Abstract
During standing balance, kinematics of postural behaviors have been previously observed to change across visual conditions, perturbation amplitudes, or perturbation frequencies. However, experimental limitations only allowed for independent investigation of such parameters. Here, we adapted a pseudorandom ternary sequence (PRTS) perturbation previously used in rotational support-surface perturbations (Peterka 2002) to a translational paradigm, allowing us to concurrently examine the effects of vision, perturbation amplitude, and frequency on balance control. Additionally, the unpredictable PRTS perturbation eliminated effects of feedforward adaptations typical of responses to sinusoidal stimuli. The PRTS perturbation contained a wide spectral bandwidth (0.08-3.67 Hz) and was scaled to 4 different peak-to-peak amplitudes (3-24 cm). Root-mean-square (RMS) of hip displacement and velocity increased relative to RMS ankle displacement and velocity in the absence of vision across all subjects, especially at higher perturbation amplitudes. Gain and phase lag of CoM sway relative to the perturbation also increased with perturbation frequency; phase lag further increased when vision was absent. Together, our results suggest that visual input, perturbation amplitude, and perturbation frequency can concurrently and independently modulate postural strategies during standing balance. Moreover, each factor contributes to the difficulty of maintaining postural stability; increased difficulty evokes a greater reliance on hip motion. Finally, despite high degrees of joint angle variation across subjects, CoM measures were relatively similar across subjects, suggesting that the CoM is an important controlled variable for balance.
Keywords: Balance, posture, kinematics, kinetics, perturbation response
INTRODUCTION
During standing balance control, different kinematic coordination patterns have been observed to stabilize the body, but the conditions under which they are used are uncertain. The “ankle strategy” and “hip strategy” have been identified during recovery of balance in response to discrete perturbations based on joint angles and joint torques (Horak and Nashner 1986; Runge et al. 1998). The ankle strategy has been described as a default mechanism during quiet standing (Winter 1995; Gatev et al. 1999), but can only generate limited ankle torque for balance recovery (Kuo and Zajac 1993). In contrast, the hip strategy is considered to move the body center of mass (CoM) more effectively (Kuo and Zajac 1993; Winter 1995), using more proximal musculature (Horak and Nashner 1986; Winter 1995; Runge et al. 1999; Gatev et al. 1999). These postural strategies are not mutually exclusive but describe a continuum of postural responses where the hip strategy is used in response to increasingly difficult perturbations (Winter 1995; Runge et al. 1999; Horak and Nashner 1986). However, it is not fully understood how biomechanical factors contribute to the selection of such strategies, and proposed boundaries based joint torques and CoM velocity and position have not been validated (Pai and Patton 1997; Pai et al. 1998). Further, more recent work has demonstrated that both hip and ankle strategies may represent co-existing “excitable modes” that are present even during quiet standing (Creath et al. 2005).
While disruption of visual input has been shown to affect joint kinematics, the effects of vision on postural strategies have been dependent upon the type of perturbations administered. Therefore, the effects of vision on discrete ramp-and-hold translations are dependent upon the particular stimulus characteristics (Maki and Ostrovski 1993a), as subjects switch from an ankle to a hip strategy with increasing perturbation amplitude (Diener et al. 1988; Horak 1996). Similarly, individuals with visual impairments tend to use increased hip motion in response to discrete rotations of the support surface (Ray et al. 2008). In sinusoidal support-surface translations, disruption of visual input has been shown to shift predominant joint motion from the ankle to the hip at specific isolated frequencies (Buchanan and Horak 1999, 2001; Jeka et al. 1998); however, a shift from ankle to hip joint motion also occurs as frequency increases at a given amplitude. Further, in sinusoidal translations of fixed amplitude and frequency, there may be changes in head stability and joint motion over the duration of a trial as subjects learn predictive feedforward strategies for postural stabilization (Buchanan and Horak 1999; Berthoz et al. 1979). This can only occur if perturbations have predictable timing, amplitude, and frequency characteristics.
Regardless of individual joint strategies used during balance, it has been shown that global, task-level variables such as the CoM are more conserved and consistently controlled across motor tasks. Here we define a task-level variable as a motor variable that cannot be directly mapped to any one sensory input or unique motor output. In order to maintain balance, the body CoM must remain over the base of support (BoS) (Massion 1992), even under conditions of altered gravity (Massion et al. 1997). A variety of studies examining kinematic variability of motor tasks demonstrate that global variables such as CoM are controlled while allowing individual joint angles to vary, including sit-to-stand (Scholz and Schoner 1999; Scholz et al. 2001; Krishnamoorthy et al. 2005; Ting 2007), standing on unstable surfaces with and without touch support (Krishnamoorthy et al. 2004), and quiet stance (Krishnamoorthy et al. 2005). In human balance control, CoM feedback has been shown to modulate joint torques (Kuo 1995) as well as muscle activity (Kuo 1995; Welch and Ting 2008; Ting et al. 2009). Control of the CoM has also been shown to change with alterations in sensory input including (but not limited to) vestibular loss (Nashner et al. 1982; Peterka and Benolken 1995; Peterka 2002), galvanic vestibular stimulation (Inglis et al. 1995), and changes in cutaneous cues (Jeka and Lackner 1994; Jeka et al. 1998).
There has been some debate as to whether vision affects task-level variables during balance such as CoM and center of pressure (CoP), which is also confounded by differences in perturbation characteristics. Lack of vision has been shown to cause increased CoP sway and larger displacements of head and hip angle in sinusoidal rotational paradigm (Diener et al. 1982). Similarly, it has been found that visual deprivation during sinusoidal platform translation caused subjects to switch control strategies from head stabilization to head oscillation (Corna et al. 1999). However, other studies have found that visual deprivation did not affect CoP in transient and continuous support-surface translations, but in such studies CoM was not analyzed (Maki and Ostrovski 1993a, b). Finally, lack of vision has also been shown to increase CoM sway in quiet stance (Krishnamoorthy et al. 2005) and in quiet stance sway-referenced visual-surround rotations (Kuo et al. 1998), but these results cannot be generalized to perturbation responses.
We hypothesized that removal of visual input would change motor strategies independent of stimulus amplitude and frequency. Prior studies have been limited to examining amplitude effects in ramp-and-hold perturbations, or examining either frequency or amplitude effects in continuous perturbations where either amplitude was varied at a single frequency (Van Ooteghem et al. 2008), or frequency was varied at a single amplitude (Buchanan and Horak 1999, 2001). By contrast, we examined the simultaneous effects of both frequency and amplitude using a pseudorandom perturbation that incorporated multiple simultaneous frequencies and amplitudes within a single trial. Specifically, we used a pseudo-random ternary sequence (PRTS) perturbation that was previously used in rotational perturbations(Peterka 2002) in which the platform made small discrete steps, resulting in a roughly sinusoidal perturbation that contains a wide spectral bandwidth and can be scaled to different peak-to-peak amplitudes. This allows us to examine balance control over a wide range of frequencies and amplitudes within a few trials and to dissociate their effects through time-domain and frequency-domain analysis. Furthermore, the PRTS has been shown to be unpredictable over many repeated cycles (Peterka 2002), eliminating the effects of feedforward strategies resulting from adaptation to a cyclic, predictable perturbation. We predicted that removal of vision would shift predominant joint motion from the ankle to the hip over all stimulus amplitudes and frequencies, causing subsequent changes in time- and frequency-domain responses of CoM displacement and velocity.
