Abstract
Background
Methods involving the analysis of nucleic acids have become widespread in the fields of traditional biology and ecology, however the storage and transport of samples collected in the field to the laboratory in such a manner to allow purification of intact nucleic acids can prove problematical.
Results
FTA® databasing paper is widely used in human forensic analysis for the storage of biological samples and for purification of nucleic acids. The possible uses of FTA® databasing paper in the purification of DNA from samples of wildlife origin were examined, with particular reference to problems expected due to the nature of samples of wildlife origin. The processing of blood and tissue samples, the possibility of excess DNA in blood samples due to nucleated erythrocytes, and the analysis of degraded samples were all examined, as was the question of long term storage of blood samples on FTA® paper. Examples of the end use of the purified DNA are given for all protocols and the rationale behind the processing procedures is also explained to allow the end user to adjust the protocols as required.
Conclusions
FTA® paper is eminently suitable for collection of, and purification of nucleic acids from, biological samples from a wide range of wildlife species. This technology makes the collection and storage of such samples much simpler.
Background
Techniques involving the analysis of DNA have become ubiquitous in many areas of wildlife research, such as systematics, pathogen detection, and studies of relatedness amongst populations. Unfortunately the transport of fresh or 'wet' samples from the point of collection involves leakage risks and the possibility of sample degradation due to temperature variation and spoilage. Extracted DNA or stored tissue may also degrade in the laboratory, resulting in problems for re-analysis of samples.
We describe here the storage of biological samples on FTA® paper (commercially available reagent-loaded papers similar to filing cards). These cards have been used for some time in forensic human biology [1,2] and their characteristics are well understood. Processing is often a simple washing in-situ, with impurities being washed away and the DNA sample being retained on the paper. The storage of material on these papers allows the re-interrogation of the DNA at any time, in addition to space-efficient room-temperature storage of samples and cheap sample transport by mail or in personal baggage. The ease of transport and storage of samples dried on paper makes it practical for researchers to acquire large reference collections throughout their careers just as they acquire collections of printed references.
This paper presents a comparison of a selection of simple procedures to purify DNA from samples on FTA® paper, and explores some of the issues in the procedures such as sample overload, sample age, and sample deterioration, particularly as they apply to wildlife samples.
Results
Purification of DNA using FTA® paper
FTA® paper is a commercial product (Whatman at http://www.whatman.co.uk/) consisting of filter paper impregnated with a proprietary mix of chemicals which serve to lyse cells, to prevent growth of bacteria, and to protect the DNA in the sample.
The basic premise of purifying DNA using FTA® paper is simple: biological samples are applied to the FTA® paper and air-dried. A small disc of the FTA® paper is then removed, and washed to remove any non-DNA material (the DNA remains entangled within the paper). Analysis can subsequently be performed on the DNA whilst still attached to the paper, or the DNA can be eluted prior to use.
Examples of washing and processing procedures, and of the utility of DNA processed in this manner, are presented in this manuscript.
Sample selection and collection
For vertebrates, the common sample of choice is generally blood or blood clots, as the cells are often still living and the DNA is usually of high quality. After processing, the resulting concentration of DNA per unit area of FTA® paper is also usually fairly constant within each species. This reliability simplifies many procedures by removing the need for DNA quantitation prior to analysis. Although more variable, cheek wipes and saliva deposited on FTA® are satisfactory samples, although such samples include some DNA that has been damaged by apoptotic nucleases as well as bacterial DNA. None of this may be important for procedures such as PCR as long as some of the DNA of the animal is intact; however it may cause amplification-variations due to the highly variable amounts of DNA present.
