Abstract
Animals display an innate preference for novelty, spending more time exploring both novel objects and familiar objects in novel locations. This increase in exploration is thought to allow the animal to gather the information necessary to encode new experiences. Despite extensive evidence that increased exploration following spatial change requires the hippocampus, the patterns of hippocampal activity that support this behavior remain unknown. We examined activity in hippocampal output area CA1 and one synapse upstream in area CA3 while freely behaving rats performed an object-place recognition task. We found that the presence of novelty substantially altered activity in CA1, but not in CA3. During exploration of displaced familiar objects and novel objects in unexpected locations, CA1 place cells showed robust increases in firing rate. These firing rate increases persisted during sharp wave ripples, when place cell representations of previous experiences are replayed. Unexpectedly, increases in CA1 activity were not spatially restricted to regions of the environment that underwent change, indicating a generalized novelty signal. We suggest that hippocampal area CA1 broadcasts the presence of novelty, rather than signaling what is novel, and simultaneously becomes more plastic, allowing the integration of new information into previously stored memories.
Keywords: Place cell, sharp wave ripple, learning and memory, memory reactivation, CA3
Introduction
The systematic investigation of novel stimuli has led many to hypothesize that exploration is fundamentally a process of information gathering (O'Keefe and Nadel, 1978; Renner, 1990). Animals eventually habituate to a novel environment, but the introduction of a novel object or the displacement of a familiar object can reinstate exploration (O'Keefe and Nadel, 1978), as if re-inspecting the environment is necessary to update internal representations. The hippocampus is necessary for the behavioral expression of re-exploration following contextual novelty, which can be defined as alterations in the conjunction of what is present when and where. Lesions or inactivation of the hippocampus prevent re-exploration following displacement of a familiar object (what was present where) or the appearance of a familiar object in an unexpected context (what was present where or when) but do not diminish exploration of an entirely novel object (what) (Save et al., 1992; Mumby et al., 2002; Oliveira et al., 2010; Barker and Warburton, 2011). Furthermore, the unexpected presence or absence of objects can modify the activity of hippocampal place cells (O'Keefe, 1976; Lenck-Santini et al., 2005; Manns and Eichenbaum, 2009; Deshmukh and Knierim, 2013). These observations have led to the widespread use of object-place recognition paradigms to elucidate the molecular mechanisms of hippocampal memory formation (for a review see: Dere et al., 2007) and to investigate hippocampal dysfunction during natural aging (Wimmer et al., 2012; Gerstein et al., 2013) or neurological diseases (Francis et al., 2012; Kleschevnikov et al., 2012; Zhang et al., 2012). Despite the widespread application of these paradigms, it remains unknown how activity within the hippocampus supports the detection and encoding of novel spatial configurations and whether hippocampal responses to novel object-place configurations drive spatially focused re-exploration.
We recorded in hippocampal output area CA1, which is thought to be essential for novelty detection (Blum and Abbott, 1996; Lisman and Otmakhova, 2001), and upstream area CA3, which is necessary for one-trial learning (Nakazawa et al., 2003; Nakashiba et al., 2008), while rats performed an object-place recognition task. We investigated the properties of CA1 and CA3 place cells and subsequent reactivation of these representations during sharp wave ripples (SWRs). We found robust novelty-dependent alterations of CA1 place cell and SWR activity that were not present one synapse upstream in CA3. Furthermore, we investigated whether CA1 novelty signals were spatially selective, as one would expect if this activity were to drive spatially focused re-exploration. Surprisingly, we found no evidence for spatial selectivity in the CA1 novelty response. Thus it is unlikely that altered place cell or SWR activity within the hippocampus drives selective re-exploration of new stimuli. Rather, we propose that the hippocampus broadcasts a generalized novelty signal to downstream regions. Furthermore, we propose that the heightened state of excitability we observe during novel experiences reflects a renewed period of hippocampal plasticity where novel information is incorporated into previously formed representations.
Materials & Methods
Seven male Long Evans rats (450–550g) were implanted with micro-drive arrays following University of California, San Francisco and National Institutes of Health guidelines (Frank et al., 2004). To allow for simultaneous recording of CA3 and CA1, 14–25 independently movable tetrodes were arranged unilaterally (n = 5) or bilaterally (n = 2; −3.60mm AP, ±3.20mm ML from bregma) with medial tetrodes targeting CA1 and lateral tetrodes targeting CA3. Over 14–18 days tetrodes were lowered to CA1 and CA3, which were identified by depth and characteristic EEG waveforms. Following data collection, electrode locations were identified histologically. Neural signals were recorded relative to an electrode in the corpus callosum and a ground screw located above the cerebellum. Two animals were initially part of another study and received injections of lentivirus (ArchT-CaMKII-EYFP, n = 1; ChR2-CaMKII-EYFP, n = 1) in the hippocampus during implantation of the arrays and had an optical fiber implanted dorsally to the hippocampus. No evoked responses were observed with optical stimulation, so animals were removed from that study and were instead exposed to the object-place recognition task. All results remained significant if these two animals were excluded from analyses, demonstrating that despite their different life experiences, no conclusions are altered by the inclusion of these animals.
Data were collected using the NSpike data acquisition system (L.M. Frank; J. MacArthur, Harvard University, Cambridge, MA). An infrared diode array was attached to the micro-drive to enable off-line behavioral tracking. Local field potentials were sampled at 1.5Khz and digitally filtered (0.5–400Hz). Spike data were sampled at 30kHz, digitally filtered (600Hz–6Khz), and threshold-crossing events were saved to disk. Tetrodes with poor unit recordings were adjusted after daily recording sessions. Peak amplitude and spike width were used to cluster individual units using custom software (MatClust, M. Karlsson). Only units with a clear refractory period were included. It was generally possible to use a single set of cluster bounds to isolate units across the entire recording session. In the minority of cases in which there was a slight shift in amplitudes across time, putative single neurons were clustered only when that shift was coherent across multiple clusters and where plots of amplitude versus time showed a smooth shift.
