in the final analysis (n = 100)
| Healthy controls | MDD | BD | Group differences | MDD versus BD | |
|---|---|---|---|---|---|
| Gender, male/female | 12/25 | 9/31 | 4/19 | χ2 = 1.93, p = 0.38 | FET = 0.75 |
| Age, years, mean (SD) | 33.09 (6.23) | 31.04 (8.04) | 33.94 (8.51) | F(2,97) = 1.28, p = 0.28 | |
| NART IQ, mean (SD) | 112.60 (6.88) | 113.33 (8.76) | 112.64 (9.20) | F(2,97) < 1 | |
| HRSD-25 score, mean (SD) | 1.86 (2.26) | 26.63 (5.70) | 24.70 (8.02) | F(2,97) = 229.30 | t(35.0) < 1 |
| YMRS score, mean (SD) | 0.51 (1.15) | 3.95 (2.50) | 4.00 (2.54) | F(2,97) = 31.16 | t(55) < 1 |
| State anxiety score, mean (SD) | 26.95 (7.17) | 56.85 (8.89) | 53.61 (12.14) | F(2,97) = 114.96 | t(35.7) = 1.12, p = 0.27 |
| Lifetime comorbid anxiety disorders, with/without | 0/37 | 25/15 | 16/7 | χ2 < 1 | |
| Lifetime substance use disorders, with/without | 0/37 | 13/27 | 10/13 | χ2 < 1 | |
| Illness duration, years, mean (SD) | N/A | 12.92 (7.25) | 17.29 (8.12) | t(55) = –2.20, p = 0.031 | |
| Illness age at onset, years, mean (SD) | N/A | 18.13 (7.21) | 16.65 (5.11) | t(55) < 1 | |
| Psychotropic medication load, mean (SD) | 0 (0) | 1.63 (1.44) | 2.30 (1.55) | t(55) = –1.75, p = 0.085 | |
| Antipsychotic, taking/not taking | 0/37 | 5/35 | 11/12 | χ2 = 11.67, p = 0.001 | |
| Antidepressant, taking/not takinga | 0/37 | 29/11 | 9/14 | χ2 = 6.79, p = 0.009 | |
| Bupropion, taking/not taking | 0/37 | 6/34 | 2/21 | FET: p = 0.47 | |
| Mood stabilizer, taking/not taking | 0/37 | 4/36 | 13/10 | χ2 = 16.04, p < 0.001 | |
| Anxiolytic, taking/not takingb | 0/37 | 11/29 | 5/18 | χ2 < 1 | |
| Nicotine use, smoking/not smoking | 4/33 | 9/31 | 12/11 | FET = 13.16, p = 0.001 | χ2 = 5.79, p = 0.016 |
Major depressive disorder (MDD) and bipolar disorder (BD) are contrasted for most of the clinical variables, due to the theoretical importance for the study. Healthy individuals are not included in the analysis of clinical variables; patient groups are contrasted if omnibus test is significant.
FET = Fisher's exact test; HRSD = Hamilton Rating Scale for Depression; N/A = not applicable; NART IQ = National Adult Reading Test intelligence quotient; SD = standard deviation; YMRS = Young Mania Rating Scale.
Includes buproprion.
Predominantly benzodiazepines.
Forty healthy adults with no previous personal or family history of psychiatric illness in first-degree relatives, age- and gender-matched with both patient groups, participated in the study as controls. All individuals were right-handed and native English speaking.
Exclusion criteria for all individuals included: (i) history of head injury (from medical records and participant report); (ii) systemic medical illness; (iii) cognitive impairment [Mini-Mental State Examination score <24 (61)]; (iv) premorbid intelligence quotient (IQ) estimate <85 [National Adult Reading Test (62)]; and (v) general exclusion criteria for magnetic resonance imaging. Further exclusion criteria for individuals with BD included rapid cycling disorder, and, for healthy individuals, previous or current alcohol/illicit substance abuse (determined by SCID-P, saliva, and urine screen). Data for 18 of the 37 control participants have been reported previously in Nusslock et al. (23). In addition, other fMRI data from participant cohorts with overlapping participants have been reported previously (63–65).
Two healthy individuals and two individuals with MDD were excluded due to excessive movement during scanning (>4 mm) or poor task performance (>6 errors). Two further individuals (one with BD and one healthy individual) were excluded due to contrast maps with abnormally high global intensity following screening using the ArtRepair toolbox (66). This resulted in groups of 37 (healthy individuals), 40 (MDD), and 23 (BD).
The participant population reflected the demographics of Pittsburgh, PA, USA and the surrounding area. The study protocol was approved by the University of Pittsburgh Institutional Review Board and written informed consent was obtained following complete description of the study to the individuals.
Medication
To examine possible effects of psychotropic medication on neuroimaging measures in individuals with BD and MDD, we computed: (i) medication load, an index that reflects the number and dose of different medications (67, 68) (see Supplementary Information), and (ii) identified medication status (taking versus not taking each of five main psycho-tropic medication subclasses: mood stabilizers/ antipsychotics/antidepressant/anxiolytics/dopaminergic-antidepressants, e.g., bupropion).