Blood
Raw blood or blood containing anti-coagulants is dropped onto storage paper and left for 3–5 minutes until dry. If uniformity of sample deposition is highly desirable (i.e. multiple analyses will be performed on the same sample) then samples should be run onto the surface quickly as cells are lysed soon after contact with the paper and a very slow delivery of sample tends to cause localised lysis and thus a non-uniform spread of DNA. Several samples of approximately 1 cm diameter (20 μl blood) can be placed on the same piece of storage paper, ideally with small unloaded 'white' areas between them as a visible assurance that the paper has not been grossly overloaded and to ensure sufficient sample separation. Blood samples with areas of only 1–2 mm are quite sufficient for at least 1 or 2 analyses, and up to 50 dependent on species.
Blood clots
Dead animals (for example, 'roadkill') or rapidly clotting samples may make blood clots the only DNA-source. Ideally, clots of a size 3–5 mm in diameter are gently squeezed into the grain of the FTA® paper between shielded finger and thumb. Cross-contamination from soiled fingers is avoided by the use of simple shields of discardable plastic sheeting such as polythene. The paper is then dried as for liquid blood samples. The distribution of DNA within a squeezed clot may be non-uniform, with most DNA in the centre 2/3 of the area.
Tissue samples
Dead animals may also yield tissue samples for analysis. Small pieces of soft tissue (3–5 mm in diameter) are placed onto the FTA® paper and squashed as for clots before being allowed to dry. Some of the fibre-content often remains on the surface of the paper, but sufficient tissue cells are lysed into the paper to allow DNA analysis.
Saliva or cheek wipes
A variety of methods may be used for collection of oral samples. Saliva may be dropped onto FTA® paper as for blood samples, although clean saliva is often a poor source of DNA. Swabs of either gums or cheeks are preferable sources of DNA, as swabbing or brushing detaches large numbers of cells. Cheeks may be wiped with a swab, or a toothbrush dipped in sterile water. The swab or brush is then wiped or, if a toothbrush, its bristle ends are tapped onto the FTA® paper to transfer a cellular deposit to the paper.
Storage, transport and integrity of samples on FTA® paper
The reagents on the FTA® paper are designed to kill most pathogens, inhibit saprophytes during drying or bouts of high humidity, and have strong buffering and free-radical-trap properties to deal with atmospheric pollutants.
Room temperature transport in folders or envelopes (by hand or mail) has been common for years. The papers protect DNA within the samples for some years at ambient conditions. The main variable is expected to be the storage atmosphere quality, particularly the content of acid gases and free-radical-generating pollutants, although FTA® paper can protect against such conditions [5]. Definite storage times can therefore only be stated with reference to the load of these agents in the atmosphere. However forensic samples have been stored on these papers for more than a decade in ordinary office filing cabinets whilst still remaining amplifiable. From the basic chemistry of DNA damage it is reasonable to expect that storage times may be considerably enhanced if atmospheric circulation is deliberately inhibited.
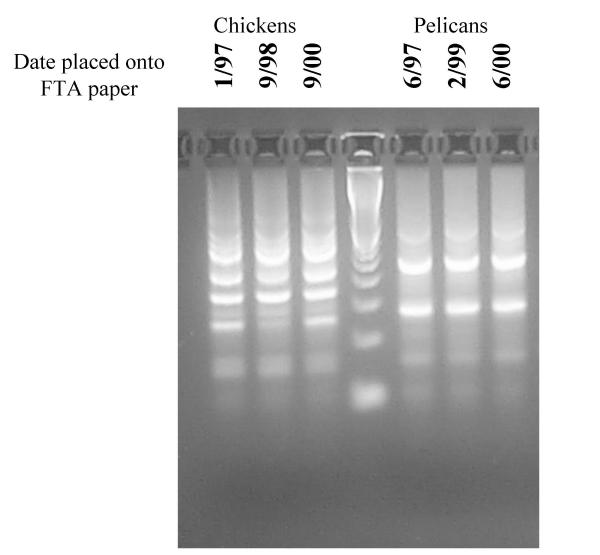
From basic principles of DNA chemistry, sample integrity would be expected to vary with the cleanliness of the storage atmosphere and the amounts of preservatives on the papers. In a western-style city in a Mediterranean climate (South Australia) avian blood samples were placed onto FTA® paper, and were stored in an ordinary filing box in a laboratory cupboard (to restrict atmospheric circulation) in the dark for up to 44 months. Air conditioning was intermittent, and therefore room temperature ranged from 18°C to 42°C. Discs were removed from these samples, processed by method #4 (see below), and used in a PCR reaction to amplify ILRC loci [4]. All samples amplified successfully, (figure 1) demonstrating that the DNA has not undergone significant degradation during storage. Thus, once dried, blood and tissue samples placed onto FTA® paper require no specialised storage or transport conditions, and the DNA within the samples remains intact for at least 4 years stored in a card file at room temperature.
