Abstract
Major advances in understanding basic bone biology and the cellular and molecular mechanisms responsible for the development of osteoporosis, over the last 20 years, have dramatically altered the management of this disease. The purpose of this mini-review is to highlight the seminal role of Wnt signaling in bone homeostasis and disease and the emergence of novel osteoporosis therapies by targeting Wnt signaling with drugs.
Keywords: osteoblasts, osteoclasts, osteocytes, RANKL, OPG, bone therapies
Introduction
The mammalian skeleton regenerates throughout life by the removal (resorption) of old bone by osteoclasts and its replacement with new bone by osteoblasts, during a process called remodeling [1]. Osteocytes – former osteoblasts which are entombed within the mineralized matrix – sense the need for regeneration in a particular anatomical site and orchestrate the process by directing the homing of osteoclasts and osteoblasts to the site that is in need of remodeling, by producing and secreting key factors that control osteoclast and osteoblast generation [2, 3]. Under physiologic conditions, bone resorption and formation are balanced with the exact same amount of bone added in the site from which it was previously resorbed. With advancing age, the balance between resorption and formation is disturbed and bone mass declines. In addition bone progressively loses mechanical strength to an extent that is greater than the decline of bone mass because of the deterioration of its microarchitecture and the quality of its matrix and mineral (by mechanisms that are not well understood) and an increase in the number of dead or dysfunctional osteocytes as well as increased cortical porosity [4, 5]. Decreased bone mass and strength lead to the bone fragility syndrome known as osteoporosis.
The traditional thinking of osteoporosis as a disease of women starting at menopause is nowadays yielding ground to the recognition that osteoporosis is a multifactorial disease that affects both sexes. The disease process begins as early as the third decade of life and age-related mechanisms intrinsic to bone cells, including oxidative stress, decreased Wnt/β-catenin signaling, increased activation of FoxO transcription factors, oxidized lipids (acting via PPARγ and increasing bone marrow adiposity), declining osteocyte autophagy, and increased osteocyte senescence, play a primary role [6]. Age-dependent changes in other organs and tissues, such the decline of ovarian function at menopause and increased glucocorticoid production and/or responsiveness, contribute to the development of osteoporosis by accelerating the effects of aging.
Wnt signaling
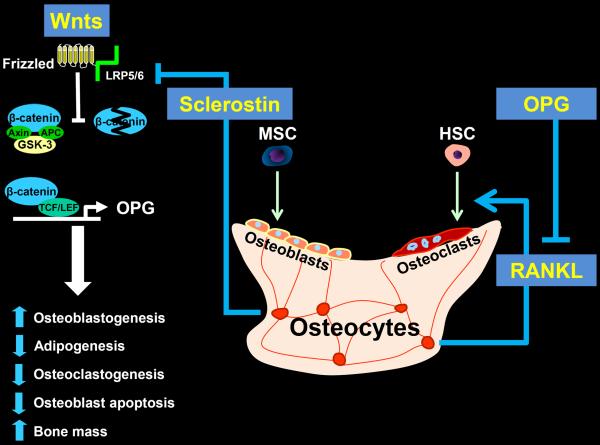
Wnts comprise a large family of secreted signaling glycoproteins that control cell proliferation, differentiation, apoptosis, survival, migration, and polarity in a plethora of cell types [7]. They play a critical role during embryonic development (including skeletal patterning) as well as in postnatal health and diseases, including cancer and degenerative disorders. To date, 19 different Wnt proteins have been found in humans and mice and some of them are specific to certain cells and tissues. Wnt proteins deliver their signal by binding to transmembrane receptor proteins. Like Wnt proteins, there are several Wnt receptors. The list includes 10 members of the Frizzled family and low density lipoprotein receptor-related protein (LRP) 5 as well as LRP6, Ror2, and Ryk. Different Wnts recognize different set of receptors and thereby selectively activate distinct intracellular pathways. Binding of Wnts to their cognate receptors activates at least three different intracellular signaling cascades: the Wnt/β-catenin pathway (also known as the canonical Wnt pathway), the non-canonical Wnt pathway, and the Wnt-calcium pathway. Activation of the Frizzled/LRP5 or Frizzle/LRP6 receptor complexes by Wnts, such as Wnt1 and Wnt3a, leads to the recruitment of Axin – a scaffold protein – to the Wnt/receptor complex on the cell membrane and causes the inactivation of glycogen synthase kinase 3β (GSK-3β) (Figure 1). Inactivation of GSK-3β, in turn, prevents the degradation of β-catenin by the proteasome. This step allows the stabilization of β-catenin in the cytoplasm and its subsequent entry into the nucleus, where it associates with the T-cell factor (TCF) lymphoid-enhancer binding factor (LEF) family of transcription factors and regulates the expression of Wnt target genes [8].
