Abstract
Methylene blue (MB, methylthioninium chloride) is a phenothiazine that crosses the blood brain barrier and acts as a redox cycler. Among its beneficial properties are its abilities to act as an antioxidant, to reduce tau protein aggregation and to improve energy metabolism. These actions are of particular interest for the treatment of neurodegenerative diseases with tau protein aggregates known as tauopathies. The present study examined the effects of MB in the P301S mouse model of tauopathy. Both 4 mg/kg MB (low dose) and 40 mg/kg MB (high dose) were administered in the diet ad libitum from 1 to 10 months of age. We assessed behavior, tau pathology, oxidative damage, inflammation and numbers of mitochondria. MB improved the behavioral abnormalities and reduced tau pathology, inflammation and oxidative damage in the P301S mice. These beneficial effects were associated with increased expression of genes regulated by NF-E2-related factor 2 (Nrf2)/antioxidant response element (ARE), which play an important role in antioxidant defenses, preventing protein aggregation, and reducing inflammation. The activation of Nrf2/ARE genes is neuroprotective in other transgenic mouse models of neurodegenerative diseases and it appears to be an important mediator of the neuroprotective effects of MB in P301S mice. Moreover, we used Nrf2 knock out fibroblasts to show that the upregulation of Nrf2/ARE genes by MB is Nrf2 dependent and not due to secondary effects of the compound. These findings provide further evidence that MB has important neuroprotective effects that may be beneficial in the treatment of human neurodegenerative diseases with tau pathology.
INTRODUCTION
Neurodegenerative diseases involving pathological tau protein aggregation are collectively known as tauopathies and include Alzheimer's disease (AD), progressive supranuclear palsy (PSP), Pick's disease (PD), frontotemporal dementia with parkinsonism linked to chromosome 17, chronic traumatic encephalopathy and ALS–Parkinsonism-dementia complex of Guam. Tau is a microtubule-associated protein (MAP) that is primarily expressed in the central nervous system (CNS) and is responsible for polymerization and stabilization of microtubules. However, in disease states tau is mutated, misfolded and forms aggregates in non-native conformations. This contributes to both a reduction in the normal physiological activity of the tau protein and to dysfunction associated with tau toxicity. Tau aggregates into both filamentous and non-filamentous inclusions, and disease manifestations vary among the tauopathies in both their brain regional specificity and their symptoms. Tau aggregates known as neurofibrillary tangles (NFTs), as well as tau oligomers, contribute to both brain dysfunction and degeneration in many age-related neurodegenerative diseases. Tau oligomers, which can be transferred between neurons, may play a critical role in tau-related diseases (1).
A number of studies show that oxidative stress and inflammation play a role in tau-associated neurodegeneration. Oxidative stress reflects a disruption in redox balance due to either an increase in production of oxidants and/or a reduction in antioxidant species. It can lead to DNA, protein and lipid damage, subsequently leading to cell dysfunction and death. Oxidative damage occurs early in AD (2) as shown by oxidative damage to DNA, proteins and lipids (3–7). Inflammation, on the other hand, is a response to cell damage. It is elevated in tauopathies and exacerbates disease pathology (8–10). Therefore, stimulating antioxidant and anti-inflammatory pathways is of tremendous therapeutic interest for the treatment of tauopathies.
Methylene blue (MB, methylthioninium chloride) is a phenothiazine known for its ability to cross the blood brain barrier and to exert neuroprotective effects (11,12), which makes it attractive as a potential treatment for neurodegenerative diseases. MB has a long history as a therapeutic agent and clinical applications have included treatment of depression, cancer, methemoglobinemia and AD (13). Among its beneficial properties, MB is a redox cycler and an electron donor (11). It blocks tau aggregation in vitro and reduces the amount of tau aggregates in a C. elegans model of tau pathology (14,15). Recently, its ability to inhibit tau aggregation was shown to be due to its ability to oxidize cysteine residues (16,17). Using NMR spectroscopy, it was shown that MB oxidized tau cysteine sulfhydryl groups to sulfenic, sulfinic and sulfonic acids, maintaining tau in a monomeric state and blocking misfolding (16). Strong evidence favoring this mechanism comes from replacing the cysteines in tau with other amino acids, which results in tau that still aggregates, but in which MB no longer blocks the aggregation. MB also inhibits nitric oxide synthase, decreases oxidative damage, induces autophagy and improves mitochondrial function and cellular respiration (11,13,18), actions that increase its therapeutic potential for the treatment of tauopathies. The initial studies showing that MB blocked tau aggregation led to phase 2 clinical trials in AD with promising initial results, and it is now in phase 3 clinical trials (15,19).
The present study evaluated the neuroprotective effects of MB [4 mg/kg (low dose) and 40 mg/kg (high dose) in the diet] in the P301S transgenic mice. P301S transgenic mice and wild-type (WT) littermates were randomly assigned to either control diet, MB low dose diet or MB high dose diet. Diets were provided ad libitum from 1 to 10 months of age. The P301S transgenic mice express the P301S mutation in the gene encoding the human microtubule-associated protein tau (MAPT). These mice develop progressive behavioral deficits and NFTs, the pathology characteristic of tauopathies (20,21). We found that MB improved behavioral abnormalities, reduced tau pathology, reduced oxidative stress and inflammation and increased mitochondrial biogenesis in P301S mice, and that these neuroprotective effects were associated with increased expression of NF-E2-related factor 2 (Nrf2)/antioxidant response element (ARE) activated genes.
RESULTS
Methylene blue improved behavioral deficits in P301S mice
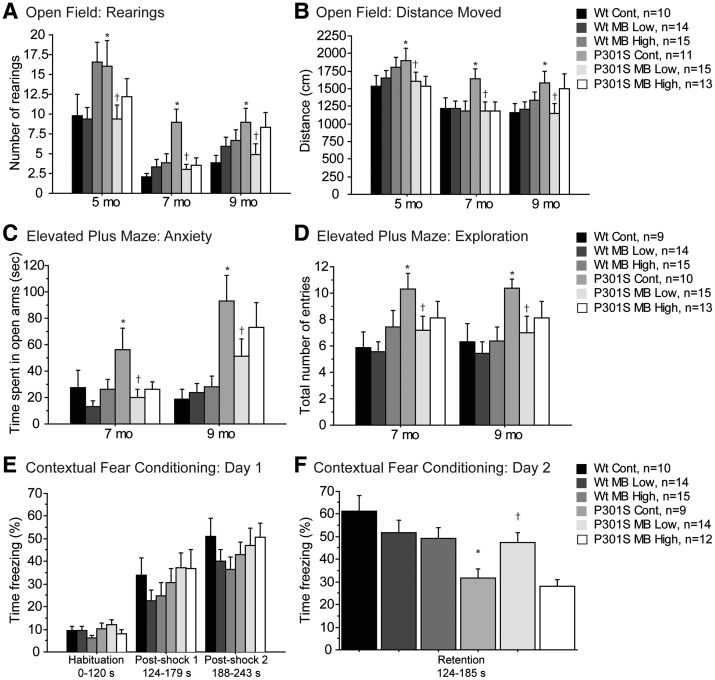
P301S mice exhibit behavioral abnormalities such as hyperactivity, disinhibition and cognitive deficits. A battery of behavioral tests, including the open field, elevated plus maze and contextual fear conditioning, was employed in order to assess the effects of MB on these behavioral abnormalities (Fig. 1). Locomotion and exploration were assessed by the open field test at 5, 7 and 9 months of age. P301S mice fed a control diet showed hyperactivity at baseline as indicated by a significantly increased number of rearings and distance moved in the open field apparatus, when compared with WT mice fed a control diet (Fig. 1A and B). This locomotor abnormality was improved by administration of the MB low dose diet (Fig. 1A and B).
Figure 1.
MB improved behavioral deficits in P301S mice. (A) Number of rearings and (B) distance moved in the open field in WT mice fed a control diet (Wt Cont, n = 10), WT mice fed the MB low dose diet (Wt MB low, n = 14), WT mice fed the MB high dose diet (Wt MB High, n = 15), P301S mice fed a control diet (P301S Cont, n = 11), P301S mice fed the MB low dose diet (P301S MB Low, n = 15) and P301S mice fed the MB high dose diet (P301S MB High, n = 13). P301S mice fed a control diet were significantly more hyperactive in the open field test when compared with WT mice fed a control diet (Fisher's PLSD, *P < 0.05). MB significantly reduced hyperactivity in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). (C) Time spent in the open arms and (D) total number of entries between arms in the elevated plus maze in Wt Cont (n = 9), Wt MB Low (n = 14), Wt MB High (n = 15), P301S Cont (n = 10), P301S MB Low (n = 15) and P301S MB High (n = 13). P301S mice fed a control diet were significantly dis-inhibited and significantly more hyperactive in the elevated plus maze when compared with WT mice fed a control diet (Fisher's PLSD, *P < 0.05). MB significantly reduced behavioral abnormalities in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). (E) Percent time freezing on day 1 and (F) day 2 of the contextual fear conditioning test in Wt Cont (n = 10), Wt MB Low (n = 14), Wt MB High (n = 15), P301S Cont (n = 9), P301S MB Low (n = 14) and P301S MB High (n = 12). There were no significant differences between groups on day 1 of testing. P301S mice fed a control diet showed a significant decrease in memory during the retention period on day 2 compared with WT mice fed a control diet (Fisher's PLSD, *P < 0.05). MB significantly increased learning and memory in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet on day 2 of testing (Fisher's PLSD, †P < 0.05).
Anxiety and exploration were measured using the elevated plus maze test at 7 and 9 months of age. Time spent in the open arms and total number of entries were measured during a 4 min trial. P301S mice fed a control diet again exhibited baseline behavioral abnormalities when compared with WT mice fed a control diet with both a significant decrease in anxiety, as evidenced by increased time spent in the open arms, and a significant increase in exploration, as evidenced by the increased number of entries into the arms of the maze (Fig. 1C and D). The MB low dose diet improved the anxiety and exploration-related behavioral abnormalities (Fig. 1C and D).
At 9 months of age, learning and memory were assessed by the contextual fear conditioning paradigm in which an aversive electrical shock stimulus was used in a neutral context. The percent time freezing was measured as an indicator of fear response. There were no significant differences between groups during the acquisition phase on day 1 of testing (Fig. 1E). However, P301S mice fed a control diet spent significantly less time freezing during the retention period on day 2 of testing when compared with WT mice fed a control diet, indicating an impaired ability to remember the association between the aversive stimulus and context (Fig. 1F). Interestingly, P301S mice fed the MB low dose diet showed a significant improvement in learning and memory when compared with P301S mice fed a control diet, as indicated by a significant increase in the percent time freezing during the retention period on day 2 of testing (Fig. 1F).