Behavioral paradigm and analysis
After fully recovering from surgery, rats were habituated to an empty open field (1 × 1m with 50cm walls) for at least 5 days, up to one hour per day. A white cue card (8.5” × 11”) on one of the black walls served as a local cue. Items in the recording room (ladder, windows, cabinets, etc.) served as distal cues. Local and distal cues remained constant across the experiment. Each behavioral run consisted of three phases—Familiarization (F), Novel Location (NL), and Novel Object & Location (NO&L)—with rest sessions before and after each session. The paradigm was run multiple times for each animal. We observed no change in the tendency of animals to exhibit re-exploration over multiple runs (see Results). During F, rats had 10 minutes to explore two identical novel objects located in diagonally opposed quadrants of the arena. Distinct sets of objects were used for each experimental run and placed in the same initial locations. Following the F session, animals were removed from the arena for a five-minute rest session. Before the NL session, objects were removed and replaced with an identical pair so that no markings could be used to distinguish them. One object remained in the same, now familiar location, while the other was placed in a previously unoccupied, novel location (NL). The choice of which object remained in the familiar location and which moved was chosen pseudo-randomly such that there was no way to predict a priori which object would move to the novel location. Animals had five minutes to explore during the NL session before another five-minute rest. For the final five-minute novel location and object (NL&O) session, the object located in the novel location was removed and a distinct novel object was placed in the previously empty quadrant. A copy of the now familiar object remained in the same location, as during F and NL. One animal was allowed only a five-minute F session and ten-minute novel sessions. All trends were the same in this animal as others. In this case, we restricted our analysis to the first five minutes of the novel sessions.
We assessed novelty preference by measuring the relative time animals spent within 10cm of the center of the objects in novel and familiar locations, although similar results were seen with other distance criteria (5, 15, 20, and 25cm). Novelty preference was defined as the difference in the time spent near the object in the novel location and time spent near the object in the familiar location divided by the sum of time spent near both objects. A preference score of one indicates that animals only visited the object in the novel location, a score of negative one indicates animals only visited the object in the familiar location, and a score of zero denotes equal time spent near each object. We excluded times when the animal was still (<1cm/s) for more than 10s from the analysis. During F sessions, novelty preference was measured between the object that would be displaced and the object that would remain stationary throughout the rest of the experiment.
Analysis of neural data
All place field analyses excluded times when animals were immobile (<1 cm/s) for more than 10s and were restricted to neurons that fired ≥100 spikes and had a peak spatial rate ≥3Hz. To compute firing rate maps, we divided the open field into 5 × 5cm spatial bins and divided the number of spikes in each bin by the amount of time spent there. To minimize behavioral sampling issues due to the large arena and short behavioral epochs, we used large spatial bins, restricted our analyses to spatial bins with an occupancy ≥1s, and restricted all comparisons of spatial firing between sessions to locations that were visited in both sessions. To determine the stability of place cells we computed the Pearson correlation between firing rate maps calculated from different behavioral sessions. To assess within session stability we computed the Pearson correlation between firing rate maps calculated from the first and second 5 minutes of the F session.
The mean rate for each cell was calculated as the total number of spikes divided by the total amount of time. We also calculated the residual firing rate for each neuron to account for differences in behavioral sampling across sessions and thus differences in place cell firing rate due to spatial tuning. The residual firing rate is the difference between the observed and expected firing rate (Singer et al., 2010). We calculated the expected firing rate by combining spiking and position data from all behavioral sessions for each neuron to compute a global firing rate map. From this global firing rate map and the animal's current position, we computed the expected firing rate in 1s time-bins. When measuring the average residual firing rate across a session, we omitted any time-bin where both the observed and expected firing rate were zero.
We also examined activity during SWRs, which were defined as times when the power of the filtered (150–250Hz) local field potential signal on any CA1 tetrode exceeded three standard deviations above the mean (Cheng and Frank, 2008; Carr et al., 2012). We calculated the rate of SWR occurrence by taking the total number of SWRs observed when rats were moving <4 cm/sec for behavioral epochs and dividing by the amount of time spent at those speeds. We restricted our analyses to experimental runs with at least one CA1 tetrode with multiple putative excitatory neurons to ensure SWRs were detected in stratum pyramidale. We calculated the activation probability per SWR for single CA1 and CA3 place cells and the co-activity z-score for place cell pairs as has been described previously (Cheng and Frank, 2008). For comparisons between pairs of behavioral sessions we used a more liberal criterion, including place cells that fired at least 100 spikes in one or both behavioral sessions. All analyses were performed using custom Matlab scripts (Mathworks).
Results
We recorded from hippocampal areas CA1 and CA3 simultaneously as rats performed an object-place recognition task that consisted of three phases (Figure 1A). During the first phase (Familiarization: F), rats explored a familiar open arena with two identical novel objects. We found no preference for one object over the other indicating no initial bias (Figure 1B; student's t-test, p>0.5; n=41 sessions). In the second phase (Novel Location: NL), one of the now familiar objects was displaced to a previously unoccupied location. Rats exhibited a robust preference for the displaced object, initially spending more time investigating the familiar object in the novel location before exhibiting habituation. Novelty preference was evident during the first two minutes of the NL session and was no longer detectable after three minutes (t-test; 1st minute p<1×10−5, 2nd p<0.01; 3rd–5th p>0.5, n = 41, Holm-Bonferonni correction for multiple comparisons). Rats showed minimal renewed exploration of the newly empty location, spending more time investigating both objects (mean time (s); displaced object = 32.6, familiar object = 23.3, newly empty = 14.7, familiar empty = 8.1). Minimal exploration of the newly empty location may be due to the fact that animals were habituated to an empty open field.