MATERIALS AND METHODS
Subjects
Eight healthy young adults ages 20-24 (mean age ± SD = 21.6 ± 1.3 yrs., 3 males and 5 females) participated in the experiment. Subjects gave informed consent in accordance with Georgia Institute of Technology and Emory University IRB protocols.
Stimulus
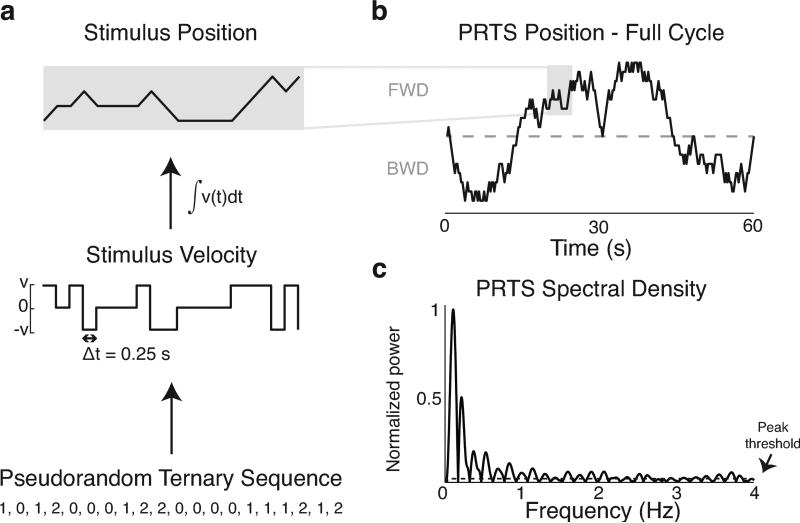
The waveform that defined the perturbation was a pseudorandom ternary sequence (PRTS) as outlined in Peterka (2002). A 5-stage shift register (n = 5) was used to generate the sequence based on modulo-3 addition and feedback (Peterka 2002). The resulting sequence was periodic with a number of values equal to 3n-5 (242, in this case). The sequence of zeros, ones, and twos was transformed into a velocity stimulus of 0, v, and –v cm/s, respectively. Each velocity value was held for 0.25 s for a total cycle time of 60.5 s. This sequence was time-integrated to yield the position waveform that specified platform motion (Figure 1a). We used the same pseudorandom trajectory for all perturbations in all subjects.
Figure 1.
Pseudorandom ternary stimuli. a, a pseudorandom ternary sequence (PRTS) was generated using a 5-stage feedback register (not shown) and was comprised of zeros, ones, and twos (bottom). This sequence was translated into unit velocities (middle) with a time step of Δt = 0.25 s. The velocity sequence was time-integrated to yield the position command to the platform (top), and the position trajectory was scaled depending on the desired peak-to-peak amplitude. b, Full 60.5 s PRTS position trajectory. Five cycles were strung together for each trial. FWD: forward platform position relative to starting position; BWD: backward platform position relative to starting position. c, Spectral density of PRTS signal. Several peaks of high spectral power were averaged across cycles to produce the smoothed spectra used for transfer function estimates.
Stimuli were scaled versions of the same PRTS-derived position sequence (3, 6, 12, and 24 cm peak-to-peak) and consisted of anterior-posterior support surface translations. The lowest perturbation amplitude was similar to the smallest (0.5°) perturbation used in Peterka (2002); both were below the perceptual threshold and induced peak-to-peak body sway of about 1°. The smallest possible movement length was 1/15 of the total peak-to-peak amplitude; namely, 2 mm for the smallest perturbation amplitude and 16 mm for the largest. Each trial contained five PRTS cycles strung together for a total time of 302.5 s. Cycles were constrained to stop and start at the same location so the platform returned to the initial position at the end of the trial. Moreover, the PRTS ended with a velocity stimulus of 0, resulting in a pause of 0.25s between cycles. Cycles were constrained to produce equal amounts of anterior and posterior excursion (Figure 1b).
Experimental Conditions
Eight trials were randomly presented to each subject, one of each amplitude under both eyes open (EC) and eyes closed (EO) conditions. The only constraint on trial order was that the largest amplitude trial (24 cm) was presented sometime after the second-largest trial condition (12 cm). We avoided placing the largest amplitude trial first so that subjects could successfully maintain their balance without stepping or falling. Because each trial was long (~6 minutes) and incorporated over 1000 individual perturbations, familiarization was not necessary as the timescales for adaptation of postural responses occur on the order of a few seconds or trials (Keshner et al. 1987). This ensured that the subject was accustomed to the platform paradigm before the most difficult stimulus. Subjects were unrestrained and required to maintain their balance with a stance width equal to the horizontal distance between their anterior superior iliac spines (inter-ASIS width). Subjects were instructed to “stand with arms folded, feet on tape markers, stare straight ahead, and maintain balance as best you can”. If a subject took a step, the trial was repeated. For blindfolded trials, subjects were instructed to put on the blindfold so that it was comfortable yet completely occluded vision. After a trial was complete, the subject was instructed to remove the blindfold. After every two trials, subjects rested for 2-5 minutes. Two quiet standing trials of 180 s were performed before moving trials, one for each visual condition.
Data Collection
Ground reaction forces were collected through two force plates (AMTI, Watertown, MA) mounted in the platform (1080 Hz). An 8-camera motion capture system (Vicon, Centennial, CO) was used to record kinematic data (120 Hz) using a 25-marker modified Helen-Hay marker set. Markers were placed bilaterally on the heel, 1st metatarsal head, lateral malleolus, mid-shank, lateral knee, ASIS, posterior superior iliac spine (PSIS), mid-thigh, spinous process of C7, acromion, with 4 markers on the head and one on the back. We also collected subject anthropometric data which was used with Vicon Plug-In Gait to compute joint centers and joint angles. CoM acceleration was calculated from force plate data and subject mass (F=ma). Kinematic data were used to calculate bilateral joint angles as well as CoM displacement and velocity based on estimates of segmental masses (Winter 1990). Although joint angles were computed bilaterally, there was no evidence of asymmetry in postural responses; therefore, only the right leg was used for analysis.