Methylene blue affected behavior in a gender dependent manner
A gender split of behavioral data further revealed that improvements in behavioral abnormalities tended to occur in male P301S mice fed MB low dose diet rather than female P301S mice fed MB low dose diet. In the open field, female P301S mice fed a control diet showed a significant increase in the number of rearings when compared with female WT mice fed a control diet (Supplementary Material, Table S1A). Male P301S mice fed the MB low dose diet showed a significant decrease in both the number of rearings and total distance moved when compared with male P301S mice fed a control diet (Supplementary Material, Table S1A). However, female P301S mice fed the MB low dose diet showed a trend for decreased rearings (P = 0.0787, Supplementary Material, Table S1A). In the elevated plus maze, both male and female P301S mice fed a control diet showed significantly increased anxiety as indicated by significantly increased time spent in the open arms of the apparatus when compared with male and female WT mice fed a control diet, respectively (Supplementary Material, Table S1A). Male P301S mice fed the MB low dose diet showed a significant decrease in anxiety when compared with male P301S mice fed a control diet (Supplementary Material, Table S1A). Male P301S mice fed a control diet were significantly more hyperactive than male WT mice fed a control diet as indicated by increased number of entries into the arms of the apparatus, while male P301S mice fed MB low dose diet showed significantly reduced hyperactivity when compared with male P301S mice fed a control diet (Supplementary Material, Table S1A). This was not seen in the female mice. In the contextual fear conditioning test, there were no significant differences between groups on day 1 even with data split by gender. During the retention period on day 2, both male and female P301S mice fed a control diet spent significantly less time freezing when compared with male and female WT mice fed a control diet (Supplementary Material, Table S1A). Male P301S mice fed the MB low dose diet showed a trend for increased time spent freezing when compared with male P301S mice fed a control diet (Supplementary Material, Table S1A). Note that a gender split was not performed for other analyses because the resultant would be too low for statistical information.
Diet containing methylene blue high dose increased body weight in male P301S mice
Dietary consumption was measured at 5, 7 and 9 months of age for all groups. There were no significant differences in food consumption between any of the groups (Supplementary Material, Fig. S1C). Body weight was also measured at 5, 7 and 9 months of age. Male P301S mice fed a control diet showed significantly decreased body weight when compared with male WT mice fed a control diet (Supplementary Material, Fig. S1A). However, administration of the MB high dose diet improved body weight in male P301S mice when compared with male P301S mice fed a control diet (Supplementary Material, Fig. S1A). There were no significant differences in body weight between the groups in female mice (Supplementary Material, Fig. S1B).
Methylene blue decreased tau pathology in P301S mice
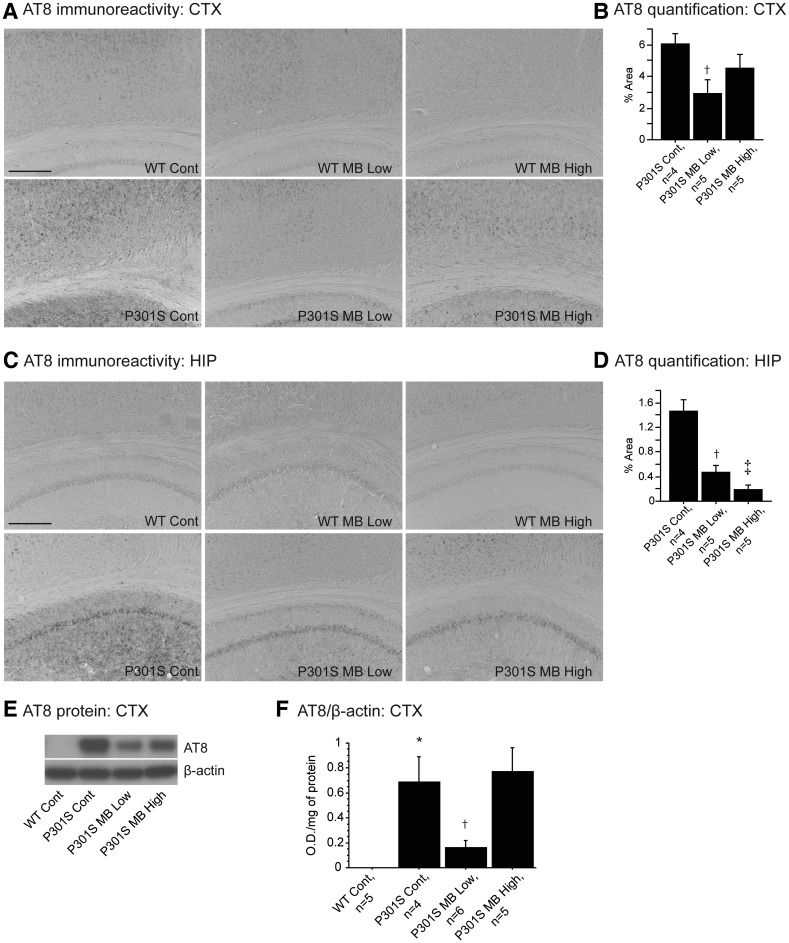
In order to assess the effects of MB on tau pathology, brain sections were stained with the AT8 antibody, a human tau antibody that detects paired helical filamentous (PHF)-tau (Ser202/Thr205; Fig. 2). AT8 immunoreactivity was higher in the P301S groups when compared with WT groups as expected. Because tau pathology is not present in WT mice, quantitative analysis of AT8 immunostaining was performed in P301S brain sections only. The P301S mice treated with the MB low dose diet showed a significant decrease in AT8 immunoreactivity in cerebral cortex when compared with P301S mice fed a control diet (Fig. 2A and B). Both P301S mice fed the MB low dose diet and the MB high dose diet showed a significant decrease in AT8 immunoreactivity in the hippocampus when compared with P301S mice fed a control diet (Fig. 2C and D). Western blot was also used to assess the level of hyperphosphorylated tau using the AT8 antibody in the cerebral cortex of WT mice fed a control diet, P301S mice fed a control diet, P301S mice fed the MB low dose diet and P301S mice fed the MB high dose diet (Fig. 2E and F). In keeping with our histological analyses, hyperphosphorylated tau was not present in WT mice fed a control diet (Fig. 2E and F). The P301S mice fed the MB low dose diet showed significantly increased phosphorylation of tau when compared with WT mice fed a control diet, while P301S mice fed the MB low dose diet showed significantly reduced phosphorylation of tau when compared with P301S mice fed a control diet (Fig. 2F). Taken together, the western blot data on AT8 protein in the cerebral cortex corroborates our immunohistochemical data of AT8 in the cerebral cortex.
Figure 2.
MB decreased tau pathology in P301S mice. (A) AT8 immunoreactivity in the cortex and (C) hippocampus of WT mice fed a control diet (Wt Cont), WT mice fed the MB low dose diet (Wt MB Low), WT mice fed the MB high dose diet (Wt MB High), P301S mice fed a control diet (P301S Cont), P301S mice fed the MB low dose diet (P301S MB Low) and P301S mice fed the MB high dose diet (P301S MB High). (B) Quantification of AT8 staining in cortex and (C) hippocampus of P301S Cont (n = 4), P301S MB Low (n = 5) and P301S MB High (n = 5). MB significantly decreased tau pathology in the cortex and hippocampus of P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05) and in hippocampus of P301S mice fed the MB high dose diet compared with P301S mice fed a control diet (Fisher's PLSD, ‡P < 0.05). (E) Western blot of AT8 and (F) quantification of hyperphosphorylated tau by optical densities normalized to β-actin. P301S mice fed a control diet showed significantly increased levels of hyperphosphorylated tau compared with WT mice fed a control diet. Mice fed MB low dose diet showed significantly decreased phosphorylation of tau when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05).
Methylene blue reduced astrogliosis in P301S mice
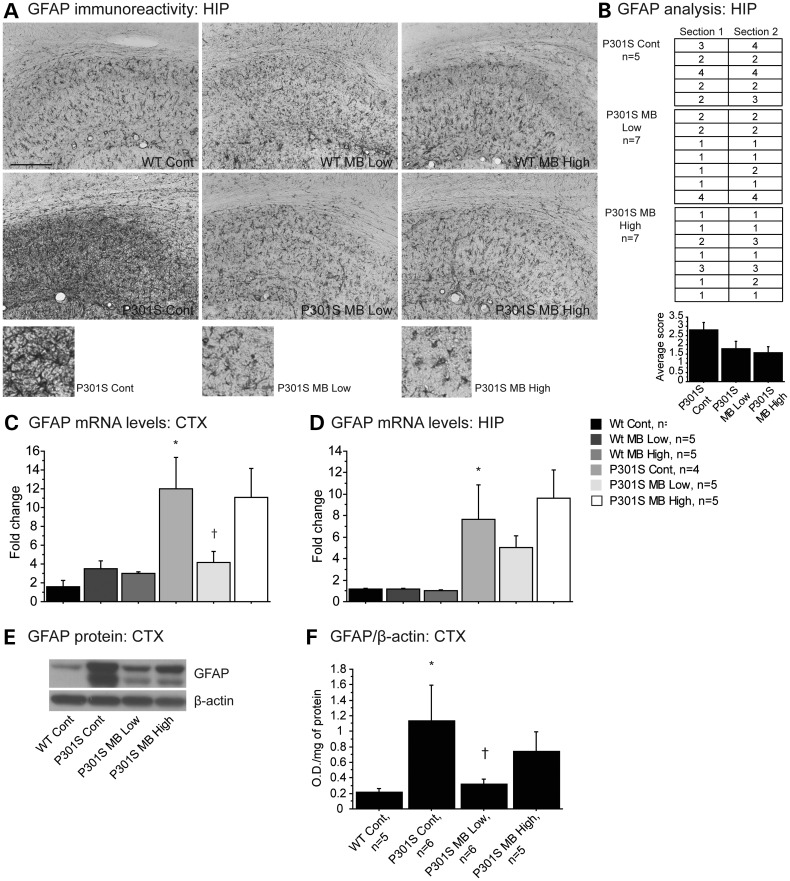
Astrocytes proliferate in response to CNS injury and inflammation. Immunohistochemical analysis of astrogliosis was performed in the cerebral cortex and hippocampus using a glial fibrillary acidic protein (GFAP) antibody (Fig. 3). There was very little positive staining for GFAP in the cerebral cortex in any of the groups (data not shown). In the hippocampus of P301S mice fed a control diet, GFAP immunoreactivity was increased when compared with each of the groups of WT mice (Fig. 3A). P301S mice fed either the MB low dose diet or the MB high dose diet showed markedly reduced GFAP immunoreactivity when compared with P301S mice fed a control diet (Fig. 3A and B). GFAP mRNA levels were measured by quantitative real-time PCR (qRT-PCR). In keeping with immunohistochemical analysis, mRNA levels of GFAP were significantly increased in the cerebral cortex and hippocampus of P301S mice fed a control diet when compared with WT mice fed a control diet (Fig. 3C and D). In the cerebral cortex of P301S mice fed the MB low dose diet, mRNA levels of GFAP were significantly reduced when compared with P301S mice fed a control diet (Fig. 3C). Western blot was used to measure GFAP protein levels in the cerebral cortex tissue. Protein levels of GFAP were significantly increased in the cerebral cortex of P301S mice fed a control diet when compared with WT mice fed a control diet (Fig. 3E and F). Mice fed the MB low dose diet showed significantly reduced levels of GFAP protein when compared with P301S mice fed a control diet (Fig. 3E and F). These data are consistent with both our immunohistochemical and qRT-PCR data in cerebral cortex tissue.
Figure 3.
