Abstract
Background
Albumin is the most abundant protein in blood plasma, and due to its ligand binding properties, serves as a circulating depot for endogenous and exogenous (e.g. drugs) compounds. Hence, the unbound drug is the pharmacologically active drug. Commercial human albumin preparations are frequently used during surgery and in critically ill patients. Recent studies have indicated that the use of pharmaceutical-grade albumin is controversial in critically ill patients. In this in vitro study we investigated the drug binding properties of pharmaceutical-grade albumins (Baxter/Immuno, Octapharma, and Pharmacia & Upjohn), native human serum, and commercially available human serum albumin from Sigma Chemical Company.
Methods
The binding properties of the various albumin solutions were tested in vitro by means of ultrafiltration. Naproxen, warfarin, and digitoxin were used as ligands. HPLC was used to quantitate the total and free drug concentrations. The data were fitted to a model of two classes of binding sites for naproxen and warfarin and one class for digitoxin, using Microsoft Excel and Graphpad Prism.
Results
The drugs were highly bound to albumin (95–99.5%). The highest affinity (lowest K1) was found with naproxen. Pharmaceutical-grade albumin solutions displayed significantly lower drug-binding capacity compared to native human serum and Sigma albumin. Thus, the free fraction was considerably higher, approximately 40 times for naproxen and 5 and 2 times for warfarin and digitoxin, respectively. The stabilisers caprylic acid and N-acetyl-DL-tryptophan used in the manufacturing procedure seem to be of importance. Adding the stabilisers to human serum and Sigma albumin reduced the binding affinity whereas charcoal treatment of the pharmaceutical-grade albumin from Octapharma almost restored the specific binding capacity.
Conclusion
This in vitro study demonstrates that the specific binding for warfarin and digitoxin is significantly reduced and for naproxen no longer detectable in pharmaceutical-grade albumin. It further shows that the addition of stabilisers may be of major importance for this effect.
Background
Albumin is the most abundant protein in blood plasma. One of its main functions is transporting endogenous and exogenous compounds, which might be toxic in the unbound state, but non-toxic as albumin-bound. Thus the albumin may serve as a circulating depot, whereas the unbound substances are the pharmacologically active moieties [1,2]. Changes in the free drug concentration due to displacement or pathological states like impaired renal or liver function and hypoalbuminaemia may increase drug effect, particularly if the drug is highly albumin-bound [3]. Albumin also plays an essential role in generating the colloid osmotic pressure, and has a long history of clinical use in colloid replacement therapy. The Cochrane injuries group albumin reviewers [4] reported a possible excess mortality after albumin administration in critically ill patients. Some mechanisms behind the harmful effect were discussed, but altered binding properties of albumin were not mentioned.
Recently, another meta-analysis by Wilkes and Navickis [5] was not able to detect any effect of albumin administration on mortality. Thus, the use of albumin in critically ill patients is controversial. Børmer et al. [6] reported that an albumin assay (Vitros "dry chemistry" bromocresol green (BCG) albumin method) underestimated the albumin concentration in pharmaceutical-grade albumin from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn, compared to serum protein electrophoresis (SPE) and a manual BCG method. This was the case also with serum samples from patients receiving significant amounts of albumin. Due to the findings [4-6] we wanted to study the drug-binding properties of pharmaceutical-grade albumins, native sera from healthy volunteers, and defatted albumin from Sigma (commercially available and without the stabilisers caprylic acid and N-acetyl-DL-tryptophan).
Naproxen, warfarin, and digitoxin were selected as test drugs. According to Sudlow et al. [7] and Sjøholm et al. [8], these drugs represent three different binding sites on the albumin molecule and all are highly albumin-bound, i.e. more than 95 % bound. During the manufacturing process of pharmaceutical-grade albumin (Cohn) the stabilisers caprylic acid (octanoic acid) and N-acetyl-DL-tryptophan are added before pasteurisation. Thus we also studied the effects of the stabilisers caprylic acid and N-acetyl-DL-tryptophan on drug binding by adding the stabilisers to stabiliser-free albumin solutions and by charcoal purification of pharmaceutical-grade albumin.
Methods
Chemicals and reagents
Tris-base, phosphate buffered saline (PBS) (D-5773), naproxen (M-4015), warfarin (A-2250), digitoxin (D-5878) and defatted human serum albumin (A-3782) were purchased from Sigma Chemical Co., St. Louis, MO, USA. HPLC grade methanol and acetonitrile were from Merck, Darmstadt, Germany. N,N-Dimethyloctylamine (DMOA) was from Aldrich, St. Louis, MO, USA. Sodium caprylate and N-acetyl-DL-tryptophan were from ICN Biomedicals Inc., Ohio, USA. Pharmaceutical-grade albumin (200 g/L) was purchased from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn. From Octapharma we also received an in-process albumin concentrate, collected just before the addition of stabilisers and final heating (in-process albumin). Human serum was obtained from five healthy volunteers (4 females, 1 male), age 25–42 years. All aqueous reagents were made up in water purified through a Milli-Q UF-PLUS system (Millipore Corp., Bedford, MA, USA).
