Abstract
Background
Early environmental exposures may help shape the development of the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis, influencing vulnerability for health problems across the lifespan. Little is known about the role of maternal sensitivity in influencing the development of the ANS in early life.
Aims
To examine associations among maternal sensitivity and infant behavioral distress and ANS and HPA axis reactivity to the Repeated Still-Face Paradigm (SFP-R), a dyadic stress task.
Study Design
Observational repeated measures study.
Subjects
Thirty-five urban, sociodemographically diverse mothers and their 6-month-old infants.
Outcome Measures
Changes in infant affective distress, heart rate, respiratory sinus arrhythmia (RSA), and T-wave amplitude (TWA) across episodes of the SFP-R were assessed. A measure of cortisol output (area under the curve) in the hour following cessation of the SFP-R was also obtained.
Results
Greater maternal insensitivity was associated with greater infant sympathetic activation (TWA) during periods of stress and tended to be associated with greater cortisol output following the SFP-R. There was also evidence for greater affective distress and less parasympathetic activation (RSA) during the SFP-R among infants of predominantly insensitive mothers.
Conclusions
Caregiving quality in early life may influence the responsiveness of the sympathetic and parasympathetic branches of the ANS as well as the HPA axis. Consideration of the ANS and HPA axis systems together provides a fuller representation of adaptive versus maladaptive stress responses. The findings highlight the importance of supporting high quality caregiving in the early years of life, which is likely to promote later health.
Keywords: Infant, Maternal Sensitivity, Autonomic Nervous System, Parasympathetic, Sympathetic, HPA Axis
A growing body of evidence supports the developmental origins of health and disease hypothesis, which purports that early environmental factors influence mental and physical health into adulthood (1). Physiological systems involved in the human stress response, particularly the endocrine system and the autonomic nervous system (ANS), have received attention for their potential malleability by early environmental influences and their hypothesized etiological involvement in a broad array of disease states (e.g., cardiovascular disease, asthma, metabolic syndrome) as well as emotional and cognitive well-being (e.g., posttraumatic stress disorder, depression) (1–3).
Maternal caregiving quality has been identified as a robust programming agent of child stress response systems in early life, with sensitive maternal behaviors, including accurate reading of the child’s signals and contingent, timely, emotionally supportive responding, linked to more optimal stress responding, and insensitive behaviors, including inaccurate interpretation of the child’s needs and withdrawn, intrusive, and hostile behaviors, linked to maladaptive stress responding throughout life (4, 5). The majority of research on caregiving effects on offspring stress response systems has focused on the hypothalamic-pituitary-adrenal (HPA) axis (5–7). Less is known about the impact of maternal sensitivity on ANS activity. Existing studies on infant ANS stress reactivity have focused on the cardiac vagal system (as part of the parasympathetic branch of the ANS), following hypotheses about its role in early life attention, communication, affect, and coping (7, 8); [however, see (9)]. In this context, cardiac vagal withdrawal is hypothesized to support the greater metabolic demand associated with an active coping response. However, work in adult cardiovascular psychophysiology suggests that overactivity of the sympathetic system in particular may be linked to long-term adverse health outcomes (10). Therefore, research is needed to understand the potential influence of caregiving behaviors on both the parasympathetic and sympathetic branches of the ANS.
Notably, evidence shows that responding across and within stress systems is not necessarily correlated (11, 12) Studies that have examined the effects of maternal behaviors on child stress reactivity have tended to study stress systems in isolation. Thus, there is a need for studies that examine maternal caregiving effects on a comprehensive assessment of infant stress reactivity, including affect/behavior, the HPA axis, and the sympathetic and parasympathetic branches of the ANS. The resultant findings may enhance our understanding of the mechanisms by which early experiences influence long-term health and inform the development of more efficacious interventions to prevent disease across the lifespan.
One of the most commonly used paradigms for assessing the infant stress response is the Still-Face Paradigm (SFP), during which the mother is asked to interact normally with the infant (baseline play episode), then withhold interaction (stressor still-face episode), and then resume interaction (recovery reunion episode) (13). The SFP has been shown to reliably produce a stress response, as reflected in increases in observable negative affect/distress and heart rate and decreases in respiratory sinus arrhythmia (RSA; interpreted as an indicator of cardiac parasympathetic activity) during the still-face episode; and increases in cortisol output following the procedure (7, 14–17). More recently, investigators have discovered that the reunion episode may provide evidence of the dyad’s ability to reduce the infant’s stress response following cessation of the still-face episode, as evident by decreases in negative affect and heart rate and increases in RSA, though not necessarily back to baseline play levels (7, 14–18). Infant affective and behavioral responses to the SFP (e.g., amount of positive and negative affect, gaze aversion) have been linked to parental caregiving history and to future adaptation (e.g., attachment quality, emotional and behavioral problems) (16).
Maternal sensitivity during the SFP can be assessed (16). The few studies specifically linking maternal sensitivity to infant affective and physiological responses to the SFP have produced mixed findings. Some studies have linked greater maternal sensitivity to lower infant negative affect and lower heart rate during the still-face and reunion episodes or to a more attenuated HPA axis response following the SFP (6, 7, 15, 19, 20). Others have found associations between lower maternal sensitivity and greater increases in infant heart rate from the still-face to the reunion episode (19). However, others have found no associations between maternal sensitivity and infant affect or cortisol (7, 15), and yet others have found results contrary to prediction, such as decreases in RSA from the play episode to the reunion episode only for infants of sensitive mothers (7). Notably, studies have been inconsistent in how maternal sensitivity is measured in the context of SFP studies. Some studies consider maternal behaviors during the play episode only (20), some focus on the reunion (6), some create composite sensitivity scores across play and reunion episodes (15), and some consider behaviors during the play and reunion episodes separately (19).
Currently there are numerous gaps in the literature assessing associations between maternal sensitivity and infant affective and physiological stress reactivity in response to the SFP. Only two studies have assessed the infant HPA axis response (15, 20), with one collecting only one cortisol measure pre-SFP and one measure 20-minutes post-SFP (15). Studies examining ANS reactivity have focused on heart rate (parasympathetically and sympathetically influenced) and RSA (parasympathetically influenced) and have not included measures of sympathetic reactivity. Furthermore, such studies have often failed to control for motor activity, impeding efforts to distinguish possible stress effects on autonomic measures from metabolic costs of increased motor activity, which often accompanies distress (21). Measures of maternal sensitivity have most frequently been examined in relation to infant responses within episodes rather than in relation to changes across episodes, though examining changes across episodes is important to understanding infant stress responses (7, 16). Finally, only one study has considered separately the impact of maternal sensitivity under both non-distress (play) and distress (reunion) conditions on infant SFP responses, though maternal behaviors under each of these conditions may differentially influence child outcomes (6, 19). For example, the developmental literature suggests that maternal sensitivity during times of distress predicts mother-infant attachment quality, problem behaviors, social competence, and physiological and behavioral regulation; that maternal sensitivity under non-distress conditions predicts child cognitive development; and that maternal sensitivity under non-distress and distress conditions together predict child affect regulation (19, 22–24). More studies are needed that consider maternal sensitivity under both non-distress and distress conditions to determine if they have differential influences on the various infant stress response systems.