MB reduced astrogliosis in P301S mice. (A) GFAP immunoreactivity in hippocampus of WT mice fed a control diet (Wt Cont), WT mice fed the MB low dose diet (Wt MB Low), WT mice fed the MB high dose diet (Wt MB High), P301S mice fed a control diet (P301S Cont), P301S mice fed the MB low dose diet (P301S MB Low) and P301S mice fed the MB high dose diet (P301S MB High). (B) Visual analysis of staining in the hippocampus of P301S Cont (n = 5), P301S MB Low (n = 7) and P301S MB High (n = 7). Data expressed as ‘1’ for minimal immunoreactivity, ‘2’ for mild immunoreactivity, ‘3’ for moderate immunoreactivity and ‘4’ for extensive immunoreactivity. MB decreased the apparent levels of GFAP in both P301S mice fed the MB low dose diet and the MB high dose diet when compared with P301S mice fed a control diet. (C) GFAP mRNA levels in cerebral cortex and (D) hippocampus of Wt Cont (n = 4), Wt MB Low (n = 5), Wt MB High (n = 5), P301S Cont (n = 4), P301S MB Low (n = 5) and P301S MB High (n = 5). P301S mice fed a control diet showed significantly increased mRNA levels of GFAP when compared with WT mice fed a control diet. MB significantly decreased GFAP in the cerebral cortex of P301S mice fed the MB low dose when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). (E) Western blot of GFAP and (F) quantification by optical densities normalized to β-actin. P301S mice fed a control diet showed significantly increased levels of GFAP protein compared with WT mice fed a control diet. Mice fed MB low dose diet showed significantly decreased GFAP protein when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05).
Methylene blue reduced oxidative stress and promoted mitochondrial biogenesis in P301S mice
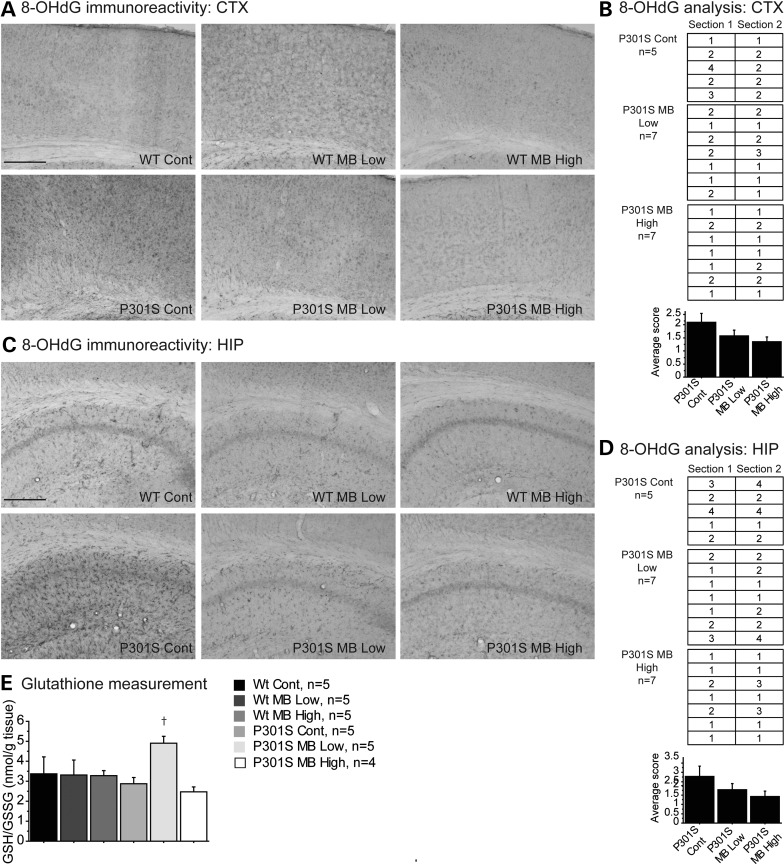
Oxidative stress is a characteristic feature of tauopathies (22,23). Immunohistochemical analysis of oxidative stress was performed in the cerebral cortex and hippocampus tissue using an 8-hydroxy-2′-deoxyguanosine (8-OHdG) antibody, an antibody that detects oxidative DNA damage (Fig. 4). In the cerebral cortex and hippocampus of P301S mice fed a control diet, there was increased 8-OHdG staining when compared with each of the groups of WT mice (Fig. 4A and C). The P301S mice fed either the MB low dose diet or the MB high dose diet showed markedly reduced 8-OHdG staining when compared with P301S mice fed a control diet in both the cerebral cortex (Fig. 4A and B) and the hippocampus (Fig. 4C and D).
Figure 4.
MB reduced oxidative stress in P301S mice. (A) 8-OHdG immunoreactivity in cortex and (C) hippocampus of WT mice fed a control diet (Wt Cont), WT mice fed the MB low dose diet (Wt MB Low), WT mice fed the MB high dose diet (Wt MB High), P301S mice fed a control diet (P301S Cont), P301S mice fed the MB low dose diet (P301S MB Low) and P301S mice fed the MB high dose diet (P301S MB High). (B) Visual analysis of staining in cortex and (D) hippocampus of P301S Cont (n = 5), P301S MB Low (n = 7) and P301S MB High (n = 7). Data expressed as ‘1’ for minimal immunoreactivity, ‘2’ for mild immunoreactivity, ‘3’ for moderate immunoreactivity and ‘4’ for extensive immunoreactivity. MB decreased the apparent oxidative DNA damage in both P301S mice fed the MB low dose diet and the MB high diet dose when compared with P301S mice fed a control diet. (E) Glutathione levels in cerebral cortex of Wt Cont (n = 5), Wt MB Low (n = 5), Wt MB High (n = 5), P301S Cont (n = 5), P301S MB Low (n = 5) and P301S MB High (n = 4). MB increased the ratio of reduced to oxidized glutathione (GSH/GSSG) in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet.
Endogenous antioxidant systems responsible for maintaining cellular redox balance were also assessed as an indicator of oxidative stress. Glutathione, an important cellular antioxidant, was measured in cerebral cortex tissue by HPLC coupled to mass spectrometry (Fig. 4). The ratio of reduced glutathione to oxidized glutathione (GSH/GSSG) was significantly increased in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet, indicating higher levels of reduced glutathione in the MB treated mice (Fig. 4E).
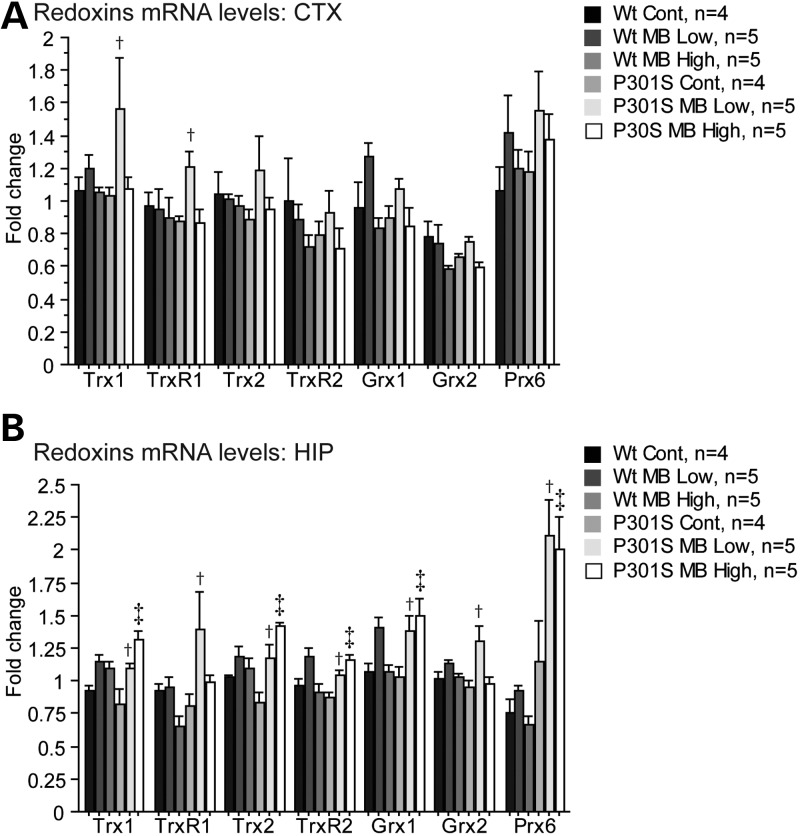
Redox proteins were also analyzed. mRNA levels of thioredoxin 1 (Trx1), thioredoxin reductase 1 (TrxR1), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TrxR2), glutaredoxin 1 (Grx1), glutaredoxin 2 (Grx2) and peroxiredoxin 6 (Prx6) were measured by qRT-PCR in both the cerebral cortex and hippocampus (Fig. 5). In the cerebral cortex, P301S mice fed the MB low dose diet showed significant increases in mRNA levels of Trx1 and TrxR1 when compared with P301S mice fed a control diet (Fig. 5A). In the hippocampus, P301S mice fed the MB low dose diet showed significant increases in mRNA levels of Trx1, TrxR1, Trx2, TrxR2, Grx1, Grx2 and Prx6 when compared with P301S mice fed a control diet, while P301S mice fed the MB high dose diet showed significant increases in the mRNA levels of Trx1, Trx2, TrxR2, Grx1 and Prx6 when compared with P301S mice fed a control diet (Fig. 5B).
Figure 5.
MB increased expression of redoxins in P301S mice. (A) Redoxins mRNA levels in the cerebral cortex and (B) hippocampus in WT mice fed a control diet (Wt Cont, n = 4), WT mice fed the MB low dose diet (Wt MB Low, n = 5), WT mice fed the MB high dose diet (Wt MB High, n = 5), P301S mice fed a control diet (P301S Cont, n = 4), P301S mice fed the MB low dose diet (P301S MB Low, n = 5) and P301S mice fed the MB high dose diet (P301S MB High, n = 5). MB significantly increased expression of Trx1 and TrxR1 in cerebral cortex tissue of P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). In hippocampus, MB also significantly increased expression of Trx1, Trx2, TrxR2, Grx1 and Prx6 in both P301S mice fed the MB low dose diet and the MB high dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05, ‡P < 0.05) and TrxR1 and Grx2 in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05).
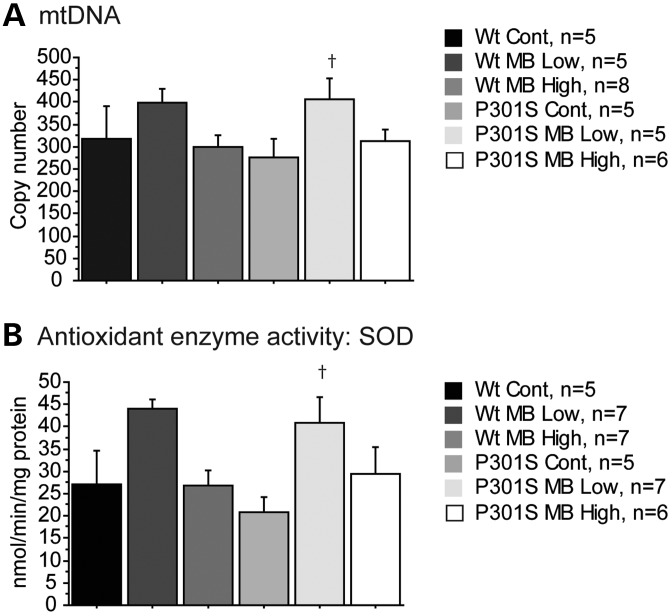
MB has been shown to increase mitochondrial function (11,13,24). Mitochondrial biogenesis was assessed by measuring mitochondrial DNA (mtDNA) copy number, which correlates with numbers of mitochondria (25). There was a significant increase in mtDNA copy number in the P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet, indicating improved mitochondrial biogenesis in these mice (Fig. 6A). The levels of activity superoxide dismutase (SOD), a key enzyme involved in cellular antioxidant defense and controlled by the Nrf2 transcription factor, were also assessed. P301S mice fed the MB low dose diet showed a significant increase in SOD activity when compared with P301S mice fed a control diet (Fig. 6B).