Paradigm
We employed a well-validated eight-min slow event-related card-guessing game (23) [adapted from Forbes et al. (33) and Holm et al. (69)] (see Fig. 1), designed to examine neural activation during anticipation and receipt of monetary reward. There were four possible trials: the expectation of a possible win, followed by a win outcome (win trials) or a no change outcome (disappointment trials); expectation of a possible loss, followed by a loss outcome (loss trials) or no change (relief trials). The task constituted one run, in which 24 trials were presented, with six trials each for win, disappointment, relief, and loss outcomes. Trials were presented in pseudorandom order with predetermined outcomes. Individuals were told that their performance would determine a monetary reward after the scan, with $1 for each win and 50 cents deducted for each loss. Total possible earnings were $3. The trial structure was as follows. First, individuals guessed via button press whether the value of a visually presented card was high or low during the four sec presentation of a question mark. An upward or downward arrow was then presented for six sec, representing possible-win or possible-loss, respectively, while the participant anticipated the outcome. The outcome then appeared for one sec (the number for 500 msec and then the feedback arrow for 500 msec) followed by a nine-sec inter-trial interval (ITI). Individuals practiced the task before the scan.
Fig. 1.
Diagram describing the sequence of events in a given trial. The paradigm consists of 24 trials: 12 are reward-expectation trials, in which the arrow points upward and the possible outcomes are a win (six trials) or no change (six trials); the remaining 12 are loss-expectation trials, in which the arrow points downward, and the possible outcomes are a loss (six trials) or no change (six trials). Reward and outcome expectancy regressors are coupled to the onset of the arrow stimulus, while the outcome and prediction error regressors are coupled to the presentation of the number and the feedback (one sec total). ITI = inter-trial interval; MRI = magnetic resonance imaging.
Neuroimaging data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Trio magnetic resonance imaging (MRI) scanner (Siemens, Erlangen, Germany) at the University of Pittsburgh. Structural three-dimensional axial magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) images were acquired in the same session [echo time (TE) = 3.29 msec; repetition time (TR) = 2,200 msec; flip angle = 9°; field of view (FOV) = 256 × 192 mm; slice thickness = 3.1 mm; matrix = 256 × 256; 192 continuous slices]. Mean BOLD images were then acquired with a gradient echo EPI (echo planar imaging) sequence during eight min covering 39 axial slices (3.1 mm thick; TR/TE = 2,000/ 28 msec; FOV = 205 × 205 mm; matrix = 64 × 64; flip angle = 90°). Three warm-up scans were discarded prior to the recording of BOLD images.
Neuroimaging data analysis
Data were preprocessed and analyzed with Statistical Parametric Mapping software, Version 8 (SPM8; Wellcome Trust Centre for Neuroimaging, University College, London, www.fil.ion.ucl.ac.uk/spm). Data for each participant were realigned to the first volume in the time series to correct for head motion. Realigned BOLD images were then co-registered with the subject's anatomical image. The anatomical image was normalized to the Montreal Neurological Institute (MNI)/International Consortium for Brain Mapping (ICBM) 152 template using a non-linear transformation and segmented into separate tissue types. BOLD images were then transformed to the same space via the segmented structural image (the unified segmentation method), and then spatially smoothed with an 8-mm Gaussian kernel. A first-level fixed-effect model was constructed for each participant. These included four regressors representing different phases of the task: response (four-sec duration, starting at the onset of the question mark), anticipation per se (six-sec duration, starting at the onset of the arrow), outcome (one-sec duration, starting at the onset of the number and including the feedback arrow), and baseline (the final three sec of the ITI). The anticipation and outcome regressors were also accompanied by parametric modulators representing reward expectancy and prediction error, respectively. Reward expectancy regressors, coupled to the anticipation period, reflected the expected value (EV) of the arrow, being set to +0.5 for the up arrow condition (given the 50% chance of winning $1) and -0.25 for the down arrow condition (given the 50% chance of losing 50 cents). Prediction error regressors, coupled to the outcome, were determined by the difference between the outcome and the EV, i.e., +0.5 for a win following an up arrow, –0.5 for no win following an up arrow, +0.25 for a no loss following a down arrow, and –0.25 for a loss following a down arrow. Our conditions of interest were reward expectancy, anticipation per se (the anticipation regressor minus the baseline regressor) and prediction error.
Another regressor was included to model omission errors, if these were made, which lasted 17 sec from the onset of the question mark and replaced other trial events during this period. The Canonical Hemodynamic Response Function was convolved with each regressor. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. A scaling factor to correct for the magnitude of the global signal, a high pass filter (128 sec), and autoregressive [AR (1)] modeling were also implemented at the first level.