The goal of the current study was to address these gaps by examining associations between maternal sensitivity under non-distress and distress conditions and infant affective and physiological stress reactivity. The recently developed repeated version of the Still-Face Paradigm (SFP-R), which includes two still-face and reunion episodes, was utilized to ensure sufficient opportunity to observe an infant stress and recovery response. Measures of infant responding over the course of the SFP-R included affective distress, heart rate, RSA (parasympathetic activity), and T-wave amplitude (sympathetic activity). In addition, cortisol was measured before and repeatedly after completion of the SFP-R to characterize the HPA axis response. We hypothesized that, on average, infants would show increases in behavioral distress, attenuation of the T-wave, and decreases in RSA during the stressful still-face episode. We further hypothesized that maternal insensitivity would be associated with greater infant distress, T-wave attenuation, and decreases in RSA during the still-face episodes; poorer recovery (i.e., more limited decreases in distress, T-wave attenuation, and increases in RSA relative to the preceding still-face episode) during the reunion episodes; and greater overall cortisol output following completion of the SFP-R. Based on the one prior study to examine the differential contributions of maternal sensitivity during the play and reunion episodes to infant biobehavioral reactivity during the SFP (19), we expected that maternal sensitivity during the reunion episode would have stronger associations with infant biobehavioral reactivity than maternal sensitivity during the play episode.
Method
Participants
Participants were mothers and their 6-month-old infants (M = 27.8 weeks, SD = 1.3 weeks) enrolled in a longitudinal study examining the impact of maternal trauma on infant emotional, behavioral, and physiological stress reactivity in the first year of life. Pregnant women receiving prenatal care at two major Boston hospitals and three affiliated urban community health centers and women attending Women, Infants and Children (WIC) programs associated with the health centers were recruited during the 1st or 2nd trimester between August 2006 and September 2009. Inclusion criteria included a) mother aged ≥18 years at the child’s birth and b) single gestation birth. Exclusion criteria included (a) mother not sufficiently fluent in English to complete study measures; (b) infant at increased risk for neurodevelopmental disorders (e.g., gestational age < 32 weeks; birth weight < 5.5 pounds; congenital abnormalities; neurological injury) (25); and (c) maternal endorsement of drinking 7 or more alcoholic drinks per week or smoking 10 or more cigarettes per day during pregnancy,1 as usage at or above these thresholds has been associated with increased risk for neurodevelopmental problems (26, 27). Maternal trauma history was not an inclusion/exclusion criterion. All procedures were approved by the relevant Institutional Review Boards, and written informed consent was obtained from mothers prior to study procedures.
Fifty mother-infant dyads participated in the 6-month laboratory assessment, with 35 providing sufficient data for these analyses. Dyads were excluded for the following reasons: infant too distressed during fitting to wear the physiological recording equipment (n = 7); protocol terminated by mother or examiner due to infant distress (n = 5) or sleepiness (n = 1); lack of usable ANS data due to excessive artifact (n = 1); video recording failure preventing the scoring of maternal sensitivity (n = 1). The demographic characteristics of the final sample are presented in Table 1. These dyads did not differ from excluded dyads on any of the demographic variables (ps > .05).
Table 1.
Sample Demographic Characteristics (N = 35)
| N | % | M | SD | |
|---|---|---|---|---|
| Maternal age at infant’s birth (years) | 27.5 | 6.6 | ||
| Maternal education attainment | ||||
| High school or less | 13 | 37 | ||
| Some college or greater | 18 | 51 | ||
| Not reported | 4 | 11 | ||
| Maternal relationship status | ||||
| Married or living with partner | 17 | 49 | ||
| Separated, divorced, never married | 14 | 40 | ||
| Not reported | 4 | 11 | ||
| Primiparous birth | 15 | 43 | ||
| Infant sex: Male | 19 | 54 | ||
| Infant ethnicity/race | ||||
| Hispanic/White | 3 | 9 | ||
| Not Hispanic/White | 8 | 23 | ||
| Hispanic/Black | 1 | 3 | ||
| Not Hispanic/Black | 13 | 37 | ||
| Hispanic/Multiracial | 5 | 14 | ||
| Not Hispanic/Multiracial | 3 | 9 | ||
| Not Hispanic/Asian | 2 | 6 | ||
| Infant gestational age (weeks) | 39.3 | 1.8 | ||
| Infant birthweight (grams) | 3410.6 | 482.2 | ||
| Infant age at assessment (weeks) | 27.6 | 1.4 | ||
Measures
Repeated Still-Face Paradigm (SFP-R) (13, 15)
Mother-infant dyads participated in the Repeated Still-Face Paradigm (SFP-R) (15). The Still-Face Paradigm (SFP) is a videotaped observational procedure well-validated in sociodemographically diverse samples to assess infant affective, behavioral, and physiological reactivity to brief, moderate levels of induced stress (16, 28). The SFP involves three 2-minute episodes during which the infant is seated in an infant seat across from the mother. The mother is instructed to play with her infant for 2 minutes (“play” = baseline), followed by a still-face episode, during which the mother is instructed to maintain a neutral facial expression and avoid touching or vocalizing at the infant (“still-face” = stressor). The still-face episode is hypothesized to be a stressor for the infant because the mother is no longer providing behavioral cues needed for the infant to maintain an organized social and affective state (17). The mother is then instructed to resume playing with the infant during a reunion episode (“reunion” = recovery), which provides an opportunity for the infant to recover, with the mother’s assistance, from stress elicited by the still-face episode. To prevent the infant from becoming excessively distressed, the still-face episode was curtailed if the infant displayed one minute of continuous fussing or 30 seconds of continuous crying. The repeated version (SFP-R) includes a second still-face episode (still-face 2) and reunion episode (reunion 2) administered immediately after cessation of the first reunion episode (reunion 1). The still-face 2 and reunion 2 episodes were only administered if the infant had returned to a non-distressed state by the end of the reunion 1 episode.
Maternal sensitivity
Independent trained coders scored maternal sensitivity during the SFP-R play and reunion episodes using a 9-point scale assessing the extent to which the mother correctly interpreted and responded to her infant’s cues and adjusted her behavior to the infant’s affective state (29). Increasing scores indicate greater sensitivity in responding, including accurately reading the infant’s obvious and subtle cues, responding promptly and appropriately to the infant’s cues, and facilitating smooth dyadic interactions. Lower scores indicate increasingly insensitive responding, including inaccurately interpreting the infant’s cues, delayed responding or refusing to respond to the infant’s cues, ending interactions abruptly while the infant is still engaged, and behaving in an intrusive or withdrawn manner. Maternal sensitivity during the play (sensitivity_p) and reunion 1 (sensitivity_r1) episodes were considered separately. Reunion 2 scores were not considered because not all dyads completed the second reunion. For both sensitivity_p and sensitivity_r1, the inter-rater reliability Pearson correlation coefficients were r = .74.