Figure 6.
MB promoted antioxidant defenses in P301S mice. (A) mtDNA copy number in WT mice fed a control diet (Wt Cont, n = 5), WT mice fed the MB low dose diet (Wt MB Low, n = 5), WT mice fed the MB high dose diet (Wt MB High, n = 8), P301S mice fed a control diet (P301S Cont, n = 5), P301S mice fed the MB low dose diet (P301S MB Low, n = 5) and P301S mice fed the MB high dose diet (P301S MB High, n = 6). MB significantly increased mtDNA copy number in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). (B) Antioxidant enzyme activity level for SOD in Wt Cont (n = 5), Wt MB Low (n = 7), Wt MB High (n = 7), P301S Cont (n = 5), P301S MB Low (n = 7) and P301S MB High (n = 6). MB significantly increased SOD activity in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05).
Methylene blue upregulated Nrf2/ARE genes in P301S mice
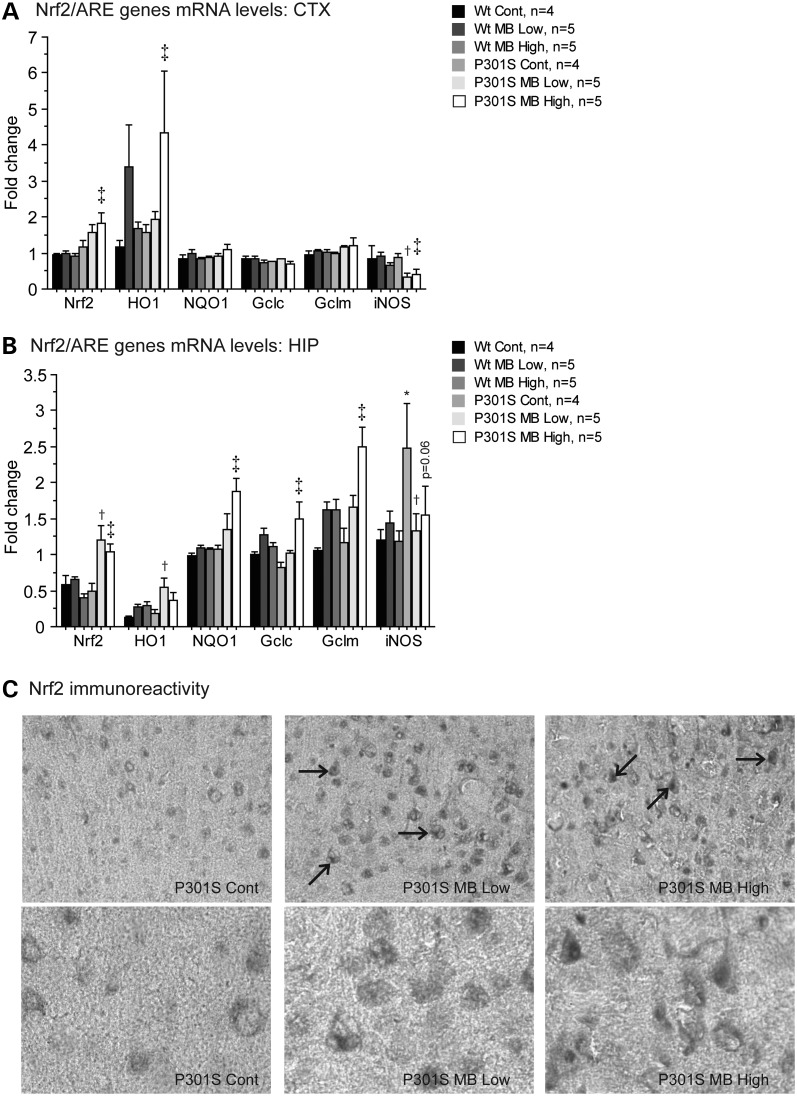
The Nrf2/ ARE pathway is involved in defense against oxidative stress and inflammation and it exerts neuroprotective effects. Conversely, inflammatory processes contribute to neurodegeneration in tauopathies (8–10). MB activated the expression of prototypical genes known to be activated by the Nrf2/ARE pathway (Fig. 7). The mRNA levels of Nrf2, heme oxygenase 1 (HO1), NAD(P)H-quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (Gclc), glutamate-cysteine ligase regulatory subunit (Gclm) and inducible nitric oxide synthase (iNOS) were measured by RT-PCR in both the cerebral cortex and hippocampus. In the cerebral cortex, both Nrf2 and HO1 mRNA levels were significantly increased in P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fig. 7A). In the hippocampus, the mRNA level of Nrf2 was significantly increased in the P301S mice fed either the MB low dose diet or the MB high dose diet when compared with P301S mice fed a control diet (Fig. 7B). The mRNA level of HO1 was significantly increased in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet, and the mRNA levels of NQO1, Gclc and Gclm were significantly increased in P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fig. 7B). In the cerebral cortex, P301S mice fed the MB low dose diet showed significantly decreased iNOS mRNA when compared with P301S mice fed a control diet (Fig. 7A). In the hippocampus, P301S mice fed a control diet showed a significant increase in iNOS mRNA when compared with WT mice fed a control diet (Fig. 7B). However, P301S mice fed the MB low dose diet again showed significantly decreased iNOS mRNA when compared with P301S mice fed a control diet (Fig. 7B). Moreover, iNOS mRNA levels were significantly reduced in the cerebral cortex and nearly significantly reduced in the hippocampus (P = 0.06) in the P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fig. 7B).
Figure 7.
MB modulated Nrf2/ARE genes in P301S mice. (A) Nrf2/ARE genes in cerebral cortex and (B) hippocampus of WT mice fed a control diet (Wt Cont, n = 4), WT mice fed the MB low dose diet (Wt MB Low, n = 5), WT mice fed the MB high dose diet (Wt MB High, n = 5), P301S mice fed a control diet (P301S Cont, n = 4), P301S mice fed the MB low dose diet (P301S MB Low, n = 5) and P301S mice fed the MB high dose diet (P301S MB High, n = 5). In the cerebral cortex, MB significantly increased expression of Nrf2, and HO1 in P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, ‡P < 0.05) and significantly decreased expression of iNOS in P301S mice fed the MB low dose diet and the MB high dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05, ‡P < 0.05). In the hippocampus, MB significantly increased expression of Nrf2 in P301S mice fed the MB low dose diet and the MB high dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05, ‡P < 0.05), and significantly increased expression of HO1 in P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05) and NQO1, Gclc and Gclm in P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, ‡P < 0.05). iNOS mRNA levels were significantly increased in the hippocampus of P301S mice fed a control diet when compared with WT mice fed a control diet (Fisher's PLSD, *P < 0.05). MB significantly decreased iNOS expression in hippocampus of P301S mice fed the MB low dose diet when compared with P301S mice fed a control diet (Fisher's PLSD, †P < 0.05). (C) Nrf2 immunoreactivity in P301S Cont, P301S MB Low and P301S MB High. MB increased the apparent Nrf2 immunoreactivity and caused nuclear translocation of Nrf2 in P301S mice fed the MB low dose diet and P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet.
Immunocytochemical staining showed that MB caused translocation of Nrf2 from the cytoplasm to the nucleus in P301S mice fed either the MB low dose diet or the MB high dose diet (Fig. 7C). Nrf2 immunoreactivity was increased in both P301S mice fed the MB low dose diet and P301S mice fed the MB high dose diet when compared with P301S mice fed a control diet (Fig. 7C). Moreover, staining appeared diffuse and cytoplasmic in P301S mice fed a control diet, while staining appeared darker and nuclear in P301S mice fed the MB low dose diet or the MB high dose diet (Fig. 7C).
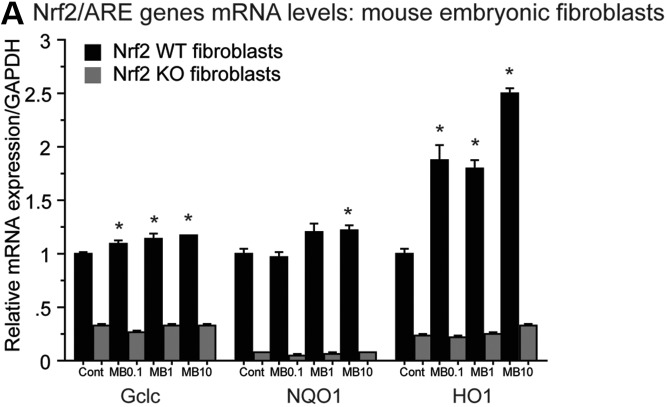
To further confirm that MB acted as Nrf2/ARE activator, we used Nrf2 WT and Nrf2 knock out (KO) mouse embryonic fibroblasts (MEFs). MB treatment induced transcription of HO1, NQO1 and Gclc in Nrf2 WT MEFs but not Nrf2 KO MEFs (Fig. 8A). Nrf2 WT MEFs treated with 0.1, 1 or 10 μm MB showed significantly increased levels of HO1 mRNA compared with Nrf2 WT MEFs treated with vehicle; however, HO1 mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB (Fig. 8A). Nrf2 WT MEFs treated with 10 μm MB showed significantly increased levels of NQO1 mRNA compared with Nrf2 WT MEFs treated with vehicle and Nrf2 WT MEFs treated with 0.1 or 1 μm MB showed a trend for increased levels of NQO1 (Fig. 8A). NQO1 mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB (Fig. 8A). Nrf2 WT MEFs treated with 0.1, 1 or 10 μm MB showed significantly increased levels of Gclc mRNA compared with Nrf2 WT MEFs treated with vehicle; however, Gclc mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB (Fig. 8A).
Figure 8.
MB acts as Nrf2 activator. (A) mRNA levels of HO1, NQO1 and Gclc in Nrf2 WT and Nrf2 KO MEFs. Nrf2 WT MEFs treated with 0.1, 1 or 10 μm MB showed significantly increased levels of HO1 mRNA compared with Nrf2 WT MEFs treated with vehicle; however, HO1 mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB (paired Student’s t-test, *P < 0.05). Nrf2 WT MEFs treated with 10 μm MB showed significantly increased levels of NQO1 mRNA compared with Nrf2 WT MEFs treated with vehicle and Nrf2 WT MEFs treated with 0.1 or 1 μm MB showed a trend for increased levels of NQO1 (paired Student’s t-test, *P < 0.05). NQO1 mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB. Nrf2 WT MEFs treated with 0.1, 1 or 10 μm MB showed significantly increased levels of Gclc mRNA compared with Nrf2 WT MEFs treated with vehicle; however, Gclc mRNA levels remained unchanged in Nrf2 KO MEFs treated with 0.1, 1 or 10 μm MB (paired Student’s t-test, *P < 0.05).
