Abstract
The epigenetic marks displayed by a cancer cell originate from two separate processes: The most prominent epigenetic signatures are associated with the cell of origin, i.e., the lineage and cell type identity imposed during development. The second set comprises those aberrant cancer-specific epigenetic marks that appear during tumor initiation or subsequent malignant progression. These are generally thought to associate with tumor-promoting pathways. As biochemical pathways regulating epigenetic mechanisms are potentially “druggable” and reversible, there is considerable interest in defining their roles in tumor genesis and growth, as they may represent therapeutic targets for treatment of human neoplasias.1 However, despite the potential importance of epigenetic modifications in human cancer, it has been difficult to determine when, where and how epigenetic disruptions occur, and if they have important functional roles in sustaining the malignant state.
Keywords: cancer, DNA methylation, epigenetics, glioblastoma, neural stem cell, polycomb, reprogramming, iPS cells
We, and others, have recently demonstrated that lineage reprogramming to pluripotency through forced expression of reprogramming transcription factors (termed induced pluripotent stem cell [iPSC] technology) can be applied to study epigenetic mechanisms in human cancer.2-6 Such experimentally induced reprogramming provides a cellular model to assess the functional contribution of both cancer-specific and lineage-associated methylation changes in maintaining the malignant cellular state. Here we summarize the major conclusions from our studies of the human brain cancer glioblastoma (GBM), and discuss the benefits and limitations of this experimental approach.4
DNA Methylation is a Cancer-Associated Epigenetic Mark
Much progress has been made during the past few decades in identifying the molecular events underlying modifications of DNA and chromatin. Methylation of cytosines at CpG dinucleotides has been strongly implicated in regulating transcription since its discovery in the 1970s (reviewed by A. Bird).7 DNA methylation is the canonical epigenetic mark, and there are well established mechanisms through which the methylated status can be inherited through DNA replication and mitosis.7 While patterns of DNA methylation are faithfully inherited through cell division, they can nevertheless undergo dynamic changes during development or disease, including cancer.8,9
DNA methylation marks can directly control gene expression at specific loci. For example, those marks set in the germ cells trigger “imprinted expression” of several transcripts (reviewed by D. Barlow).10 DNA methylation of loci necessary for activation of transposable elements also provides an example of direct control of transcription.11 However, only a minority of CpG islands in promoter regions are methylated, and it remains unclear whether DNA methylation plays a general causal role in gene silencing, or instead reinforces repression that was established through other chromatin-based mechanisms.12 Genetically modified mice in which the levels of the de novo DNA methylases Dnmt3a or Dnmt3b are modulated display altered patterns of tumorigenesis, and provide support for a role for DNA methylation in cancer initiation and progression.13,14 Nevertheless, how these alterations in DNA methylation relate to those observed in human primary tumor samples, and whether they are critical for driving malignant cellular behavior is unclear.
Rapid improvements and falling costs of technologies enabling genome-wide analysis has meant it has become feasible to define DNA methylation patterns at unprecedented scale and resolution.15,16 Consequently many laboratories and international research consortia are working intensively to produce genome-wide maps of DNA methylation across a range of cell and tissue types, including human cancers and cancer cell lines.15 Such studies are aiding efforts to define stem and progenitor cell states during development and adult tissues (e.g., between pluripotent and somatic stem cells or during hematopoietic lineage choices,17,18 and are vital in providing a foundation for future studies. Inevitably, however, such descriptive studies cannot provide functional insights.
One observation from genome-wide analysis of primary human tumor samples has been that DNA hypermethylation of tumor suppressor genes commonly mutated in diverse human cancers (e.g., TP53, PTEN) is not frequently observed; therefore, the accumulation of DNA methylation at such genes following long-term in vitro expansion is likely a tissue culture artifact.19 Instead, a different gene set is hypermethylated in human cancers, including genes that might act as direct tumor suppressors, as well as hundreds of polycomb-associated PRC2 target genes, which have been implicated in stem cell lineage choice.20,21 A simplistic explanation for these observations is that tumor cells, possibly early in their development, ensure that their tissue stem cell identity becomes “locked in,” restricting alternative lineage choices and terminal differentiation. This view would, however, run opposite to the observation that human cancers frequently display anaplasia and wildly aberrant programs of gene expression not typically observed in normal cell types. How can such discrepant views (epigenetic “lock-in” vs. epigenetic plasticity) be reconciled? We would speculate that the perturbation of polycomb regulated genes through DNA methylation changes is an important step early in tumor development—perhaps in benign tumors or pre-neoplastic growths. This would be consistent with the “epigenetic progenitor” model of tumorigenesis,22 which proposes that functionally relevant epigenetic alterations appear at the earliest stages of tumor initiation in (pre-cancerous) progenitor cells. The corruption of tissue identity and global epigenetic relaxation may then occur at later stages alongside or downstream of malignant transformation following accumulation of catastrophic widespread genetic damage.23 Consequently, it is possible that many epigenetic alterations detected in mature tumors might not play a causal role in driving disease.