Canonical Wnt/β-catenin signaling, osteocyte-derived sclerostin and RANKL, and the generation of osteoblasts and osteoclasts.
Activation of the Frizzled/LRP5/6 receptor complex by Wnt proteins prevents the degradation of β-catenin, which then enters the nucleus and together with TCF/LEF stimulates the transcription of several genes that promote osteoblastogenesis, thereby increasing bone mass. In addition, β-catenin and TCF/LEF stimulate the transcription of the OPG gene, which is the natural antagonist of RANKL – the sine qua non factor for osteoclastogenesis – made primarily by osteocytes. Osteocyte-derived sclerostin blocks the binding of Wnts to their receptor complex. MSC = mesenchymal stem cell; HSC = hematopoietic stem cell.
Wnt/β-catenin signaling in bone health and disease
Bone-forming osteoblasts and bone-resorbing osteoclasts are terminally differentiated cells with short lives. Therefore, both need to be continuously replaced with new ones originating from stem cells of the mesenchymal and hematopoietic lineage, respectively [9, 10]. The supply of differentiated cells of either lineage is up- or down-regulated, in order to meet the demand. This is accomplished primarily through an increase or decrease of the replication of lineage-committed descendants of the respective stem cells [11, 12]. Aberrant osteoblast and/or osteoclast number underlines most acquired metabolic bone diseases, including osteoporosis, and it results from changes in their supply as well as their lifespan [1].
Starting about twelve years ago, genetic studies of four patient families – three with unusually high and one with low bone mass – revealed that activating or deactivating mutations of Wnt signaling were responsible for their high and low bone mass, respectively. In two of the families, the mutations were located on the LRP5 gene [13, 14]. In the other two, the mutations were located on the SOST gene, which encodes for sclerostin – an antagonist of the Wnt signaling that is made and secreted primarily by osteocytes [15, 16]. These discoveries established that lack of sclerostin expression in bone is the cause of Sclerosteosis and Van Buchem disease – two rare bone sclerosing dysplasias; whereas the loss of function mutation of the LRP5 gene is the cause of the osteoporosis-pseudoglioma syndrome. Following these genetic studies, an intensive research effort has revealed that Wnt signaling is indeed a key regulator of bone health and disease and can, therefore, be targeted to develop new therapies for osteoporosis. For an extensive discussion of the subject, the reader is referred to the excellent review by Baron and Kneissel [17].
Wnt/β-catenin signaling stimulates the generation of osteoblasts by promoting commitment and differentiation of pluripotential mesenchymal stem cells (MSCs) towards the osteoblast lineage, while simultaneously suppressing commitment to the chondrogenic and adipogenic lineage [12]. In particular, Wnt/β-catenin signaling promotes the progression of Osterix1 (Osx1)-expressing cells to bone producing osteoblasts. In addition, Wnts prevent the apoptosis of mature osteoblasts and thereby prolong their lifespan by both β-catenin-dependent and independent pathways [18].
In addition to its effects on osteoblasts, Wnt/β-catenin signaling decreases osteoclast differentiation by stimulating the production and secretion of osteoprotegerin (OPG) [19] – a natural antagonist of the receptor activator of nuclear factor-B ligand (RANKL) [20]. RANKL is indispensable for the differentiation, survival, and function of osteoclasts; thereby critical for bone resorption. RANKL is produced primarily by osteocytes [21]. During the process of osteoclast generation, bone marrow macrophages (BMMs) differentiate into tartrate-resistant acid phosphatase (TRAP)-positive pre-osteoclasts, which then fuse with each other to form mature osteoclasts. RANKL and macrophage colony–stimulating factor, provide the two necessary and sufficient signals for osteoclast differentiation [22]. In addition to their indirect effects on osteoclastogenesis that are mediated by controlling OPG expression and secretion by osteoblasts/osteocytes, Wnts act directly on osteoclasts. However, the biological significance of the direct effects is less clear. In any event, deletion of β-catenin in osteoclasts increases osteoclast number and bone resorption and decreases bone mass [23].