Stock solutions and standards
Stock solutions of naproxen (0.1 mol/L) and of warfarin (0.1 mol/L) were prepared in 0.110 mol/L NaOH. Stock solutions of digitoxin (0.01 mol/L) were prepared in ethanol. These stock solutions were stored in glass vials at +4°C, and new dilutions for HPLC calibrations or binding experiments were prepared each day in the appropriate matrix. PBS was prepared in purified water and pH adjusted to 7.35 with HCl, then filtered through a 0.22 µm filter into a sterilised container and pH controlled to be 7.40.
Experimental design
The albumin concentrations of the human sera used in the experiments were 0.606 (±0.023) mmol/L (39.4 g/L). Pharmaceutical-grade albumin concentrates (200 g/L) and Sigma albumin were diluted with PBS to 0.615 mmol/L (40 g/L). Both human serum and the albumin solutions were adjusted to pH 7.40 with H3PO4 and NaOH respectively, filtered (0.22 µm filter), stored in aliquots (11 mL) and frozen at -70°C [9]. Before use the solutions were thawed and heated to +37°C in a water bath before final pH-control. The albumin solutions were divided into 10 separate samples of 1 ml each before addition of 10 different drug concentrations. After drug admixture albumin samples were pipetted into pre-heated Amicon Centrifree filter units. Duplicates of 10 µL were removed for determination of total drug concentration. To produce ultrafiltrates with unbound drug, the samples were equilibrated on the Amicon Centrifree filters (membrane pore size 30 000 molecular weight cut-off) at +37°C in a hot-air oven for 30 minutes prior to centrifugation. Centrifugations were done at +37°C, albumin solutions for 5 min at 1 000 × g and human serum for 10 min at 1 640 × g according to the method of Borgå & Borgå [9], resulting in filtrate volumes of 0.25–0.33 mL. The pH of the solution was controlled before and after ultrafiltration without finding any significant effects, as stated by Borgå & Borgå [9].
Each experiment with one matrix included ten drug concentrations covering a broad concentration range. The total drug concentrations (mmol/L) tested were: naproxen 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8, 1.0, 1.5, 2.0; warfarin 0.05, 0.1, 0.2, 0.3, 0.4, 0.6, 1.0, 1.5, 2.0, 2.5; digitoxin 0.02, 0.03, 0.04, 0.05, 0.1, 0.2, 0.25, 0.30, 0.35, 0.40. In separate experiments when specific binding was investigated, the 5 lowest concentrations of naproxen and warfarin were used. The isolated effects of the stabilisers caprylic acid and N-acetyl-DL-tryptophan, on binding capacity of naproxen, warfarin, and digitoxin were investigated by adding the stabilisers to Sigma albumin solutions and human serum and by charcoal treatment of the pharmaceutical-grade and an in-process albumin solution from Octapharma.
Analyses
Albumin concentrations were measured immunonephelometrically on a Beckman Immage Immunochemistry System (Beckman Instruments) [6]. The unbound (free) and total drug concentrations in solutions were determined by reversed phase High Performance Liquid Chromatography (HPLC). For naproxen, according to Borgå & Borgå [9] with some modifications: In brief, the mobile phase was a 25 mmol/L potassium phosphate buffer pH 2.50 (62.5+37.5 acetonitrile), the column used was a Purospher Star RP-18e, 55 × 4 mm with 3 µm particles (Merck, Darmstadt, Germany), and naproxen was detected by native fluorescence [10]. Warfarin was quantified with a modified version of the method described by Wong et al. [11]: The mobile phase was a 25 mmol/L Tris-HCl buffer pH 7 (65+35 acetonitrile) with the ion-pairing agent DMOA added to the buffer/acetonitrile mixture to a final concentration of 10 mmol/L.
The addition of DMOA raised the pH of the mobile phase to approximately 8.5, sufficiently high to be able to detect warfarin by native fluorescence. The column used for separation was a Purospher Star RP-18e, 55 × 4 mm with 3 µm particles. Digitoxin was separated and detected as described by Plum et al. [12] with minor modifications. Sample preparation was adapted from Dasgupta et al. [13] and the procedure for the Abbot TDx Digitoxin assay. Determination of tryptophan, N-acetyl-DL-tryptophan, and the internal standard N-formyl-DL-tryptophan were performed using perchloric acid to precipitate proteins before separation on a C-8 column using octanesulfonic acid as ion-pairing agent. The compounds of interest were detected by native fluorescence [14-16].