Infant affective distress
The infants’ affective states throughout the SFP-R were coded second-by-second from videos by independent trained coders blind to maternal sensitivity status. Codes were mutually exclusive and consisted of the following: hard crying, crying, fussing/negative affect without vocalizations (called “fussing” from this point forward), neutral, positive, very positive, mixture of positive and negative, unclassifiable, unobservable/asleep, and autonomic indicator (i.e., yawn, sneeze). Forty-four percent of videos were coded for inter-rater reliability (weighted kappa = 0.77). For the current analyses, a distress score was calculated based on the percentage of time spent fussing, crying, or hard crying for each SFP-R episode. The calculation for distress included weightings for the level of distress to account for intensity (fussing + 2x crying + 3x hard crying). Possible scores ranged from 0 (no distress) to 300 (hard crying for entire episode).
Infant autonomic nervous system (ANS) measurement
Infant respiration and cardiac activity were measured during the SFP-R using the LifeShirt System (VivoMetrics, Inc., Ventura, CA), a non-invasive ambulatory respiratory inductance plethysmography device (30) adapted to infants that collects continuous measures of multiple physiological parameters, with a particular focus on detailed analysis of cardiac and respiratory functioning. A sleeveless Velcro shirt with built-in inductance bands and cables for a 3-lead ECG was fitted to the infant, with electrodes attached to the sternum, the lower left rib, and the left clavicle. Raw signals from the two inductance bands and the ECG leads were amplified, A/D converted, and stored continuously either in an attached PDA-size recorder or in real time on an IBM compatible laptop. For volume calibration of the inductance band output, the Qualitative Diagnostic Calibration procedure (31) was applied. During the course of the study, the system was modified to increase the ECG sampling rate from 200 Hz to 1000 Hz for greater accuracy of R-wave detection, given infants’ higher heart rates relative to adults; 19 (54%) of the infants were assessed with the 200 Hz system, and 16 (46%) with the 1000 Hz system. The specific ANS parameters assessed and analyzed include heart rate (HR), respiratory sinus arrhythmia (RSA), and T-wave amplitude (TWA).
Heart rate (HR)
Continuous ECG signals were examined for artifacts by extensively trained raters. Artifacts were removed using the VivoLogic software (VivoMetrics, Inc., Ventura, CA) for analysis of LifeShirt System data. HR means were then extracted for each of the SFP-R episodes. The mean rate of edited artifacts across SFP-R episodes was 6% (SD = 8%) and did not differ between the 200 and 1000 Hz LifeShirt Systems.
Respiratory sinus arrhythmia (RSA)
RSA was extracted to estimate cardiac vagal activity as an indicator of parasympathetic influences on the heart. The time-domain peak-valley method (32) was used by extracting modulation of the cardiac interbeat interval (IBI, in ms) breath-by-breath with the customized rsaToolbox software (33). The time-domain peak-valley index is typically correlated with the frequency-domain high-frequency heart-rate variability index to r = .92 and above (34). RSA was set to “missing” when a breath was too short to accommodate at least two full IBIs (TTOT < IBIt1+IBIt2, or HR/2> fR, where t1 is the first IBI associated with the onset of inspiration, and t2 is the subsequent IBI) (9, 35). Breaths that did not adhere to the basic peak-valley criterion of IBIminimum preceding IBImaximum were set to zero. influenced by the respiratory pattern (9, 36, 37), with both longer and deeper breaths increasing RSA potentially independent of cardiac vagal activity changes, an additional within-individual correction of RSA was employed (38). LogRSA was normalized by VT (logRSA/VT) and residualized for TTOT, a strategy that has been shown to improve the estimation of cardiac vagal activity in adults (32, 39, 40). The grand mean of unadjusted logRSA/VT was added to the residual to obtain the respiration-corrected RSA (RSAc). Results are reported both for RSAc, which we have previously shown to be a more robust measure of infant stress-elicited changes in RSA (38), and for respiration-uncorrected RSA (RSAu), to allow comparison with the existing infant literature, which has generally not controlled for respiration. Higher RSA scores indicate greater cardiac parasympathetic activity, and decreases in RSA scores indicate cardiac parasympathetic withdrawal. One infant wore the electrodes but refused to wear the LifeShirt vest and thus was missing respiration data needed to calculate RSAu and RSAc.
T-wave amplitude (TWA)
TWA of the ECG was employed as an index of cardiac sympathetic activity, as prior studies have suggested a sensitivity to sympathetic influences [e.g., (41, 42)]. Although measures from impedance cardiography are typically used to estimate cardiac sympathetic effects (43–45), implementation of this technique was not possible in this study because of potential interference with the respiratory inductance plethysmography measurements. In adults, T-wave attenuation has been observed repeatedly in response to stressful laboratory challenges [e.g., (46–50)]. The T-wave was extracted using the ECG boundary location function (51) embedded in the AcqKnowledge software package (Version 4.1; Biopac Systems, Inc., Goleta, CA). There were level differences in T-wave amplitude scores between the 200 Hz and 1000 Hz LifeShirt Systems; therefore, T-wave scores were adjusted by z-transforming scores within each LifeShirt System. A decrease in the T-wave score (T-wave attenuation) indicates sympathetic activation.
Infant activity level
Independent trained coders scored infant activity level during the SFP-R second-by-second from videos using a 4-point scale [modified from (52)]: 0 = quiet motor (e.g., no movement other than slow moving of fingers), 1 = slow/mild movements (e.g., slow bending but not lifting of limbs), 2 = moderate movements (e.g., slow lifting of limbs), 3 = pronounced movements (e.g., forceful lifting of limbs). Twenty-eight percent of videos were coded for inter-rater reliability (ICC = .92). The percentage of time an infant spent in each activity level for each SFP-R episode was calculated, multiplied by the activity level value, and summed, resulting in a range from 0 (entire episode in “quiet motor”) to 300 (entire episode in “pronounced”).
Infant hypothalamic-pituitary-adrenal (HPA) axis measurement
Infant cortisol was assessed from five saliva samples collected over the course of the laboratory visit: prior to beginning the SFP-R, immediately after cessation of the SFP-R, and three samples in successive 20-minute increments following cessation of the SFP-R. At each collection, a dental cotton roll treated with a few crystals of pre-sweetened cherry Kool-Aid was placed in the infant’s mouth until it was saturated (approximately 1 minute); the saliva was then expressed via needleless syringes into sealed vials and frozen at −80° Celsius. Prior studies have shown that salivary cortisol accurately reflects blood concentrations (53) and that saliva collection with Kool-Aid treated cotton rolls enhances infant compliance while introducing minimal interference into analyses (54, 55). Before beginning data collection, the cortisol assessment method was tested for possible interference by use of the cotton rolls and/or Kool-Aid, which were found to have minimal impact on the amount of cortisol recovered. Samples were assayed together in the same batch in duplicate employing a chemiluminescence assay (CLIA) with high sensitivity of ~0.16 ng/ml (IBL; Hamburg, Germany, Clemens Kirschbaum). Control sera covering at least three levels of analyte were run during each 24-hour period. Samples exceeding the highest calibrator value were diluted prior to further testing. Samples with levels below the minimum detectable levels were assigned values of 0.5 nmol/l. Intra- and interassay variabilities ranged between 5% and 12%.