Figure 1.
PCR amplification of DNA purified from archived avian blood. Blood samples were dried onto FTA paper on the dates indicated, and DNA purified and PCR amplified on 23/8/00. All samples still yield amplifiable DNA.
FTA® paper processing and DNA purification
In most methods a disc of appropriate size is first punched from the tissue-containing paper; 1 mm diameter for PCR of mammalian DNA from a blood or clot, and up to 5 mm in diameter for a RFLP study. The paper has to be completely dry in order to punch a disc cleanly, and the disc is then washed and dried. For most PCR applications the DNA is amplified in situ; for RFLP or cloning of DNA, the DNA is digested while still attached to the small washed paper discs. Cross contamination due to paper carryover by the punch is not a problem as paper fibres do not adhere to the sample punch, but 'clean' discs may be punched between samples if required.
The examples below involve sample processing by simple washing carried out in eppendorf tubes, multiwell plates or similar. Wash fluid is dropped on and the washes are thrown away while the DNA remains on the paper until either removed or PCR amplified in-situ. Fine tipped needles or pipettes are best used for aspirating wash solutions as with coarse tipped devices the small discs may also be aspirated. Experience has shown that often neither the volumes or the times quoted below are critical and should thus be viewed as suggestions. During the washing, only gentle rocking is needed and violent agitation is undesirable. Indeed, with the 1 mm discs rocking may be dispensed with if extra changes of solution are added to the protocol, as the disturbance from the extra solution changes is sufficient agitation. The discs are usually dried in an ordinary lab heating block or oven for 15~20 min.
Processing method #1 – A 1 mm disc of FTA® paper was placed in 200 μl 1% SDS, (sodium dodecyl sulphate), 2 mm EDTA (pH 8.0) for 10 minutes. The SDS was removed with three changes of 200 μl Tris-EDTA buffer (TE) buffer (5 minutes each wash) and the TE was removed with a few seconds wash with water followed by 200 μl ethanol or isopropanol.
Processing method #2 – A 1 mm disc of FTA® was washed twice with 500 μl 10 mm NaOH (each wash for 15 minutes) and then washed with 500 μl TE:water (1:5) containing 1 mg/ml BSA for 15 minutes.
Processing method #3 – A 1 mm disc was washed twice (5 minutes each) with 200 μl liquid phenol (Commercial available water-saturated phenol). The disc was washed twice in 250 μl 80% isopropanol containing 5% tris (free-base) and then dried at 55°C.
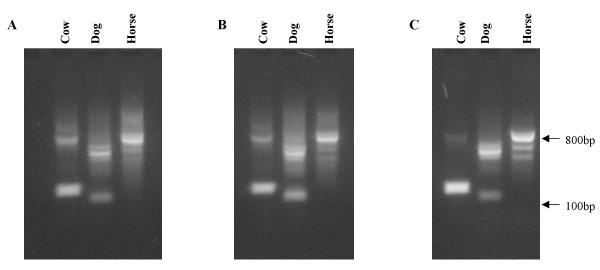
Examples of the end use of DNA processed by these methods can be seen in the amplification of ILRC PCR products (ILRCs are multi-locus markers, which may be considered as similar to microsatellites) from mammalian blood (figure 2a,2b,2c).
Figure 2.
ILRC PCR products from mammalian blood samples, purified on FTA paper using A) method #1 (SDS wash) B) method #2 (NaOH wash) and C) method #3 (phenol wash). All methods yielded amplifiable DNA.