Apparatus, chromatography
Chromatographic equipments were from Shimadzu Corp., Tokyo, Japan. The solvent delivery system consisted of a DGU-3A on-line degasser coupled to a LC-9A quartenary gradient pump. Column temperature was maintained using a CTO-6A column oven and on-line solvent preheater. Samples were injected with a SIL-9A autoinjector. Digitoxin was detected by an SPD-6AV variable wavelength detector, N-acetyl-DL-tryptophan, warfarin, and naproxen were detected by a RF-551 fluorescence detector. Data acquisition and integration were performed by a Class-VP 4.2 computer-based integration system.
Data treatment
The free fraction (fraction of unbound drug) was calculated as the ratio of the ultrafiltrate (free) concentration and the total concentration determined in the HPLC analysis. The drug concentrations were plotted according to Scatchard [17] where the abscissa represents the binding r (the number of molecules of drug bound per molecule of albumin), and the ordinate r/x (x = free drug concentration). The data of r and x were fitted by linear least squares regression analysis when number of binding sites (n1 and n2) and dissociation constants (K1 and K2) for high (n1 and K1) and low (n2 and K2) affinity binding were calculated. The drug concentrations representing the specific binding range were additionally evaluated in a double reciprocal plot of r and x according to Lineweaver-Burk [18]. Results given are means (SDs) of three to five separate experiments. Microsoft Excel and GraphPad Prism were used for calculating the results. Wilcoxon's signed rank test was used for statistical analysis.
Results
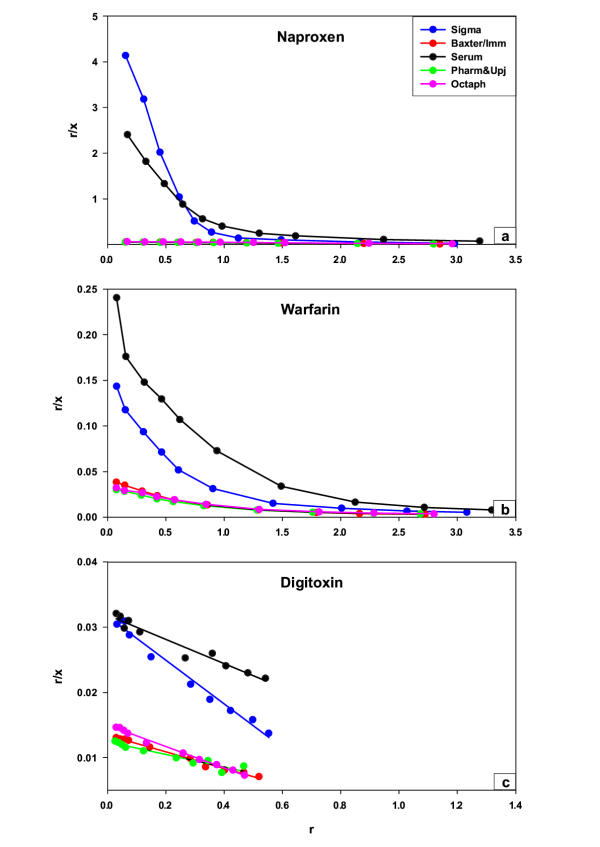
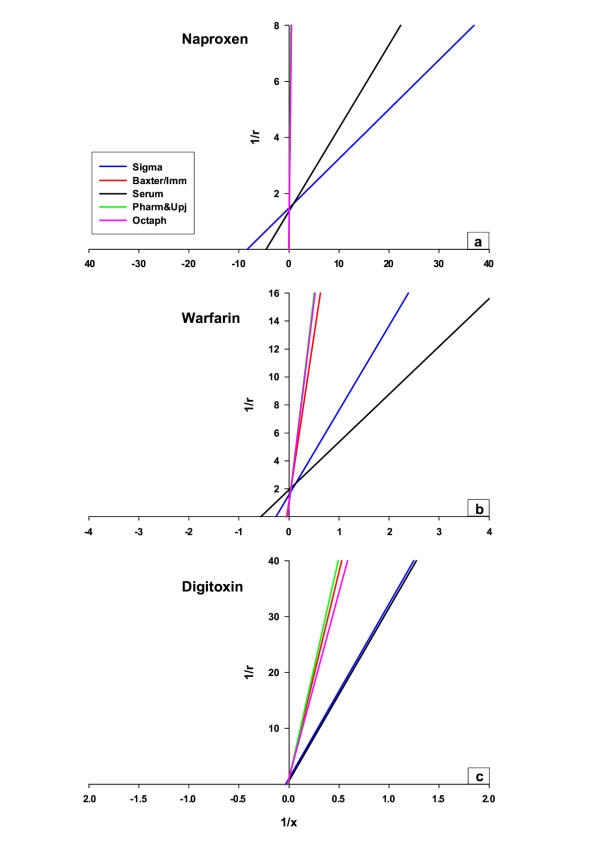
Naproxen, warfarin, and digitoxin were highly bound in solutions of Sigma albumin and native human serum, yielding free fractions of approximately 0.05, 1.0, and 5% respectively (Table 1). The free fractions were considerably higher in albumin solutions from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn, approximately 40-times for naproxen and 5 and 2 times for warfarin and digitoxin (Table 1). In figure 1 the results of the binding studies with various concentrations of naproxen (fig 1a), warfarin (fig 1b), and digitoxin (fig 1c) in different albumin solutions, plotted according to Scatchard [17], are shown. In native human serum and Sigma albumin, the binding of naproxen and warfarin yielded a biphasic curve with approximately one specific albumin binding site (n1) each, while the number of unspecific binding sites (n2) ranged from 3 to 5. Digitoxin displayed a straight line, indicating specific binding only, as intended at the concentrations selected. Naproxen elicited the highest affinity with the lowest dissociation constant (K1) approximately 0.1–0.4 µmol/L. The K1 of warfarin and digitoxin were approximately 10 and 100 times higher (Table 2). In albumin solutions from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn, the specific binding of naproxen was abolished. Both n1 and K1 increased to the levels of n2 and K2 (Table 2 and 3). The correlation coefficients (r2) of the Scatchard plots (all 10 drug concentrations of naproxen) representing the binding of albumin in the products from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn were 0.99, 0.99, and 0.99 respectively. Thus the high-affinity binding site for naproxen in pharmaceutical-grade albumin was no longer detectable with the methods we have used. For warfarin and digitoxin the binding was reduced without changes in the number of binding sites, but decreasing affinity (K1 increased). Table 2 summarises the binding parameters obtained. Non-linear regression analysis was performed to verify the results obtained in the Scatchardanalysis, giving almost identical results (data not shown). Figure 2 shows the results of the binding studies with naproxen (fig 2a), warfarin (fig 2b), and digitoxin (fig 2c), in concentrations representing the specific binding presented in a double reciprocal plot (of r and x) according to Lineweaver-Burk [18]. As can be seen, the y-axis intercepts were similar with the various ligands while the x-axis intercepts varied. When caprylic acid and N-acetyl-DL-tryptophan (stabilisers used in preparing pharmaceutical-grade albumin) were added to solutions of native human serum and Sigma albumin (Table 3) to the final concentrations of 3.3 and 3.2 mmol/L respectively, the binding characteristics of naproxen, warfarin, and digitoxin were similar to that found in pharmaceutical-grade albumin solutions (Table 2). Charcoal treatment of the in-process albumin solution from Octapharma, without stabilisers and not subjected to pasteurisation (Table 4), with similar binding properties as human sera and Sigma albumin (Table 2 and 3), decreased/impaired the albumin binding capacity of naproxen (K1 increased from 0.1 ± 0.01 µmol/L to 0.2 ± 0.02 µmol/L, p < 0.05) whereas the warfarin and digitoxin binding were unaffected (Table 4). Charcoal treatment of the pharmaceutical-grade albumin from Octapharma restored the binding capacity of naproxen to the level obtained with charcoal treated in-process albumin, without stabilisers and pasteurisation. The dissociation constant K1, and the number of binding sites n1 decreased from 75.5 ± 3.9 µmol/L and 5.7 ± 0.3 to 0.2 ± 0.02 µmol/L and 1.1 ± 0.1 (n = 5, p < 0.05, Table 4).
Table 1.
Percentage unbound (free) drug concentrations of naproxen, warfarin, and digitoxin in various albumin solutions
| Naproxen 0.1 mmol/L | Warfarin 0.05 mmol/L | Digitoxin 0.02 mmol/L | |
| Human serum | 0.07 (0.02)% | 0.7 (0.2)% | 4.9 (0.2)% |
| Sigma albumin | 0.04 (0.01)% | 1.1 (0.2)% | 5.1 (0.4)% |
| Baxter/Immuno | 2.77 (0.09)%* | 4.1 (0.2)%* | 11.1 (0.3)%* |
| Pharmacia & Upjohn | 3.15 (0.17)%* | 5.2 (0.5)%* | 11.5 (0.4)%* |
| Octapharma | 2.61 (0.10)%* | 4.8 (0.3)%* | 10.0 (0.4)%* |
Percent free drug concentrations of naproxen, warfarin, and digitoxin calculated from the results obtained by ultrafiltration of the albumin solutions and human serum. The solutions were diluted to the final albumin concentration of 0.615 mmol/L. Values are means (SDs) of five separate experiments. * p < 0.05 compared with Sigma albumin and human serum.