Procedure
Appointments were scheduled to accommodate the families’ needs, and thus there was variability in the time of day of assessments, with 10% of dyads completing the SFP-R in the morning (9am–11am), 42% in the early afternoon (11am–2pm) and 48% in the late afternoon (2pm–6pm). Approximately 45 minutes after arrival to the laboratory, the first saliva sample was obtained. Infants were then fitted with the LifeShirt and placed in the infant seat, where they remained for the duration of the SFP-R. Following the SFP-R, infants and mothers engaged in a 10-minute unstructured free play on the floor, mothers completed questionnaires (not included here), and the remaining saliva samples were collected.
Data Analytic Plan
First, descriptive analyses regarding maternal sensitivity and the percentage of dyads completing all versus part of the SFP-R are presented. Chi-square analyses/Fisher’s exact tests, t-tests, ANOVAs, and correlational analyses assessed whether maternal sensitivity and completion of the SFP-R were associated with relevant study variables. A series of mixed effects ANOVAs were used to explore the associations of maternal sensitivity with infant distress and ANS responding over the course of the SFP-R. Analyses included tests for main effects of SFP-R episode and maternal sensitivity within the same model and, in a subsequent step, the interaction between episode and maternal sensitivity in predicting infant distress and ANS responding. The interaction term tested whether maternal sensitivity had a differential association with infant responding by SFP-R episode. Separate analyses considered maternal sensitivity during the play episode (sensitivity_p) and during the reunion 1 episode (sensitivity_r1). If the interaction term was significant, contrast analyses were conducted to specify the nature of the interaction. Mixed effects ANOVAs that incorporated an ANS parameter (HR, RSAu, RSAc, TWA) as the dependent variable included infant activity level as a covariate to allow for testing of the effects of psychological stress on ANS responding independent of physical activity. Analyses included all infants who completed at least the first still-face test (play, still-face 1, reunion 1). Estimation for the mixed effects ANOVAs was via full information maximum likelihood to account for missing data, which produces unbiased results if missing data are missing at random. Degrees of freedom were calculated using the Satterthwaite method.
Cortisol values were examined for outliers, defined as > 100 nmol/l or > 3 standard deviations (SD) from the mean following standardization. No subject had any value exceeding 100 nmol/l, and two subjects had values exceeding > 3 SD from the mean. Due to non-normality of the data, scores were log transformed prior to creating the area under the curve (AUC) variable. To capture the change in cortisol output following the SFP-R, AUC was computed using the trapezoidal rule (56) to measure total cortisol output in the hour following completion of the SFP-R. Specifically, for each participant, a line depicting cortisol values at each of the four post-test time points of data collection (0, 20, 40, 60 minutes post SFP-R) was plotted, and the AUC value was calculated as the sum of the areas of the three trapezoids below the line. Higher AUC values reflect greater cortisol release over the assessment period. Calculation of AUC required data for samples obtained 0, 20, 40, and 60 minutes post-SFP-R; 23 subjects had complete data and thus were included in the cortisol analyses. Data were missing for various reasons, including values exceeding outlier criteria and participants needing to leave the laboratory before providing all samples. Participants who did and did not provide complete cortisol data did not differ on maternal sensitivity during the play or reunion episodes (ps > .40), infant distress during play, still-face 1, or reunion 1 (ps > .40), or completion of both still-face tests (p = .736). Partial correlations that controlled for time of day of SFP-R administration tested whether infant HPA axis responding was associated with maternal sensitivity during the play or reunion 1 episode.
Results
Descriptive Analyses
Maternal sensitivity scores ranged from 2 to 8 during play, M = 5.43, SD = 4.63, and from 2 to 9 during reunion 1, M = 4.63, SD =1.90. There was a significant association between sensitivity during the play and reunion 1 episodes, r = .76, p < .001. Maternal sensitivity during the play episode was associated with maternal age, r = .34, p = .046, and with parity, r = .34, p = .044, with younger maternal age and primiparity associated with lower sensitivity. Notably, maternal age and parity were highly related, r = .54, p <.001. Neither maternal sensitivity during play nor maternal sensitivity during reunion 1 was associated with infant sex, age, birthweight, race, or ethnicity or maternal educational attainment or relationship status (ps > .15).
The majority of infants (63%, n = 22) completed all five episodes of the SFP-R, with 26% (n = 9) terminating after reunion 1 and 11% (n = 4) after still-face 2. Completers and non-completers did not differ on infant sex, birthweight, gestational age, or age at assessment or by LifeShirt system (200 or 1000 Hz) used (ps > .25). Infant birthweight, gestational age, age at assessment, and sex did not predict infant distress or ANS responding (HR, RSAu, RSAc, TWA) during the baseline play episode (ps > .05).
Infant Distress and ANS Responding to the SFP-R
The series of mixed effects ANOVAs testing the main effects of SFP-R episode (Figure 1) revealed main effects in predicting infant distress, F(4, 37) = 20.59, p < .001, activity level, F(4,52) = 48.50, p < .001, HR, F(4, 48) = 7.15, p < .001, and RSAc, F(4, 49) = 6.12, p < .001 over the course of the protocol. There was no main effect for SFP-R episode in predicting RSAu or TWA, ps > .70.
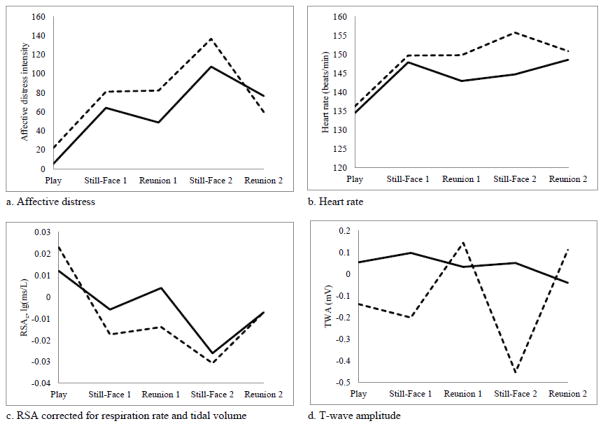
Figure 1.
Infant affective distress, heart rate, RSAc, and TWA during the Repeated Still-Face Paradigm (SFP-R) by maternal sensitivity during the play episode. Solid lines indicate infants of sensitive mothers (scored ≥5 on sensitivity measure), with 25 dyads completing the play, still-face 1, and reunion 1 episodes, 19 dyads completing the still-face 2 episode, and 18 dyads completing the reunion 2 episode. Dotted lines indicate infants of insensitive mothers (scored < 5 on sensitivity measure), with 10 dyads completing the play, still-face 1, and reunion 1 episodes, 7 dyads completing the still-face 2 episode, and 4 dyads completing the reunion 2 episode. Using either a continuous or dichotomized measure of maternal sensitivity, the interaction term SFP-R Episode x Maternal Sensitivity was significant for TWA. In addition, main effects were significant for SFP-R episode for distress, heart rate, and RSAc, and main effects were significant for maternal sensitivity (dichotomized) for distress and RSAc. RSAc = respiratory sinus arrhythmia corrected for respiration rate and tidal volume. TWA = T-wave amplitude.