This first-level model was fitted to each voxel using restricted maximum likelihood estimation, for each participant, and resulting parameter maps were analyzed at the second level using directional, voxelwise t-tests. To test our main hypotheses regarding group differences, we examined neural activation related to parameter estimates in a priori-defined ROIs: ACC, left VLPFC, and VS reward expectancy-related activation; left VLPFC anticipation per se-related activation; and ventral striatal prediction error activation. As these regions are either large or heterogeneous, we focused on four ROIs, following other studies: a region of the left VLPFC (x = –43, y = 30, z = –11) (see 48); a region of the dorsal ACC (x = 2, [C0]y = 28, z = 30) (see 15); and left and right VS (x = –10, y = 10, z = –2 and x = 12, y = 14, z = –4) (see 15). As our goal was to analyze detailed patterns of data in these regions, we extracted mean BOLD signal from an 8-mm sphere centered on each of these peak coordinates. These extracted data were analyzed using analysis of variance to identify any main effect of group upon activation in each ROI, followed up using Games–Howell post-hoc tests. To provide additional information regarding the peak location of the relevant activations in between-group comparisons, we performed planned voxel-wise between-group tests for all ROIs (VS, ACC and VLPFC), employing a family-wise error (FWE)-corrected peak threshold of p < 0.05 using small volume correction (SVC) within an 8-mm-radius sphere around the above peak coordinates to define the ROI masks. To investigate the amygdala, we used the tripartite anatomical specification of Amunts and colleagues (56), from the SPM anatomy toolbox (70), to conduct ROI analysis. This resulted in six dependent measures per contrast: each region (basolateral, superficial, and centromedian) within both hemispheres. The main effect of group and interactions were investigated within a repeated-measures ANOVA for each contrast (anticipation per se, reward expectancy, and prediction error).
In secondary analyses, groups were compared at the whole-brain level for all three of the critical contrasts, using a cluster-forming threshold of p < 0.005 uncorrected and an FWE cluster threshold of p < 0.05. Whole-brain tests within each group and across all groups were also performed: these were tests of the main effect of the three critical conditions in each group, using the same significance threshold. Whole-brain maps thresholded at a voxelwise uncorrected threshold of p < 0.005 with a 10-voxel cluster threshold are reported in the Supplementary Information to provide a more complete picture of the data.
In addition, focused analysis of covariance, t-tests or correlational analyses were performed to examine the extent to which hypothesized relationships within above-defined a priori neuroimaging measures might be influenced by potentially confounding factors such as medication, illness severity, history of comorbid diagnoses and demographic variables (see Supplementary Information). Finally, to test further assumptions of our first-level modeling strategy, we report two further analyses of outcome-related activation in the Supplementary Information.
Results
Demographic and clinical measures
Nineteen individuals with BD and 31 individuals with MDD were taking at least one psychotropic medication, which is representative of mood-disordered populations (71). Psychotropic medication load was slightly higher in individuals with BD than in individuals with MDD. Individuals with BD had higher usage of mood stabilizers and anti- psychotics, and lower usage of antidepressants, relative to individuals with MDD. The two groups were equivalent with regard to use of benzodiaze-pines (Table 1). History of substance abuse and anxiety were more prevalent in the patient groups compared to the controls, but similar between patient groups.
Behavioral analyses
As expected, individuals with BD, those with MDD, and healthy individuals did not differ in reaction time during the task [F(2,97) < 1]. Groups were also matched for total omission errors [F(2,97) = 1.34, p = 0.27], of which there were few (all group means <1).
BOLD response: reward expectancy (Fig. 2)
Fig. 2.
Main effect of reward expectancy in each of the three groups [top left = healthy individuals; top right = individuals with major depressive disorder (MDD); bottom left = individuals with bipolar disorder (BD); bottom right = controls versus patients; colored bars above the images reflect T statistic scale] thresholded at p < 0.005 uncorrected. The inset plot represents the parameter estimates obtained from the anterior cingulate cortex (ACC) region [8-mm sphere centered at: x = 2, y = 28, z = 30 for each group]. Error bars represent standard error of the mean.
ROI analyses
The reward expectancy parameter estimate-related mean BOLD signal extracted from our ACC ROI yielded a main effect of group [F (2,97) = 3.37, p = 0.039], with post-hoc tests yielding a significant difference between the healthy and BD groups (p = 0.027), but no significant difference between the healthy and MDD groups (p = 0.15), or between the two patient groups (p = 0.73). A basic contrast of the healthy individuals and patients using a t-test revealed a signifi-cant effect [t(98) = 2.50, p = 0.014]: the healthy individuals showed significantly greater activation than zero [t(36) = 3.53, p = 0.001] but the patients did not (all t < 1 within each of the patient groups and in both patient groups combined). Voxelwise analyses within the ACC ROI revealed a trend toward a difference in activation between healthy individuals and the MDD group (peak voxel: x = 3, y = 32, z = 37; t = 2.61, p = 0.076, SVC), and similarly between healthy individuals and the BD group (peak voxel: x = 0, y = 23, z = 31; t = 2.69, p = 0.065, SVC). If the patient groups were collapsed, a significant difference in this region was observed (peak voxel: x = –3, y = 32, z = 34; t = 2.98, p = 0.035, SVC). By contrast, no significant findings were observed in the left VLPFC: neither an effect of group [F(2,97) = 1.014, p = 0.37], nor was the activation significantly different from zero in any of the groups or across all participants (all t < 1.46; all p < 0.15).
We also extracted reward expectancy-related activation from the bilateral VS ROI. Significant activation was not observed in any group (t < 1.76 in all cases), and no main effect of group was observed [F(2,97) < 1]. Likewise, no significant (SVC) voxels were found within the left or right VS within any of the groups, or all participants. However, we also investigated whether there might be a relationship in activation between the ACC and VS ROIs, given that they are thought to co-activate during reward expectancy. Across both patient groups, there were highly significant positive correlations between parameter estimates from the ACC and bilateral VS [BD: (n = 23), r = 0.71, p < 0.001; MDD: (n = 40), r = 0.44, p = 0.004], but not in healthy individuals [(n = 37), r = 0.00, p = 0.99]. The difference between the correlation coeffcient observed across both patient groups [(n = 63), r = 0.51, p < 0.001] and that observed in healthy individuals was significant (z = 2.62, p = 0.0088).