Figure 1. Behavior.
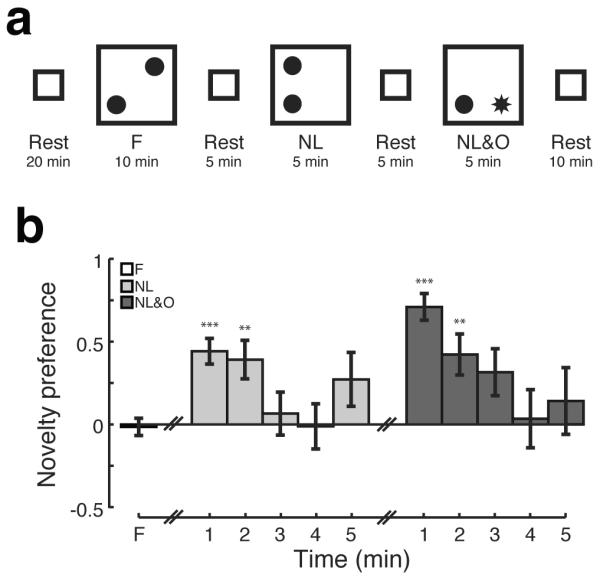
A) The object-place recognition task consisted of three phases with rest periods interleaved. During the first phase (Familiarization: F), rats explored a familiar open field with two identical novel objects (filled circles). In the second phase (Novel Location: NL), one of the now familiar objects was displaced to a previously unoccupied location. In the final phase (Novel Location & Object: NL&O), the familiar object remained in place and a distinct novel object (star) was placed in a previously empty location. B) Novelty preference as a function of time. Bars show mean, error bars show SEM; **p<0.01; ***p<1×10−5.
In the final phase (Novel Location & Object: NL&O), the familiar object remained in place and a distinct novel object was placed in a previously empty location. Animals exhibited a strong preference for the novel object that persisted for the first two minutes of the NL&O session (t-test; 1st minute p<1×10−5; 2nd p<0.01; 3rd p>0.1; 4th–5th p>0.5; n = 37; Holm-Bonferonni correction for multiple comparisons). As during the NL session, rats did not spend much time investigating the newly empty location, rather devoting their attention to the novel object (mean time (s); novel object = 42.6, familiar object = 14.4, newly empty = 7.5, familiar empty = 5.5). The object-place recognition task was run multiple times for each rat, raising the possibility that over repeated runs of the experiment there would be a change in the tendency of animals to exhibit re-exploration. To address this possibility, we compared the novelty preference during the first two minutes of NL and NL&O sessions between the first and last experimental run for each animal. We found no sign of habituation over repeated runs of the object-place recognition task (Wilcoxon signed-rank test; first vs. last experimental run; NL p>0.5, n = 7 animals, NL&O p>0.5, n = 6).
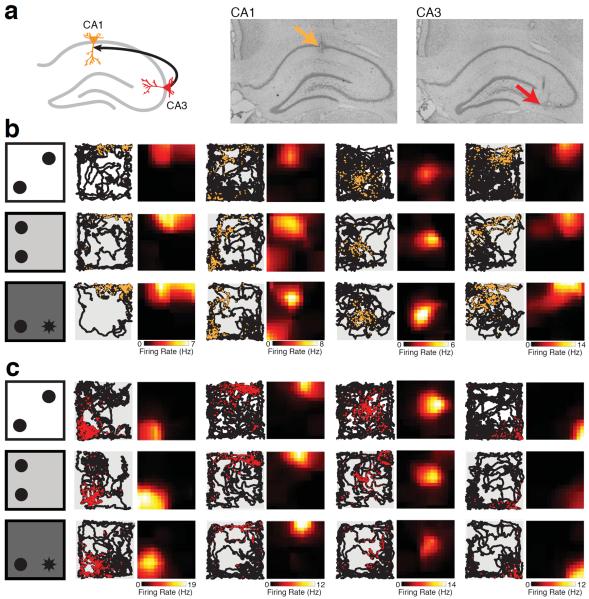
We recorded a total of 254 well-isolated neurons (average isolation distance; CA1 = 37.31; CA3 = 43.75; no significant difference between CA1 and CA3; student's t-test, p>0.15) with place fields in at least one behavioral session from CA1 (n = 192 place cells) and upstream area CA3 (n = 62; Figure 2A). Neurons in both CA1 (Figure 2B) and CA3 (Figure 2C) had place fields throughout the open field that showed relatively stable spatial tuning across behavioral sessions. We noticed that in many cases the overall firing rate appeared to increase from F to NL and NL&O sessions for CA1 neurons (Figure 2B). Although some CA3 neurons showed an increase in rate over time (Figure 2C, second neuron from left), others showed decreases in firing rate over time (Figure 2C, third neuron from left) suggesting that there was no consistent rate changes in CA3 place field activity. While the detection of spatial change during the NL session is thought to depend on the hippocampus, the detection of a novel object in an unexpected location likely relies on distributed brain regions such as the parietal and perirhinal cortices in addition to the hippocampal circuit (Save et al., 1992; Mumby et al., 2002; Barker and Warburton, 2011). Since the response to novelty in the hippocampus may be different between these two types of novel exploration, we quantified novelty signals for CA1 and CA3 place cells relative to the F baseline in the NL and NL&O sessions separately.
Figure 2. Place fields recorded in CA1 and CA3.