In t-test analyses comparing dyads who did and did not complete the SFP-R, infants in early terminating dyads demonstrated greater distress during still-face 1, t(33) = −4.13, p < .001, and reunion 1, t(13.19) = −4.73, p < .001, and greater HR during reunion 1, t(33) = −2.45, p = .020. Dyads who did and did not complete the SFP-R did not differ on infant distress during play, HR during play or still-face 1, or RSAu, RSAc, or TWA during play, still-face 1, or reunion 1 (ps > .10).
Infant Distress and ANS Responding to the SFP-R by Maternal Sensitivity
Pearson correlational analyses tested whether infants’ level of distress or ANS responding differed during the baseline play episode by maternal sensitivity during the play episode. Maternal sensitivity during play was not associated with any infant behavioral or ANS parameters during the play episode (ps ≥.15). In t-test analyses, maternal sensitivity scores during reunion 1 were significantly greater among dyads who completed the SFP-R (M = 5.14, SD = 1.75) than among dyads who terminated early (M = 3.77, SD = 1.88), t(33) = 2.17, p = .037. Maternal sensitivity scores during the play episode did not distinguish SFP-R completers from non-completers, t(33) = 1.11, p = .273.
The series of mixed effects ANOVAs testing the main effects of SFP-R episode and maternal sensitivity did not show any main effects for maternal sensitivity during play or during reunion 1 in predicting overall differences in infant behavioral or ANS indices, ps > .30.
The Episode x Maternal Sensitivity_p interaction term was significant in predicting TWA, F(4, 108) = 5.64, p < .001, but not any of the other behavioral or ANS indices, ps > .26. The Episode x Maternal Sensitivity_r1 interaction term was significant in predicting TWA, F(4, 109) = 2.46, p = .050, but not any of the other ANS indices, ps > .17, and showed a trend towards significance for infant distress, F(4, 40) = 2.37, p = .069.
Follow-up contrasts examined the specificity of the significant Episode x Maternal Sensitivity interaction terms predicting TWA. Contrasts tested maternal sensitivity differences in TWA between the play and still-face episodes and between each still-face and subsequent reunion episode. Contrasts comparing TWA during still-face 1 and reunion 1 were significant for maternal sensitivity_p, t(75) = −2.07, p = .042, but not for maternal sensitivity_r1, t(77) = −.97, p = . 337. Contrasts comparing differences in TWA during still-face 2 and reunion 2 were significant for maternal sensitivity_p, t(60) = 3.13, p = .003, and for maternal sensitivity_r1, t(58) = 2.35, p = .022. As shown in Figure 1d, lower maternal sensitivity was associated with larger TWA attenuation during the still-face episodes. Contrasts comparing differences in TWA between the play and still-face episodes were not significant for maternal sensitivity_p or for maternal sensitivity_r1, ps > .30.
Infant HPA Axis Responding by Maternal Sensitivity
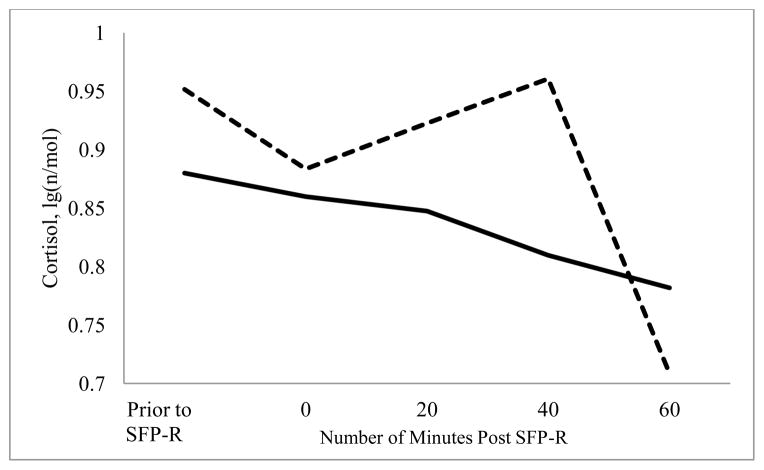
Neither maternal sensitivity_p nor maternal sensitivity_r1 was associated with infant cortisol levels prior to the initiation of the SFP-R or immediately after completion of the SFP-R (ps > .50). Greater maternal sensitivity during play tended to be associated with lower AUC (pr = −.39, p = .071; Figure 2). Maternal sensitivity during reunion 1 was not significantly associated with AUC (pr = −.24, p = .286).
Figure 2.
Infant cortisol prior to and following administration of the Repeated Still-Face Paradigm (SFP-R) by maternal sensitivity during the play episode.
Supplementary Analysis: Maternal Sensitivity as a Categorical Variable
We conducted supplementary analysis of infant stress responding by maternal sensitivity scores dichotomized into sensitive and insensitive categories (82% agreement between inter-rater reliability coders on sensitive/insensitive classification for both the play and the reunion 1 episodes). Previous studies have created high and low maternal sensitivity groups from continuous scores, noting that this approach has been shown to be an effective method for identifying caregiver influences on infant stress measures (15, 57). The scores were dichotomized on the basis of behavioral meaning, with scores of 5 or greater indicating predominantly sensitive responding (n = 25 during play, n = 17 during reunion 1) and scores below 5 indicating predominantly insensitive responding (n = 10 during play, n = 18 during reunion 1). Mothers either retained their sensitivity/insensitivity rating across episodes or changed from sensitive during play to insensitive during reunion 1. Analyses using the dichotomized sensitivity scores yielded largely comparable findings to those reported using continuous sensitivity scores, with greater infant T-wave attenuation during the still-face episodes associated with maternal insensitivity during play and reunion 1, and greater cortisol output following the SFP-R tending to be associated with maternal sensitivity during play. In addition, infants of mothers categorized as insensitive during the play episode showed greater distress, F(1, 33) = 8.86, p = .006, and lower levels of RSAc, F(1, 59) = 4.44, p = .039, over the course of the SFP-R (i.e., significant main effects of maternal sensitivity_p on infant distress and RSAc). The Episode x Maternal Sensitivity_p interaction term approached significance in predicting RSAc over the SFP-R, F(4, 40) = 2.41, p = .065.
Discussion
The goals of this study were to (a) examine multiple indicators of infant stress reactivity, with a focus on detailed assessment of affective, autonomic, and HPA axis responses, to a standardized mother-infant stressor, the Repeated Still-Face Paradigm (SFP-R) and (b) to test the modifying effects of maternal sensitivity on infant response patterns. Analyses tested maternal sensitivity during the SFP-R play and reunion 1 episodes separately, as prior research has not established which measure is more predictive of infant biobehavioral reactivity. Moreover, maternal sensitivity during non-stressful (i.e., play) and stressful (i.e., reunion) conditions may differentially contribute to child outcomes and thus each be worthy of consideration (6, 19).