DISCUSSION
Reduction and oxidation of the intracellular environment, known as redox, influences cellular signaling and can induce cellular proliferation, differentiation and death. Redox imbalance, involving increased oxidative species and decreased antioxidant defenses, causes oxidative stress that is injurious to DNA, proteins and lipids. Brain tissue is particularly susceptible to oxidative stress because it has a markedly increased rate of oxygen consumption, higher lipid content and fewer antioxidant species in comparison with other tissues (26). This is corroborated by the mounting evidence associating both aging and age-related dementias with oxidative damage (2). HO1, a stress induced protein, and other markers of oxidative damage have been localized to the lesions associated with AD, frontotemporal dementia, corticobasal degeneration and PSP, indicating that oxidative stress occurs in both AD and other tauopathies (27,28). Similarly, the p38 pathway is activated by oxidative stress and levels of phospho-p38 are elevated in PD and PSP, and it localizes to Pick bodies, NFTs and neuropil threads (29). Inflammatory processes also contribute to the progression of AD (30). Although dietary antioxidants and anti-inflammatory compounds combat the adverse effects of oxidative stress, they have thus far been unsuccessful in the treatment tauopathies. Transcriptional stimulation of both endogenous antioxidant and anti-inflammatory systems to produce a coordinated response may therefore be a more effective approach.
In the present study, we evaluated the effects of MB on disease progression in the P301S transgenic mice. These mice overexpress a mutant form of human MAPT and develop progressive behavioral deficits including hyperactivity and disinhibition, and exhibit tau-related neuropathology (20,21). Synaptic deficits, microglial activation, oxidative damage and mitochondrial dysfunction precede NFT formation in this model (20,31).
The phenothiazine MB (methylthioninium chloride) is known for its redox chemistry, and can function as an electron donor and acceptor (11,12). Because it readily crosses the blood brain barrier, it is of tremendous interest for the treatment of neurodegenerative diseases. MB has a hormetic dose–response in which its beneficial effects are optimal in the lower to intermediate range (11,13). It stimulates energy metabolism and has antioxidant effects at lower doses, whereas it decreases energy metabolism and acts as a pro-oxidant at higher doses (32). Similarly, dose-dependent memory improvement and increased brain oxygen consumption were seen in rats treated with 4 mg/kg MB, but were less consistent at 10 mg/kg MB (33).
Several recent reports examined the efficacy of MB in reducing tau pathology in vivo. In a zebra fish model, MB did not influence tau pathology, neuron loss or swimming behavior (34). In C. elegans expressing pro-aggregant tau, MB reduced detergent insoluble tau and improved locomotion (14). In P301L transgenic mice, 1 mm MB infused into the hippocampus improved learning in the Morris Water Maze (MWM) test (35). In another experiment, two groups of P301L mice were treated with 10 mg/kg of MB or vehicle in the drinking water for 12 weeks and the MB treatment significantly improved performance in the MWM test (35). This was accompanied by a reduction in soluble tau levels; however, there was no change in tau pathology. MB also improved learning and memory, induced proteasome activity and reduced amyloid-beta (Aβ) burden in 3xTg-AD mice, although there was no effect on tau (36). In a recent study, MB was administered orally to P301L transgenic mice for 5 months, beginning at 8–11 months of age, and there was a reduction in the detergent-insoluble phospho-tau and MC1-immunoreactive tau aggregates (37). Another recent study showed that MB induced autophagy and reduced both total tau and phospho-tau levels in P301L transgenic mice (18).
In the present experiments, P301S mice and WT littermates were fed either a control diet, a diet containing 4 mg/kg MB (low dose) or a diet containing 40 mg/kg MB (high dose) ad libitum from 1 to 10 months of age. MB low dose diet improved the behavioral abnormalities that are characteristic of P301S mice, namely, hyperactivity, disinhibition and learning and memory impairment. Hyperactivity and disinhibition were rescued in the P301S mice fed the MB low dose diet in both the open field and elevated plus maze. Moreover, treatment with the MB low dose diet improved learning and memory in the contextual fear conditioning test. Overall, the 4 mg/kg low dose of MB was efficacious at improving all aspects of the behavioral phenotype of the P301S mice, whereas the 40 mg/kg high dose was less effective, consistent with the hormetic dose response effect of MB seen in previous studies. Of note, the behavioral improvements were seen primarily in male P301S mice fed MB low dose diet when behavioral data were split by gender. The gender split was not performed for other analyses because the resultant would be too low for statistical information. Gender differences have been reported in human and animal studies of neurodegenerative diseases. We have previously shown that male P301S mice exhibit more deficits than female P301S mice, perhaps due to the potential protective effect of female hormones like estrogen (20).
Following behavioral assessment, tau pathology was evaluated in the cerebral cortex and hippocampus using the AT8 antibody. Tau accumulation and NFTs increase with age in P301S mice, and they are significantly increased by 10 months of age (20). The AT8 immunoreactivity was decreased by 34% in the cerebral cortex and by 67% in the hippocampus of P301S mice fed the MB low dose diet, and decreased by 87% in the hippocampus of P301S mice fed the MB high dose diet. Western blot analysis also revealed a significant reduction in tau phosphorylation in the cerebral cortex of P301S mice fed MB low dose diet when compared with P301S mice fed a control diet. MB treatment therefore results in a marked reduction in both phospho-tau immunoreactive neurons using an antibody that recognizes human NFTs and PHF-tau protein levels, consistent with previous reports showing that MB reduces tau and phosphor-tau levels both in vitro and in vivo (18).
Astrogliosis was assessed in the P301S mice as a marker of inflammation. Astrocytes are highly reactive and proliferate rapidly in response to CNS injury and inflammation. Immunohistochemical analysis of the P301S mice fed a control diet showed increased astrogliosis when compared with the WT mice, indicating a pronounced increase in reactive astrocytes, consistent with a previous study (31). There is a correlation between increased numbers of reactive astrocytes and mutant tau protein accumulation in TgR406W transgenic mice, another animal model of tauopathy (38). In the present study, both P301S mice fed the MB low dose diet and the MB high dose diet showed markedly reduced astrogliosis when compared with P301S mice fed a control diet, using immunohistochemical analyses. Biochemical analyses showed that P301S mice fed the MB low dose diet have reduced astrogliosis in cerebral cortex tissue as evidenced by both qRT-PCR and western blot.
Because oxidative stress is believed to be a key component in tau-related neurodegeneration, immunohistochemical analysis of oxidative stress was performed in the cerebral cortex and hippocampus using an 8-OHdG antibody, which is a marker of oxidative damage to DNA. Glutathione levels, a major tissue antioxidant, were measured by HPLC. We previously showed that oxidative damage precedes tau pathology in the P301S mice, and it also occurs in a hippocampal slice model of tau toxicity (20,39). The copper (II) beta-amyloid (Cu(II)-Aβ) complex, a toxic form of Aβ, increases free radical generation and produces hyperphosphorylated tau-containing axonal swellings (40). This indicates that oxidative stress can drive tau pathology, which is substantiated by the finding that the B-27 supplement, a cocktail of antioxidants and the antioxidant curcumin prevent the formation of these large axonal swellings (40). In P301S mice, both the MB low dose diet and MB high dose diet decreased the immunoreactivity of 8-OHdG in the cerebral cortex and the hippocampus. Similarly, treatment with the MB low dose diet led to an increase in the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG; GSH/GSSG ratio), which is consistent with Nrf2 activation. Collectively, these data show that MB reduces oxidative stress.
Among the antioxidant proteins are redoxins, which combat the deleterious effects of oxidative stress by regulation of gene expression, anti-apoptotic activities and direct antioxidant effects (41,42). Thioredoxins (Trxs), thioredoxin reductases (TrxRs), glutaredoxins (Grxs) and peroxiredoxins (Prxs) are thiol-containing proteins with various activities related to cell viability and redox regulation. Notably, thioredoxins regulate apoptosis, modulate transcription factors and contribute to the maintenance of protein folding, while peroxiredoxins reduce hydrogen peroxide (H2O2) and regulate redox cellular signaling, and glutaredoxins counteract apoptosis and regulate cellular differentiation (43). Moreover, Prx6-positive astrocytes are increased in AD brain when compared with controls (44). Grx1 expression levels are decreased after cerebral ischemia, and Grx1 mRNA levels are decreased in NFT-bearing neurons, suggesting that oxidative stress plays a role in the disease (45). In the present study, MB increased mRNA expression of several redoxins including Trx1, TrxR1, Trx2, TrxR2, Grx1, Grx2 and Prx6. Interestingly, upregulation of the redoxins was most pronounced in the hippocampus of the P301S mice. Other studies showed that transgenic mice that overexpress thioredoxin (Trx) have a decreased susceptibility to oxidative stress and an increased life span (46). Overexpression of Grx2 and Prx6 leads to increased cell survival and proliferation in cells exposed to hypoxia and reoxygenation (47). Similarly, in a cellular model of AD, overexpression of Grx1 and Trx1 led to protection against Aβ-mediated toxicity (48). In P301S mice, astrocytes that localized to areas of hyperphosphorylated tau and neuronal death showed increased expression of Prx6, indicating that upregulation of this protein may occur in response to oxidative stress (49). Of interest, the thioredoxin reductase system, consisting of Trx, TrxR and NAPDH, is able to restore function after peroxynitrite induced damage to tau and the microtubule-associated protein 2 (MAP2) (50).
Mitochondria are particularly vulnerable to oxidative stress due to the fact that mtDNA is localized to sites of high free radical production, there is a lack of histones and it does not encode repair enzymes (51). The mitochondrial damage hypothesis suggests that early inflammatory events, and increased production of free radicals from impaired mitochondria, lead to proteasome inhibition and subsequent accumulation of damaged proteins (51). Mitochondrial dysfunction occurs concomitantly with oxidative stress in a mouse model of tauopathy (52), and tau hyperphosphorylation occurs as a consequence of mitochondrial oxidative stress when there is a reduction in MnSOD (53). Overexpression of P301L mutant tau, which is phosphorylated more readily than WT tau, results in mitochondrial dysfunction including morphological changes, decreased fission and fusion, decreased ATP levels and increased vulnerability to oxidative stress (54). Expression of human tau in a Drosophila model of tauopathy causes mitochondrial elongation, mitochondrial dysfunction and neurotoxicity, which are linked to excess actin stabilization and DRP1 mislocalization (55).
In the present study, P301S mice treated with the MB low dose diet showed an increase in mtDNA copy number, which suggests increased mitochondrial biogenesis. This is consistent with previous reports showing that MB modulates and improves mitochondrial function by increasing heme synthesis and mitochondrial respiration in AD-damaged brain regions such as the hippocampus (56,57). MB induced improvements in learning and memory in rats correlate with increased cytochrome oxidase activity (58). We also observed that the MB low dose diet increased SOD activity, which may contribute to its neuroprotective effects since MnSOD deficiency increased Aβ and tau pathology in a mouse model of AD (59). Additionally, overexpression of MnSOD in the Tg19959 transgenic mouse model of AD reduced oxidative stress, reduced amyloid deposition and improved memory impairments (60).