In all methods the disc can be placed directly in the PCR mix after processing, and PCR amplified in situ.
The above methods are older procedures that have been reported to work well with microbial [6] and mammalian DNA with direct PCR.
Method #4 is a method that can cope with the very high levels of DNA found in blood from vertebrates with nucleated erythrocytes (e.g. birds, fish and reptiles). Variations of this method, as required dependant on the source of the sample and the final use of the DNA, are described in Table 1 (see additional file 1).
This procedure involves a more thorough deproteinisation than the previous procedures because the DNA is to be removed from the disc or digested in-situ.
Discs of FTA® paper containing avian blood samples were washed with 1 ml (100 mM Tris (free base), 0.1% SDS), agitating gently for 30 minutes. The discs were then washed in 500 μl 5 M guanidine thiocyanate or one of the commercial DNA purifying solutions such as DNAzol© (Life Technologies at http://www.lifetech.com). Finally the discs were washed 3 times with 500 μl water and once with 500 μl 95% ethanol. The DNA-loaded discs were now ready for procedures such as restriction digestion or DNA elution.
Dealing with DNA overload from nucleated erythrocytes – Avian blood
Bird blood contains too much DNA for even the smallest punched discs to be used directly in PCR reactions. For example six blood samples from Australian pelicans (Pelecanus conspicilattus) were processed by method #4 and three of the 1 mm discs were placed into 50 μl water and heated to 90°C for 10 minutes to release much of the DNA. Eluant (1 μl) was used in the subsequent PCR. The three remaining discs were used directly in the PCR as is usual practice with mammalian samples. PCR amplification of the CHD gene was performed, and all six samples then analysed by gel electrophoresis (figure 3a). The samples with the 1 μl eluant as template (that is to say only one fiftieth or less of the total DNA on a disc) amplified well while direct amplification on the FTA® punches (with the full load of DNA) was poor or failed resulting in smearing on the gel. This is the opposite of the case with mammalian blood (non-nucleated erythrocytes) and was apparently due to the gross excess of DNA introduced into the PCR reaction from the nucleated red blood cells.
Figure 3.
Processing pelican blood samples on FTA paper. A) PCR on DNA eluted from FTA paper (1–3) and directly on a 1 mm punch (4–6). B) Sexing of pelicans by PCR (processed on FTA paper and DNA eluted), followed by HaeIII restriction digestion. Digestion products (2 smaller bands) indicate heterogametic sex (female = ZW, male = ZZ).
FTA® punches from 24 pelican blood samples were processed by method #4 and extracted for 10 minutes with 50 μl water at 90°C. Eluted DNA (1 μl) was then used as template in a subsequent DNA amplification to identify the sex of the birds by amplification of the CHD gene and subsequent restriction digestion.
All but one sample yielded a clear result (figure 3b) at the first amplification (this sample was subsequently successfully amplified), with the total time required for sex identification (processing, PCR, restriction of product and analytical gel) being less than 8 hours.
DNA release issues
With samples containing nucleated erythrocytes, heating to 90°C in water was suitable for detaching DNA from the FTA® paper for PCR, although once released from the paper the DNA may not be stable (either at 4°C or -20°C) in water for long periods (> 2 weeks). Once the sample has been well washed with deproteinising agents such as guanidine thiocyanate, DNA is readily digestable and may be released by restriction endonucleases in-situ for RFLP analysis or cloning. The release of digested DNA from the paper was examined using a cocktail of restriction enzymes, to completely digest any DNA present. After digestion the DNA fragments should be stripped from the discs using centrifugation, as even very low molecular weight DNA diffuses too slowly to leave the paper completely in short time periods.