As expected, there were main effects for SFP-R episode on affective distress and ANS functioning, suggesting that the procedure was successful in eliciting a stress response. On average, infants showed a pattern of elevated distress and physical activity during each still-face episode, with some recovery during the reunion episodes. Heart rate increased in response to the first still-face episode and remained elevated over the remainder of the procedure. RSA corrected for respiration decreased during the still-face episodes relative to the baseline play and increased during the reunion episodes, though not back to initial baseline values.
The most robust and consistent effects for maternal sensitivity on infant stress reactivity was found for sympathetic activation. Maternal sensitivity during the play and reunion 1 episodes were each associated with changes in T-wave amplitude (TWA) during the SFP-R. Lower maternal sensitivity was associated with greater T-wave attenuation during both still-face episodes relative to the reunion episodes. Maternal sensitivity during play also tended to be associated with infant HPA axis reactivity following the SFP-R, with lower maternal sensitivity associated with greater cortisol output. When maternal sensitivity was considered as a dichotomous variable (predominantly sensitive versus predominantly insensitive), maternal sensitivity during the play episode was associated with infant affective distress over the course of the SFP-R. Although infants on average demonstrated increases in affective distress during the still-face episodes and decreases during the reunion episodes, infants of predominantly sensitive mothers showed lower levels of distress across episodes. In addition, infants of predominantly sensitive and predominantly insensitive mothers showed similar changes in RSA corrected for respiration over the course of the SFP-R, including relative decreases during the still-face episodes and relative increases during the reunion episodes; however, infants of predominantly sensitive mothers generally showed higher levels of RSA.
The pattern of findings suggests that maternal sensitivity may buffer infant stress reactivity across stress systems. Although infants on average showed affective distress in response to the SFP-R, infants of sensitive mothers showed minimal changes in TWA, indicating limited sympathetic reactivity to the stressor, and minimal changes in cortisol, indicating limited HPA axis reactivity. These results are consistent with previous studies showing that behavioral and physiological indicators of stress are not necessarily correlated in early life and that caregiving quality may influence the effective functional integration of stress systems over development (7, 15, 17, 21, 58). Behavioral signals of distress, such as fussing, crying, and directed vocalizations at the caregiver, may be adaptive under conditions of stress, eliciting a response from the caregiver, particularly if the caregiver is sensitive to the infant’s signals and responds in a timely and appropriate manner. The caregiver may then offer comforting that relieves the infant’s distress and prevents activation of physiological stress systems. Infants who do not expect, based on the quality of prior maternal interactions, or who do not receive a sensitive maternal response to their behavioral indicators of distress may experience activation of other stress systems, including the sympathetic nervous system and HPA axis. A stress response marked by behavioral distress and concomitant reactivity of the sympathetic nervous system and the HPA axis may result in greater “wear and tear” on the body and worse health (7). The buffering effect of maternal sensitivity on physiological stress reactivity may have particular consequence for children at risk for maladaptive stress responses, for example due to underlying genetic vulnerabilities (59) or prenatal exposure to maternal psychopathology (6).
Our findings suggest that the consequences of maternal insensitivity during both play and in response to the stressful experience of the still-face are more apparent in cardiac sympathetic activation than in vagal withdrawal. Problematic long-term health outcomes in the cardiovascular system have been tied specifically to sympathetic activation (10). Whereas the main role of the cardiac parasympathetic system is the immediate and direct adaptation of cardiac activity to changes in metabolic demand arising through physical activity and environmental challenges, the additional boost of the sympathetic system, especially when it goes beyond what is needed for covering metabolic demand (10, 60), is hypothesized to be one of the major harmful components of the stress response, especially if maintained or activated repeatedly over long periods. Notably, our supplementary analysis of the dichotomized maternal sensitivity variable showed evidence for an additional overall modulation of RSA corrected for respiration by maternal sensitivity during the play episode. These results are consistent with earlier research focused on parasympathetic activity, which has shown significant modulation of RSA by maternal sensitivity (7), although not necessarily compatible in its direction with the present findings. The findings should be interpreted carefully considering the relatively loose association between RSA and overall cardiac vagal activity between individuals (61). Studies with larger samples are needed to solidify the evidence on associations of maternal sensitivity with cardiac sympathetic versus parasympathetic stress responding in infants.
The somewhat different pattern of findings that emerged when maternal sensitivity was considered as a continuous versus a dichotomized score may be the result of several factors. Notably, maternal sensitivity was a significant predictor of infant TWA responding regardless of how it was operationalized (during play or reunion 1, continuous or dichotomized). These results suggest a rather robust association between maternal sensitivity and infant sympathetic reactivity. Maternal sensitivity was only related to infant affective distress and RSA corrected for respiration when considered as a dichotomized variable, suggesting that the relation between maternal behaviors and infant parasympathetic reactivity may be weaker than with sympathetic reactivity. The results may also indicate that the sympathetic system may be more vulnerable to subtle changes in maternal behavior, whereas infant affective distress and parasympathetic reactivity are influenced by more global measures of functioning, i.e., whether the mother is predominantly sensitive or insensitive, and finer distinctions in behavior are not as influential on infant responding.
On average, maternal sensitivity scores decreased and the number of mothers classified as predominantly insensitive increased from the play episode to the reunion 1 episode. Specifically, all mothers rated as predominantly insensitive during the play episode were rated as predominantly insensitive during the reunion 1 episode, and some mothers rated as predominantly sensitive during the play episode were rated as predominantly insensitive during the reunion 1 episode. This pattern likely reflects the effects of the intervening still-face episode on the dyad, with some mothers capable of sensitivity toward their infant under non-stress conditions but not under stressful conditions. Increases in infant distress following the still-face episode may have challenged some mothers’ abilities to respond sensitively, possibly due to their own stress reactivity and available coping resources. A consistent pattern of insensitive behaviors across episodes may reflect general caregiving behaviors. Thus, infants of mothers who were predominantly insensitive during both presumably non-stressful (play) and stressful (reunion) episodes may be more likely to be exposed to insensitive caregiving behaviors in their daily life compared to infants of mothers who only appeared insensitive during the stressful reunion episode. Exposure to such insensitive caregiving behaviors may increase risk for the development of heightened reactivity of the ANS, HPA axis, and affective systems in response to stress and may thus explain why maternal sensitivity during the play episode was more consistently associated with infant stress reactivity than maternal sensitivity during the reunion 1 episode. Also, maternal sensitivity during the play episode may have set the tone for the remainder of the procedure, influencing infants’ distress and ANS reactivity during the subsequent SFP-R episodes. Notably, these results are inconsistent with the one other study that examined associations of maternal sensitivity during both the play and reunion episodes with infant biobehavioral responding during the SFP. That study found stronger effects for maternal sensitivity during the reunion, though somewhat different infant responses were examined (HR, RSA, attentional engagement, resistant behaviors) (19). Further investigation into the independent and joint contributions of maternal sensitivity during nondistress and distress conditions to infant biobehavioral reactivity and other developmental outcomes (e.g., neurocognitive development, socioemotional functioning) is needed.