Figure 1.
Scatchard plots of naproxen (a), warfarin (b), and digitoxin (c) binding in various albumin solutions Scatchard plots of naproxen (a), warfarin (b), and digitoxin (c) binding in native human serum, defatted human albumin from Sigma, and three pharmaceutical-grade solutions from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn. The symbols are given in separate box. The albumin solutions contained 0.615 mmol/L albumin. The experiments were performed with ultrafiltration and the results of the calculated parameters are found in table 2.
Table 2.
Binding parameters of naproxen, warfarin, and digitoxin in various albumin solutions
| Specific binding | Non Specific binding | |||||||||
| n1 | K1 (µmol/L) | n2 | K2 (µmol/L) | |||||||
| Napr. | Warf. | Dig. | Napr. | Warf. | Dig. | Napr. | Warf. | Napr. | Warf. | |
| Human serum | 1.0 (0.1) | 1.1 (0.1) | 1.8 (0.5) | 0.4 (0.4) | 5.1 (1.9) | 56.5 (21.0) | 3.9 (0.2) | 3.7 (0.1) | 11.3 (0.8) | 72.1 (8.2) |
| Sigma albumin | 0.8 (0.1) | 0.9 (0.1) | 1.0 (0.1) | 0.2 (0.1) | 6.3 (1.3) | 30.0 (2.2) | 3.2 (0.2) | 3.9 (0.3) | 16.3 (1.9) | 173.5 (31.2) |
| Baxter/Immuno | 3.3* (0.6) | 1.0 (0.1) | 1.1 (0.1) | 55.2* (10.9) | 25.4* (2.4) | 79.2# (6.9) | 3.8 (0.2) | 3.6 (0.2) | 70.5* (2.0) | 316.3* (27.4) |
| Pharmacia & Upjohn | 3.5* (1.3) | 1.2 (0.1) | 1.4 (0.3) | 66.9* (28.2) | 37.6* (6.4) | 113.0# (33.7) | 3.8 (0.3) | 3.8 (0.3) | 78.5* (9.2) | 342.1* (45.4) |
| Octapharma | 4.5* (0.8) | 1.3 (0.2) | 0.9 (0.03) | 70.0* (14.0) | 39.6* (7.6) | 61.2# (3.1) | 4.0 (0.3) | 3.9 (0.2) | 60.2* (8.4) | 321.1* (42.0) |
The binding studies with naproxen, warfarin, and digitoxin were performed with ultrafiltration of the various albumin solutions containing 0.615 mmol/L albumin. The binding parameters: binding sites, specific (n1) and non-specific (n2) and dissociation constants K1 and K2 were calculated according to Scatchard [17]. The values are means (SDs) of five separate experiments. * p < 0.05 compared with human serum and Sigma albumin. # p < 0.05 compared with Sigma albumin.
Table 3.
Effects of caprylate and/or N-acetyl-DL-tryptophan on naproxen, warfarin, and digitoxin binding
| n1 | K1 (µmol/L) | |||||||
| No | C | T | C+T | No | C | T | C+T | |
| Naproxen (n = 5) | ||||||||
| Native serum | 1.0 (0.1) | 5.7* (1.4) | 2.6* (0.4) | 3.2* (0.6) | 0.5 (0.1) | 67.9* (19.3) | 10.4* (2.1) | 49.9* (7.6) |
| Sigma albumin | 0.8 (0.04) | 2.4* (0.6) | 1.4* (0.1) | 2.1* (0.4) | 0.1 (0.03) | 43.9* (11.5) | 6.3* (0.5) | 47.8* (11.7) |
| Warfarin (n = 3) | ||||||||
| Native serum | 1.6 (0.4) | 1.3 (0.1) | 1.2 (0.02) | 1.0 (0.2) | 10.4 (3.4) | 20.3 (2.9) | 9.4 (0.9) | 18.1 (3.2) |
| Sigma albumin | 1.6 (0.2) | 1.4 (0.3) | 1.4 (0.2) | 1.6 (0.1) | 11.4 (2.1) | 30.9 (7.0) | 13.5 (3.1) | 47.6 (3.6) |
| Digitoxin (n = 3) | ||||||||
| Native serum | 1.6 (0.2) | 1.4 (0.4) | 1.4 (0.5) | 1.2 (0.3) | 54.2 (8.1) | 68.6 (23.2) | 50.1 (28.4) | 63.4 (28.4) |
| Sigma albumin | 1.3 (0.1) | 1.2 (0.1) | 1.0 (0.3) | 1.0 (0.2) | 41.0 (11.0) | 66.8 (14.7) | 28.3 (18.3) | 57.2 (32.3) |
The effects of the stabilisers caprylate (C) and N-acetyl-DL-tryptophan (T) and both (C+T) on naproxen, warfarin, and digitoxin binding were tested with ultrafiltration of human serum and Sigma albumin after addition of the stabilisers and ligands. The results were calculated according to Scatchard [17] and expressed by specific binding sites (n1) and dissociation constants (K1). The final concentrations of caprylate and N-acetyl-DL-tryptophan were each 3.2 and 3.3 mmol/L respectively. The values are means (SDs) of 5 separate experiments with naproxen and 3 with warfarin and digitoxin. *p < 0.05 compared with no stabiliser (No).