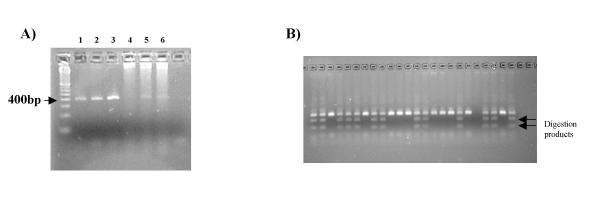
A stripping exercise was performed to illustrate these issues: A 1 mm disc of FTA® paper containing chicken blood was washed as normal (method #4). After digestion with a cocktail of restriction enzymes in a 50 μl volume (to completely digest the entangled DNA) the bottom of the reaction-tube was punctured with the tip of a scalpel blade, the tube placed into a larger tube, and centrifuged in a standard bench centrifuge at 13 000 rpm for 30 seconds to strip all solution from the paper. TE (50 μl) was then added to the paper (twice) and the tube re-centrifuged. The paper was subsequently transferred into a fresh tube, 50 μl water added and heated to 90°C for 10 minutes, to remove any large DNA fragments still attached to the paper. Finally, the residual paper was sealed into the well of an agarose gel. All DNA samples were analysed by agarose gel electrophoresis (figure 4). From the gel tracks it can be seen that most DNA was recovered by a simple spin elution (Lane 1), with little extra DNA being yielded by the subsequent spin elutions with TE (Lanes 2a,2b). Heating to 90°C released some extra DNA, particularly larger fragments (Lane 3) such as restriction-site-deficient macrosatellite DNA which had remained entangled in the paper, whilst even after this procedure some DNA was retained on the paper (Lane 4) again, presumably, macrosatellite DNA.
Figure 4.
DNA release from FTA paper. After complete digestion with multiple restriction enzymes DNA was spin eluted from the paper (Lane 1). The paper was washed and spin eluted twice with TE (Lanes 2a and b), heated to 90°C in water (Lane 3), and sealed in the well of the agarose gel before electrophoresis (Lane 4). Some residual large DNA fragments are retained on the paper
This apparent size-selection may have an application in the enrichment of DNA for particular restriction resistant DNA fragments.
Processing a range of wildlife samples
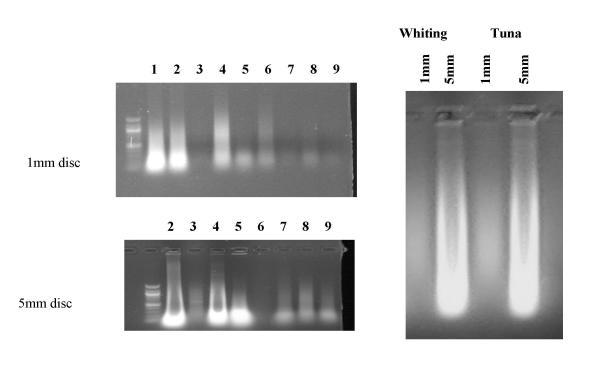
Blood samples from chicken, cow, crested pigeon (Geophaps lophotes) and sleepy lizard (Tiliqua rugiosa) were spotted onto FTA® paper as normal. The blood samples from the chicken and cow contained anticoagulant (heparin). Small tissue fragments (generally muscle) approximately 2 to 4 mm in diameter from mallee fowl (Leipoa ocellata), frog (Rana sp.), yabbie (an Australian fresh water crustacean, Cherax tenuimanus), abalone (Haliotis laevigata) and blue swimmer crab (Portunus pelagicus) were placed onto FTA® paper and manually crushed into the weave of the paper using a microscope slide to shield the thumb from the sample. Two sizes of disc (1 mm and 5 mm diameter) were first processed following method #4, and the discs digested overnight with 10 units of AluI (nominally an amount capable of digesting 10 μg of DNA per hour.) The centrifugally-stripped DNA was analysed by agarose gel electrophoresis (figure 5a). As expected, observable amounts of DNA were only observed from the 1 mm discs from species that have nucleated red blood cells (birds, lizard, fish) although only small amounts of DNA were observed from frogs. Observable amounts of DNA were eluted from 5 mm punches from all species, nucleated or not, from blood samples and other tissues. The 5 mm punches of blood samples from some species with nucleated red blood resulted in incompletely digested DNA, presumably due to the large amounts of DNA present on the disc. King George Whiting (Sillaginodes punctata) and tuna (Thunnus maccoyii) blood samples were also processed similarly. Observable amounts of DNA were eluted from 1 mm discs, and large amounts of incompletely digested DNA were eluted from 5 mm discs.