Limitations of the current study include a relatively small sample size and loss of subjects or incomplete data due to distress/early termination of the protocol, lowering the power to detect effects. Of 50 eligible participants, 12 were excluded due to excessive distress during fitting of the equipment or during the initial SFP-R episodes, and 13 failed to complete the second still-face test. Compared to infants who completed the SFP-R, infants who did not complete the second still-face test did not differ on affective distress or ANS reactivity during the baseline play episode but did demonstrate greater affective distress and HR during the reunion 1 episode. Also, mothers of infants who failed to complete the second still-face test had significantly lower sensitivity scores during reunion 1 compared to mothers of infants who completed the second still-face test. Thus, the dyads providing data for the second still-face test may have been more “robust” in terms of reduced infant stress reactivity and increased maternal sensitivity. Ratings of maternal sensitivity did not distinguish between types of insensitive behaviors (e.g., intrusiveness, withdrawal); specific patterns of maternal behavior may have differential effects on child outcomes (62). Future research should explore how different caregiving behaviors influence infant responding across stress systems. Infant distress was not separated into distress type, such as sadness and anger. Infants may vary in their specific emotional responses to the still-face, and different responses may be associated with different physiological profiles [e.g., sadness related to cortisol increase and anger related to sympathetic activation (63, 64)]. The study design did not include a measure of infant stress reactivity outside the presence of the mother. Thus, determining whether infants of insensitive mothers show greater stress reactivity across contexts or whether this pattern only occurs in response to insensitive caregiving was not possible. Because maternal sensitivity and infant stress reactivity were assessed at the same time, causal direction cannot be definitively determined; for example, mothers of infants who displayed less stress reactivity may have appeared more sensitive due to their infants’ behaviors, and behaving sensitively toward a highly reactive infant may be more difficult than toward a less distressed infant.
Our findings support the hypothesis that caregiving quality is associated with infant responding across stress systems. This is the first study to test associations between maternal sensitivity and a wide-range of stress response measures, including indicators of the sympathetic and parasympathetic branches of the ANS and the HPA axis. The pattern of results suggests that the responses of the individual systems may not be correlated, but that consideration of all of the systems together may provide the most accurate representation of an adaptive versus maladaptive stress response. Given the involvement of these systems in a range of long-term physical and mental health outcomes (1), the findings have public health implications, underscoring the importance of supporting high quality caregiving in the early years of life to promote health across the lifespan.
Acknowledgments
The research was supported by grants from the National Institute of Mental Health (K08MH074588; Bosquet Enlow), the National Institute of Environmental Health Sciences (R01ES010932; Wright) and the National Heart, Lung & Blood Institute (R01HL080674; Wright). During preparation of this manuscript, the authors were supported by K08MH074588 and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital (Bosquet Enlow); R01MD006086 (Bosquet Enlow and Wright); and R01DA027533 (Rosenfield). None of the funding agencies had any role in the study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency.
Abbreviations
- ANS
autonomic nervous system
- HPA
hypothalamic-pituitary-adrenal
- HR
heart rate
- RSA
respiratory sinus arrhythmia
- RSAc
respiratory sinus arrhythmia corrected for respiration rate and tidal volume
- RSAu
respiratory sinus arrhythmia uncorrected for respiration rate and tidal volume
- SFP
Still-Face Paradigm
- SFP-R
Repeated Still-Face Paradigm
- TWA
T-wave amplitude
Footnotes
Throughout the course of the study, mothers were re-interviewed about their use of substances during pregnancy. During later interviews, one mother (3%) endorsed averaging one alcoholic drink per day during the first trimester, and 4 mothers (11%) endorsed smoking 10 or more cigarettes per day at some point during pregnancy, with one only prior to pregnancy recognition, two only during the first trimester, and one throughout the pregnancy. No mother endorsed the use of marijuana, opioids, cocaine/crack, or stimulants at any point during pregnancy. Prenatal substance use was not significantly associated with any of the analyses and is therefore not considered further.
Conflict of Interest Statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Den Bergh BRH. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Developmental Medicine & Child Neurology. 2011;53(Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010 Apr;84(1):46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Prog Brain Res. 2008;167:121–35. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- 4.Sroufe LA, Egeland B, Carlson EA, Collins WA. The development of the person. New York: Guilford Press; 2005. [Google Scholar]
- 5.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014 Jan;140(1):256–82. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant KA, McMahon C, Reilly N, Austin MP. Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behavior and Development. 2010;33(4):453–62. doi: 10.1016/j.infbeh.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face Still-Face Paradigm is moderated by maternal sensitivity. Child Dev. 2009 Jan-Feb;80(1):209–23. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 8.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995 Jul;32(4):301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 9.Grossman P, Taylor E. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution, and biobehavioral functions. Biol Psychol. 2007;74(2):263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Obrist PA. The cardiovascular-behavioral interaction--as it appears today. Psychophysiology. 1976 Mar;13(2):95–107. doi: 10.1111/j.1469-8986.1976.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991 Oct;98(4):459–87. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- 12.Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998 May 1;840:664–73. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- 13.Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry. 1978 Winter;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 14.Enlow MB, Kullowatz A, Staudenmayer J, Spasojevic J, Ritz T, Wright RJ. Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosom Med. 2009 Jul;71(6):607–14. doi: 10.1097/PSY.0b013e3181ad1c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haley DW, Stansbury K. Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Dev. 2003 Sep-Oct;74(5):1534–46. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- 16.Mesman J, van Ijzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review. 2009;29(2):120–62. [Google Scholar]
- 17.Weinberg MK, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Dev. 1996 Jun;67(3):905–14. [PubMed] [Google Scholar]
- 18.Moore GA, Calkins SD. Infants’ vagal regulation in the Still-Face Paradigm is related to dyadic coordination of mother-infant interaction. Dev Psychol. 2004 Nov;40(6):1068–80. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- 19.Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development. 2010;33(3):251–65. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Developmental Psychobiology. 2009;51(8):625–37. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- 21.Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Dev. 2001 Sep-Oct;72(5):1314–26. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein MH, Tamis-LeMonda CS. Maternal responsiveness and infant mental abilities: Specific predictive relations. Infant Behavior & Development. 1997;20(3):283–96. [Google Scholar]