Figure 2.
Lineweaver-Burk plots of naproxen (a), warfarin (b) and digitoxin (c) binding in various albumin solutions Lineweaver-Burk plots of naproxen (a), warfarin (b) and digitoxin (c) binding in various albumin solutions. The figures are based on the results representing the specific binding, presented in total in table 2 and figure 1.
Table 4.
Effects of charcoal-treatment on naproxen, warfarin, and digitoxin binding in albumin solutions with/without stabilisers#
| Not charcoal treated albumin | Charcoal treated albumin | |||
| In-process | Final product | In-process | Final product | |
| Naproxen | ||||
| K1 (µmol/L) | 0.1* (0.01) | 75.5 (3.9) | 0.2 (0.02) | 0.2 (0.02) |
| n1 | 0.9* (0.2) | 5.7 (0.3) | 0.9 (0.1) | 1.1 (0.1) |
| Warfarin | ||||
| K1 (µmol/L) | 13.1* (1.6) | 43.1 (7.1) | 13.5 (0.6) | 14.6 (2.4) |
| n1 | 1.9 (0.2) | 1.7 (0.2) | 1.7 (0.1) | 2.0 (0.2) |
| Digitoxin | ||||
| K1 (µmol/L) | 19.7* (1.0) | 67.6 (6.8) | 20.8 (1.0) | 31.0 (4.2) |
| n1 | 0.8 (0.03) | 1.1 (0.1) | 0.8 (0.03) | 1.3 (0.2) |
Effects of charcoal treatment on naproxen, warfarin, and digitoxin binding in two different albumin solutions. The in-process albumin solution (collected from the albumin manufacturing process before addition of the stabilisers and final heating) and the final product. Values are means (SDs) of 5 separate experiments. #: The concentration of N-acetyl-DL-tryptophan was 3 mmol/L in the final product before charcoal treatment, but not detectable (<0.2 µmol/L) after charcoal treatment (separate analysis). *p < 0.05 compared with the final product not charcoal treated.
Discussion
Several mechanisms may lead to an increase in free fraction of drugs or other ligands. Hypoalbuminaemia is characterised by a reduction in the quantity of binding protein available, and when this decreases, so does the amount of bound drug [3,19]. Competitive and non-competitive displacement of other ligands is another mechanism to explain increased free fraction of ligands. Kragh-Hansen [20] studied possible competitive binding between L-tryptophan, octanoate, and diazepam. During simultaneous presence of L-tryptophan and octanoate or diazepam plus octanoate, pronounced mutual reductions in binding were observed. Lagrange et al. [21] studied binding of ketoprofen enantiomers in various human albumin preparations. The effects of hippuric acid (in concentrations found in uraemic patients) and octanoic acid (one of the stabilisers used in pharmaceutical-grade albumin) on binding characteristics of ketoprofen enantiomers were tested. Both hippuric and octanoic acid induced a significant increase in the free fraction of ketoprofen enantiomers, most pronounced with octanoic acid. Alterations in albumin binding may significantly change pharmacokinetics and pharmacodynamics, especially for highly albumin-bound drugs. Pharmaceutical-grade albumin is obtained from blood or plasma from healthy human donors by fractionation according to the cold Cohn ethanol process [22]. Stabilisers (N-acetyl-DL-tryptophan and Na-caprylate) are then added before pasteurisation for 10 h at 60°C to inactivate among others human immunodeficiency and hepatitis virus.