A) Schematic (left) and representative histological sections (right) of the hippocampus showing CA3 (red) and CA1 (orange) recording locations. B) Schematic of behavioral paradigm (left) and representative place cells from four CA1 neurons are shown for the F (top), NL (middle), and NL&O (bottom) sessions with the location of detected spikes (orange) superimposed on the animal's trajectory (black). The occupancy normalized place field is shown to the right of the raw data for each session. The color bar below each column indicates the peak spatial firing rate for each neuron. C) Representative place cells from four CA3 neurons during F, NL, and NL&O sessions as in B).
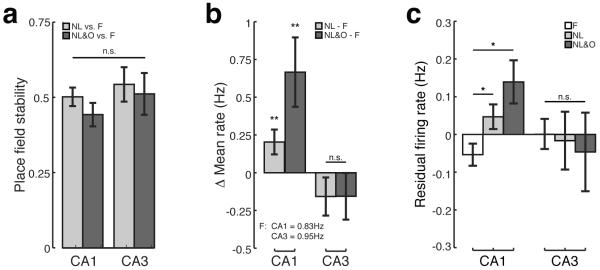
To quantify the observation that both CA1 and CA3 place fields appeared stable across behavioral sessions we computed the correlation between place fields measured during the familiarization session with those measured during novel sessions (see Methods). There was no difference in the stability of CA1 and CA3 place fields during either the NL or NL&O sessions as compared to the F session (Figure 3A; two-way ANOVA; no effect of region: CA1 vs. CA3, F(1,234) = 1.28, p>0.25; no effect of session: NL relative to F vs. NL&O relative to F, F(1,234) = 0.87, p>0.35). Thus neither the displacement of a familiar object nor the introduction of a novel object in a novel location led to instability in the CA1 or CA3 place cell map.
Figure 3. Novelty is associated with increases in CA1, but not CA3, place cell firing rate.
A) Average Pearson's correlation between place fields recorded during F session and either NL (light grey) or NL&O (dark grey) sessions for both CA1 and CA3. B) Within cell changes in mean firing rate between the NL and F sessions (light grey) or between the NL&O and F sessions (dark grey) for both CA1 and CA3. C) The average residual firing rate during F (white), NL (light grey), and NL&O (dark grey) sessions for CA1 and CA3. Bars show mean, error bars show SEM; *p<0.05, **p<0.01.
Exploration of novel environments has been shown to lead to increased firing rates in CA1, but not CA3 neurons (Karlsson and Frank, 2008). Firing rate increases in CA1 during new learning has been proposed to signal the presence of novelty to downstream regions. To quantify whether similar increases in firing rates occurred during the object-place recognition task we took two approaches. First, we measured the difference in mean firing rate for CA1 and CA3 place cells in NL and NL&O versus the F session. We found a significant increase in mean firing rate of CA1 place cells during both novel sessions as compared to F (Figure 3B; Wilcoxon signed-rank test; NL vs. F, p<0.01, n = 104 neurons; NL&O vs. F, p<0.01, n = 75). In contrast, there was no effect of novelty on firing rate one synapse upstream in CA3 (Figure 3B; Wilcoxon signed-rank test; NL vs. F, p>0.2, n = 30 neurons; NL&O vs. F, p>0.2, n = 32).
We also observed evidence for increases in rates when measuring changes in the residual firing rate across sessions (see Methods). As the residual firing rate only considers deviations from the average firing rate for locations visited during both sessions, this method controls for tuning properties of place cells and differences in behavioral sampling across sessions. As we observed using the first approach to measure firing rate changes, we found that CA1 place cells have increased residual firing rates in both novel sessions relative to F (Figure 3C; one-way ANOVA: main effect of session: F vs. NL vs. NL&O, F(2,344) = 10.89, p<0.01; post-hoc comparisons; residual firing rate NL>F, p<0.05; NL&O>F, p<0.05). In contrast, there was no indication that novelty had an effect on the firing rate of place cells in CA3 (Figure 3C; one-way ANOVA; F(2,112) = 0.16, p>0.5). Increases in CA1 firing rate, as measured by both mean firing rate (Figure 3B) and residual firing rate (Figure 3C), were robust across multiple runs of the behavioral experiment: we found no difference in the firing rate increases observed during the first and last experimental run (Mann–Whitney U test; change in mean firing rate first exposure vs. last exposure; NL: p>0.5, n = 16 CA1 neurons in first session, n = 24 CA1 neurons in last session; NL&O: p>0.5, n = 14 neurons in first session, 16 neurons in last session; residual firing rate first exposure vs. last exposure; NL: p>0.5, n = 17 CA1 neurons in first session, n = 29 CA1 neurons in last session; NL&O: p>0.5, n = 16 neurons in first session, n = 17 neurons in last session).
We next asked whether the increase in CA1 firing rate we observed from the familiarization session to the NL and NL&O sessions reflects the detection of change from the rat's most recent experience or rather, is due to a gradual increase in firing rate over time unrelated to the task at hand. Three lines of evidence suggest that increases in CA1 firing rate reflect a detection of change rather than a generalized increase in rate over time. First, we directly tested whether there was an increase in rate over time by looking at within session changes in firing rate. There was no increase in the mean firing rate of CA1 neurons between the first and second five minutes of the F session (Mann–Whitney U test; change in CA1 mean firing rate first half F – second half F, p>0.4, n = 138 CA1 neurons; mean change in firing rate = 0.004 Hz). Furthermore, when taking into account differences in behavioral sampling by comparing the residual firing rate between the first and second five minutes of the F session we found a significant decrease in firing rate (Mann–Whitney U test; change in CA1 residual firing rate first half F – second half F, p<0.05, n = 140 neurons; mean change in residual firing rate = 0.04Hz). This demonstrates that CA1 firing rates are not simply increasing over time and further suggests that novelty leads to an increase in firing rate at the beginning of the F session that decays over a period of minutes.