Whole-brain voxelwise analyses
Within healthy individuals alone, ACC activation was observed at FWE-cluster corrected significance (peak voxel: x = –3, y = 32, z = 34; t = 3.67, p < 0.001 uncorrected; cluster size 259 voxels, p = 0.027 corrected). However, across all participants or within each patient group, no activation was observed at this significance level (see Supplementary Information Table 4.1. for further information).
BOLD response: anticipation per se—baseline (Fig. 3)
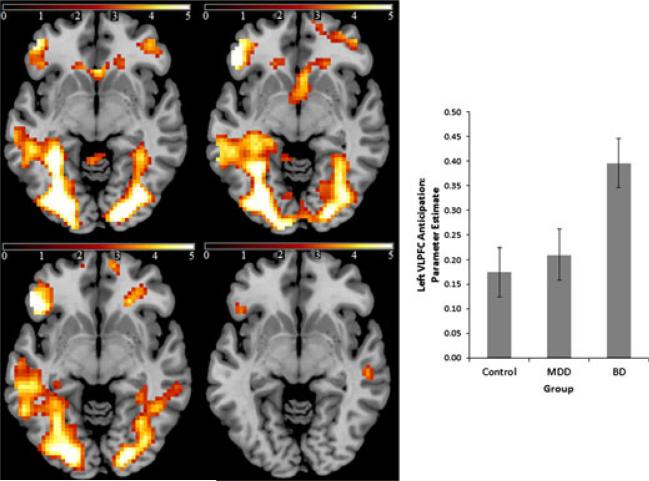
Fig. 3.
Main effect of anticipation per se in all three groups [top left = healthy individuals; top right = individuals with major depressive disorder (MDD); bottom left = individuals with bipolar disorder (BD); bottom right = BD versus non-BD; colored bars above the images reflect T statistic scale] thresholded at p < 0.005 uncorrected (z = 5). The inset plot represents the parameter estimates obtained from the left ventrolateral prefrontal cortex (VLPFC) region [8-mm sphere centered at: x = –43, y = 30, z = –11 for each group]. Error bars represent standard error of the mean.
ROI analyses
The anticipation per se parameter estimate-related mean BOLD signal extracted within our left VLPFC ROI revealed a main effect of group [F(2,97) = 4.15, p = 0.019]. Post-hoc tests revealed that the BD group showed significantly greater activation in this region relative to the MDD group (p = 0.033) and healthy individuals (p = 0.008), while the latter two groups were very similar (p = 0.88). All three groups showed signifi-cant activation in this region for this contrast (t > 3.46, p < 0.002 in all cases). These findings were also observed at the voxel level in the left VLPFC ROI. Here, direct contrast of the BD and MDD groups yielded a significant difference in the left VLPFC (peak voxel: x = –45, y = 26, z = –8; t = 3.00, p = 0.034, SVC), and similarly following contrast of the BD and healthy groups (peak voxel: x = –45, y = 26, z = –5; t = 3.37, p = 0.013, SVC). When the BD group was contrasted with the other two groups, a similar peak voxel was observed (peak voxel: x = –45, y = 23, z = –8; t = 3.27, p = 0.017, SVC).
Whole-brain voxelwise analyses
Across all groups, a widespread network of brain regions was activated by anticipation per se, including the occipital cortex, dorsomedial prefrontal cortex, and predominantly left lateral prefrontal cortex at corrected significance levels (dorsal and ventral) (see Fig. 3 and Supplementary Table 4.2). Parietal and temporal cortical activation was also observed, the former also being more clearly observed on the left. Individual group data broadly reproduced the same pattern of findings. Due to the extensive nature of these within-group activation maps, these findings are reported at a voxelwise corrected significance threshold in the Supplementary Information to provide more specific information about the extent of activation clusters.
BOLD response: prediction error (Fig. 4)
Fig. 4.
Main effect of prediction error activation in all three groups [top = healthy individuals; middle = individuals with major depressive disorder (MDD); bottom = individuals with bipolar disorder]. Regional brain activation (left) thresholded at p < 0.005 uncorrected. The inset displays data extracted from the ventral striatum for each group: top graph = all participants in each group; bottom graph = groups stratified by antipsychotic (AP) medications. Error bars represent standard error of the mean.
ROI analyses
We evaluated the response of the VS to the difference between the value of the outcome and the expected value (signed prediction errors). The mean extracted BOLD signal from the left and right VS showed that the effect of prediction error in this region was similar in all groups (main effect of group: F(2,97) < 1; within each group: t > 1.88, p < 0.074 in all cases). Parameter estimates in the BD group were numerically smaller than in the other two groups, likely due to the fact that VS prediction error response was attenuated in individuals with BD taking antipsychotic medication compared with those who were not (see Supplementary Information), but there were no significant differences between the groups.
This pattern of findings was corroborated at the voxel level within the VS ROIs: bilateral VS activation was observed in healthy individuals (left, peak voxel: x = –9, y = 11, z = –2; t = 3.65, p = 0.006, SVC; right, peak voxel: x = 12, y = 11, z = –8; t = 4.23, p = 0.001, SVC) and individuals with MDD (left, peak voxel: x = –9, y = 17, z = –5; t = 3.75, p = 0.005, SVC; right, peak voxel: x = 12, y = 14, z = –8; t = 4.03, p = 0.002, SVC). Similar findings were not observed in the BD group.