A further observation is that the relationship between DNA methylation to the transcriptional state of adjacent genes is less clear than originally thought; a situation made further complicated by the discovery of new DNA modifications, such as 5-hydroxymethylcytosine. Although correlations between DNA methylomes and transcriptomes have been reported, these are often usually weak and hold poor predictive value, arguing against a widespread generic role in transcriptional repression. New experimental approaches would clearly be helpful to enable functional insights into the interplay between cancer genome and epigenome.24,25 This has prompted us,4 and others,2,3,6 to explore whether strategies to experimentally reprogram cell fate might be useful to study the functional consequences of human cancer-specific epigenetic changes.
Epigenetic Resetting of Human Glioblastoma
The transcriptional networks that configure the pluripotent cell state are present within the pre-implantation epiblast and germ cells,26 but do not normally occur in somatic cells. However, somatic cells can be forced into a pluripotent state using techniques such as nuclear transfer, cell fusion or transcription factor-mediated reprogramming—i.e., iPSC technology (reviewed by S. Yamanaka and H. Blau27). It is well established that somatic cells undergo a widespread resetting of DNA methylation marks as they transit from a differentiated state toward pluripotency,28 thereby potentially providing an experimental tool to reconfigure the epigenetic restrictions that are acquired through normal development.
Glioblastoma (GBM), also termed high-grade astrocytoma, is the most common and aggressive type of primary brain tumor. Serum-free cell culture conditions developed for the propagation of normal human neural stem cells can be successfully applied to GBM, enabling propagation of primary cultures from these tumors.29-31 Given the accessibility and expandability of primary human GBM cells, as well as the possibility to use genetically normal cultured neural stem cells as reference controls,32 we reasoned this would be a useful experimental system to explore cancer epigenetics using iPSC technology. Methylation patterns found in cultured glioblastoma-derived neural stem cell (GNS) cells mirror those found in the human disease, including some of the most frequent epigenetic anomalies observed in primary GBM tumors, such as hypermethylation of thousands of PRC2 target sites and the tumor suppressor genes cyclin-dependent kinase inhibitor 1C (CDKN1C, encoding p57KIP2) and TES.33
Previous studies had indicated that mouse and human cancer cell lines might be amenable to experimentally induced reprogramming, using either nuclear transfer,34,35 or more recently forced expression of reprogramming transcription factors C-MYC, OCT4 (POU5F1), SOX2, and KLF4.2,3 Normal neural stem cells can be efficiently reprogrammed using two factors, OCT4 and KLF436 (the other “Yamanaka factors,” SOX2 and C-MYC, are already expressed). We reasoned that such “two factor” reprogramming might therefore be sufficient for reprogramming of GBM stem cells. A further incentive to explore iPSC reprogramming using human GBM stem cells, is that well defined protocols exist to steer the resulting iPSCs along the neural lineage,37,38 thus enabling analysis of the functional consequences of a cancer-specific epigenetic resetting in the appropriate lineage context. Glioblastoma iPSCs (GiPSCs) would also be useful to explore how the GBM genome behaves in distinct non-neural lineages, and if they are able to engage in distinct differentiation programs.
Despite the extensive genetic and karyotypic changes in GBMs, we found that a subset of GNS cells were amenable to transcription factor mediated reprogramming, through forced expression of only OCT4 and KLF4.4 Reprogramming was no more efficient than control NS cells, and switching cells into hESC culture conditions did not result in iPSC conversion. Prior to reprogramming, we also failed to observe transcription of key pluripotency markers in the GBM stem cells, and the promoter regions of OCT4 and NANOG were hypermethylated. Thus, the core pluripotency network driven by OCT4 and NANOG is not likely to be a feature of human GBM; an earlier study suggested that this was the case,39 but has since been challenged by the finding that only the MYC-driven transcriptional module is shared.40
GiPSCs gained expression of pluripotency-associated genes and extinguished neural lineage marker gene expression. This process led to the resetting of a large proportion of developmentally defined and cancer-associated DNA methylation marks. G-iPSCs remained capable of commitment to neural lineages allowing us to explore the consequences of the epigenomic resetting of cancer specific modifications in the context of appropriate developmental lineage—something that has not been reported in iPSC studies of human cancers.