We have used the ultrafiltration technique in this study, mainly due to the simple and rapid technique [23]. According to Borgå & Borgå [9], using the acidification procedure with phosphoric acid allows CO2 to escape prior to the ultrafiltration, keeping the pH constant during the experiments. The binding parameters like number of binding sites and dissociation constants (=the inverse value of the association constant) for the ligands (Table 1, 2 and 3) are quite similar and in agreement with values reported by others [9,19,24,25]. The present study demonstrates that the specific binding of warfarin and digitoxin is significantly reduced and for naproxen abolished in pharmaceutical-grade albumin from Baxter/Immuno, Octapharma, and Pharmacia & Upjohn. Furthermore, our study shows that caprylic acid and N-acetyl-DL-tryptophan may be of major importance in affecting the binding properties. It has previously been shown that both caprylic acid and tryptophan are highly bound to albumin [20] and that the specific binding site of these substances is on site II, according to Sudlow et al. [7], the binding site for naproxen. The finding (fig 2) that the intercepts on the y-axis were almost identical whereas the intercepts on the x-axis were quite different strongly indicates a competition mechanism. The molar concentrations of caprylic acid and N-acetyl-DL-tryptophan used to stabilise albumin during the pasteurisation process are several times higher than the albumin concentration. In our study, binding parameters like number of binding sites and dissociation constants obtained for the specific binding of naproxen was no longer detectable in pharmaceutical-grade albumin (Table 2 and 3) with the methods we have used, whereas for warfarin and digitoxin the dissociation constant only increased.
Thus, displacement effects of the stabilisers are most likely. If the findings of Børmer et.al. and the present results reflect changed binding capacity of albumin also in vivo, it raises the possibility that altered albumin binding may contribute to the possible detrimental effect of albumin transfusions. Patients with either hypoalbuminaemia or patients receiving large amounts of pharmaceutical-grade albumin might be at special risk, as discussed recently in a meta-analysis that demonstrated no overall effect of albumin transfusion on mortality [5]. The report from the Cochrane injuries group albumin reviewers [4] indicated possible harmful effects and excess mortality after albumin administration to critically ill patients. However, the validity of the Cochrane meta-analysis has been challenged by a recent meta-analysis [5]. Naproxen, warfarin, and digitoxin are drugs used in post-operative and intensive care units. Increased free fraction of such drugs may cause enhanced adverse effects and possibly serious adverse effects. Thus, it could be hypothesised that the combination of transfusing large amounts of pharmaceutical-grade albumin solutions and certain highly albumin-bound drugs might precipitate detrimental effects in critically ill patients. However, further in vitro and in vivo studies will be needed to examine if these results can have consequences for patients receiving large amounts of pharmaceutical-grade albumin and certain highly albumin-bound drugs.
Conclusions
Previous studies have indicated that transfusion of pharmaceutical-grade albumin to critically ill patients is controversial. The present in vitro studies of drug binding capacity of pharmaceutical-grade albumin demonstrate that the specific binding of naproxen is no longer detectable and the specific binding of warfarin and digitoxin is significantly reduced. The studies further shows that caprylic acid and N-acetyl-DL-tryptophan, used as stabilisers in the purification process when manufacturing pharmaceutical albumin, are of importance of this effect, and that the impairing or displacing effect might be of competitive nature.
Competing interests
None declared.
Authors' contributions
HO was the main designer and coordinator of the study and drafted the manuscript. AA and AN participated in designing the study, drug analysis, statistical analysis, and manuscript preparation. UEK and OPB participated in designing the study, results interpretation and manuscript drafting. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The technical work of Ann-Kristin Thøgersen and Linda Therese Frønes is highly appreciated and acknowledged. The volunteers donating blood for this study are highly acknowledged.
The study was financially supported by an unrestricted grant from Octapharma AG, Switzerland. Octapharma AG also donated some of the albumin solutions used in this study.
Contributor Information
Harald Olsen, Email: har-ol@online.no.
Anders Andersen, Email: anders.andersen@klinmed.uio.no.
Arve Nordbø, Email: arve.nordbo@siv.no.
Ulf E Kongsgaard, Email: ulf.kongsgaard@klinmed.uio.no.
Ole P Børmer, Email: ole.bormer@labmed.uio.no.