Figure 5.
Digestion of DNA from blood (1–4) and tissue (5–9) samples on FTA paper. Samples were crested pigeon (1), chicken (2), cow (3), sleepy lizard (4), Mallee fowl (5), frog (6), yabbie (7), abalone (8) and blue crab (9). Samples were processed, and digested overnight with AluI Fish blood samples. 1 mm and 5 mm discs from King George Whiting blood and tuna blood were processed, and digested overnight with AluI. A 1 mm disc yields less DNA than in birds, and a 5 mm disc yields incompletely digested DNA.
Thus, as a general principle, restricting DNA from FTA® to be used in cloning should be preceded by observing a trial restriction of a 1 mm disc so as to determine the amount of DNA present in the particular organism's tissue sample.
Mammalian samples
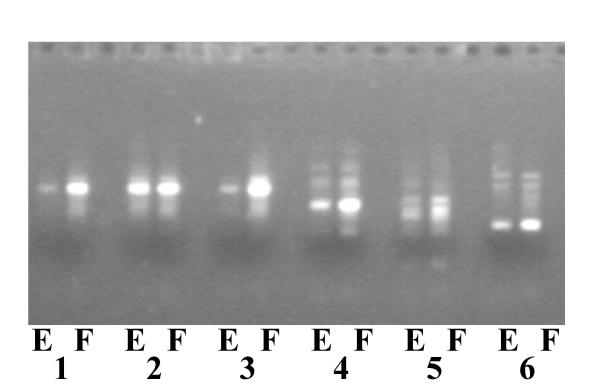
Mammalian samples may be processed by the standard forensic procedures developed for humans [1,2,8]. In addition to methods #1, #2, and #3 described above, method #4 (developed for wildlife samples) can also be used. Duplicate 1 mm discs from anti-coagulated blood samples of several mammalian species (i.e. containing non-nucleated red blood cells) were processed by method #4 on FTA® paper. One disc was heated to 90°C in 50 μl water, and 1 μl eluted DNA used in subsequent PCR reaction. The other disc was used directly in the PCR reaction. PCR reactions were performed to amplify Inverted Line Repeat Clusters (ILRCs) in the mammalian genome [4]. Identical PCR products were amplified from both eluted DNA and the FTA® paper (figure 6), although, unlike the avian blood, direct amplification from the DNA in FTA® paper was more efficient than using the eluted DNA. This is in accord with the expectation that the amount of DNA on a 1 mm disc cut from a mammalian bloodstain is many fold less than that of a bloodstain from a bird.
Figure 6.
PCR amplification of mammalian ILRCs either on eluted DNA (E) or directly on 1 mm disc of FTA paper (F). Species used (all blood samples): Human (1,2,3), Cat (4), Dog (5), Mouse (6).
Taphology: Handling samples that have suffered degradation before loading on the FTA® paper
Blood samples were collected from pelicans, and plasma removed. The residual blood cells were then allowed to dry at room temperature for several days under non-sterile conditions before being resuspended in water and placed onto FTA® paper. Using method #4 initial attempts at amplification failed, indicating that significant degradation of the sample had occurred. However, by omitting the deproteinisation wash (and increasing the number of cycles in the PCR), PCR amplification of the CHD gene was possible (figure 7a) suggesting that residual protein was serving to hold DNA fragments onto the paper during the processing procedure.
Figure 7.