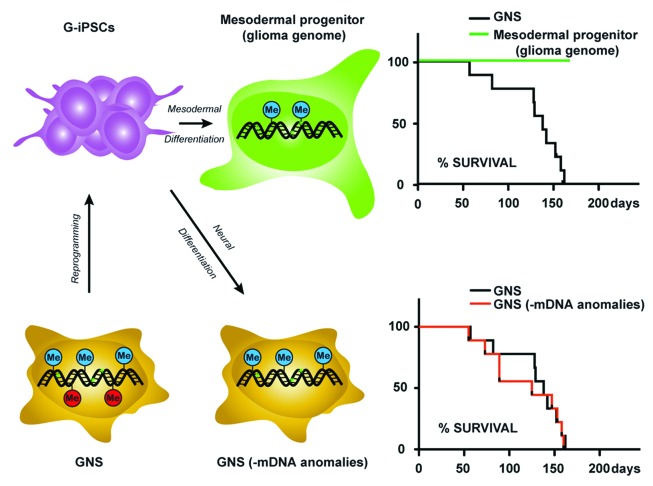
Following conversion of GiPSCs to a neural stem cell identity, only a minority of the DNA methylation changes were reacquired in these reprogrammed and redifferentiated cells. This strongly suggests that the genetically driven cancer pathways do not immediately enforce the epigenetic disruptions associated with GBM. We found that the widespread resetting of DNA methylation alone had no detectable effect on the tumorigenicity of these cells (Fig. 1), and cells remained highly proliferative and infiltrative. Thus, widespread resetting of cancer-associated DNA methylation is not sufficient to suppress malignant cellular behavior. These results are consistent with the view that the critical functional roles of epigenetic alterations occur at an early stage of tumor development or initiation, prior to accumulation of oncogenic mutations—or “genetic gatekeepers”—that promote tumor genetic evolution and potentially highly malignant cellular phenotypes.23
Figure 1. iPSC reprogramming technology can be used to explore both cancer-specific and tissue-associated epigenetic mechanisms in human glioblastoma. During reprogramming, around half of the cancer-specific DNA methylation anomalies, including those on tumor suppressor genes and PRC2 target gene (red lollipop) are reset. Irreversible genetic abnormalities are illustrated (green stars). Steering glioblastoma iPSCs along alternative lineages (mesodermal progenitor with glioma genome) suppresses the malignant behavior. Resetting DNA methylation anomalies alone (GNS–mDNA anomalies) in the neural stem cell lineage is not sufficient to restore normal cellular behavior.
We also found that for some tumor suppressors (e.g., TES) the removal of methylation did not immediately result in reactivation of expression.4 This result is consistent with a view that the function of DNA methylation is not as a primary switch controlling gene activation or repression and also suggests that the mechanism of tumor suppressor gene silencing is not dependent on DNA methylation changes. This is supported by the findings that agents such as 5-Azacytidine do not necessarily lead to transcriptional upregulation of hypermethylated genes,41 and that epigenetically silenced genes accumulate DNA methylation marks usually after they are already repressed as part of the normal tissue-specific patterns of gene expression.42,43
A caveat of cancer iPS reprogramming studies, including ours, is that not all cancer-associated epigenetic defects (as defined by comparing normal and tumor cell populations) were reset. We found that around 50% were not stably reset. Although these did not include common disease relevant loci (they may reflect the difficulties in defining what is “cancer specific” and inherent variability between independent cultures), it is still possible that these include some critical functional epigenetic disruptions. Furthermore, for practical reasons only a limited number of different patient samples can be explored and there is inherent variability in the reprogramming process. For example, the majority of lines we tested failed reprogramming, which may either highlight the incompatibility of highly aneuploidy cancer cell lines with an iPSC state, or potentially some other roadblock to reprogramming that has disease relevance (e.g., mutations in the core epigenetic reprogramming machinery). Thus, the iPSC reprogramming approach may be better suited to study tumors that display low levels of structural genetic disruptions and mutations, and potentially have clear epigenetic disruptions associated with malignancy, such as pediatric GBM,44,45 ependymomas,46 or Wilms Tumor.