Time-Domain Analysis
Root-mean-square (RMS) was used to quantify variability in CoM and joint angle measures and to compare body motion during EO versus EC conditions. CoM sway was calculated as CoM position minus platform position, and sway velocity was calculated as CoM velocity minus platform velocity. Sagittal ankle, knee, and hip joint angles were calculated. As changes in knee angle were negligible over the course of a trial, we excluded them from analyses. Because joint angles and CoM sway fluctuated around certain “set points”, switching between a few of these points over the course of a trial. The set points were identified using a 10 s median-filter on the joint angle data. We then subtracted the set point trajectory from the original joint angle data. Therefore shifts of more than 10 s were considered to be set points and eliminated from the computation of RMS values. This allowed us to use the RMS values to quantify higher-frequency changes in the joint angles about the set points. We computed an RMS value for each of the 5 cycles of the PRTS perturbation in each trial.
Using RMS values over the course of each PRTS cycle, four-way repeated measures ANOVA (visual condition ⨯ amplitude ⨯ subject ⨯cycle) was performed to determine statistically significant differences between amplitudes and visual conditions. Six variables were compared across conditions (CoM sway, sway velocity, hip angle and angular velocity, and ankle angle and angular velocity) for a total of six ANOVAs. A Bonferroni correction was applied to adjust for multiple ANOVA comparisons at α = .05, yielding a final value of α = .0083. Tukey-Kramer post-hoc tests (α = .05) were used to assess individual differences between visual conditions and stimulus amplitude.
We further compared ankle to hip RMS values using regression analysis. We did not find any effect of cycle using four-way ANOVA (F[4,319] = 1.07, P = 0.37); thus, we included RMS values for individual cycles in our regression analysis (5 values per trial). Using data points for RMS of each cycle for each amplitude, separate linear fits were applied to both EO and EC conditions, yielding slopes (m) and coefficients of determination (R2) for each fit. We compared the mean magnitudes of the slopes across subjects in EO and EC conditions using one-tailed t-tests with Bonferroni correction for multiple comparisons (α = .025).
Frequency-Domain Analysis
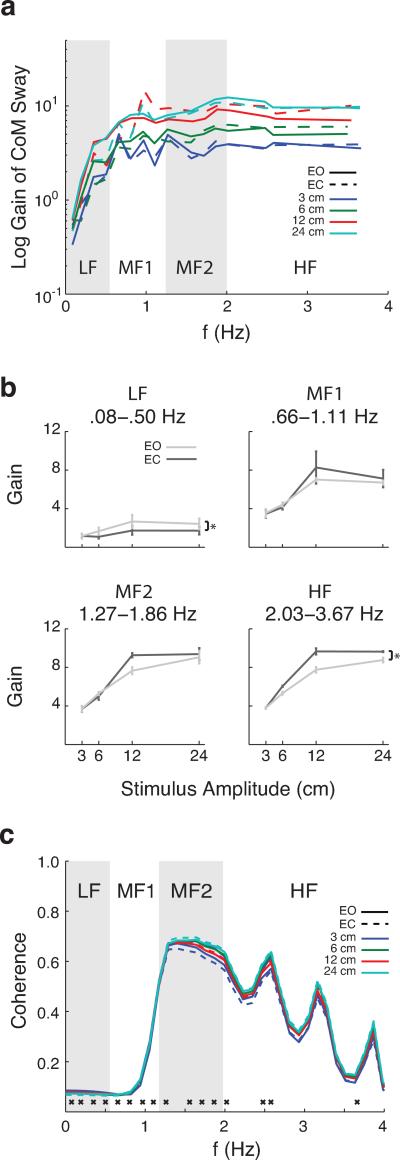
Transfer functions were used to characterize the dynamic behavior of the subject's body in response to platform stimuli (Maki et al. 1987; Oie et al. 2002; Peterka 2002; Milton et al. 2009) across visual conditions and stimulus amplitudes. A transfer function describes the relationship between the input and output of a system that is assumed to be linear and time-invariant (Bendat and Piersol 2010), which does not change as a function of the amplitude of the input signal. However, we expected nonlinearities in the frequency-domain relationship between the spectral power of the input signal (PRTS perturbation) and output signal (CoM sway). Thus, we numerically computed the value of transfer functions at each frequency and amplitude to describe the nonlinear relationships between input and output signals across frequencies and amplitudes. The changes in the gain and phase of the transfer function across perturbation amplitude reveal these nonlinearities (Peterka 2002). The PRTS position waveform was decomposed into its Fourier frequency components, which contain several peaks in the power spectrum (Figure 1c). CoM sway was similarly decomposed into spectral components. Sixteen spectral peaks with high power were selected between 0.08 and 3.55 Hz, as the maximum translation frequency of the platform was 4 Hz. These peaks were initially identified by thresholding the power spectra at 0.001 to find all the points above the noise floor, resulting in 16 discrete frequency regions (corresponding to each peak) found in all perturbation amplitudes (c.f. Figure 1c). For each frequency region, a mean power and standard deviation was found. Because each frequency region in the power spectra was of different power and width, we only included spectral points in each frequency region that had magnitude greater than the mean + 0.2 SD for that frequency region for transfer function analysis. Gain and phase values describing the transfer functions were averaged at each frequency peak across subjects to produce smoothed spectra (Peterka 2002).
Both gain and phase of the transfer functions were analyzed to compare responses between EO and EC conditions. Gain measures how the subject's body sway responds to certain stimulus frequencies. Phase measures the subject's tendency to lead or lag behind platform motion. To characterize trends in the frequency responses across perturbation amplitudes, data were binned into four frequency ranges for analysis: low (LF, 0.08-0.50 Hz), two middle (MF1, 0.66-1.11 Hz; MF2, 1.27-1.86 Hz), and high (HF, 2.02-3.66 Hz). Each range was chosen to have the same number of frequency peaks (four). Four-way ANOVAs (visual condition ⨯ frequency ⨯ amplitude ⨯ subject) were performed for each frequency range (Matlab ANOVAN). Subject was treated as a random effects variable. To address the validity of our transfer function results, we also performed a coherence function on smoothed spectral data. Values of the coherence function range from 0 to 1, where 0 indicates no linear correlation between platform motion and CoM sway, and 1 indicates a perfect linear correlation.
RESULTS
Time-Domain
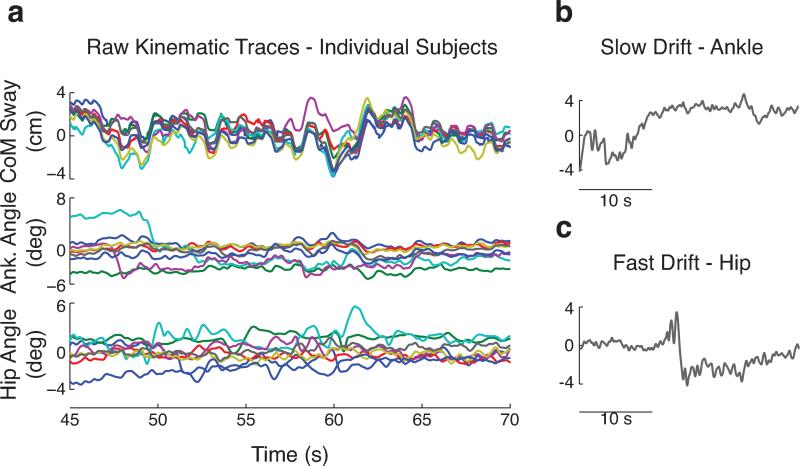
Although ankle and hip angle trajectories over time were different across subjects, CoM trajectories were observed to be quite similar across subjects (Figure 2a). This effect was independent of EO or EC conditions and stimulus amplitudes. Some similarities were observed in ankle angle but not hip angle traces across trials and subjects, likely because the ankle is more directly coupled to perturbation platform motion. On some trials, ankle or hip angles would fluctuate about multiple set points over the course of a trial. This manifested as either a slow drift from one set point to another (over tens of seconds; Figure 2b) or a quick shift to a new set point (< 3 seconds; Figure 2c). No such shifts were observed for CoM sway kinematics.