The molecular chaperones of the heat shock protein 70 (Hsp70) family are involved in proteostasis, the folding, trafficking and degradation of other proteins. Heat shock protein 72 (Hsp72) is an Hsp70 isoform that is stress-inducible (61,62). MB reduces the ATPase activity of Hsp72 in vitro and promotes clearance of the Hsp72 substrate tau in a dose-dependent manner (62). It was also shown that Hsp72, but not the nearly identical constitutively expressed isoform heat shock cognate 70 (Hsc70), is irreversibly inactivated by MB oxidation of cysteine 306 (Cys306), which is absent in Hsc70 (61). The authors also found that Hsp72 became resistant to MB by mutation of the key cysteines Cys267 and/or Cys306 in vitro, and overexpression of the C306S mutant suppressed MB-mediated tau degradation in a cellular model. The authors suggested that oxidation of specific cysteine residues that were modified by MB played a critical role in the loss of Hsp72 ATPase activity. Prominent effects of MB in modifying cysteines were also linked to the ability of MB to prevent tau aggregation in vitro (16,17). Using NMR spectroscopy, it was shown that MB oxidized cysteines in tau to sulfenic, sulfinic and sulfonic acids, which prevented tau aggregation (16).
Among the endogenous defenses against oxidative stress are antioxidant and anti-inflammatory signaling pathways. The NF-E2-related factor 2 (Nrf2)/ARE signaling pathway is involved in both antioxidant and anti-inflammatory activities. Our findings that MB activates the Nrf2/ARE signaling pathway are consistent with an effect on cysteine oxidation. The Nrf2 transcriptional activator is normally bound to the protein Keap1 in the cytoplasm by cysteine bridges. Keap1 targets Nrf2 to the Cullin3-E3 ligase complex for ubiquitination and subsequent degradation by the ubiquitin proteasome system. Oxidative stress and/or exposure to electrophilic compounds cause the release and nuclear translocation of Nrf2. In complex with Maf proteins, Nrf2 then binds to AREs in the promoters of antioxidant and anti-inflammatory genes, inducing transcriptional activation of genes such as HO1, NAD(P)H-quinone oxidoreducatse 1 (NQO1), thioredoxins, Gclc and Gclm (63–65). Nrf2 signaling also downregulates pro-inflammatory proteins such as iNOS and cyclooxygenase 2 (COX2), which are increased in AD brains (30,66). Thus, therapies with the potential to activate the Nrf2/ARE pathway and curb pro-inflammatory proteins are of particular interest in combating the oxidative damage and inflammation associated with tauopathies. Nrf2 overexpressing astrocytes protect against H2O2-induced cell death in primary cortical neurons in vitro and against malonate-mediated neurotoxicity in mouse brain in vivo (67). We measured the mRNA levels of Nrf2/ARE genes Nrf2, HO1, NQO1, Gclc, Gclm and iNOS following MB treatment. MB treatment upregulated Nrf2/ARE genes, including Nrf2, HO1, NQO1, Gclc and Gclm, and it decreased the pro-inflammatory gene iNOS. MB low dose diet and MB high dose diet also promoted the translocation of Nrf2 to the nucleus, indicating that Nrf2 was transcriptionally active and able to affect downstream target genes. Our findings that MB upregulates redoxins are also consistent with Nrf2/ARE activation. The genes encoding Trx and Prxs are among the genes that are ARE-activated (41,42,68,69). By inducing Gclc and Gclm, Nrf2 signaling increases glutathione levels consistent with the increase in the GSH/GSSG ratio that we observed, and the reduction in 8-OHdG levels. Curcumin, which is also known to activate the Nrf2/ARE pathway, reduces soluble tau dimers and corrects synaptic and behavioral deficits in aged P301L tau transgenic mice (70–72).
In order to confirm that the neuroprotective effects of MB were dependent on Nrf2 transcriptional signaling, we assessed the effects of MB on activation of Nrf2-dependent genes in WT and Nrf2 KO MEFs. Our data show that MB upregulated Nrf2/ARE genes in an Nrf2-dependent manner such that Nrf2 WT MEFs, but not Nrf2 KO MEFs, showed significantly increased levels of HO1, NQO1 and Gclc following MB treatment. We therefore believe that the induction of Nrf2/ARE transcriptional signaling may play a critical role in the neuroprotective effects of MB that we observed in the P301S transgenic mice. The results are in keeping with related studies on Nrf2/ARE signaling in neurodegenerative diseases. Intra-hippocampal injection of Nrf2 induces HO1 expression and improves learning and memory in a mouse model of AD (73). Triterpenoids upregulate Nrf2/ARE-related genes, including HO1 and NQO1, decrease oxidative damage and exert neuroprotective effects in transgenic mouse models of AD (74), Huntington's disease (75) and ALS (76). We also showed that triterpenoids protect against the toxicity and oxidative damage produced by either MPTP or 3-nitropropionic acid, which are mitochondrial toxins (77,78).
MB has numerous hormetic effects at the behavioral and biochemical levels (reviewed in 32), which are frequently thought to be associated with oxidative promiscuity of this compound. On the one hand, low doses of MB are thought to be able to rescue mitochondria with damaged respiratory chains and to restore ATP production. By competing for oxygen at the iron–sulfur cluster of xanthine oxidase MB at low micromolar concentrations suppresses the enzyme's superoxide production without inhibiting its natural catalytic activity (79–81). However, such an ‘antioxidant’ property is a double edged sword because as mentioned above, the reduced MB is re-oxidized by oxygen to H2O2 (80,81). This can stimulate unproductive oxygen consumption (uncouple mitochondria) and impair the efficiency of ATP synthesis. These potentially deleterious effects might be expected at high doses of accumulated MB.
Due to its low mildly positive midpoint redox potential (0.01 V), MB can readily accept electrons from reduced glutathione (−0.28 V) (82). MB was shown to directly oxidize glutathione in both aerobic and anaerobic conditions in vitro (82) and most likely does so in vivo. It is known that tau fibril formation is prevented by the oxidation of cysteine residues in the protein, which can be done by oxidized MB. It was therefore suggested (17) that MB should not be able to prevent tau fibril formation in the cellular environment because of an abundance of reduced glutathione. This conclusion was reached on the basis of an in vitro experiment in which oxidized MB was not able to oxidize tau protein's cysteine residues in the presence of 5 mm reduced glutathione. However, we think the importance of this in vitro finding might not be so clear when applied to in situ conditions, since it is known that MB enters the cytosol after being reduced at the cell surface, whereas glutathione does not (83). Thus, at least in erythrocytes, the oxidized MB concentration is much higher than that of reduced MB; and even more of a disbalance toward the oxidized form can be expected in cells containing mitochondria which can actively oxidize MB. Therefore, in vitro experiments with the protein and glutathione suspended in a tube with no-diffusion-barriers and 5 mm reduced glutathione may not accurately reflect the cytosol conditions where tau fibrils form.
Our present results therefore demonstrate that MB improves the behavioral impairments and brain pathology found in P301S mice and that this is associated with increased expression of Nrf2/ARE genes, which is more likely to explain the beneficial effects of MB than its direct anti-oxidant effects. The most striking results were seen with the MB low dose diet (4 mg/kg), consistent with the hormetic dose response of the drug. We observed improvements in behavior and tau-related pathology that were associated with decreased phospho-tau accumulation, increased expression of antioxidant defenses, decreased inflammation and increased mitochondrial biogenesis. Therefore, these data provide further evidence that compounds such as MB, which increase expression of Nrf2/ARE-dependent genes, may be effective for the treatment of tauopathies and related neurodegenerative diseases.
MATERIALS AND METHODS
Animals and treatment
Male P301S transgenic mice, which express the P301S mutant human MAPT, and female B6C3F1/J WT mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and bred. Offspring were genotyped by PCR of tail DNA. At 1 month of age, P301S transgenic mice and WT littermates were randomly assigned to either control diet (LabDiet #5002, Purina-Mills, Richmond, IN, USA), diet containing 4 mg/kg MB (low dose) or diet containing 40 mg/kg MB (high dose). Diets were provided ad libitum from 1 to 10 months of age. MB (3,7-bis(Dimethylamino)phenazathionium chloride, C16H18ClN3S · 3H2O) was received from Sigma-Aldrich (St Louis, MO, USA) and diets were pelleted by Purina-Mills.
Behavioral analysis was performed on all mice at 5, 7 and 9 months of age. Mice were then divided into two groups, those intended for histology and those intended for biochemistry. Histological and biochemical analyses were performed at 10 months of age. All experiments were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Behavioral assessment
A battery of behavioral analyses, including the open field test, elevated plus maze, and body weight and consumption measurements, was conducted at 5, 7 and 9 months of age. The open field test was used to assess locomotion and exploration as previously described (84). Briefly, mice were placed in the open field for a 5 min trial during which distance moved and rearing frequency were recorded by a video tracking system (Ethovision 3.1, Noldus Technology, Attleborough, MA, USA). The elevated plus maze was used at 7 and 9 months of age to measure both anxiety, as indicated by time spent in the open arms of the apparatus, and exploration, as indicated by total number of entries into the arms of the apparatus. Body weight and consumption were also measured at 5, 7 and 9 months to ensure that there were no adverse effects of the diets on weight or appetite.
During the 9-month behavioral assessment, an additional behavioral measurement was used to assess learning and memory. Contextual fear conditioning was conducted over a 2-day period. On day 1, mice were introduced to a neutral context during a habituation period. Electrical shock was used as a negative stimulus within the neutral context, and two electrical shocks were delivered during the trial on day 1 of testing. The percent time spent freezing was recorded (Coulbourn Instruments, Allentown, PA, USA). The degree of learning and memory was then assessed by the percent time spent freezing during the trial on day 2, during which no shocks were delivered by the apparatus.
Tissue collection and storage
At 10 months of age, mice intended for biochemical studies were sacrificed by decapitation. Brain tissues were collected, dissected and snap frozen in liquid nitrogen. All tissues were stored at −80°C until processing.
Mice intended for immunohistochemistry and histology studies were also sacrificed at 10 months of age. Sodium pentobarbital was used to deeply anesthetize the mice before transcardial perfusion with ice-cold 0.9% sodium chloride followed by 4% paraformaldehyde. Brains were collected and stored in 4% paraformaldehyde followed by 30% sucrose and, finally, cryoprotectant solution.
Immunohistochemistry and histology
Paraformaldehyde-fixed brain tissue was sectioned at 40 μm thickness. Sections were stained with the AT8 mouse monoclonal anti-human tau pSer202/Thr205 antibody (1:500, Thermo Fisher Scientific, Rockford, IL, USA), anti-8-hydroxydeoxyguanosine antibody (8-OHdG, 1:200, Millipore, Billerica, MA, USA), anti-GFAP antibody (GFAP; 1:500, DAKO, Carpinteria, CA, USA) or anti-NF-E2-related factor 2 antibody (Nrf2, 1:50, Abcam, Cambridge, MA, USA). Immunolabeling was detected with the streptavidin-horseradish peroxidase method and visualized with diaminobenzidine visualization system (Vector, Burlingame, CA, USA). Quantification of AT8 was done using two 40 μm serial non-adjacent sections per animal (480 μm apart, from bregma –1.34 through bregma –2.84 through the cerebral cortex and hippocampus). The percentage area occupied by AT8 was measured using Scion Image (Scion Corp., Frederick, MD, USA). For the cerebral cortex, measures were determined within a 0.9 mm2 area encompassing the primary (M1) and secondary (M2) motor cortex and the threshold was set to 60. For the hippocampus, the percent area occupied by AT8-labeled structures in the CA1 was determined and the threshold was set to 90.