To date, several of the Wnt proteins have been shown to play a role in skeletal development and homeostasis as well as joint formation in humans and mice, including Wnt1, Wnt3a, Wnt4, Wnt5, Wnt5a, Wnt7a, Wnt10b, and Wnt14. Of those, Wnt10b seems to be the most critical positive modulator of bone formation in adult bone [24, 25]. In addition to Wnt proteins, mammals produce enhancers of Wnt/β-catenin signaling, such as the four R-sponding proteins. Recently, missense mutations in the Wnt1 gene were identified in a form of autosomal dominant early-onset osteoporosis and a severe form of osteogenesis imperfecta [26].
Wnt signaling, osteocytes, and the mechanical adaptation of the skeleton
Wnt signaling in bone is fine-tuned by several secreted glycoproteins that act as Wnt antagonists [27]. The most potent and best recognized of these are sclerostin, Wise, and the Dickkopf (DKK) proteins 1 and 2. Sclerostin binds to LRP5 and LRP6 and inhibits canonical Wnt signaling by blocking the binding of Wnt proteins to the extracellular regions of LRP5 and LRP6. Interference with the binding of Wnts to LRP6 seems to be functionally most significant in this respect. Sclerostin deficiency, on the other hand, unleashes Wnt signaling and dramatically increase bone mass in mice and humans.
The skeleton adapts to meet mechanical needs. This is best exemplified by the rapid and dramatic loss of bone that occurs with immobilization or weightlessness during space flights. The bone cells that are responsible for both sensing mechanical strains and orchestrating the adaptation of the skeleton to changing strains are the osteocytes [3]. Mechanical stimulation of bone reduces osteocyte expression of SOST-sclerostin [28]. Conversely, sclerostin expression increases during immobilization [29].
Wnt/β-catenin signaling, the FoxO transcription factors, and the pathogenesis of osteoporosis
The hallmarks of age-related osteoporosis are a decrease in bone formation and an increase in bone marrow adiposity [6]. Recent research findings from the mouse model suggest that attenuation of Wnt/β-catenin signaling may be responsible for these changes [30]. The decline of bone mass and increase of marrow adiposity with advancing age is associated with a progressive increase in oxidative stress (OS) [31]. In the last few years, members of the subclass of the forkhead family of transcription factors, called FoxOs, have emerged as an important defense mechanism against OS and growth factor deprivation – another accompaniment of old age. In the setting of OS or growth factor deprivation, FoxOs translocate from the cell cytoplasm to the nucleus where they stimulate the transcription of antioxidant enzymes as well as genes involved in cell cycle, DNA repair, and lifespan [32, 33]. Importantly, β-catenin is an essential co-activator of FoxOs, in addition to its role in TCF/LEF-transcription [34]. In the setting of OS or nutrient depletion, the limited pool of β-catenin in osteoblast progenitors is diverted from Wnt/TCF- to FoxO-mediated transcription [35]. Through this mechanism attenuation of canonical Wnt/β-catenin signaling decreases the progression of Osx1 expressing cells to bone producing osteoblasts, and thereby it decreases bone mass and simultaneously increases adipogenesis [30]. The diversion of β-catenin from TCF- to FoxO-mediated transcription may also contribute to several other age-related pathologies [34]. Thus, similar to several other defense responses against aging, FoxO activation eventually aggravates the effects of aging on bone and becomes a culprit of involutional osteoporosis [36].
Targeting Wnt signaling for the development of a novel bone anabolic therapy for osteoporosis
Heterozygous carriers of the SOST mutation have high normal or increased BMD without any of the abnormal traits of the full carriers of the mutation, indicating that decreasing the levels of the gene product sclerostin can modestly unleash Wnt signaling and increase bone formation without undesirable side effects [37]. Importantly, parathyroid hormone (PTH) decreases the production of sclerostin by osteocytes [38]. Intermittent administration of a recombinant form of parathyroid hormone (PTH) is currently the only approved therapy for the treatment of osteoporosis that can increase bone mass de novo. These and similar considerations have paved the way for an attempt by the pharmaceutical industry to develop a novel anabolic therapy for osteoporosis based on antibodies that neutralize sclerostin. Preclinical studies with the sclerostin neutralizing antibody have convincingly shown that sclerostin inhibition leads to increased bone formation, gain of bone mass, and increased bone strength in rodents and monkeys [39, 40]. Moreover, a multicenter, randomized, placebo-control study of post-menopausal women with low BMD have shown that romosozumab, a monoclonal sclerostin-neutralized antibody, increases BMD and bone formation and decreases bone resorption, within a year, to a greater extent (11.3%) than the bisphosphonate alendronate (4.1%) or PTH (teriparatide) (7.1%) [41]. Unexpectedly, nonetheless, the effect of romosozumab on bone turnover markers suggested a rapid, marked, but transient increase in bone formation and a moderate but more sustained decrease in bone resorption. The reason for the unpredictable changes in bone turnover markers is unclear. In any event, denosumab, a neutralizing antibody against RANKL – another osteocyte-derived protein – is an approved and very effective anti-resorptive therapy for osteoporosis [42].