Figure 2.
Kinematic data during a 12 cm eyes-open trial. a, CoM sway, ankle angle, and hip angle over a 25-s segment of a trial for all subjects. Each subject is represented by a different color, and traces have been demeaned to assess common features across subjects. CoM sway (top) was more similar across subjects than individual joint angles. b, example of slow drift in ankle angle “set point” for one subject. c, example of fast drift in hip angle “set point” for one subject.
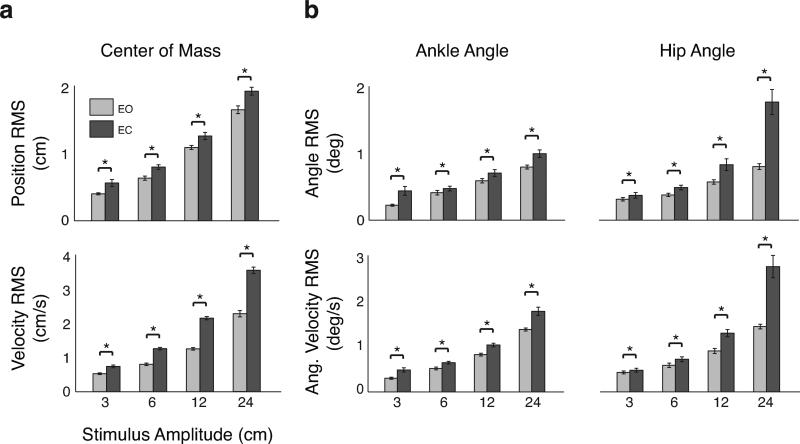
With EC at the highest perturbation amplitude, hip angular displacement and velocity RMS values increased much more than expected based on the independent effects of increasing perturbation amplitude or removing vision, suggesting that a hip strategy is employed in the most difficult perturbation conditions. We found significant effects of vision and perturbation amplitude on RMS values for CoM, ankle, and hip kinematics (all P < 10−12, ANOVA). Independent of stimulus amplitude, RMS of all kinematic variables was significantly increased in EC versus EO conditions (all P < 0.01, Tukey-Kramer post-hoc tests; Figure 3). Moreover, RMS of all kinematic variables was significantly increased for between 6 cm to 12 cm, and between 12 cm to 24 cm conditions regardless of visual condition (all P < 10−12, Tukey-Kramer post-hoc tests). RMS changes between 3 cm and 6 cm conditions was not significant for any kinematic variable. We also found an interaction effect of vision and perturbation amplitude on RMS values for CoM sway velocity, hip velocity, hip angular displacement (all P < 10−12, ANOVA), and ankle velocity (P < 0.001, ANOVA).
Figure 3.
Root-mean-square averages over all subjects (± SEM) for each kinematic variable measured. RMS values were calculated over a single PRTS cycle and averaged. Significant differences between EO and EC conditions at α = .05 are indicated with an asterisk. Significant differences between amplitudes not shown. a, CoM sway and sway velocity (top and bottom, respectively). b, Ankle and hip angle (top left and top right) and angular velocity (bottom left and right).
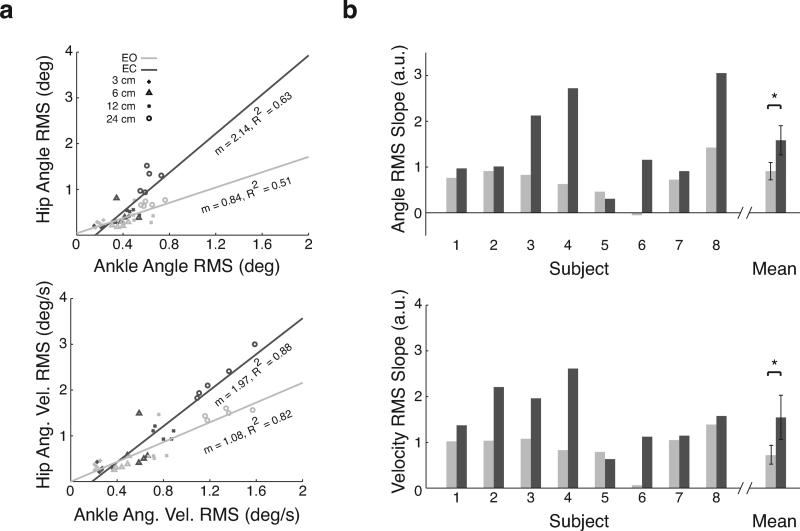
The ratio of hip to ankle kinematic variables revealed that there was more hip motion relative to ankle motion in EC versus EO conditions at higher stimulus amplitudes (Figure 4). This general trend was observed for seven of eight subjects, although variation in hip angle and angular velocity RMS values were seen across subjects. Linear regression was used to identify the slopes between hip and ankle angular displacements and velocities across perturbation amplitudes in each subject (e.g. Figure 4). Mean R2 values across subjects for EO conditions were 0.53 ± 0.26 and 0.67 ± 0.30 for RMS position and velocity, respectively. In EC conditions, mean R2 values were 0.59 ± 0.24 and 0.84 ± 0.08 for RMS position and velocity, respectively. Slopes of hip motion relative to ankle motion were consistently larger in EC compared to EO conditions across subjects (Figure 4b). Only one subject (subject 5) appeared to use more ankle strategy in EC conditions, having a slightly smaller slope in EC conditions for position and velocity RMS. Across all subjects, slopes of hip motion relative to ankle motion were significantly larger in EC conditions (P < 0.025 for RMS position and velocity; Figure 4b).
Figure 4.
Hip versus ankle RMS values in EO (dark gray) and EC (light gray) conditions. a, angle and angular velocity RMS for a representative subject. Each data point represents RMS for one cycle within one trial (40 points total). Linear fits are shown to both EC and EO conditions with slopes (m) and coefficients of determination (R2). b, angle and angular velocity RMS slopes (m) for individual subjects and averaged across subjects. RMS slopes are larger in EC conditions. Significant differences between EO and EC conditions at α = .025 are indicated with an asterisk. a.u. = arbitrary units.