Protein measurement by western blot
Snap frozen cerebral cortex tissues were homogenized in RIPA buffer with protease and phosphatase inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Equal amounts of protein were electrophoresed through 4–20% Criterion TGX Precast Gels (Bio-Rad, Hercules, CA, USA). After transfer to polyvinylidene fluoride, membranes were blocked in 5% non-fat dry milk in phosphate buffer saline with 0.05% Tween 20 (PBST) and exposed overnight to the primary antibody at 4°C. Horseradish peroxidase-conjugated secondary antibody binding was visualized with enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Primary antibodies and concentrations used for western blotting were AT8 mouse monoclonal anti-human tau pSer202/Thr205 (1:500, Thermo Fisher Scientific), anti-GFAP antibody (GFAP; 1:5000, DAKO) and mouse monoclonal anti-β-actin (1:10 000, Sigma, St Louis, MO, USA). Quantitative analysis was performed using NIH-based Scion Image software (Scion Corp.). Statistical analysis was performed using ratios of the densitometric value of each band normalized to β-actin as loading control.
Glutathione measurement by high-performance liquid chromatography (HPLC) mass spectrometry
Cerebral cortex tissue samples were homogenized with a buffer containing 200 mm methane sulphonic acid with 5 mm DTPAC as previously described (85). Briefly, 20 vol of buffer were added to each sample. Samples were homogenized and centrifuged for 30 min at 14000g at 4°C. Supernatants were collected and filtrated in an eppendorf UltraFree 5 kDa filter. An Agilent 1290 LC system coupled to an ESI-Q-TOF MS/MS 6520 instrument (Agilent Technologies, Barcelona, Spain) was used to analyze the samples. Two microliters of extracted sample was applied to a reversed-phase column (Zorbax SB-Aq 1.8 µm 2.1 × 50 mm; Agilent Technologies) equipped with a pre-column (Zorba-SB-C8 Rapid Resolution Cartridge 2.1 × 30 mm 3.5 µm; Agilent Technologies) with a column temperature of 60°C. The flow rate was 0.6 ml/min. Solvent A was composed of water containing 0.2% acetic acid and solvent B was composed of methanol 0.2% acetic acid. The gradient started in 2% B and increased to 98% B in 13 min and hold at 98% B during 6 min. Pot-time was established in 5 min. Data were collected in positive electrospray mode TOF operated in full-scan mode at 50–1600 m/z in an extended dynamic range (2 GHz), using N2 as the nebulizer gas (10 l/min, 350°C). The capillary voltage was 4000 V with a scan rate of 1.5 scan/s. The ESI source used a separate nebulizer for the continuous, low-level (10 l/min) introduction of reference mass compounds: 121.050873, 922.009798, which were used for continuous, online mass calibration. MassHunter Qualitative Analysis Software (Agilent Technologies) was employed for integration and extraction of peak intensities. The m/z values used for quantification were: m/z 308.0966 [M+H]+ for GSH and m/z 613.1594 [M+H]+ for GSSG. Interassay and intra-assay CV were below 5%.
Gene expression by qRT-PCR
Snap frozen cerebral cortex and hippocampus tissues were processed for RNA extraction (Qiagen RNeasy Mini Kit, Valencia, CA, USA). qRT-PCR was performed at the Weill Cornell Medical College Microarray Core Facility using the ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). The following genes were analyzed using SybrGreen assays: GAPDH, GFAP, Trx1, TrxR1, Trx2, TrxR2, Grx1, Grx2, Prx6, Nrf2, HO1, NQO1, Gclc, Gclm and iNOS.
mtDNA copy number
Fresh, non-fixed, snap-frozen cerebral cortex tissues stored at −80°C were processed for mtDNA copy number as previously described (86). Briefly, DNA extraction was performed according to the manufacturer's protocol (Qiagen Kit, Valencia, CA, USA). The relative mtDNA copy number was determined by qRT-PCR on an ABI PRISM 7900H Sequence Detection System (Applied Biosystems) using the TaqMan® Universal PCR Master mix and predeveloped TaqMan® Gene Expression Assay primers/probes (Applied Biosystems) for mitochondrial cytochrome oxidase 2 and β-actin (nuclear DNA control). Results were calculated from the threshold cycle values and expressed as the 2−ΔCT of cytochrome oxidase 2 to β-actin.
SOD activity in enriched mitochondrial fractions
Fresh, non-fixed, snap-frozen frontal lobe samples (∼30–55 mg) were stored frozen at −80°C until assaying. Tissue samples were thawed on ice and homogenized with Dounce-type 2 ml homogenizer (glass vessel/glass pestle) before assays. The homogenate was centrifuged at 1000g × 5 min to get rid of nuclear fraction and cell debris. The resulting supernatant was centrifuged at 14 000g × 5 min. The pellet was collected and centrifuged again at 14 000g × 5 min and the final pellet was re-suspended in 20 mm HEPES (pH 7.8) and used for all assays. All samples were assayed for SOD activity (Superoxide Dismutase Assay Kit, Cayman Chemical, Ann Arbor, MI, USA). All activities were normalized by protein content (BCA Protein Assay, Thermo Scientific, FL, USA).
Cell culture and treatments
Nrf2 WT and KO MEFs were received from Dr Thomas W. Kensler (University of Pittsburgh) (87). Cells were maintained in the IMDM medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, in a humidified incubator set at 37°C 5% CO2. MB (Sigma-Aldrich) was dissolved in DMSO. Cells were treated with 0.1, 1 or 10 μm MB or the solvent for 3 or 8 h in triplicates.
Total RNA was prepared from MB treated and control cells using Trizol reagent (Life Technologies, Carlsbad, CA, USA). Half microgram of total RNA was reverse transcribed using High Capacity Reverse Transcription Kit (Life Technologies). cDNA was diluted and 100 ng was used to measure relative expression of genes with respect to control WT, normalized to the expression of GAPDH. Specific primers listed below and SyBR Select® kit (Life Technologies) was used to amplify cDNA in an ABI Prism 7900HT RT-PCR. Relative gene expression was determined using the ΔΔCt method compared with that of WT control, all values normalized to GAPDH expression.
Statistical analysis
Analysis of variance and post hoc Fisher's PLSD test were used for multiple comparisons (comparing six groups: WT mice fed a control diet, WT mice fed a the MB low dose diet, WT mice fed a the MB high dose diet, P301S mice fed a control diet, P301S mice fed the MB low dose diet and P301S mice fed the MB high dose diet) for all data sets (Statview 5.0.1, SAS Institute Inc., Cary, NC, USA). For cell culture experiments, significance was set at P < 0.05 as determined by two-tailed, paired Student’s t-test. All presented data were expressed as means ± standard errors of the means.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Tau Consortium (M.F.B.), NIH grant NS060885, Michael J Fox Foundation for Parkinson's Disease Research, and Par Fore Parkinson's (B.T.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Noel Calingasan (Weill Cornell Medical College, New York, USA) for his help throughout this study. We thank the Institut Professeur Baulieu for its support to D.T.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Walker L.C., Diamond M.I., Duff K.E., Hyman B.T. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S., et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 3.Sultana R., Butterfield D.A. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J. Bioenerg. Biomembr. 2009;41:441–446. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sultana R., Perluigi M., Butterfield D.A. Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppede F., Migliore L. DNA damage and repair in Alzheimer's disease. Curr. Alzheimer Res. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- 6.Markesbery W.R., Kryscio R.J., Lovell M.A., Morrow J.D. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 7.Williams T.I., Lynn B.C., Markesbery W.R., Lovell M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol. Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 9.Wyss-Coray T., Rogers J. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad S., Sung B., Aggarwal B.B. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev. Med. 2012;54(suppl):S29–S37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas J.C., Bruchey A.K., Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter-Sack I., Rengelshausen J., Oberwittler H., Burhenne J., Mueller O., Meissner P., Mikus G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009;65:179–189. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]
- 13.Schirmer R.H., Adler H., Pickhardt M., Mandelkow E. ‘Lest we forget you—methylene blue…. Neurobiol. Aging. 2011;32:2325 e7–2325 e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Fatouros C., Pir G.J., Biernat J., Koushika S.P., Mandelkow E., Mandelkow E.M., Schmidt E., Baumeister R. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum. Mol. Genet. 2012;21:3587–3603. doi: 10.1093/hmg/dds190. [DOI] [PubMed] [Google Scholar]
- 15.Wischik C.M., Edwards P.C., Lai R.Y., Roth M., Harrington C.R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl Acad. Sci. USA. 1996;93:11213–11128. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akoury E., Pickhardt M., Gajda M., Biernat J., Mandelkow E., Zweckstetter M. Mechanistic basis of phenothiazine-driven inhibition of tau aggregation. Angew. Chem. Int. Ed. Engl. 2013;52:3511–3515. doi: 10.1002/anie.201208290. [DOI] [PubMed] [Google Scholar]
- 17.Crowe A., James M.J., Lee V.M., Smith A.B., 3rd, Trojanowski J.Q., Ballatore C., Brunden K.R. Aminothienopyridazines and methylene blue affect tau fibrillization via cysteine oxidation. J. Biol. Chem. 2013;288:11024–11037. doi: 10.1074/jbc.M112.436006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Congdon E.E., Wu J.W., Myeku N., Figueroa Y.H., Herman M., Marinec P.S., Gestwicki J.E., Dickey C.A., Yu W.H., Duff K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landhuis E. Will tau drug show its true colors in phase 3 trials? Alzforum. 2012 [Google Scholar]
- 20.Dumont M., Stack C., Elipenahli C., Jainuddin S., Gerges M., Starkova N.N., Yang L., Starkov A.A., Beal F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J. 2011;25:4063–4072. doi: 10.1096/fj.11-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi H., Iba M., Inoue H., Higuchi M., Takao K., Tsukita K., Karatsu Y., Iwamoto Y., Miyakawa T., Suhara T., et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE. 2011;6:e21050. doi: 10.1371/journal.pone.0021050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beal M.F. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 23.Romano A.D., Serviddio G., de Matthaeis A., Bellanti F., Vendemiale G. Oxidative stress and aging. J. Nephrol. 2010;23(Suppl. 15):S29–S36. [PubMed] [Google Scholar]
- 24.Kumar R., Atamna H. Therapeutic approaches to delay the onset of Alzheimer's disease. J. Aging Res. 2011;2011:820903. doi: 10.4061/2011/820903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Aurelio M., Vives-Bauza C., Davidson M.M., Manfredi G. Mitochondrial DNA background modifies the bioenergetics of NARP/MILS ATP6 mutant cells. Hum. Mol. Genet. 2010;19:374–386. doi: 10.1093/hmg/ddp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markesbery W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 27.Castellani R., Smith M.A., Richey P.L., Kalaria R., Gambetti P., Perry G. Evidence for oxidative stress in Pick disease and corticobasal degeneration. Brain Res. 1995;696:268–271. doi: 10.1016/0006-8993(95)00535-x. [DOI] [PubMed] [Google Scholar]