References
- Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan Med Bull. 1990;37:57–84. [PubMed] [Google Scholar]
- Vorum H. Reversible ligand binding to human serum albumin. Theoretical and clinical aspects. Dan Med Bull. 1999;46:379–399. [PubMed] [Google Scholar]
- Tillement JP, Lhoste F, Giudicelli JF. Diseases and drug protein binding. Clin Pharmacokinet. 1978;3:144–154. doi: 10.2165/00003088-197803020-00004. [DOI] [PubMed] [Google Scholar]
- Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes MM, Navickis RJ. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:149–164. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- Bormer OP, Amlie LM, Paus E, Kongsgard U. Automated albumin method underestimates pharmaceutical-grade albumin in vivo. Clin Chem. 1999;45:1082–1084. [PubMed] [Google Scholar]
- Sudlow G, Birkett DJ, Wade DN. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–832. [PubMed] [Google Scholar]
- Sjoholm I, Ekman B, Kober A, Ljungstedt-Pahlman I, Seiving B, Sjodin T. Binding of drugs to human serum albumin:XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol Pharmacol. 1979;16:767–777. [PubMed] [Google Scholar]
- Borga O, Borga B. Serum protein binding of nonsteroidal antiinflammatory drugs: a comparative study. J Pharmacokinet Biopharm. 1997;25:63–77. doi: 10.1023/A:1025719827072. [DOI] [PubMed] [Google Scholar]
- Mortensen A, Jensen EB, Petersen PB, Husted S, Andreasen F. The determination of naproxen by spectrofluorometry and its binding to serum proteins. Acta Pharmacol Toxicol (Copenh) 1979;44:277–283. doi: 10.1111/j.1600-0773.1979.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Wong YW, Davis PJ. Analysis of warfarin and its metabolites by reversed-phase ion-pair liquid chromatography with fluorescence detection. J Chromatogr. 1989;469:281–291. doi: 10.1016/S0021-9673(01)96463-5. [DOI] [PubMed] [Google Scholar]
- Plum J, Daldrup T. Detection of digoxin, digitoxin, their cardioactive metabolites and derivatives by high-performance liquid chromatography and high-performance liquid chromatography-radioimmunoassay. J Chromatogr. 1986;377:221–231. doi: 10.1016/S0378-4347(00)80777-X. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Vega AE, Wells A, Datta P. Sensitive methods for determination of free digitoxin concentration using digitoxin immunoassays: demonstration of elevated free digitoxin concentration caused by digitoxin-phenytoin interaction by applying these new techniques. Ther Drug Monit. 1999;21:625–630. doi: 10.1097/00007691-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Riley CM, Tomlinson E, Jefferies TM, Redfern PH. Surfactant ion-pair high-performance liquid chromatography of tryptophan and some of its metabolites in biological fluids. J Chromatogr. 1979;162:153–161. doi: 10.1016/S0378-4347(00)81908-8. [DOI] [PubMed] [Google Scholar]
- Herve C, Beyne P, Jamault H, Delacoux E. Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J Chromatogr B Biomed Appl. 1996;675:157–161. doi: 10.1016/0378-4347(95)00341-X. [DOI] [PubMed] [Google Scholar]
- Nelis HJ, Lefevere MF, Baert E, D'Hoore W, De Leenheer AP. Chromatographic determination of N-acetyl-DL-tryptophan and octanoic acid in human albumin solutions. J Chromatogr. 1985;333:381–387. doi: 10.1016/S0021-9673(01)87367-2. [DOI] [PubMed] [Google Scholar]
- Scatchard G. The attraction of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51:660–672. [Google Scholar]
- Goldstein A, Aronow L, Kalman SM. In Principles of Drug Action. New York: Jonh Wiley & Sons, Inc; 1974. Molecular Mechanisms of Drug Action; pp. 1–127. [Google Scholar]
- Brors O, Fremstad D, Poulsson C. The affinity of human serum albumin for [3H]-digitoxin is dependent on albumin concentration. Pharmacol Toxicol. 1993;72:310–313. doi: 10.1111/j.1600-0773.1993.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U. Octanoate binding to the indole- and benzodiazepine-binding region of human serum albumin. Biochem J. 1991;273:641–644. doi: 10.1042/bj2730641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange F, Penhourcq F, Matoga M, Bannwarth B. Binding of ketoprofen enantiomers in various human albumin preparations. J Pharm Biomed Anal. 2000;23:793–802. doi: 10.1016/S0731-7085(00)00380-0. [DOI] [PubMed] [Google Scholar]
- Matejtschuk P, Dash CH, Gascoigne EW. Production of human albumin solution: a continually developing colloid. Br J Anaesth. 2000;85:887–895. doi: 10.1093/bja/85.6.887. [DOI] [PubMed] [Google Scholar]
- Pacifici GM, Viani A. Methods of determining plasma and tissue binding of drugs. Pharmacokinetic consequences. Clin Pharmacokinet. 1992;23:449–468. doi: 10.2165/00003088-199223060-00005. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U. Relations between high-affinity binding sites of markers for binding regions on human serum albumin. Biochem J. 1985;225:629–638. doi: 10.1042/bj2250629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RA. The binding of sodium warfarin to plasma albumin and its displacement by phenylbutazone. Ann N Y Acad Sci. 1973;226:293–308. doi: 10.1111/j.1749-6632.1973.tb20491.x. [DOI] [PubMed] [Google Scholar]