- 23.McElwain NL, Booth-Laforce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. J Fam Psychol. 2006 Jun;20(2):247–55. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- 24.Leerkes EM, Nayena Blankson A, O’Brien M. Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Dev. 2009 May-Jun;80(3):762–75. doi: 10.1111/j.1467-8624.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minde K. Prematurity and serious medical conditions in infancy: Implications for development, behavior, and intervention. In: Zeanah C, editor. Handbook of Infant Mental Health. 2. New York, NY: Guilford; 2000. [Google Scholar]
- 26.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: A meta-analytical review. Alcohol Alcohol. 2003 Jul-Aug;38(4):295–304. doi: 10.1093/alcalc/agg087. [DOI] [PubMed] [Google Scholar]
- 27.Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002 Jun;92(6):966–74. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamson LB, Frick JE. The Still Face: A history of a shared experimental paradigm. Infancy. 2003;4(4):451–73. [Google Scholar]
- 29.Ainsworth MD. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 1969 Dec;40(4):969–1025. [PubMed] [Google Scholar]
- 30.Ritz T, Dahme B, Dubois AB, Folgering H, Fritz GK, Harver A, et al. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology. 2002 Sep;39(5):546–67. doi: 10.1017.S0048577202010715. [DOI] [PubMed] [Google Scholar]
- 31.Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, et al. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol (1985) 1989 Jan;66(1):410–20. doi: 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- 32.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991 Mar;28(2):201–16. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 33.Schulz SM, Ayala E, Dahme B, Ritz T. A MATLAB toolbox for correcting within-individual effects of respiration rate and tidal volume on respiratory sinus arrhythmia during variable breathing. Behav Res Methods. 2009 Nov;41(4):1121–6. doi: 10.3758/BRM.41.4.1121. [DOI] [PubMed] [Google Scholar]
- 34.Grossman P, van Beek J, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990 Nov;27(6):702–14. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 35.Rother M, Witte H, Zwiener U, Eiselt M, Fischer P. Cardiac aliasing--a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Hum Dev. 1989 Sep;20(1):1–12. doi: 10.1016/0378-3782(89)90068-6. [DOI] [PubMed] [Google Scholar]
- 36.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985) 1993 Nov;75(5):2310–7. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 37.Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: Practice against better evidence? Psychosom Med. 2006 Jul-Aug;68(4):617–27. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- 38.Ritz T, Bosquet Enlow M, Schulz S, Kitts R, Staudenmayer J, Wright RJ. Respiratory sinus arrhythmia as an index of vagal activity during stress in infants: Respiratory influences and their control. PLoS One. 2012;7(12):e52729. doi: 10.1371/journal.pone.0052729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saul JP, Berger RD, Chen MH, Cohen RJ. Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am J Physiol. 1989 Jan;256(1 Pt 2):H153–61. doi: 10.1152/ajpheart.1989.256.1.H153. [DOI] [PubMed] [Google Scholar]
- 40.Ritz T, Thons M, Dahme B. Modulation of respiratory sinus arrhythmia by respiration rate and volume: stability across posture and volume variations. Psychophysiology. 2001 Sep;38(5):858–62. [PubMed] [Google Scholar]
- 41.Contrada RJ, Krantz DS, Durel LA, Levy L, LaRiccia PJ, Anderson JR, et al. Effects of beta-adrenergic activity on T-wave amplitude. Psychophysiology. 1989 Jul;26(4):488–92. doi: 10.1111/j.1469-8986.1989.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 42.Rau H. Responses of the T-wave amplitude as a function of active and passive tasks and beta-adrenergic blockade. Psychophysiology. 1991 Mar;28(2):231–9. doi: 10.1111/j.1469-8986.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 43.Alkon A, Lippert S, Vujan N, Rodriquez ME, Boyce WT, Eskenazi B. The ontogeny of autonomic measures in 6- and 12-month-old infants. Dev Psychobiol. 2006 Apr;48(3):197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- 44.Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006 Jul;43(4):357–65. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 45.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990 Jan;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 46.Bunnell DE, Bevier WC, Horvath SM. Effects of exhaustive submaximal exercise on cardiovascular function during sleep. J Appl Physiol (1985) 1985 Jun;58(6):1909–13. doi: 10.1152/jappl.1985.58.6.1909. [DOI] [PubMed] [Google Scholar]
- 47.Heslegrave RJ, Furedy JJ. Sensitivities of HR and T-wave amplitude for detecting cognitive and anticipatory stress. Physiol Behav. 1979 Jan;22(1):17–23. doi: 10.1016/0031-9384(79)90397-4. [DOI] [PubMed] [Google Scholar]
- 48.Kline KP, Ginsburg GP, Johnston JR. T-wave amplitude: relationships to phasic RSA and heart period changes. Int J Psychophysiol. 1998 Aug;29(3):291–301. doi: 10.1016/s0167-8760(98)00021-x. [DOI] [PubMed] [Google Scholar]
- 49.Montoya P, Brody S, Beck K, Veit R, Rau H. Differential beta- and alpha-adrenergic activation during psychological stress. Eur J Appl Physiol Occup Physiol. 1997;75(3):256–62. doi: 10.1007/s004210050157. [DOI] [PubMed] [Google Scholar]
- 50.Scher H, Furedy JJ, Heslegrave RJ. Phasic T-wave amplitude and heart rate changes as indices of mental effort and task incentive. Psychophysiology. 1984 May;21(3):326–33. doi: 10.1111/j.1469-8986.1984.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 51.Laguna P, Jane R, Caminal P. Automatic detection of wave boundaries in multilead ECG signals: validation with the CSE database. Comput Biomed Res. 1994 Feb;27(1):45–60. doi: 10.1006/cbmr.1994.1006. [DOI] [PubMed] [Google Scholar]
- 52.Bazhenova OV, Stroganova TA, Doussard-Roosevelt JA, Posikera IA, Porges SW. Physiological responses of 5-month-old infants to smiling and blank faces. Int J Psychophysiol. 2007 Jan;63(1):64–76. doi: 10.1016/j.ijpsycho.2006.08.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 54.Gordon MK, Peloso E, Auker A, Dozier M. Effect of flavored beverage crystals on salivary cortisol enzyme-immunoreactive assay measurements. Developmental Psychobiology. 2005;47(2):189–95. doi: 10.1002/dev.20081. [DOI] [PubMed] [Google Scholar]
- 55.Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: error introduced by oral stimulants in salivary cortisol research. Dev Psychobiol. 2005 Dec;47(4):369–76. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- 56.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Human Development. 2008;84(4):249–56. doi: 10.1016/j.earlhumdev.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunnar M, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–63. [Google Scholar]
- 59.Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, et al. Gene-environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Dev. 2008 Sep-Oct;79(5):1377–94. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 60.Carroll D, Phillips AC, Balanos GM. Metabolically exaggerated cardiac reactions to acute psychological stress revisited. Psychophysiology. 2009 Mar;46(2):270–5. doi: 10.1111/j.1469-8986.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 61.Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30(5):486–95. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 62.Bosquet Enlow M, Egeland B, Carlson E, Blood E, Wright RJ. Mother-infant attachment and the intergenerational transmission of posttraumatic stress disorder. Dev Psychopathol. 2013;(1):1–26. doi: 10.1017/S0954579413000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis M, Ramsay D. Infant emotional and cortisol responses to goal blockage. Child Dev. 2005 Mar-Apr;76(2):518–30. doi: 10.1111/j.1467-8624.2005.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis M, Ramsay DS, Sullivan MW. The relation of ANS and HPA activation to infant anger and sadness response to goal blockage. Developmental Psychobiology. 2006;48(5):397–405. doi: 10.1002/dev.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]