Second, in three cases two experimental runs occurred on the same day allowing us to compare the mean firing rate between two F sessions that occurred within a few hours of one another. If the firing rate of CA1 neurons simply increase over time then we would expect that the second F session of the day would have a higher mean firing rate than the first. In contrast, if the mean firing rate of CA1 neurons reflects a detection of change then, as both F sessions are equally novel, we would expect both sessions to have similar firing rates. We observed no difference in the mean rate between the first and second F session (Wilcoxon signed-rank test; mean rate first F session vs. second F session, p>0.5, n = 11 CA1 neurons first F session, n = 12 neurons second F session; mean rate first F session = 1.11Hz; second F session = 1.05Hz), further supporting the hypothesis that increases in CA1 firing rate reflect the detection of change rather and a generalized increase over time.
Third, previous reports of novelty related firing rate increases in CA1 have shown that firing rates are initially high in a novel environment and then decrease over a period of time similar in duration to the present experiment (Karlsson and Frank, 2008). Furthermore, upon repeated exposure to familiar environments neither CA1 nor CA3 show changes in mean firing rate over a period of two days of recording (Mankin et al., 2012). These previous reports demonstrate that CA1 neurons do not always show a general increase in firing rate over time and suggest that the increases in CA1 firing rate we observe during the object-place recognition task is more likely to reflect novelty detection. Based on these three lines of evidence, we conclude that the observed increase in CA1 firing rate between F and NL and between F and NL&O most likely reflects the detection of change from the rat's most recent experience. Thus CA1, but not CA3, shows a robust increase in firing rate during exploration of displaced familiar objects and novel objects in unexpected locations; consistent with the idea that CA1 signals the presence of novelty to downstream structures.
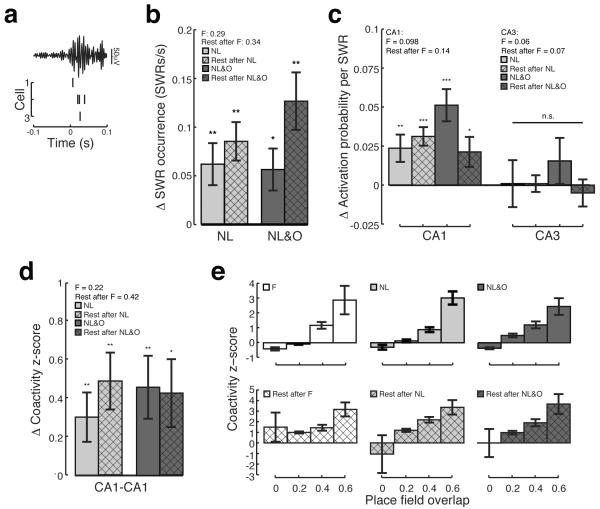
To investigate whether exploration of displaced familiar objects and novel objects in unexpected locations alters SWR activity (Figure 4A), we first measured the rate of SWR occurrence during behavioral sessions and subsequent rest sessions. We observed a large increase in the rate of SWR occurrence in both NL and NL&O sessions relative to F (Figure 4B; Wilcoxon signed-rank test; NL vs. F, p<0.01, n = 34 sessions; NL&O vs. F, p<0.05, n = 29 sessions). This increase in SWR occurrence persisted into subsequent rest sessions (Figure 4B; Wilcoxon signed-rank test; rest after NL vs. rest after F, p<0.001, n = 34 sessions; rest after NL&O vs. rest after F, p<0.001, n = 30 sessions).
Figure 4. Sharp wave ripple activity increases during novelty and is consistent with memory replay.
A) An example SWR recorded during an NL session showing a filtered (150–250Hz) local field potential and spikes from three CA1 neurons that were active during this SWR. B) Rate of SWR occurrence during NL (light grey) and NL&O (dark grey) relative to the F session and rate of SWR occurrence during rest after NL (light grey hatched) and rest after NL&O (dark grey hatched) relative to rest after F. The average rate of SWR occurrence during F and the rest after F are shown in the key. C) Activation probability of CA1 and CA3 neurons during NL (light grey) and NL&O (dark grey) relative to F and activation probability during rest after NL (light grey hatched) and rest after NL&O (dark grey hatched) relative to rest after F. The average activation probability during F and rest after F are shown in the key. D) Co-activity z-score of CA1 place cell pairs during NL (light grey) and NL&O (dark grey) relative to F and during rest after NL (light grey hatched) and rest after NL&O (dark grey hatched) relative to the rest after F. The average co-activity z-score measured during F and rest after F are shown in the key. E) Co-activity z-score of CA1 place cell pairs shown as a function of two-dimensional place field overlap. Bars show mean, error bars show SEM; *p<0.05; **p<0.01; ***p<1×10−5.
To determine whether CA1 and CA3 place cells were more active during SWRs associated with novelty we measured the activation probability during all behavioral sessions and in the subsequent rest sessions. We found that CA1 place cells were significantly more likely to fire in an SWR during NL and NL&O relative to F (Figure 4C; Wilcoxon signed-rank test; NL vs. F, p<0.001, n = 142 neurons; NL&O vs. F, p<1×10−5, n = 112 neurons). This increase persisted into subsequent rest sessions (Figure 4C; Wilcoxon signed-rank test; rest after NL vs. rest after F, p<1×10−5, n = 140 neurons; rest after NL&O vs. rest after F, p<0.05, n = 116 neurons). In contrast, novelty had no detectable effect on CA3 place cell firing during SWRs (Figure 4C; Wilcoxon signed-rank test; NL vs. F, p>0.4, n=37; NL&O vs. F, p>0.08, n = 37 neurons; rest after NL vs. rest after F, p>0.4, n = 50 neurons; rest after NL&O vs. rest after F, p>0.4, n = 45 neurons).