Frequency-Domain
In general, transfer function gains between the platform stimulus and CoM sway displayed a characteristic saturation at values greater than 1 at higher frequencies (Figure 5). Gains of greater than 1 indicate that the CoM sway motion was greater than the amplitude of the perturbation, while a gain of 1 would indicate that the CoM sway motion was identical to platform motion. A gain of zero indicates that the subject's CoM remained static in space while the platform moved. Gains tended to be even higher as stimulus amplitude increased, although this difference became less pronounced between the 12 and 24 cm perturbations. For all perturbation amplitudes, the gain increased and reached a peak between 1-1.5 Hz and reached a plateau for all higher frequencies (Figure 5a). Coherence values were largest between 1 and 2 Hz (Figure 5c), indicating that platform input and CoM sway output were most highly correlated at these frequencies.
Figure 5.
Transfer function gain estimates of platform input to CoM sway output. a, Averaged transfer function gains across subjects over the full range of measured frequencies (16 total). EO conditions are represented by solid lines and EC by dashed lines. b, Gains versus stimulus amplitude in four frequency regions for both EO and EC conditions. Significant overall differences between EO and EC conditions are indicated by an asterisk in the lowest (LF: top left) and highest (HF: bottom right) frequency ranges. c, Averaged coherence function between platform input to CoM sway output. EO conditions are represented by solid lines and EC by dashed lines. ⨯ – measured frequencies used for gain estimates (16 total).
Differences between EO and EC conditions were observed in CoM sway gain at the lowest and highest frequency ranges. The general shape of the averaged transfer function gains was preserved in the EC condition, but EC conditions displayed lower gains at low frequencies and higher gains at high frequencies compared to EO conditions (α = .05). For LF and HF, there was significant effect of vision on gain over all trial amplitudes (F[1, 255] = 19.09, P < 10−4; F[1, 255] = 6.19, P = 0.014, respectively). There was no effect of vision for MF1 and MF2 (F[1, 255] = 0.50, P = 0.48; F[1, 255] = 1.21, P = 0.27, respectively).
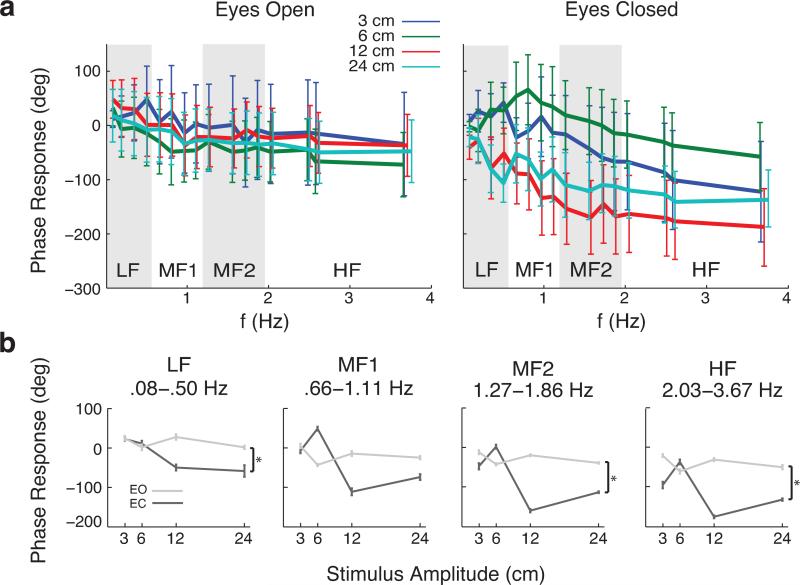
Phase responses changed more dramatically in EC compared to EO conditions, further suggesting a switch from ankle to hip strategies at higher frequencies. Removal of vision changed the nature of phase responses, which measure the subject's tendency to lead or lag behind a platform stimulus (Figure 6). In EO conditions, over all amplitudes, subjects tended to lead the platform at very low frequencies (<~0.5 Hz) but lagged with higher frequencies (Figure 6a, left). In EC conditions, the lowest two amplitudes (3 and 6 cm) displayed a lead at low frequencies (<~1 Hz) while the highest two amplitudes (12 and 24 cm) displayed a lag over all frequencies (Figure 6a, right). Within each frequency range EO conditions showed a relatively constant lag of CoM behind the platform (Figure 6b). There was a significant effect of vision on phase lag for LF (F[1, 255] = 4.74, P = 0.030), MF2 (F[1, 255] = 2.17, P = 0.024), and HF (F[1, 255] = 8.81, P = 0.003), but not MF1 (F[1, 255] = 0.64, P = 0.426).
Figure 6.
Phase responses of platform input to CoM sway output. a, phase responses over the full range of measured frequencies (16 total). b, phase responses versus stimulus amplitude in four frequency regions as in Figure 5. EC conditions display higher degrees of variability between amplitudes and larger lags in three of the four frequency ranges measured (P < .05).
DISCUSSION
The pseudorandom perturbations employed allowed us to unify prior findings from disparate experimental conditions. Previously, the effects of vision, perturbation amplitude, and perturbation frequencies were individually tested using discrete or sinusoidal perturbations. In contrast, pseudorandom perturbations allowed us to simultaneously test the effects of vision on kinematic strategies across a wide range of perturbation amplitudes and frequencies in a relatively small number of trials. Additionally, the PRTS has been shown to be unpredictable over many repeated cycles (Peterka 2002), eliminating the effects of changing feedforward strategies resulting from adaptation to a predictable perturbation that are seen in sinusoidal perturbations (Dietz et al. 1993). Finally, the PRTS could be scaled down to amplitudes that are similar to sway during quiet standing, suggesting that common mechanisms may act across quiet and perturbed standing.
Ankle versus hip strategies in postural control
Our results suggest that visual input, perturbation amplitude, and perturbation frequency can concurrently and independently modulate postural strategies during standing balance. Previous results demonstrate that disruption of the visual surround (Dokka et al. 2009) or visual deprivation (Day et al. 1993; Krizkova et al. 1993; Gatev et al. 1999) can cause the postural strategy to shift from a single link to a multi-link system. Accordingly, we demonstrated that removal of visual input caused increases in the ratio of hip to ankle kinematic motion consistent with a shift from ankle to hip strategy (Figure 4). We also found increased hip versus ankle kinematics as perturbation amplitude increased (Figure 3), consistent with previous results demonstrating that the body was controlled more like a multi-link system as perturbation amplitude increased (Runge et al. 1999). Finally, we showed an increased hip strategy with increasing frequency, as TF gains and phase lag of CoM sway increased at higher frequencies (Figures 5 and 6), consistent with prior results (Creath et al. 2005). We were able to demonstrate this shift in the context of an unpredictable translational perturbation as opposed to prior studies in which a feedforward shift in postural strategy was observed due to the predictable nature of the perturbations (Berthoz et al. 1979; Diener et al. 1982; Buchanan and Horak 1999). Therefore, the unpredictable nature of our pseudorandom perturbations allowed us to examine the effects of vision and perturbation characteristics independent of adaptive mechanisms seen in purely sinusoidal perturbations.