- 28.Schipper H.M. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Hartzler A.W., Zhu X., Siedlak S.L., Castellani R.J., Avila J., Perry G., Smith M.A. The p38 pathway is activated in Pick disease and progressive supranuclear palsy: a mechanistic link between mitogenic pathways, oxidative stress, and tau. Neurobiol. Aging. 2002;23:855–859. doi: 10.1016/s0197-4580(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 30.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 31.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Bruchey A.K., Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am. J. Pharmacol. Toxicol. 2008;3:72–79. doi: 10.3844/ajptsp.2008.72.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riha P.D., Bruchey A.K., Echevarria D.J., Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur. J. Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 34.van Bebber F., Paquet D., Hruscha A., Schmid B., Haass C. Methylene blue fails to inhibit Tau and polyglutamine protein dependent toxicity in zebrafish. Neurobiol. Dis. 2010;39:265–271. doi: 10.1016/j.nbd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary J.C., 3rd, Li Q., Marinec P., Blair L.J., Congdon E.E., Johnson A.G., Jinwal U.K., Koren J., 3rd, Jones J.R., Kraft C., et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol. Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina D.X., Caccamo A., Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosokawa M., Arai T., Masuda-Suzukake M., Nonaka T., Yamashita M., Akiyama H., Hasegawa M. Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS ONE. 2012;7:e52389. doi: 10.1371/journal.pone.0052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda M., Shoji M., Kawarai T., Kawarabayashi T., Matsubara E., Murakami T., Sasaki A., Tomidokoro Y., Ikarashi Y., Kuribara H., et al. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am. J. Pathol. 2005;166:521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messing L., Decker J.M., Joseph M., Mandelkow E., Mandelkow E.M. Cascade of tau toxicity in inducible hippocampal brain slices and prevention by aggregation inhibitors. Neurobiol. Aging. 2013;34:1343–1354. doi: 10.1016/j.neurobiolaging.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howells C., Saar K., Eaton E., Ray S., Palumaa P., Shabala L., Adlard P.A., Bennett W., West A.K., Guillemin G.J., et al. Redox-active Cu(II)-Abeta causes substantial changes in axonal integrity in cultured cortical neurons in an oxidative-stress dependent manner. Exp. Neurol. 2012;237:499–506. doi: 10.1016/j.expneurol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Calabrese V., Guagliano E., Sapienza M., Panebianco M., Calafato S., Puleo E., Pennisi G., Mancuso C., Butterfield D.A., Stella A.G. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem. Res. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 42.Patenaude A., Murthy M.R., Mirault M.E. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol. Life Sci. 2005;62:1063–1080. doi: 10.1007/s00018-005-4541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalinina E.V., Chernov N.N., Saprin A.N. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc) 2008;73:1493–1510. doi: 10.1134/s0006297908130099. [DOI] [PubMed] [Google Scholar]
- 44.Power J.H., Asad S., Chataway T.K., Chegini F., Manavis J., Temlett J.A., Jensen P.H., Blumbergs P.C., Gai W.P. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lillig C.H., Berndt C., Holmgren A. Glutaredoxin systems. Biochim. Biophys. Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida T., Nakamura H., Masutani H., Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann. N Y Acad. Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]
- 47.Godoy J.R., Oesteritz S., Hanschmann E.M., Ockenga W., Ackermann W., Lillig C.H. Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radic. Biol. Med. 2011;51:552–561. doi: 10.1016/j.freeradbiomed.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Akterin S., Cowburn R.F., Miranda-Vizuete A., Jimenez A., Bogdanovic N., Winblad B., Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 49.Yata K., Oikawa S., Sasaki R., Shindo A., Yang R., Murata M., Kanamaru K., Tomimoto H. Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau-transgenic mouse. Brain Res. 2011;1410:12–23. doi: 10.1016/j.brainres.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 50.Landino L.M., Skreslet T.E., Alston J.A. Cysteine oxidation of tau and microtubule-associated protein-2 by peroxynitrite: modulation of microtubule assembly kinetics by the thioredoxin reductase system. J. Biol. Chem. 2004;279:35101–35105. doi: 10.1074/jbc.M405471200. [DOI] [PubMed] [Google Scholar]
- 51.Prasad K.N., Cole W.C., Prasad K.C. Risk factors for Alzheimer's disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J. Am. Coll. Nutr. 2002;21:506–522. doi: 10.1080/07315724.2002.10719249. [DOI] [PubMed] [Google Scholar]
- 52.David D.C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Drose S., Brandt U., Muller W.E., et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 53.Melov S., Adlard P.A., Morten K., Johnson F., Golden T.R., Hinerfeld D., Schilling B., Mavros C., Masters C.L., Volitakis I., et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulz K.L., Eckert A., Rhein V., Mai S., Haase W., Reichert A.S., Jendrach M., Muller W.E., Leuner K. A new link to mitochondrial impairment in tauopathies. Mol. Neurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 55.DuBoff B., Gotz J., Feany M.B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atamna H., Kumar R. Protective role of methylene blue in Alzheimer's disease via mitochondria and cytochrome c oxidase. J. Alzheimers Dis. 2010;20(Suppl. 2):S439–S452. doi: 10.3233/JAD-2010-100414. [DOI] [PubMed] [Google Scholar]
- 57.Lin A.L., Poteet E., Du F., Gourav R.C., Liu R., Wen Y., Bresnen A., Huang S., Fox P.T., Yang S.H., et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS ONE. 2012;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrubel K.M., Riha P.D., Maldonado M.A., McCollum D., Gonzalez-Lima F. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol. Biochem. Behav. 2007;86:712–717. doi: 10.1016/j.pbb.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami K., Murata N., Noda Y., Tahara S., Kaneko T., Kinoshita N., Hatsuta H., Murayama S., Barnham K.J., Irie K., et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dumont M., Wille E., Stack C., Calingasan N.Y., Beal M.F., Lin M.T. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyata Y., Rauch J.N., Jinwal U.K., Thompson A.D., Srinivasan S., Dickey C.A., Gestwicki J.E. Cysteine reactivity distinguishes redox sensing by the heat-inducible and constitutive forms of heat shock protein 70. Chem. Biol. 2012;19:1391–1399. doi: 10.1016/j.chembiol.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jinwal U.K., Miyata Y., Koren J., 3rd, Jones J.R., Trotter J.H., Chang L., O'Leary J., Morgan D., Lee D.C., Shults C.L., et al. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J. Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hybertson B.M., Gao B., Bose S.K., McCord J.M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Lu S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanito M., Agbaga M.P., Anderson R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Korolainen M.A., Auriola S., Nyman T.A., Alafuzoff I., Pirttila T. Proteomic analysis of glial fibrillary acidic protein in Alzheimer's disease and aging brain. Neurobiol. Dis. 2005;20:858–870. doi: 10.1016/j.nbd.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Johnson J.A., Johnson D.A., Kraft A.D., Calkins M.J., Jakel R.J., Vargas M.R., Chen P.C. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann. N Y Acad. Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maher P. Redox control of neural function: background, mechanisms, and significance. Antioxid. Redox. Signal. 2006;8:1941–1970. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.C., Yamaguchi Y., Kondo N., Masutani H., Yodoi J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene. 2003;22:1860–1865. doi: 10.1038/sj.onc.1206369. [DOI] [PubMed] [Google Scholar]
- 70.Ma Q.L., Zuo X., Yang F., Ubeda O.J., Gant D.J., Alaverdyan M., Teng E., Hu S., Chen P.P., Maiti P., et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013;288:4056–4065. doi: 10.1074/jbc.M112.393751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickering A.M., Linder R.A., Zhang H., Forman H.J., Davies K.J. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., Yla-Herttuala S., Tanila H., Levonen A.L., Koistinaho M., et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dumont M., Wille E., Calingasan N.Y., Tampellini D., Williams C., Gouras G.K., Liby K., Sporn M., Nathan C., Flint Beal M., et al. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J. Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stack C., Ho D., Wille E., Calingasan N.Y., Williams C., Liby K., Sporn M., Dumont M., Beal M.F. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic. Biol. Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neymotin A., Calingasan N.Y., Wille E., Naseri N., Petri S., Damiano M., Liby K.T., Risingsong R., Sporn M., Beal M.F., et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang L., Calingasan N.Y., Thomas B., Chaturvedi R.K., Kiaei M., Wille E.J., Liby K.T., Williams C., Royce D., Risingsong R., et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaidery N.A., Banerjee R., Yang L., Smirnova N.A., Hushpulian D.M., Liby K.T., Williams C.R., Yamamoto M., Kensler T.W., Ratan R.R., et al. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson's disease. Antioxid. Redox. Signal. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCord J.M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J. Biol. Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- 80.Kelner M.J., Bagnell R., Hale B., Alexander N.M. Methylene blue competes with paraquat for reduction by flavo-enzymes resulting in decreased superoxide production in the presence of heme proteins. Arch. Biochem. Biophys. 1988;262:422–426. doi: 10.1016/0003-9861(88)90393-1. [DOI] [PubMed] [Google Scholar]
- 81.Salaris S.C., Babbs C.F., Voorhees W.D., 3rd Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem. Pharmacol. 1991;42:499–506. doi: 10.1016/0006-2952(91)90311-r. [DOI] [PubMed] [Google Scholar]
- 82.Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 83.Kelner M.J., Alexander N.M. Methylene blue directly oxidizes glutathione without the intermediate formation of hydrogen peroxide. J. Biol. Chem. 1985;260:15168–15171. [PubMed] [Google Scholar]
- 84.Bongard R.D., Merker M.P., Shundo R., Okamoto Y., Roerig D.L., Linehan J.H., Dawson C.A. Reduction of thiazine dyes by bovine pulmonary arterial endothelial cells in culture. Am. J. Physiol. 1995;269:L78–L84. doi: 10.1152/ajplung.1995.269.1.L78. [DOI] [PubMed] [Google Scholar]
- 85.May J.M., Qu Z.C., Cobb C.E. Reduction and uptake of methylene blue by human erythrocytes. Am. J. Physiol. Cell Physiol. 2004;286:C1390–C1398. doi: 10.1152/ajpcell.00512.2003. [DOI] [PubMed] [Google Scholar]
- 86.Dumont M., Wille E., Calingasan N.Y., Nathan C., Flint Beal M., Lin M.T. N-iminoethyl-L-lysine improves memory and reduces amyloid pathology in a transgenic mouse model of amyloid deposition. Neurochem. Int. 2010;56:345–351. doi: 10.1016/j.neuint.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Galino J., Ruiz M., Fourcade S., Schluter A., Lopez-Erauskin J., Guilera C., Jove M., Naudi A., Garcia-Arumi E., Andreu A.L., et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of X-adrenoleukodystrophy. Antioxid. Redox. Signal. 2011;15:2095–2107. doi: 10.1089/ars.2010.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.