We next investigated whether pairs of CA1 place cells were more likely to show coordinated SWR activity during novel sessions as compared to the F session. To exhibit coordinated SWR activity, place cell pairs must be co-active during SWRs above and beyond what would be expected given the activation probability of each neuron. CA1 place cell pairs showed increased coordinated SWR activity during both NL and NL&O sessions relative to F and this increase persisted into subsequent rest sessions (Figure 4D; Wilcoxon sign-rank test; NL vs. F, p<0.01, n = 238 CA1-CA1 neuron pairs; NL&O vs. F, p<0.01, n = 202 CA1-CA1 neuron pairs; rest after NL vs. rest after F, p<0.01, n = 136 CA1-CA1 neuron pairs; rest after NL&O vs. rest after F, p<0.05, n = 160 CA1-CA1 neuron pairs). Thus exploration of both displaced familiar objects and novel objects in unexpected locations is sufficient to drive enhanced coordination of CA1 neurons during SWRs.
The increase in coordinated SWR activity observed in CA1 was consistent with the ordered replay of place cell sequences. Non-overlapping cells were relatively unlikely to fire together during SWRs. Furthermore, increases in place field overlap were associated with increases in the amount of coordinated SWR activity during both active exploration and rest periods (Figure 4E; Spearman correlation place field overlap vs. co-activity z-score; F, r = 0.29, n = 351 CA1-CA1 neuron pairs; NL, r = 0.36, n = 267 CA1-CA1 neuron pairs; NL&O, r = 0.28, n = 224 CA1-CA1 neuron pairs; rest after F, r = 0.23, n = 319 CA1-CA1 neuron pairs; rest after NL, r = 0.38, n = 201 CA1-CA1 neuron pairs; rest after NL&O, r=0.35, n = 170 CA1-CA1 neuron pairs; all p's<1×10−5). Taken together, these findings are consistent with the idea that novelty leads to increased memory replay during SWRs, which may initiate consolidation of the newly formed hippocampal representation (Buzsáki, 1989).
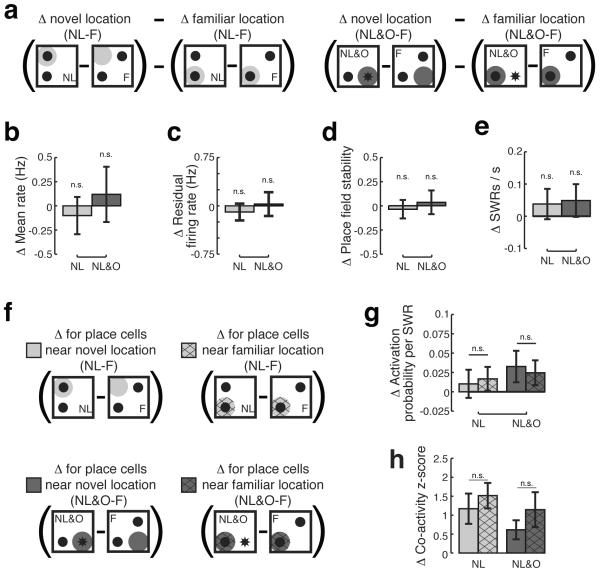
Finally, we asked whether the novelty signals we observed in hippocampal area CA1 were localized in space or were generalized across the environment. We initially hypothesized that novelty signals would be more pronounced near novel as compared to familiar locations (Figure 5A, 5F). To our surprise, we found no evidence for a spatially localized novelty signal.
Figure 5. There is no evidence for a spatially localized novelty signal in CA1.
A) Schematic demonstrating the comparisons for 5B – 5E. For each measure, changes in the familiar location between NL and F (light grey) or NL&O and F (dark grey) were subtracted from changes observed in the novel location. Positive values indicate increased change near the novel location, negative values indicate increased change near the familiar location, and values near zero indicate no difference. Change in B) mean firing rate, C) residual firing rate, D) Pearson's correlation in novel as compared to familiar locations for CA1 place cells in NL (light grey) and NL&O (dark grey) sessions. E) rate of SWR occurrence in novel as compared to familiar locations in NL (light grey) and NL&O (dark grey) sessions. F) Schematic demonstrating the comparisons for 5G and 5H. For each measure, changes in activity of place fields with a peak spatial rate within 25cm of the center of the displaced familiar object during NL (light grey) or from the center of the novel object during NL&O (dark grey) were compared to changes in the activity of place fields with a peak spatial rate within 25cm of the center of the familiar object during NL (light grey hatched) or NL&O (dark grey hatched). G) Activation probability of CA1 place cells during SWRs in novel and familiar locations. F) Co-activity z-score of CA1 place cell pairs during SWRs. Bars show mean, error bars show SEM.