One implication of the temporal difference model, sometimes used to describe prediction error-related activation in the VS, is that greater activation in response to a cue, predictive of reward, corresponds to a weaker activation at the time of the predicted reward, given fixed experimental conditions. We investigated whether this was the case across individuals by examining the correlation between reward expectancy and prediction error related activation. In healthy individuals, the predicted negative relationship was observed [(n = 37), r = 0.37, p = 0.023], but this was not observed in the patient groups [MDD: (n = 40), r = 0.043, p = 0.79; BD: (n = 23), r = 0.039, p = 0.86]. However, the difference between the correlation coeffcients of the healthy group and the collapsed patient group [(n = 63), r = 0.038, p = 0.77] did not reach significance (z = 1.65, p = 0.099).
Whole-brain voxelwise analyses
Across all individuals, a cluster centered on the bilateral VS was activated at FWE-cluster corrected significance [right VS: peak coordinate (x = 12, y = 14, z = –8); left VS: peak coordinate (x = –6, y = 11, z = –5); cluster volume 271 voxels; p = 0.022 corrected]. In addition, a cluster within the left rostral prefrontal cortex was also observed (peak voxel: x = –12, y = 65, z = 7; cluster volume 253 voxels; p = 0.029 corrected), which spread into the medial prefrontal cortex (x = 9, y = 47, z = 13). Activations did not reach whole-brain corrected significance within each subgroup. Whole-brain coordinates for each group are reported in Supplementary Table 4.3.
The influence of group and task on the activation of amygdala subregions
The anticipation per se condition yielded a significant main effect of region [F(2,194) = 15.56, p < 0.001] and significant main effect of hemisphere [F(1,97) = 4.25, p = 0.042]. The region by hemisphere interaction approached significance [F(2,194) = 2.92, p = 0.057]. Exploratory post-hoc t-tests across all participants revealed that the main effect of region was mostly driven by greater activation in the basolateral region relative to the other regions of the same hemisphere (all t > 2.83; all p < 0.006), while activation was slightly greater on the right than on the left (particularly in centromedian and basolateral regions: all p < 0.046). Basolateral activation was significantly greater than zero across both hemispheres [right: t(99) = 4.19, p < 0.001; left: t(99) = 2.44, p = 0.016], but not other subregions [all n.s., other than left centromedian which was less than zero: t(99) = –2.28, p = 0.025]. Importantly, however, the main effect of group and all group interactions were not significant (F < 1 in all cases).
A similar analytic approach was applied to the reward expectancy and prediction error conditions, but no significant findings were observed (p > 0.070 in all cases).
Effect of medications: analysis of individuals treated with antipsychotic medication
Antipsychotic medication was particularly prevalent in the BD group (Table 1). We investigated whether this confound accounted for our between-group effects. Across all individuals (patients and healthy individuals), antipsychotic medications were associated with a non-significant increase in anticipation-related left VLPFC activation [t(98) = 1.26, p = 0.21]. Interestingly, within the BD group alone, in which this medication use was common, these medications, if anything, were associated with a non-significant reduction in left VLPFC anticipation-related activation [t(21) = 1.12, p = 0.28]. Consequently, when excluding individuals on antipsychotic medication, the main effect of group [F(2,80) = 4.24, p = 0.018] and the post-hoc tests (BD versus control: p = 0.007; MDD versus control: p = 0.012) were significant as before.
Consistent with previous findings, we observed that prediction error-related activation in the VS was also reduced in patients taking antipsychotic medication, compared with those who were not [BD: t(21) = 1.58, p = 0.13; MDD: t(38) = 2.92, p = 0.006; both patient groups combined: t(61) = 3.13, p = 0.003] (see Fig. 4). Anticipation and prediction error VS responses remained uncorrelated in patients when individuals taking anti-psychotic medication were excluded [(n = 46), r = –0.21, p = 0.17]. ACC reward expectancy-related activation did not differ between patients medicated or unmedicated with antipsychotic medication in either patient group (all t < 1.58), and including antipsychotics as a covariate did not affect the significance of the main effect of group on ACC activation [F(2,96) = 4.71, p = 0.011] or the patient versus control difference [F(1,97) = 7.51, p = 0.007].
Exploratory analysis: effect of comorbidity and other variables
We did not observe any of the group differences in neural activation to be driven by potentially confounding variables such as medication, general measures of illness severity or demographic measures. Within the combined patient group alone, longer illness duration was associated with reduced ACC reward expectancy activation [(n = 63), ρ = –0.30, p = 0.015], but was not associated with left VLPFC anticipation-related activation [(n = 63), ρ = 0.015, p = 0.91]. Within the depressed subgroups or across all patients, illness age of onset was not associated with either anticipation-related left VLPFC activation or reward expectancy-related ACC activation (all p > 0.61). Including either illness duration or age of onset as a covariate in a comparison of MDD and BD anticipation-related left VLPFC activation had no effect on the significance of this group difference (all F > 5.63; all p < 0.022).