We propose that visual deprivation, increased perturbation amplitude, and increased perturbation frequency all contribute to postural “difficulty” which can influence postural strategies. We found significant interaction effects between visual condition and amplitude on RMS values in the time domain (Figure 3), and effects of frequency on transfer function gain and phase (Figures 5 and 6). Prior studies have shown interactions between perturbation amplitude and vision (Maki and Ostrovski 1993a) as well as perturbation frequency and vision (Diener et al. 1982; Dietz et al. 1993; Buchanan and Horak 1999; Corna et al. 1999; Schmid et al. 2011), and perturbation amplitude and frequency (Peterka 2002). Postural difficulty has also been shown to increase due to disruption of vestibular or somatosensory information (Horak et al. 1990), distance from CoM position-velocity stability limits (Pai and Patton 1997), and changes in stance width (Winter et al. 1998; Gatev et al. 1999; Bingham et al. 2011). Here we focused on a shift from ankle to hip strategy, although increasing difficulty could also involve knee flexion (Dokka et al. 2009; Allum et al. 2008), arm raising (Allum et al. 2008), as well as stepping responses (McIlroy and Maki 1993; Ting et al. 2009; Chvatal et al. 2011). The ankle strategy may be a preferred strategy for less difficult perturbations despite its limited biomechanical efficacy in restoring balance (Kuo and Zajac 1993). Thus, employing alternative strategies may be necessitated as perturbations become more difficult and the resulting effects of one particular postural strategy become saturated. Accordingly, our results showed that ankle angle was the least affected variable due to changes in visual condition.
Our results further demonstrate that the precise “difficulty” at which a shift from ankle to hip strategy takes hold depends on individual biomechanical and neural factors. We saw different ratios of hip to ankle motion as visual input was removed and as perturbation amplitude increased across individuals (evidenced by different slopes of hip to ankle motion across subjects, Figure 4). This could reflect individual biomechanics, as subjects were of unequal height and weight and were allowed to select a preferred stance width, which can all affect sensorimotor feedback for balance (Goodworth and Peterka 2010). Differences in sensory reweighting may have also contributed to the changes in postural strategies (Peterka 2002; Fetsch et al. 2009; Keshner 2003; Bair et al. 2007). Dynamic reweighting of vestibular and visual inputs varies in proportion to the perceived reliability of the sensory modality, which may differ among subjects (Fetsch et al. 2009). Similarly, individuals with somatosensory loss rely predominantly on a hip strategy, whereas those with vestibular loss rely predominantly on the ankle strategy (Horak et al. 1990; Peterka 2002). Although inter-subject neuromechanical variability may influence the threshold of strategy changes, all subjects exhibited the common pattern of increasing hip motion as “difficulty” increased.
Effects of vision on CoM control
We found differential effects of vision on balance strategies at low and high frequencies across all perturbation amplitudes. At low frequencies (0.08 - 0.5 Hz), we showed that CoM sway relative to the perturbation decreased when vision was removed (Figure 5b). This suggests that subjects were more dependent upon proprioceptive information that allowed them to orient their posture with respect to the support surface, essentially “riding” the platform at low frequencies (Peterka 2002). Previously, no effect of vision was found on CoM sway at low (0.1 Hz) frequencies during sinusoidal translational perturbations (Buchanan and Horak 1999). Similar reliance on proprioceptive cues to maintain posture with respect to the support surface are found in vestibular loss, even in the presence of vision (Peterka 2002; Macpherson et al. 2007) At high frequencies (≥ 2.03 Hz), CoM sway relative to the platform increased in the presence of vision (Figure 5b), consistent with an increased reliance on dynamic stabilization using the hip strategy when ankle strategy is insufficient (Horak 1987). However, removal of vision further increased CoM sway gains at high frequencies, likely due to uncertainty in subjects’ ability to estimate vertical orientation. Similarly, it has been reported in sinusoidal translational perturbations that subjects switch from “riding” the platform at low frequencies to stabilizing the head at higher frequencies (Diener et al. 1982; Buchanan and Horak 1999; Kuo et al. 1998), a process that depends on estimating vertical orientation. Increases in CoM phase lag at high frequencies when vision was removed were also consistent with increased hip strategy, which has been described as being anti-phase (Creath et al. 2005). Similar to a prior study examining pseudorandom translations (van der Kooij and de Vlugt 2007), we found no effect of vision on gains at intermediate frequencies, indicating that responses in the MF range were delayed in EC conditions but not damped or amplified.
The translational PRTS perturbations appear to have some limitation in their implementation and interpretation compared to previously-studied rotational PRTS perturbations during standing balance. At low frequencies we observed near unity gains in translations, as has been observed in rotations; a unity gain means that the subject's body is oriented perpendicular to the support surface. However, as frequency increases in rotations, CoM gains decrease toward zero, as subjects tend to orient more toward the vertical, relying more heavily on visual and vestibular information (Peterka 2002). In contrast, for translations, we found at higher frequencies elicited gains approaching 10 in the largest-amplitude case. Especially at high amplitudes, the translation perturbation imposes horizontal CoM motion relative to the feet whereas the rotational perturbation does not. Transfer function gains between 1 and 10 have been observed previously in a rotational paradigm only frequencies less than ~1 Hz (Peterka 2002). Further, the coherence between the CoM and platform translation in the PRTS perturbation (Figure 5c) was decreased compared to those reported for rotational perturbations, possibly resulting in less accurate transfer function gains (Bendat and Piersol 2010). Our reason for selecting such frequencies was that the platform perturbation contained distinct spectral peaks that were exactly repeatable from experiment to experiment. It is possible that transfer function estimates based on frequencies with high cross-correlation or coherence values could produce different results, but these would need to be calculated on a subject-by-subject basis. As such, it would be difficult to average transfer function estimates or compare trends across subjects. Finally, our analysis assumes a time-invariant system, but subjects may adapt their responses during each trial and across multiple trials (Buchanan and Horak 2001). However our perturbation cycles were quite long in relation to the relatively fast dynamics of postural adaptations (Keshner et al. 1987); we did not observe differences in our results when examining results across cycles within a trial.