Summary
Appreciation of the critical role of osteocytes in the orchestration of bone remodeling and the discoveries of the seminal role of RANKL and the Wnt/β-catenin signaling and its antagonist sclerostin, have provided a much improved understanding of the pathophysiology of osteoporosis. Furthermore, these developments have paved the way for the development of rational and effective new therapeutic strategies for the treatment of osteoporosis.
Research Agenda
The phase 3 clinical trial with romosozumab is on its way and should provide definitive answers on whether the effect of this therapy on BMD, shown in the phase 2 trial, will translate into anti-fracture efficacy; and whether, of course, romosozumab will be safe for long-term use. Future research aiming to elucidate age-related mechanisms affecting more than one tissue could lead to the development of even more advanced therapies that could simultaneously combat osteoporosis and other degenerative disorders, for example sarcopenia, resulting from shared mechanisms of aging.
Acknowledgements
The author thanks Leah Elrod for help with the preparation of the manuscript, and Maria Almeida for advice and reviewing the manuscript. The author's research is supported by the NIH (P01 AG13918 and R01 AR56679) and the Biomedical Laboratory Research and Development Service of the Veterans Administration Office of Research and Development (I01 BX001405).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest The author declares no competing interests.
Reference List
- [1].Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000 Apr;21(2):115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- [2].Manolagas SC, Parfitt AM. For whom the bell tolls: Distress signals from long-lived osteocytes and the pathogenesis of metabolic bone diseases. Bone. 2012 Sep 23; doi: 10.1016/j.bone.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011 Feb;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010 Mar 9;21(6):369–74. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010 May 15;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- [6].Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009 Mar;19(3):119–29. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [8].Clevers H, Nusse R. Wnt/beta-Catenin Signaling and Disease. Cell. 2012 Jun 8;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [9].Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012 Jan;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- [10].Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003 Aug;4(8):638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- [11].Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012 Mar 2;10(3):259–72. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006 Aug;133(16):3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- [13].Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001 Nov 16;107(4):513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- [14].Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- [15].Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001 Mar 1;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- [16].Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002 Feb;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013 Feb;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- [18].Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005 Dec 16;280(50):41342–51. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- [19].Glass DA, Bialek P, Ahn JD, et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005 May;8(5):751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [20].Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998 Apr 17;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- [21].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nature Medicine. 2011;17(10):1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003 May 15;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- [23].Wei W, Zeve D, Suh JM, et al. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol. 2011 Dec;31(23):4706–19. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007 Dec;22(12):1924–32. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- [25].Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010 Oct;25(10):2138–47. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013 May 9;368(19):1809–16. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013 Mar;5(3):a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008 Feb 29;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- [29].Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010 May;95(5):2248–53. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- [30].Iyer S, Ambrogini E, Bartell SM, et al. FoxOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123(8):3404–19. doi: 10.1172/JCI68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007 Sep 14;282(37):27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007 Jun;8(6):440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- [33].Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008 Apr;20(2):126–36. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007 Nov;21(11):2605–14. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- [35].Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007 Sep 14;282(37):27298–305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- [36].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Lierop AH, Hamdy NA, Hamersma H, et al. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011 Dec;26(12):2804–11. doi: 10.1002/jbmr.474. [DOI] [PubMed] [Google Scholar]
- [38].O'Brien CA, Plotkin LI, Galli C, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009 Apr;24(4):578–88. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- [40].Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010 May;25(5):948–59. doi: 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]
- [41].McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014 Jan 30;370(5):412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- [42].Cummings SR, San MJ, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009 Aug 20;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]