We investigated the effects of lifetime incidence of substance use disorders and comorbid anxiety disorders. Neither variable was associated with reward expectancy-related ACC activation or anticipation-related left VLPFC activation in either depressed subgroup (all t < 1.76; all p > 0.086). A lifetime history of comorbid anxiety disorders was associated with a non-significant increase in anticipation-related left VLPFC activation in the MDD group and a non-signficant reduction in the BD group, thus reducing rather than augmenting the observed effect of group on activation in this region. Including the variable in an ANCOVA model reduced the significance of the three-group main effect on left VLPFC activation to trend level [F(2,96) = 2.82, p = 0.064]. A history of substance use disorders did not influence the significance of the same group contrast [F(2,96) = 4.17, p = 0.018]. However, a history of substance use did slightly reduce the effect of group on ACC reward expectancy activation [F(2,96)= 2.68, p = 0.074] but did not affect the basic patient versus control contrast [F(1,97) = 4.87, p = 0.030].
Further analysis of medication, comorbidities and demographic variables is presented in the Supplementary Information.
Discussion
In the present study, we investigated the dynamics of reward and punishment processing in individuals with mood disorders and healthy individuals by evaluating the coupling of neural activation measured by fMRI to model-based parameters of reward expectancy, anticipation per se and prediction error. There were three main findings. First, individuals with MDD and those with BD depression showed attenuated reward expectancy-related activation in the ACC relative to healthy individuals. Although individual comparisons between either depressed group and healthy individuals were only marginally significant, significant effects were observed when the two depressed groups were combined. Secondly, individuals with BD depression showed increased anticipation-related activation in the left VLPFC relative to both MDD depressed individuals and healthy individuals. Thirdly, similar (signed) prediction error-related activation was observed in the VS in all three groups.
We interpreted these three main findings as follows. The disruption by depression of reward expectancy-related activation in the ACC provides support for models of depression in which antici patory affective responses are disrupted (72). While healthy individuals showed reward expectancy-related activation in regions involved in the representation of value, including the ACC (73, 74), similar patterns of activation were not observed clearly in either patient group at the same significance threshold. This aspect of our results is inconsistent with a previous report of reward expectancy-related activation in the VS and ACC during the monetary incentive delay task in unmedicated patients with MDD (18), in which information about anticipated rewards was represented differently in the ACC of the two groups but was not diminished overall in the patients. A possible area of reconciliation between the two studies arises from our observation of a robust correlation between VS and ACC reward expectancy-related activation in the depressed individuals, which was not seen in healthy individuals. This finding suggests that the transmission of information between the two regions may be altered in depression (perhaps in concert with other regions). Thus, variability in patient and healthy individual differences in simple task activations across distinct reward paradigms might reflect a consistent abnormality in the functional interactions of the ACC and VS.
Together with the observations of Nusslock and colleagues (23), our findings suggest that there is a consistent anticipation-related abnormality in the left VLPFC in individuals with BD, regardless of mood state. Moreover, they corroborate other observations of functional abnormalities in the left ventral prefrontal cortex in individuals with BD (54, 75–77) and first-degree relatives (45), as well as a reward-related abnormality in this region in individuals with BD-II (78). Together, these findings suggest that there may be a trait-level, and perhaps endophenotypic, abnormality in this region. The precise function of the VLPFC region is still widely debated. However, it is notable that a variety of fMRI studies in which both positive- and negative-valenced emotional stimuli are collapsed into a single regressor, perhaps reflecting the arousing properties of the stimuli (79), often yield activation in a similar region of the VLPFC (35, 36, 80). Our method of modeling both anticipation per se and reward expectancy as separate regressors is compatible with this approach, and the resulting anticipation regressor was robustly coupled with BOLD activity in the left VLPFC region across all groups (but particularly in the BD group). An interpretation of these findings is that anticipation per se may elicit an arousal response. However, other studies attempting to manipulate the arousal dimension while controlling for the presence of valenced stimuli have undermined the notion that the left VLPFC is associated specifically with arousal (37, 38). Thus, whether altered left VLPFC activation in individuals with BD is related to arousal (35), salience (38), or other accounts including semantic elaboration (40) remains to be examined in more detail. We also observed that the amygdala was engaged by the anticipation per se contrast, consistent with the notion that the amygdala and VLPFC are part of a functionally interacting network (81). However, no group differences were observed in this region, in agreement with previous reports (27). Our analysis of the amygdala employed similar but not identical preprocessing parameters to a study of Hurlemann and colleagues (82), which investigated the effect of facial emotion on the activation of amygdala subregions using fMRI. We identified the basolateral subregion of the amygdala to be engaged by anticipation per se compared to the other subregions. This finding does not represent strong evidence of a selective engagement of the basolateral region by the anticipation contrast, due to possible unequal resolution or sensitivity of the different amygdala sub-regions. High-resolution imaging studies would be required to demonstrate a specific role for this sub-region (83).
Across four studies of reward (including the present study) in BD (23, 24, 78), regions of the left prefrontal cortex have been shown to be abnormally engaged. The left lateralization of the finding in the VLPFC is consistent with previous electroencephalography (EEG) studies of BD. For example, we previously reported that adults with BD-II displayed abnormally elevated left lateral prefrontal cortical activation, during reward expectancy (9), and that elevated left frontal cortical activation at rest prospectively predicted conversion from BD-II to BD-I (i.e., manic episode onset: 84). Although, as a whole, the fMRI literature suggests that there are functional abnormalities in this region across both hemispheres in BD (54), our findings provide evidence supporting the existence of a left lateralized functional abnormality in individuals with BD during reward processing and/or guessing tasks. The presence of a reward-related abnormality in BD has provided inspiration for possible psychotherapeutic strategies (85), and the presence of an appropriate, state-independent biomarker may facilitate the provision of such therapies.