Specifically, we found no evidence for location-specific increases in CA1 firing rate (Figure 5B; Wilcoxon sign-rank test; change in mean rate near novel locations – change in mean rate near familiar locations; NL vs. F, p>0.5, n = 101 neurons; NL&O vs. F, p>0.5, n = 75 neurons)—even when controlling for differences in behavioral sampling (Figure 5C; Wilcoxon sign-rank test; change in residual firing rate near novel locations – change in residual firing rate near familiar locations; NL vs. F, p>0.45, n = 113 neurons; NL&O vs. F, p>0.5, n = 89 neurons). In addition, there was no difference in the stability of CA1 place fields near novel as compared to familiar locations (Figure 5D; Wilcoxon sign-rank test; stability near novel locations – stability near familiar locations; NL vs. F, p>0.5, n = 24 neurons; NL&O vs. F, p>0.5, n = 14 neurons), demonstrating that displacing an object did not result in localized instability. As for these measures of place cell activity, we found no differences in the rate of SWR occurrence between novel and familiar locations (Figure 5E; Wilcoxon sign-rank test; change in rate of SWR occurrence near novel locations – change in rate of SWR occurrence near familiar locations; NL vs. F, p>0.37, n = 34 sessions; NL&O–F, p>0.3, n = 29 sessions). Furthermore, neurons with place fields near novel and familiar locations (Figure 5F) were equally likely to be reactivated during SWRs (Figure 5G; Mann–Whitney U test; NL, p>0.5, n = 29 place cells with spatial peaks near familiar location, n = 44 place cells with spatial peaks near novel location; NL&O, p>0.5, n = 30 place cells with spatial peaks near familiar location, n = 28 place cells with spatial peaks near novel location) and equally likely to show coordinated reactivation during SWRs (Figure 5H; Mann–Whitney U test; NL, p>0.5, n = 16 CA1-CA1 neurons pairs both with spatial peaks near familiar location, n = 22 CA1-CA1 neuron pairs both with spatial peaks near novel location; NL&O, p>0.5, n = 13 CA1-CA1 neuron pairs both with spatial peaks near familiar location, n = 26 CA1-CA1 neuron pairs both with spatial peaks near novel location). Thus we find no evidence for a spatially localized novelty signal in CA1 during exploration of either displaced familiar objects or novel objects in unexpected locations. Rather, we find that there is a robust increase in CA1 firing rate and SWR activity during novelty that is generalized across the environment.
Discussion
We have shown that novelty-induced re-exploration of objects is associated with robust novelty signals in hippocampal area CA1, but not one synapse upstream in CA3. During re-exploration of displaced familiar objects and novel objects in unexpected locations we find robust increases in the firing rate of CA1, but not CA3, place cells, suggesting that novelty-dependent increases in firing rate originate within CA1 (Karlsson and Frank, 2008; VanElzakker et al., 2008). The increased CA1 excitability we observe is consistent with previous studies in novel environments showing that CA1 pyramidal cells increase, whereas CA1 interneurons decrease, their overall firing rate (Wilson and McNaughton, 1993; Nitz and McNaughton, 2004; Csicsvari et al., 2007; Karlsson and Frank, 2008; VanElzakker et al., 2008). In addition to novelty-dependent increases in place cell firing rate, we found that re-exploration following object displacement was associated with increases in the incidence of SWRs, the firing rate of CA1 neurons during SWRs, and the coordinated reinstatement of previous experiences. These results are consistent with previous reports that exploration of novel environments and novel reward contingencies are associated with prolonged increases in SWR activity that can persist long after the novel experience (Cheng and Frank, 2008; Eschenko et al., 2008; Karlsson and Frank, 2008; O'Neill et al., 2008; Ramadan et al., 2009; Singer and Frank, 2009; Dupret et al., 2010). Just as we observed for increases in place cell firing rate, novelty led to an increase in CA1, but not CA3, activation probability during SWRs. SWRs are thought to originate with population bursts in CA3 (Ylinen et al., 1995; Nakashiba et al., 2009), suggesting that the effect of novelty is to increase the ability of CA3 to drive CA1 activity (Kemere et al., 2013).
Importantly, we find similar increases in CA1 activity from F to both the NL and NL&O sessions even though detection of a novel object in an unexpected location could rely on circuits known to support the detection of novel objects such as the parietal or perirhinal cortices instead of the hippocampal circuit (Save et al., 1992; Mumby et al., 2002; Barker and Warburton, 2011). Furthermore, we find similar responses to novelty during the object-place recognition task as in previous studies of novel environments and novel tasks even though what must be identified and encoded in these different conditions varies substantially. Although the process of familiarization to a novel environment may persist over many days (Karlsson and Frank, 2008), we have shown that following even brief periods of familiarization, displacement of objects and the appearance of a novel object in an unexpected location are associated with novelty responses in CA1 place cells above and beyond any changes that may be occurring during the F session. These results suggest that any deviation from expectation, whether developed over minutes or weeks, results in similar novelty signals in CA1.
Surprisingly, we found no evidence for spatial selectivity in the CA1 novelty response. Our results provide an important contrast to previous findings showing spatially localized increases in SWR activity in a novel arm of a radial maze (Cheng and Frank, 2008) and spatially localized remapping in a multi-compartment foraging task (Spiers et al., 2013). We hypothesize that novelty leads to a renewed period of plasticity within the hippocampus where new information is incorporated into previously stabilized representations. Furthermore, only memories that must be updated with new information would need to undergo this period of increased excitability. It may be that in the present experiment, knowledge about the location of the displaced object must be incorporated into the representation of the arena as a whole. In contrast, in pervious studies that showed localized hippocampal novelty signals, novel and familiar regions were compartmentalized in space; suggesting that when novelty is contained within a region, the hippocampal response to novelty is also localized in space. In support of this hypothesis, Nitz and McNaughton (2004) describe how novelty-related alterations in firing rate permeate into familiar regions of an environment that border novel regions. Furthermore, Dupret and colleagues (2010) demonstrate that CA1 re-organizes to represent novel goal locations only when spatial learning is required. In addition, Dupret and colleagues (2010) show that subsequent memory performance is associated with the degree of remapping during learning, suggesting that the extent to which novelty can drive renewed periods of plasticity is related to the successful incorporation of new information into memory.
The generalized novelty signals we observe during spatially focused re-exploration suggest that while hippocampal activity may be necessary for learning and remembering spatial configurations (Save et al., 1992; Mumby et al., 2002; Oliveira et al., 2010), novelty signals within the hippocampus are unlikely to drive the behavioral expression of novelty induced re-exploration. Rather than signaling what should be explored, we propose that novelty-dependent changes in CA1 activity signal to downstream regions that something is novel and requires investigation. This hypothesis is consistent with recent work demonstrating that coordinated reactivation during SWRs can be used to predict correct decisions in a hippocampal-dependent alternation task (Singer et al., 2013). Singer and colleagues (2013) showed that while the content of what was reactivated during SWRs was indistinguishable between correct and incorrect decisions, correct decisions were associated with substantial increases in the amount of coordinated reactivation during SWRs.