CoM as a control variable
Our results lend support to the idea that CoM is a task-variable of critical importance to balance, and further, that CoM is controlled despite high degrees of joint angle variation. Raw kinematic traces qualitatively showed more similarities in CoM control between subjects than either joint angle measured. Inter-subject similarities in CoM sway over the entire time-course of the perturbation were also qualitatively noted despite modulation of sensory input and perturbation amplitude, and differences in anthropometrics (base of support width, CoM height, mass distribution/inertia). This is consistent with other studies in movement control demonstrating lower variability in task-level variables such as CoM and hand location compared to joint angle variables (Scholz and Schoner 1999; Krishnamoorthy et al. 2005; Black et al. 2007; Wu et al. 2009) The larger qualitative similarities of CoM variables across subjects compared to joint variables suggest that CoM may be a critical controlled variable for the neural control of balance. Similarly, it has been demonstrated that a common sensorimotor feedback mechanism based on task-level error can give rise to variable temporal patterns of muscle activity over a variety of perturbation amplitudes (Welch and Ting 2009; Lockhart and Ting 2007) and directions (Safavynia and Ting 2013). Our study suggests that subjects may differently use ankle and hip strategies to achieve similar control of CoM across perturbation difficulty. Finally, it is likely that other task-level variables such as trunk or head location and orientation may also govern the neural control of balance, as suggested previously (Buchanan and Horak 1999; Horak and Macpherson 1996; Massion 1994). We were unable to identify any consistencies in head kinematics due to high intra- and inter-subject variability of head marker position throughout the task, which may reflect different strategies employed by subjects. However, we cannot exclude the possibility that multiple control strategies may be used simultaneously over varying timescales, consistent with different control mechanisms for different body segments (Keshner and Dhaher 2008). Thus, while vision influences postural strategies at the joint level, regardless of the observed kinematic patterns, such postural strategies subserve the ability of the nervous system to regulate task-variables.
Acknowledgements
We thank J. Lucas McKay, Jeff Bingham and Hongchul Sohn for ideas and guidance with Matlab, data analysis, and advanced dynamics concepts. We thank Robert Perterka for the PRTS code. This work was supported by NIH R01 NS058322 to Lena H. Ting, NIH 5 T32 GM08169-24 to Seyed A. Safavynia, a Petit Undergraduate Research Scholars Award and a Georgia Tech President's Undergraduate Research Award to D. Joseph Jilk.
Contributor Information
D. Joseph Jilk, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, Georgia, USA.
Seyed A. Safavynia, Neuroscience Program, Emory University, Atlanta, Georgia, USA Medical Scientist Training Program, Emory University School of Medicine, Atlanta, Georgia, USA
Lena H. Ting, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, Georgia, USA.
References
- Allum JH, Oude Nijhuis LB, Carpenter MG. Differences in coding provided by proprioceptive and vestibular sensory signals may contribute to lateral instability in vestibular loss subjects. Exp Brain Res. 2008;184(3):391–410. doi: 10.1007/s00221-007-1112-z. [DOI] [PubMed] [Google Scholar]
- Bair W-N, Kiemel T, Jeka J, Clark J. Development of multisensory reweighting for posture control in children. Experimental Brain Research. 2007;183(4):435–446. doi: 10.1007/s00221-007-1057-2. doi:10.1007/s00221-007-1057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random Data: Analysis and Measurement Procedures. John Wiley & Sons; 2010. [Google Scholar]
- Berthoz A, Lacour M, Soechting JF, Vidal PP. The role of vision in the control of posture during linear motion. Progress in brain research. 1979;50:197–209. doi: 10.1016/S0079-6123(08)60820-1. doi:10.1016/S0079-6123(08)60820-1. [DOI] [PubMed] [Google Scholar]
- Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. Journal of neurophysiology. 2011;106(1):437–448. doi: 10.1152/jn.00010.2011. doi:10.1152/jn.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D, Smith B, Wu J, Ulrich B. Uncontrolled manifold analysis of segmental angle variability during walking: preadolescents with and without Down syndrome. Experimental Brain Research. 2007;183(4):511–521. doi: 10.1007/s00221-007-1066-1. doi:10.1007/s00221-007-1066-1. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol. 1999;81(5):2325–2339. doi: 10.1152/jn.1999.81.5.2325. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Transitions in a postural task: do the recruitment and suppression of degrees of freedom stabilize posture? Experimental Brain Research. 2001;139(4):482–494. doi: 10.1007/s002210100798. [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol. 2011;106(2):999–1015. doi: 10.1152/jn.00549.2010. doi:10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corna S, Tarantola J, Nardone A, Giordano A, Schieppati M. Standing on a continuously moving platform: is body inertia counteracted or exploited? Exp Brain Res. 1999;124(3):331–341. doi: 10.1007/s002210050630. [DOI] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett. 2005;377(2):75–80. doi: 10.1016/j.neulet.2004.11.071. [DOI] [PubMed] [Google Scholar]
- Day BL, Steiger MJ, Thompson PD, Marsden CD. Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J Physiol. 1993;469:479–499. doi: 10.1113/jphysiol.1993.sp019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bruzek W, Selinka H. Stabilization of human posture during induced oscillations of the body. Exp Brain Res. 1982;45(1-2):126–132. doi: 10.1007/BF00235771. [DOI] [PubMed] [Google Scholar]
- Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol. 1988;59(6):1888–1905. doi: 10.1152/jn.1988.59.6.1888. [DOI] [PubMed] [Google Scholar]
- Dietz V, Trippel M, Ibrahim IK, Berger W. Human stance on a sinusoidally translating platform: balance control by feedforward and feedback mechanisms. Exp Brain Res. 1993;93(2):352–362. doi: 10.1007/BF00228405. [DOI] [PubMed] [Google Scholar]
- Dokka K, Kenyon RV, Keshner EA. Influence of visual scene velocity on segmental kinematics during stance. Gait & Posture. 2009;30(2):211–216. doi: 10.1016/j.gaitpost.2009.05.001. doi:10.1016/j.gaitpost.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29(49):15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. doi:29/49/15601 [pii] 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514(Pt 3):915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodworth AD, Peterka RJ. Influence of stance width on frontal plane postural dynamics and coordination in human balance control. J Neurophysiol. 2010;104(2):1103–1118. doi: 10.1152/jn.00916.2009. doi:10.1152/jn.00916.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB. Clinical Measurement of Postural Control in Adults. Physical Therapy. 1987;67(12):1881–1885. doi: 10.1093/ptj/67.12.1881. [DOI] [PubMed] [Google Scholar]
- Horak FB. Adaptation of automatic postural responses. In: Bloedel JR, Ebner TJ, Wise SP, editors. The Acquisition of Motor Behavior in Vertebrates. Cambridge. The MIT Press; 1996. pp. 57–85. [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Handbook of Physiology, Section 12. Exercise: Regulation and Integration of Multiple Systems. American Physiological Society. New York. 1996:255–292. [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55(6):1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82(1):167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J Neurophysiol. 1995;73(2):896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Jeka J, Oie K, Schoner G, Dijkstra T, Henson E. Position and velocity coupling of postural sway to somatosensory drive. J Neurophysiol. 1998;79(4):1661–1674. doi: 10.1152/jn.1998.79.4.1661. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Exp Brain Res. 1994;100(3):495–502. doi: 10.1007/BF02738408. [DOI] [PubMed] [Google Scholar]