The observation of similar VS prediction error activation across all groups contrasts with previous findings of reduced VS activation in MDD during reward receipt or feedback (19, 20, 22, 86). Although there are a variety of possible explana tions for these discrepant findings, we suspect that an important area of difference concerns signed prediction error and reward. While the affective response to an outcome is predominantly dependent on relative change from the expected value rather than absolute values (87, 88), it is possible that VS activation also reflects outcome magnitude (34), and that this coding may be selectively disrupted by depression. Indeed, there is behavioral evidence that affective responses driven by relative change are intact in MDD (89). Other studies have used the temporal difference (TD) model to explain variation in BOLD signal, and found disrupted VS activation in MDD (19). This model provides a unifying explanation of processing to the predictive cue and the outcome, in accordance with theories of midbrain dopamine neuron firing properties (90). Although we did not apply this model directly to our data, there was some evidence in favor of a TD model in the healthy individuals but not the patients, insofar as, across the group, healthy individuals showed a negative relationship between reward expectancy-coupled and prediction error-coupled VS activation, but the patient group did not. For a given trial, a TD model predicts that a larger response coupled to reward expectancy reflects an increased predicted value of the outcome, perhaps caused by more rapid associative learning. Thus, the appearance of a positive outcome following optimistic value prediction should be less surprising, and consequently reduced prediction error. Although the contrast of this correlation between patients and healthy individuals only reached trend level, the findings are consistent with disrupted TD encoding in the VS in depression (19).
A key limitation of the present study was the presence of medication, but this seemed to play little part in influencing the pattern of group differences of neuroimaging measures. The majority of depressed BD or MDD individuals were receiving psychotropic medications, and in certain cases, these differed between groups. However, there was little evidence from our exploratory analyses that these played a crucial role in determining the group differences in neural activation that we observed (see Supplementary Information). However, we did observe that patients taking antipsychotic medication showed reduced VS prediction error-related activation compared with those who were not, consistent with previous observations (48, 49, 51). In addition, while some demographic and clinical variables also differed between the groups, no differences relating to these factors were observed. Although substance abuse history and risk factors have been shown to influence reward-related brain activation (e.g., 91, 92), we found no evidence that a history of substance abuse influenced our key variables of interest in our sample. Moreover, none of the participants had a current diagnosis of substance abuse, nor did history thereof differ between the BD and MDD groups. For the effect of substance abuse history on neural activation in BD to be precisely delineated, further studies on patients with particular abuse histories should be conducted, as distinct risk factors may influence different patterns of abuse and relapse which may be diffcult to identify in heterogeneous samples. A further limitation of the study was the relatively small number of trials in each condition, although this may avoid possible confounds such as habituation or task disengagement (33, 93) which can be difficult to control experimentally. Our first-level model ensured that all trials contributed to the estimation of each of the first-level parameters, and therefore that the BOLD time series was used as efficiently as possible. Assumptions of our modeling approach were subject to further tests, the results of which supported our theoretical approach (see Supplementary Information).
The present study is the first to compare activation in neural circuitry supporting distinct components of reward processing in individuals with BD versus those with MDD depression. Our findings suggest dissociable patterns of functional abnormality in key frontal regions during reward expectancy and anticipation per se in depressed individuals with BD and MDD relative to healthy individuals. Furthermore, our finding of abnormally elevated left VLPFC activation during anti-cipation per se in depressed individuals with BD versus MDD provides evidence for a biological marker reflecting underlying pathophysiologic processes that may distinguish the two types of depressive illness. Future studies can aim to replicate these findings in independent groups of depressed individuals, and determine the extent to which these neuroimaging measures of functional abnormalities in neural regions supporting distinct reward subprocesses may act as markers to help predict future clinical outcome in these individuals.
Supplementary Material
Acknowledgement
This study was supported by National Institute of Mental Health (NIMH) grant R01 MH076971 (MLP).
Footnotes
Disclosures The authors of this paper do not declare any financial or other conflicts of interest that might have biased this work.