Despite these limitations, iPSC programming experiments provide insights into individual tumors. We can still conclude that for certain GBMs the removal of a large proportion of cancer specific DNA methylation defects, including some of the most frequently associated with human GBM, is not sufficient to override the genetically driven cancer pathways. Therefore, any new therapeutic strategy that seeks to reverse these errors is unlikely to have dramatic effects.
For any specific cell, lineage identity (i.e., patterns of gene expression) reflects those transcriptional and epigenetic events that were encountered through its developmental history. Lineage identity is largely erased during reprogramming to pluripotency, but can be re-established through in vitro differentiation of the iPSCs, as cells can be directed along different differentiation paths. Despite the extensive genetic disruptions in GiPSCs we were able to observe differentiation along non-neural lineages in the context of teratomas. For example, albeit infrequent, we did observe the presence of hair follicles, cartilage, muscle, and epithelial tissues. What are the consequences of forcing a human GBM cancer genome to operate in the context of a distinct lineage? Can lineage reprogramming suppress features of the malignant brain tumor cells? To explore this issue more rigorously we also directed GiPSCs in vitro along the mesodermal lineage—generating proliferative cartilage progenitors that were then transplanted in vivo. We found that these cells lost the ability to form malignant brain tumors. Reconfiguration of the network of “cell fate” transcription factors47 and consequently the downstream developmental epigenetic mechanisms, could effectively silence cancer-promoting pathways that were essential for the cells to display uncontrolled proliferation and brain infiltration. This indicates that the glioblastoma genome can be suppressed through resetting of lineage-affiliated epigenetic programmes. Thus, the developmental, transcriptional and associated epigenetic mechanisms that define tissue types represent powerful routes to reconfigure the chromatin landscape, and these can be sufficient to suppress the activity of the aberrant genetic pathways disrupted in GBM.
Future Perspectives
The aforementioned studies using iPSC technology to study human cancer highlight the utility of the approach. This provides new and accessible human cellular models that will enable further functional studies of the relative contribution of genetics and epigenetics in tumor initiation, progression and following therapeutic intervention. GBM is one of the few cancer types for which both malignant and normal tissue stem cell counterparts can be expanded continuously in identical and defined conditions. We were therefore well placed to apply iPSC techniques to GBM. Also, established protocols exist for the efficient differentiation of iPSCs to their original cell identity (neural stem cells), and for a xenograft transplantation to test their potency and malignancy (intracranial transplants). For many human cancers, several of these criteria are not met and this potentially limits current applicability of iPSC technology to explore the cancer epigenome. A major bottleneck associated with this technology is the time-consuming nature of reprogramming experiments and variability between clonal lines, which affect its efficiency, limiting the number of tumors that can be assessed and, consequently, the generalization of the results. As protocols for the expansion of tissue stem cells and their malignant counterparts are improved, more human tumor types will become amenable to such studies. Moreover, improved protocols and novel approaches to reprogramming may soon improve the currently poor efficiency and fidelity of reprogramming.48,49
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Prof Stefan Beck, Prof Richard Meehan and Dr Paul Bertone for their helpful comments on the manuscript. SMP is supported by Cancer Research UK and The Brain Tumour Charity (Alex Bolt Research Fellowship).
References
- 1.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Carette JE, Pruszak J, Varadarajan M, Blomen VA, Gokhale S, Camargo FD, Wernig M, Jaenisch R, Brummelkamp TR. Generation of iPSCs from cultured human malignant cells. Blood. 2010;115:4039–42. doi: 10.1182/blood-2009-07-231845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci USA. 2009 ed. 2010 Jan 5;107(1):40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stricker SH, Feber A, Engström PG, Carén H, Kurian KM, Takashima Y, Watts C, Way M, Dirks P, Bertone P, et al. Widespread resetting of DNA methylation in glioblastoma-initiating cells suppresses malignant cellular behavior in a lineage-dependent manner. Genes Dev. 2013;27:654–69. doi: 10.1101/gad.212662.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumano K, Arai S, Kurokawa M. Generation of iPS cells from normal and malignant hematopoietic cells. Int J Hematol. Springer Japan. 2013;98:145–52. doi: 10.1007/s12185-013-1385-x. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Hoffman JP, Alpaugh RK, Rhim AD, Reichert M, Stanger BZ, Furth EE, Sepulveda AR, Yuan CX, Won KJ, et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep. 2013;3:2088–99. doi: 10.1016/j.celrep.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird A. DNA methylation patterns and epigenetic memory. Genes & Development. Cold Spring Harbor Lab. 2002;16:6, 21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 10.Barlow DP. Genomic imprinting: a mammalian epigenetic discovery model. Annu Rev Genet. 2011;45:379–403. doi: 10.1146/annurev-genet-110410-132459. [DOI] [PubMed] [Google Scholar]