The diverging responses of CA1 and CA3 suggest that novelty responses are not generated within the tri-synaptic pathway but rather emerge either within CA1 itself or through projections from the entorhinal cortex directly to CA1 or indirectly via CA2. We hypothesize that the generalized novelty signal broadcast by hippocampal output area CA1 may initiate spatially focused re-exploration through bi-directional interactions with the lateral entorhinal cortex (LEC). The LEC is required for re-exploration of both displaced familiar objects and familiar objects located in novel contexts (Cauter et al., 2013; Wilson et al., 2013a, 2013b). A subset of neurons in LEC is responsive to both novel and familiar objects in a context specific manner (Deshmukh and Knierim, 2011; Tsao et al., 2013). Furthermore, a recently described subset of LEC neurons show a memory trace for displaced objects (Tsao et al., 2013). Together with the reciprocally connected anterior cingulate cortex (ACC; Burwell and Amaral, 1998; Jones and Witter, 2007), where neurons display coding for both objects and previous locations of familiar objects (Weible et al., 2009, 2012), LEC responses may provide a signal of what should be explored. Thus, we propose that in the presence of novelty, hippocampal area CA1 signals that there is information to gather by returning to a period of increased excitability, enabling previously stored internal representations to be updated with recently gathered information. In addition, CA1 broadcasts this generalized novelty signal to downstream regions such as the LEC-ACC circuit, which in turn, may identify where animals must focus their renewed exploration.
Acknowledgments
This work was supported by:
Grant sponsor: National Science Foundation; Graduate Research Fellowship to M.C.L.
Grant sponsor: National Institutes of Health; Grant numbers: RO1MH080283, RO1MH090188, and F31093067.
References
- Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8:85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauter TV, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. Distinct Roles of Medial and Lateral Entorhinal Cortex in Spatial Cognition. Cereb Cortex. 2013;23:451–459. doi: 10.1093/cercor/bhs033. [DOI] [PubMed] [Google Scholar]
- Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57:303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, O'Neill J, Allen K, Senior T. Place-selective firing contributes to the reverse-order reactivation of CA1 pyramidal cells during sharp waves in open-field exploration. Eur J Neurosci. 2007;26:704–716. doi: 10.1111/j.1460-9568.2007.05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus. 2013;23:253–267. doi: 10.1002/hipo.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15:222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis BM, Kim J, Barakat ME, Fraenkl S, Yücel YH, Peng S, Michalski B, Fahnestock M, McLaurin J, Mount HTJ. Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging. 2012;33:555–563. doi: 10.1016/j.neurobiolaging.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24:7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein H, Hullinger R, Lindstrom MJ, Burger C. A behavioral paradigm to evaluate hippocampal performance in aged rodents for pharmacological and genetic target validation. PloS One. 2013;8:e62360. doi: 10.1371/journal.pone.0062360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Witter MP. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus. 2007;17:957–976. doi: 10.1002/hipo.20330. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemere C, Carr MF, Karlsson MP, Frank LM. Rapid and Continuous Modulation of Hippocampal Network State during Exploration of New Places. PloS One. 2013;8:e73114. doi: 10.1371/journal.pone.0073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, Mobley WC. Deficits in Cognition and Synaptic Plasticity in a Mouse Model of Down Syndrome Ameliorated by GABAB Receptor Antagonists. J Neurosci. 2012;32:9217–9227. doi: 10.1523/JNEUROSCI.1673-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini P-P, Rivard B, Muller RU, Poucet B. Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus. 2005;15:356–369. doi: 10.1002/hipo.20060. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem Cold Spring Harb N. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nitz D, McNaughton B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol. 2004;91:863–872. doi: 10.1152/jn.00614.2003. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat Neurosci. 2008;11:209–215. doi: 10.1038/nn2037. [DOI] [PubMed] [Google Scholar]
- Oliveira AMM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PloS One. 2009;4:e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ. Neglected aspects of exploratory and investigatory behavior. Psychobiology. 1990;18:16–22. [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30:11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Hayman RMA, Jovalekic A, Marozzi E, Jeffery KJ. Place Field Repetition and Purely Local Remapping in a Multicompartment Environment. Cereb Cortex New York N. 2013;1991 doi: 10.1093/cercor/bht198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser M-B, Moser EI. Traces of experience in the lateral entorhinal cortex. Curr Biol CB. 2013;23:399–405. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15:899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. Neural correlates of long-term object memory in the mouse anterior cingulate cortex. J Neurosci. 2012;32:5598–5608. doi: 10.1523/JNEUROSCI.5265-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Pang R, Kentros C. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J Neurophysiol. 2009;102:2055–2068. doi: 10.1152/jn.00214.2009. [DOI] [PubMed] [Google Scholar]
- Wilson DIG, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus. 2013a;23:352–366. doi: 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DIG, Watanabe S, Milner H, Ainge JA. Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus. 2013b;23:1280–1290. doi: 10.1002/hipo.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wimmer M, Hernandez P, Blackwell J, Abel T. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging. 2012;33:2220–2224. doi: 10.1016/j.neurobiolaging.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltész I, Bragin A, Penttonen M, Sik A, Buzsáki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Zhang R, Xue G, Wang S, Zhang L, Shi C, Xie X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse model. J Alzheimers Dis JAD. 2012;31:801–812. doi: 10.3233/JAD-2012-120151. [DOI] [PubMed] [Google Scholar]