- Keshner EA. Head-trunk coordination during linear anterior-posterior translations. J Neurophysiol. 2003;89(4):1891–1901. doi: 10.1152/jn.00836.2001. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Pfaltz CR. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res. 1987;69(1):77–92. doi: 10.1007/BF00247031. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Dhaher Y. Characterizing head motion in three planes during combined visual and base of support disturbances in healthy and visually sensitive subjects. Gait Posture. 2008;28(1):127–134. doi: 10.1016/j.gaitpost.2007.11.003. doi:10.1016/j.gaitpost.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle modes during shifts of the center of pressure by standing persons: effect of instability and additional support. Experimental Brain Research. 2004;157(1):18–31. doi: 10.1007/s00221-003-1812-y. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Yang JF, Scholz JP. Joint coordination during quiet stance: effects of vision. Exp Brain Res. 2005;164(1):1–17. doi: 10.1007/s00221-004-2205-6. [DOI] [PubMed] [Google Scholar]
- Krizkova M, Hlavacka F, Gatev P. Visual control of human stance on a narrow and soft support surface. Physiol Res. 1993;42(4):267–272. [PubMed] [Google Scholar]
- Kuo AD. An optimal control model for analyzing human postural balance. IEEE Trans Biomed Eng. 1995;42(1):87–101. doi: 10.1109/10.362914. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Speers RA, Peterka RJ, Horak FB. Effect of altered sensory conditions on multivariate descriptors of human postural sway. Exp Brain Res. 1998;122(2):185–195. doi: 10.1007/s002210050506. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Zajac FE. Human standing posture: multi-joint movement strategies based on biomechanical constraints. Prog Brain Res. 1993;97:349–358. doi: 10.1016/s0079-6123(08)62294-3. [DOI] [PubMed] [Google Scholar]
- Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nature Neuroscience. 2007;10(10):1329–1336. doi: 10.1038/nn1986. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J Neurophysiol. 2007;97(6):4357–4367. doi: 10.1152/jn.01338.2006. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Fernie GR. A Posture Control Model and Balance Test for the Prediction of Relative Postural Stability. Biomedical Engineering, IEEE Transactions on BME-34. 1987;(10):797–810. doi: 10.1109/tbme.1987.325922. doi:10.1109/tbme.1987.325922. [DOI] [PubMed] [Google Scholar]
- Maki BE, Ostrovski G. Do postural responses to transient and continuous perturbations show similar vision and amplitude dependence? J Biomech. 1993a;26(10):1181–1190. doi: 10.1016/0021-9290(93)90066-n. [DOI] [PubMed] [Google Scholar]
- Maki BE, Ostrovski G. Scaling of postural responses to transient and continuous perturbations. Gait Posture. 1993b;1(2):93–104. doi: 10.1016/0021-9290(93)90066-n. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Massion J. Postural constrol system. Curr Opin Neurobiol. 1994;4:877–887. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Massion J, Popov K, Fabre JC, Rage P, Gurfinkel V. Is the erect posture in microgravity based on the control of trunk orientation or center of mass position? Experimental Brain Research. 1997;114(2):384–389. doi: 10.1007/pl00005647. doi:10.1007/pl00005647. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Task constraints on foot movement and the incidence of compensatory stepping following perturbation of upright stance. Brain Res. 1993;616(1-2):30–38. doi: 10.1016/0006-8993(93)90188-s. doi:0006-8993(93)90188-S [pii] [DOI] [PubMed] [Google Scholar]
- Milton J, Cabrera JL, Ohira T, Tajima S, Tonosaki Y, Eurich CW, Campbell SA. The time-delayed inverted pendulum: Implications for human balance control. Chaos. 2009;19(2):026110. doi: 10.1063/1.3141429. doi:10.1063/1.3141429. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Black FO, Wall C., 3rd Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2(5):536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cognitive Brain Research. 2002;14(1):164–176. doi: 10.1016/s0926-6410(02)00071-x. doi:10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- Pai Y-C, Rogers MW, Patton J, Cain TD, Hanke TA. Static versus dynamic predictions of protective stepping following waist-pull perturbations in young and older adults. Journal of biomechanics. 1998;31(12):1111–1118. doi: 10.1016/s0021-9290(98)00124-9. doi:10.1016/s0021-9290(98)00124-9. [DOI] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30(4):347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res. 1995;105(1):101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- Ray CT, Horvat M, Croce R, Christopher Mason R, Wolf SL. The impact of vision loss on postural stability and balance strategies in individuals with profound vision loss. Gait & Posture. 2008;28(1):58–61. doi: 10.1016/j.gaitpost.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Runge CF, Shupert CL, Horak FB, Zajac FE. Role of vestibular information in initiation of rapid postural responses. Exp Brain Res. 1998;122(4):403–412. doi: 10.1007/s002210050528. [DOI] [PubMed] [Google Scholar]
- Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait Posture. 1999;10(2):161–170. doi: 10.1016/s0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol. 2013;109(1):31–45. doi: 10.1152/jn.00684.2012. doi:10.1152/jn.00684.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Bottaro A, Sozzi S, Schieppati M. Adaptation to continuous perturbation of balance: progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum Mov Sci. 2011;30(2):262–278. doi: 10.1016/j.humov.2011.02.002. doi:10.1016/j.humov.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Reisman D, Schoner G. Effects of varying task constraints on solutions to joint coordination in a sit-to-stand task. Exp Brain Res. 2001;141(4):485–500. doi: 10.1007/s002210100878. doi:10.1007/s002210100878. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126(3):289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, van Antwerp KW, Scrivens JE, McKay JL, Welch TD, Bingham JT, DeWeerth SP. Neuromechanical tuning of nonlinear postural control dynamics. Chaos. 2009;19(2):026111. doi: 10.1063/1.3142245. doi:10.1063/1.3142245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij H, de Vlugt E. Postural Responses Evoked by Platform Pertubations Are Dominated by Continuous Feedback. J Neurophysiol. 2007;98(2):730–743. doi: 10.1152/jn.00457.2006. doi:10.1152/jn.00457.2006. [DOI] [PubMed] [Google Scholar]
- Van Ooteghem K, Frank JS, Allard F, Buchanan JJ, Oates AR, Horak FB. Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res. 2008;187(4):603–611. doi: 10.1007/s00221-008-1329-5. doi:10.1007/s00221-008-1329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model reproduces muscle activity during human postural responses to support-surface translations. J Neurophysiol. 2008;99(2):1032–1038. doi: 10.1152/jn.01110.2007. [DOI] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J Neurophysiol. 2009;101(6):3294–3309. doi: 10.1152/jn.90775.2008. doi:90775.2008 [pii] 10.1152/jn.90775.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. 2nd edn. John Wiley & Sons; New York: 1990. [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait & Posture. 1995;3(4):193–214. doi:10.1016/0966-6362(96)82849-9. [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80(3):1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Wu J, McKay S, Angulo-Barroso R. Center of mass control and multi-segment coordination in children during quiet stance. Experimental Brain Research. 2009;196(3):329–339. doi: 10.1007/s00221-009-1852-z. doi:10.1007/s00221-009-1852-z. [DOI] [PubMed] [Google Scholar]