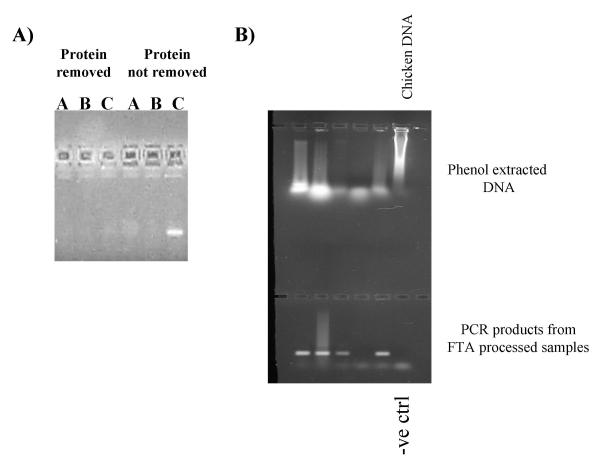
Degraded blood samples were placed onto FTA paper. A) Samples were processed with (TP) and without (NGP) a deproteinising wash, and the paper placed directly into a PCR reaction. PCR was performed for 30 (A), 40 (B) or 50 (C) cycles. B) Phenol extracted DNA from degraded samples and PCR amplification after purification using FTA paper.
Fractions of some of the original blood samples were phenol:chloroform extracted, and the DNA examined by gel electrophoresis (figure 7b). The DNA in all samples was almost totally degraded. However processing of these blood samples on FTA® paper, without deproteinisation, followed by PCR did result in amplification of the CHD gene (and sex identification) in 4 out of the 5 samples. Therefore, collection of moderately decayed samples, while not reliable, may be a worthwhile procedure.
Discussion
To yield nucleic acids of sufficient quality for analysis, standard methods of sample storage and transport generally involve refrigeration or fixation, and can prove cumbersome and expensive. Blood samples have routinely been taken onto filter paper for analysis [7,8]. Unfortunately, such samples may not be suitable for long term storage as they do not protect the sample from spoiling and degradation. FTA® paper is a commercial product which consists of filter paper impregnated with a proprietary mix of chemicals. Previously published work using FTA® paper has used processing conditions suggested by the manufacturer[1,9,10]. The methods in this paper have been adjusted to take into account the problems likely to be faced by the wildlife researcher e.g. the different types of sample available, the presence of anti-coagulants, sample overload on the paper or even different DNA contents of the applied samples. All of the methods follow the same basic procedure:
1. The sample is applied to the FTA® paper and allowed to dry: at this point, the cellular material lyses and the nucleic acid becomes entangled in the paper
2. A small disc of the FTA® paper is washed several times to remove contaminants and DNA binding proteins.
At this point the DNA is available for analysis either in situ (i.e. on the paper) or it can be eluted. The size of FTA® disc, composition of wash solutions and whether or not the DNA is eluted varies depending on the sample being analysed.
The stability of DNA when left unprocessed on FTA® paper, and the small area of the paper which is processed at each time, means that the same samples can be analysed many times over a period of several years with no concerns that DNA degradation has adversely affected the results. As long-term stability of the DNA once it has been eluted from the FTA® paper has not yet been investigated, it is preferable to only process the samples as required. The storage of multiple samples on one piece of FTA® paper with no fear of cross contamination also means that many thousands of samples can be stored in the drawer of a filing cabinet.
FTA® paper may also been used to purify DNA from plant tissues [11] and RNA may also be purified from samples with subtle alterations to the washing procedures [12].
Methods
PCR methods
PCR for the amplification of the avian CHD gene was performed as described [3]. PCR for amplification of avian and mammalian DNA repeat sequences (ILRCs) was as previously described [4]. Gels are standard 1% agarose TBE gels.
Restriction digests
Digestions were performed in 50 μl volumes, in buffers appropriate to the restriction enzyme as supplied by the manufacturer. The processed FTA® discs were placed directly into the enzyme-containing buffer and incubated overnight (generally at 37°C, but as appropriate for the restriction enzyme being used).
Authors' Contributions
LMS performed all processing and molecular biology.
LAB designed and supervised the study.