References
- 1.Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003. 64:161–174. [PubMed] [Google Scholar]
- 3.Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135–144. doi: 10.1016/s0165-0327(98)00076-7. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso de Almeida JR, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73:111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Ledoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 9.Harmon-Jones E, Abramson LY, Nusslock R, et al. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task diffculty. Biol Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 10.MacKinnon DF. Bipolar disorder as maladaptive arousal: a behavioral model and evidence. Ann N Y Acad Sci. 2008;1129:185–189. doi: 10.1196/annals.1417.000. [DOI] [PubMed] [Google Scholar]
- 11.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 13.Seymour B, O'Doherty JP, Dayan P, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- 14.Klein-Flugge MC, Hunt LT, Bach DR, Dolan RJ, Behrens TE. Dissociable reward and timing signals in human mid-brain and ventral striatum. Neuron. 2011;72:654–664. doi: 10.1016/j.neuron.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude: an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Peters J, Buchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 18.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 20.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced cau-date and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- 23.Nusslock R, Almeida JR, Forbes EE, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermpohl F, Kahnt T, Dalanay U, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 27.Foland-Ross LC, Bookheimer SY, Lieberman MD, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Alloy LB, Abramson LY, Walshaw PD, et al. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 30.Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behav Assess. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J Psychopathol Behav Assess. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 33.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt L, Clery-Melin ML, Lafargue G, et al. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci. 2009;29:9450–9457. doi: 10.1523/JNEUROSCI.1951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 43.van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Pompei F, Jogia J, Tatarelli R, et al. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage. 2011;56:1677–1684. doi: 10.1016/j.neuroimage.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 46.Robinson JL, Monkul ES, Tordesillas-Gutierrez D, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- 49.Menon M, Jensen J, Vitcu I, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biol Psychiatry. 2007;62:765–772. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worbe Y, Palminteri S, Hartmann A, Vidailhet M, Lehericy S, Pessiglione M. Reinforcement learning and Gilles de la Tourette syndrome: dissociation of clinical phenotypes and pharmacological treatments. Arch Gen Psychiatry. 2011;68:1257–1266. doi: 10.1001/archgenpsychiatry.2011.137. [DOI] [PubMed] [Google Scholar]
- 52.Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. J Neurosci. 2012;32:6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielen MM, Heslenfeld DJ, Heinen K, et al. Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain Cogn. 2009;71:387–396. doi: 10.1016/j.bandc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Grotegerd D, Suslow T, Bauer J, et al. Discriminating uni-polar and bipolar depression by means of fMRI and pattern classification: a pilot study. Eur Arch Psychiatry Clin Neurosci. 2013;263:119–131. doi: 10.1007/s00406-012-0329-4. [DOI] [PubMed] [Google Scholar]
- 56.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 57.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- 58.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 59.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 60.Spielberger CD, Gorsuch RL, Lushene R. State-Trait Anxiety Invenstory Test Manual Form Y. Consulting Psychological Press; Palo Alto, CA: 1983. [Google Scholar]
- 61.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 62.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 63.Bertocci MA, Bebko GM, Mullin BC, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from uni-polar depressed females. Psychol Med. 2012;42:1417–1428. doi: 10.1017/S003329171100242X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fournier JC, Keener MT, Mullin BC, et al. Heterogeneity of amygdala response in major depressive disorder: the impact of lifetime subthreshold mania. Psychol Med. 2013;43:293–302. doi: 10.1017/S0033291712000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perlman SB, Almeida JR, Kronhaus DM, et al. Amygdala activity and prefrontal cortex–amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disord. 2012;14:162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazaika PK, Whitfield S, Cooper JC. Detection and repair of transient artifacts in fMRI data. Neuroimage. 2005;26:S36. [Google Scholar]
- 67.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassel S, Almeida JR, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 71.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai X, Padoa-Schioppa C. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J Neurosci. 2012;32:3791–3808. doi: 10.1523/JNEUROSCI.3864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 76.Kronhaus DM, Lawrence NS, Williams AM, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 77.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral pre-frontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuppens P, Tuerlinckx F, Russell JA, Barrett LF. The relation between valence and arousal in subjective experience. Psychol Bull. 2013;139:917–940. doi: 10.1037/a0030811. [DOI] [PubMed] [Google Scholar]
- 80.Smolka MN, Schumann G, Wrase J, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dima D, Stephan KE, Roiser JP, Friston KJ, Frangou S. Effective connectivity during processing of facial affect: evidence for multiple parallel pathways. J Neurosci. 2011;31:14378–14385. doi: 10.1523/JNEUROSCI.2400-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurlemann R, Rehme AK, Diessel M, et al. Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. J Neurosci Methods. 2008;172:13–20. doi: 10.1016/j.jneumeth.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Prevost C, McCabe JA, Jessup RK, Bossaerts P, O'Doherty JP. Differentiable contributions of human amygdalar subregions in the computations underlying reward and avoidance learning. Eur J Neurosci. 2011;34:134–145. doi: 10.1111/j.1460-9568.2011.07686.x. [DOI] [PubMed] [Google Scholar]
- 84.Nusslock R, Harmon-Jones E, Alloy LB, Urosevic S, Goldstein K, Abramson LY. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. J Abnorm Psychol. 2012;121:592–601. doi: 10.1037/a0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Coan JA. Psychosocial interventions for bipolar disorder: perspective from the Behavioral Approach System (BAS) Dysregulation Theory. Clin Psychol. 2009;16:449–469. doi: 10.1111/j.1468-2850.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- 87.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 88.Mellers BA. Choice and the relative pleasure of consequences. Psychol Bull. 2000;126:910–924. doi: 10.1037/0033-2909.126.6.910. [DOI] [PubMed] [Google Scholar]
- 89.Chase HW, Camille N, Michael A, Bullmore ET, Robbins TW, Sahakian BJ. Regret and the negative evaluation of decision outcomes in major depression. Cogn Affect Behav Neurosci. 2010;10:406–413. doi: 10.3758/CABN.10.3.406. [DOI] [PubMed] [Google Scholar]
- 90.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 91.Peters J, Bromberg U, Schneider S, et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- 92.Schneider S, Peters J, Bromberg U, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 93.Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.