- 11.Hackett JA, Reddington JP, Nestor CE, Dunican DS, Branco MR, Reichmann J, Reik W, Surani MA, Adams IR, Meehan RR. Promoter DNA methylation couples genome-defence mechanisms to epigenetic reprogramming in the mouse germline. Development. 2012;139:3623–32. doi: 10.1242/dev.081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh CP, Bestor TH. Cytosine methylation and mammalian development. Genes Dev. 1999;13:26–34. doi: 10.1101/gad.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linhart HG, Lin H, Yamada Y, Moran E, Steine EJ, Gokhale S, Lo G, Cantu E, Ehrich M, He T, et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–22. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, Steine EJ, Barrasa MI, Hockemeyer D, Pawlak M, Fu D, Reddy S, Bell GW, Jaenisch R. Deletion of the de novo DNA methyltransferase Dnmt3a promotes lung tumor progression. Proc Natl Acad Sci U S A. 2011;108:18061–6. doi: 10.1073/pnas.1114946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck S, Rakyan VK. The methylome: approaches for global DNA methylation profiling. Trends Genet. 2008;24:231–7. doi: 10.1016/j.tig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Laird PW. Principles and challenges of genome-wide DNA methylation analysis. Nat Rev Genet. 2010;11:191–203. doi: 10.1038/nrg2732. [DOI] [PubMed] [Google Scholar]
- 17.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28:1079–88. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Ehrlich LIR, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–42. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestor TH. Unanswered questions about the role of promoter methylation in carcinogenesis. Ann N Y Acad Sci. 2003;983:22–7. doi: 10.1111/j.1749-6632.2003.tb05959.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–42. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, Weisenberger DJ, Campan M, Young J, Jacobs I, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–8. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 23.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153:38–55. doi: 10.1016/j.cell.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Leitch HG, Smith A. The mammalian germline as a pluripotency cycle. Development. The Company of Biologists Limited. 2013;140:2495–501. doi: 10.1242/dev.091603. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–12. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–23. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–80. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Engström PG, Tommei D, Stricker SH, Ender C, Pollard SM, Bertone P. Digital transcriptome profiling of normal and glioblastoma-derived neural stem cells identifies genes associated with patient survival. Genome Med. 2012;4:76. doi: 10.1186/gm377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G, Esteller M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–64. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 34.Blelloch RH, Hochedlinger K, Yamada Y, Brennan C, Kim M, Mintz B, Chin L, Jaenisch R. Nuclear cloning of embryonal carcinoma cells. Proc Natl Acad Sci U S A. 2004;101:13985–90. doi: 10.1073/pnas.0405015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, et al. Reprogramming of a melanoma genome by nuclear transplantation. . Genes & development. 2004;18:1875–85. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard SM, Benchoua A, Lowell S. Neural stem cells, neurons, and glia. Methods in enzymology. 2006;418:151–69. doi: 10.1016/S0076-6879(06)18010-6. [DOI] [PubMed] [Google Scholar]
- 38.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–24. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–9. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 42.Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ, Meehan RR, Sims AH, Ramsahoye BH. Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci U S A. 2011;108:4364–9. doi: 10.1073/pnas.1013224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sproul D, Kitchen RR, Nestor CE, Dixon JM, Sims AH, Harrison DJ, Ramsahoye BH, Meehan RR. Tissue of origin determines cancer-associated CpG island promoter hypermethylation patterns. Genome Biol. 2012;13:R84. doi: 10.1186/gb-2012-13-10-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartzentruber J, Korshunov A, Liu X-Y, Jones DTW, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 45.Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, et al. St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stütz AM, Wang X, Gallo M, Garzia L, Zayne K, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–50. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009;9:773, 84. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- 48.Obokata H, Wakayama T, Sasai Y, Kojima K, Vacanti MP, Niwa H, Yamato M, Vacanti CA. Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature. 2014;505:641–7. doi: 10.1038/nature12968. [DOI] [PubMed] [Google Scholar]
- 49.Di Stefano B, Sardina JL, van Oevelen C, Collombet S, Kallin EM, Vicent GP, Lu J, Thieffry D, Beato M, Graf T. C/EBPα poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature. 2014;506:235–9. doi: 10.1038/nature12885. [DOI] [PubMed] [Google Scholar]