Supplementary Material
This file contains the table referred to in the manuscript. It contains a summary of suggested protocols for processing samples collected on FTA paper, according to sample type and end-use.
Acknowledgments
Acknowledgements
The FTA® paper used in this study was provided by Whatman.
Samples were provided by Dr J Robertson, Dr G Johnston.
Contributor Information
LM Smith, Email: LMSmith@cyllene.uwa.edu.au.
LA Burgoyne, Email: leigh.burgoyne@flinders.edu.au.
References
- Vanek D, Hradil R, Budowle B. Czech population data on 10 short tandem repeat loci of SGM Plus system kit using DNA purified in FTA cards. Forensic Science International. 2001;119:107. doi: 10.1016/S0379-0738(00)00384-4. [DOI] [PubMed] [Google Scholar]
- Hsiao KM, Lin HM, Pan H, Li TC, Chen SS, Jou SB, Chiu YL, Wu MF, Lin CC, Li SY. Application of FTA® Sample Collection and DNA Purification System on the Determination of CTG Trinucleotide Repeat Size by PCR-Based Southern Blotting. Journal of Clinical Laboratory Analysis. 1999;13:188–193. doi: 10.1002/(SICI)1098-2825(1999)13:4<188::AID-JCLA8>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJ. A DNA test to sex most birds. Molecular Ecology. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Smith LM, Burgoyne LA. Species identity: conserved inverted LINE repeat clusters (ILRC) in the vertebrate genome as indicators of population boundaries. Gene. 2001;271:273–283. doi: 10.1016/S0378-1119(01)00507-8. [DOI] [PubMed] [Google Scholar]
- Seah LH, Burgoyne LA. DNA data-basing on FTA® paper :Biological assault and techniques for measuring photogenic damage. In: GF Sensabaugh, PJ Lincoln, OB, editor. Progress in Forensic Genetics. Vol. 8 2000. [Google Scholar]
- Rogers CD, Burgoyne LA. Bacterial Typing: Storing and Processing of Stabilized Reference Bacteria for Polymerase Chain Reaction Without Preparing DNA – An Example of an Automatable Procedure. Analytical Biochemistry. 1997;247:223–227. doi: 10.1006/abio.1997.2031. [DOI] [PubMed] [Google Scholar]
- Geysen G, Bishop R, Skilton R, Dolan TT, Morzaria S. Molecular epidemiology of Theileria parva in the field. Tropical Medicine and International Health. 1999;4:A21–A27. doi: 10.1046/j.1365-3156.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Panteleeff DD, John G, Nduati R, Mbori-Ngacha D, Richardson B, Kreiss J, Overbaugh J. Rapid Method for Screening Dried Blood Samples on Filter Paper for Human Immunodeficiency Virus Type 1 DNA. Journal of Clinical Microbiology. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LJ, Maddigan MN, Carter AB, Earls L. Use of FTA Gene Guard Filter Paper for the Storage and Transportation of Tumour Cells for Molecular Testing. Archives of Pathology and Laboratory Medicine. 2002;126:56–63. doi: 10.5858/2002-126-0056-UOFGGF. [DOI] [PubMed] [Google Scholar]
- Devost NC, Choy FY. Mutation analysis of Gaucher disease using dot-blood samples on FTA® filter paper. American Journal of Medical Genetics. 2001;94:417–420. doi: 10.1002/1096-8628(20001023)94:5<417::AID-AJMG14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lin J-J, Fleming R, Kuo J, Matthews BF, Saunders JA. Detection of Plant Genes Using a Rapid, Nonorganic DNA Purification Method. Biotechniques. 2000;28:346–50. doi: 10.2144/00282pf01. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Burgoyne LA. Reverse Transcription of an RNA Genome from Databasing Paper: FTA©. Applied Biochemistry & Biotechnology. 2000;31:219–224. doi: 10.1042/BA19990113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the table referred to in the manuscript. It contains a summary of suggested protocols for processing samples collected on FTA paper, according to sample type and end-use.